Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Estrogen-containing or progestin-only hormonal therapy is not associated with increased recurrent VTE risk in women on anticoagulant therapy.
Abnormal uterine bleeding occurred more frequently with rivaroxaban than with enoxaparin/VKAs.
Abstract
Women receiving vitamin K antagonists (VKAs) require adequate contraception because of the potential for fetal complications. It is unknown whether the use of hormonal therapy, especially those containing estrogens, is associated with recurrent venous thromboembolism (VTE) during anticoagulation. Despite the absence of data, World Health Organization guidelines state that use of estrogen-containing contraceptives confers an “unacceptable health risk” during established anticoagulation for VTE. We compared the incidences of recurrent VTE and abnormal uterine bleeding with and without concomitant hormonal therapy in women aged <60 years who were receiving anticoagulation with rivaroxaban or enoxaparin/VKA for confirmed VTE. Incidence densities in percentage per year were computed for the on and off estrogen-containing or progestin-only therapy periods. Cox regression models were fitted, with hormonal therapy (on vs off) as a time-dependent variable to derive the hazard ratio (HR) for the effects on recurrent VTE and abnormal uterine bleeding. In total, 1888 women were included. VTE incidence densities on and off hormonal therapy were 3.7%/year and 4.7%/year (adjusted HR, 0.56; 95% confidence interval [CI], 0.23-1.39), respectively, and were 3.7%/year and 3.8%/year, respectively, for estrogen-containing and progestin-only therapy. The adjusted HR for all abnormal uterine bleeding (on vs off hormonal therapy) was 1.02 (95% CI, 0.66-1.57). Abnormal uterine bleeding occurred more frequently with rivaroxaban than with enoxaparin/VKA (HR, 2.13; 95% CI, 1.57-2.89). Hormonal therapy was not associated with an increased risk of recurrent VTE in women receiving therapeutic anticoagulation. The observed increased risk of abnormal uterine bleeding with rivaroxaban needs further exploration.
Introduction
Patients with acute deep vein thrombosis (DVT) or pulmonary embolism (PE) require anticoagulation to prevent recurrent venous thromboembolism (VTE).1 In women of childbearing potential who receive anticoagulation with vitamin K antagonists (VKAs), adequate contraception is required because these drugs cross the placenta, potentially leading to bleeding in the fetus and/or severe embryopathy.2-4 Likewise, adequate contraception is indicated for women receiving direct oral anticoagulants owing to the ability of these small molecules to cross the placenta, potentially causing fetal bleeding and as yet unknown adverse effects on fetal development.5
There is reluctance among physicians to prescribe estrogen-containing contraceptives or postmenopausal hormone replacement to women who use anticoagulants for VTE because of the documented increased risk of VTE with these hormonal agents.6-15 Product labels of combined oral contraceptives, as a class, generally state that their use is contraindicated in patients with an active or prior VTE event, although no reference is made to the concomitant use with anticoagulation. To the best of our knowledge, there is an absence of data in the medical literature on the risk of recurrent VTE during anticoagulant treatment in women using hormonal therapies. Nevertheless, the World Health Organization (WHO) 2010 guidelines state that use of estrogen-containing contraceptives confers an “unacceptable health risk” during established anticoagulant treatment of VTE.16 By contrast, the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis recommends that women diagnosed with a hormone-associated VTE continue oral contraceptive and estrogen-replacement hormonal therapy until they discontinue anticoagulant therapy,17 because any prothrombotic effect of hormonal therapy is likely to be suppressed by therapeutic-intensity anticoagulation, whereas the risk of menorrhagia associated with stopping hormonal therapy could be intensified by anticoagulants.
Indeed, assessment of uterine bleeding in women using anticoagulants is important because cessation of hormonal therapy may contribute to increased uterine blood loss, whereas its use could be an option to prevent or treat uterine blood loss.18 A recent meta-analysis suggested that use of rivaroxaban, apixaban, and ximelagatran for acute or extended VTE treatment was associated with an increased rate of bleeding in women.19
Therefore, we used the large EINSTEIN DVT and PE study cohort to compare the incidences of recurrent VTE and abnormal uterine bleeding in women with and without concomitant hormonal therapy receiving anticoagulant treatment with either rivaroxaban or enoxaparin/VKAs for confirmed symptomatic DVT and/or PE.20,21
Patients and methods
Between 2007 and 2011, the EINSTEIN DVT and PE trials evaluated the efficacy and safety of 3-, 6-, or 12-month courses of oral rivaroxaban vs subcutaneous enoxaparin overlapping with and followed by VKA therapy in patients with acute symptomatic DVT, PE, or both. Patients were followed for the intended treatment period and assessed at fixed intervals that were identical in the 2 treatment groups. At each visit, patients were systematically questioned for symptoms or signs of recurrent VTE and bleeding, including uterine bleeding.
In cases of suspected recurrent DVT/PE, a workup using objective diagnostic tests was required. Symptomatic recurrent VTE was defined as a composite of fatal or nonfatal DVT or PE.20,21 All suspected recurrent VTE events were reviewed by the central independent adjudication committee. All investigator-reported abnormal uterine bleeding and uterine bleeding leading to ≥1 unit of blood transfusion were collected. Details on severity and treatment of abnormal uterine bleeding were obtained both from bleeding event forms and adverse event documentation.
In EINSTEIN DVT and PE, women of childbearing potential were instructed to use adequate methods of contraception. The protocol did not include guidelines for choice of the contraceptive method or the use of hormonal replacement therapy. Information on concomitant medications taken during the study was collected at each visit on an electronic case record form, and 100% source verification was carried out.
Hormonal therapy included estrogen-only pills, combined estrogen-progestogen contraceptives, (including pills, patches, vaginal rings, and injectables), and progestin-only contraceptives (including pills, implants, injectables, and intrauterine devices), which were coded according to the WHO Drug Dictionary Version 2005/Q3 and defined via Anatomical-Therapeutic-Chemical codes.
The incidences of recurrent VTE, all abnormal uterine bleeding, and uterine bleeding leading to transfusion were calculated during the periods on and off hormonal therapy (at-risk periods). The at-risk period for each outcome was defined as the period between randomization and last intake of rivaroxaban or enoxaparin/VKA plus 2 days, or, in case of occurrence of an outcome during this period, the onset date of the first: confirmed recurrent DVT/PE; any abnormal uterine bleeding; or uterine bleeding leading to transfusion; respectively. Person-time was accumulated per patient from randomization until the end of the at-risk period. Patients who were on and off hormonal therapy during the at-risk periods contributed person-time to both (on and off) categories of exposure. For the VTE analysis, events during a period of 1 week after the hormonal therapy stop date were included. For the abnormal uterine bleeding analyses, no lag period was considered because abnormal uterine bleeding is often treated with hormonal therapy.
All analyses took into account only women <60 years of age (2 patients with gender reassignment were excluded) and were performed for all hormonal therapies and for estrogen-containing therapy (ie, estrogen-only or combined) and progestin-only therapy separately. Hysterectomized women were removed from the analyses of uterine bleeding.
Incidence densities in percentage per year and corresponding 95% confidence intervals (CIs) were computed for the on and off hormonal therapy periods. Then, Cox regression models were fitted, with hormonal therapy (on vs off) as a time-dependent variable, to derive the hazard ratio (HR) and corresponding 95% CI for the effects of hormonal therapies on the rates of recurrent VTE; any abnormal uterine bleeding; and uterine bleeding leading to transfusion. All Cox models were stratified for age (20-year groups) and adjusted for prior use of hormonal therapy and assigned anticoagulant treatment. For recurrent VTE, an additional adjustment was made for any cancer at baseline, whereas for abnormal uterine bleeding, additional adjustments were made for baseline presentation with anemia and gynecologic disorders (ie, uterine fibroids, adenomyosis, gynecologic cancer, and/or history of abnormal uterine bleeding). P values for interaction between anticoagulant treatment and hormonal therapy were calculated.
The EINSTEIN DVT (#NCT00440193) and PE (#NCT00439777) studies were conducted in a manner consistent with the principles of the Declaration of Helsinki. Institutional review board approval was obtained in all participating centers, and all patients provided written informed consent. The data were collected and maintained by the sponsor. All suspected outcome events were classified by a central adjudication committee whose members were unaware of the treatment assignments. Drs Martinelli, Lensing, Gebel, and Prins had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Results
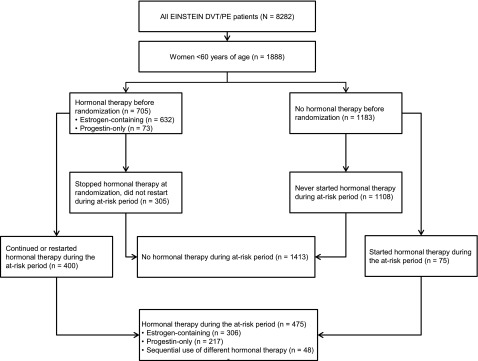
In total, 1888 women aged <60 years included in the EINSTEIN DVT and PE program qualified for these analyses (Figure 1). Of these women (mean age, 41.3 years), 925 received rivaroxaban and 963 received enoxaparin/VKA. Of the 705 women (37.3%) who were receiving hormonal therapy when their DVT and/or PE was diagnosed, 303 had stopped all hormonal therapy at the time of randomization. Thus, 402 women used hormonal therapy at some time during the analysis period. Of the 1183 women who were not using any hormonal therapy when their DVT and/or PE was diagnosed, 73 (6.2%) started hormonal therapy during the analysis period. Baseline characteristics in relation to hormonal therapy use after randomization are shown in Table 1. Women who used hormonal therapy were younger and less frequently had active cancer or a history of VTE. Accumulated patient-years at risk with and without hormonal therapy for recurrent VTE, all abnormal uterine bleeding, and uterine bleeding leading to transfusion are shown in Tables 2–4, respectively.
Figure 1.
CONSORT diagram summarizing women aged <60 years studied in the EINSTEIN DVT and PE program.
Table 1.
Demographic and clinical characteristics of study patients (women aged <60 years) at study entry
| Characteristic | No hormonal use during period at risk (n = 1413) | Any hormonal use during period at risk (n = 475) | Estrogen-containing therapy (n = 306)* | Progestin-only therapy (n = 217)* |
|---|---|---|---|---|
| Age, years, mean (SD) | 42.9 (11.3) | 36.6 (10.4) | 37.3 (10.3) | 35.4 (10.1) |
| Age, n (%) | ||||
| <40 years | 494 (35.0) | 270 (56.8) | 167 (54.6) | 135 (62.2) |
| ≥40 years | 919 (65.0) | 205 (43.2) | 139 (45.4) | 82 (37.8) |
| Creatinine clearance, ml/min, mean (SD) | 119.2 (41.3) | 127.6 (40.4) | 127.2 (39.9) | 130.4 (40.8) |
| BMI, mean (SD) | 28.4 (7.3) | 27.3 (7.0) | 27.2 (6.4) | 27.8 (7.8) |
| Index event, n (%) | ||||
| DVT | 644 (45.6) | 193 (40.6) | 139 (45.4) | 67 (30.9) |
| PE ± DVT | 769 (54.4) | 282 (59.4) | 167 (54.6) | 150 (69.1) |
| Planned treatment duration, n (%) | ||||
| 3 months | 146 (10.3) | 54 (11.4) | 37 (12.1) | 20 (9.2) |
| 6 months | 880 (62.3) | 330 (69.5) | 215 (70.3) | 149 (68.7) |
| 12 months | 387 (27.4) | 91 (19.2) | 54 (17.6) | 48 (22.1) |
| Active cancer (%) | 61 (4.3) | 6 (1.3) | 6 (2.0) | 0 |
| History of DVT/PE (%) | 230 (16.3) | 41 (8.6) | 14 (4.6) | 29 (13.4) |
| Use during at-risk period (%) | ||||
| Antiplatelet therapy | 110 (7.8) | 15 (3.2) | 7 (2.3) | 10 (4.6) |
| NSAIDs | 301 (21.3) | 94 (19.8) | 54 (17.6) | 53 (24.4) |
| Antifibrinolytic therapy | 11 (0.8) | 2 (0.4) | 1 (0.3) | 1 (0.5) |
| Anemia (%) | 406 (28.7) | 98 (20.6) | 60 (19.6) | 42 (19.4) |
| Gynecologic disorders (%)† | 66 (4.7) | 17 (3.6) | 13 (4.2) | 5 (2.3) |
BMI, body mass index; NSAID, nonsteroidal anti-inflammatory drug; SD, standard deviation.
Some patients received estrogen-containing and progestin-only therapies consecutively.
Defined as presence at baseline of uterine fibroids/adenomyosis, gynecologic cancer, and/or abnormal uterine bleeding.
Table 2.
Recurrent VTE during the at-risk period in women with and without concomitant hormonal therapy
| Characteristic | No hormone use | All hormonal therapies | Estrogen-containing therapy | Progestin-only therapy | ||||
|---|---|---|---|---|---|---|---|---|
| Events/patient-years | %/year (95% CI) | Events/patient-years | %/year (95% CI) | Events/patient-years | %/year (95% CI) | Events/patient-years | %/year (95% CI) | |
| All patients | 38/811.0 | 4.7 (3.3-6.4) | 7/187.5 | 3.7 (1.5-7.7) | 4/109.5 | 3.7 (1.0-9.4) | 3/78.0 | 3.8 (0.8-11.2) |
| Age | ||||||||
| <40 years | 19/287.7 | 6.6 (4.0-10.3) | 2/107.4 | 1.9 (0.2-6.7) | 1/57.1 | 1.8 (0.0-9.8) | 1/50.3 | 2.0 (0.1-11.1) |
| ≥40 years | 19/523.4 | 3.6 (2.2-5.7) | 5/80.0 | 6.3 (2.0-14.6) | 3/52.3 | 5.7 (1.2-16.8) | 2/27.7 | 7.2 (0.9-26.1) |
| Time period after randomization | ||||||||
| Days 1-30 | 27/121.0 | 22.3 (14.7-32.5) | 5/28.3 | 17.7 (5.7-41.2) | 4/21.1 | 19.0 (5.2-48.5) | 1/7.2 | 13.9 (0.4-77.4) |
| Days 31-90 | 7/229.9 | 3.1 (1.2-6.3) | 1/56.5 | 1.8 (0.0-9.9) | 0/34.6 | 0.0 (0.0-10.7) | 1/21.9 | 4.6 (0.1-25.4) |
| Days 91-180 | 3/300.1 | 1.0 (0.2-2.9) | 1/73.8 | 1.4 (0.0-7.6) | 0/39.1 | 0.0 (0.0-9.4) | 1/34.7 | 2.9 (0.1-16.1) |
| Days 181-end | 1/160.0 | 0.6 (0.0-3.5) | 0/28.9 | 0.0 (0.0-12.8) | 0/14.7 | 0.0 (0.0-25.1) | 0/14.2 | 0.0 (0.0-26.0) |
| History of DVT/PE before index event | ||||||||
| Yes | 4/162.3 | 2.5 (0.7-6.3) | 0/18.7 | 0.0 (0.0-19.7) | 0/5.8 | 0.0 (0.0-63.1) | 0/12.9 | 0.0 (0.0-28.6) |
| No | 34/648.7 | 5.2 (3.6-7.3) | 7/168.7 | 4.2 (1.7-8.6) | 4/103.6 | 3.9 (1.1-9.9) | 3/65.1 | 4.6 (1.0-13.5) |
| Presentation of index event | ||||||||
| DVT | 21/342.4 | 6.1 (3.8-9.4) | 4/69.6 | 5.7 (1.6-14.7) | 3/48.5 | 6.2 (1.3-18.1) | 1/21.2 | 4.7 (0.1-26.3) |
| PE ± DVT | 17/468.6.4 | 3.6 (2.1-5.8) | 3/117.8 | 2.6 (0.5-7.4) | 1/61.0 | 1.6 (0.0-9.1) | 2/56.8 | 3.5 (0.4-12.7) |
| Prior use of hormonal therapy | ||||||||
| Yes | 13/200.0 | 6.5 (3.5-11.1) | 7/162.0 | 4.3 (1.7-8.9) | 4/103.1 | 3.9 (1.1-9.9) | 3/58.8 | 5.1 (1.1-14.9) |
| No | 25/611.0 | 4.1 (2.7-6.0) | 0/25.5 | 0.0 (0.0-14.5) | 0/6.3 | 0.0 (0.0,58.2) | 0/19.2 | 0.0 (0.0-19.3) |
| Randomized treatment | ||||||||
| Rivaroxaban | 21/390.2 | 5.4 (3.3-8.2) | 3/98.1 | 3.1 (0.6-8.9) | 1/56.2 | 1.8 (0.1-9.9) | 2/41.9 | 4.8 (0.6-17.2) |
| Enoxaparin/VKA | 17/420.8 | 4.0 (2.4-6.5) | 4/89.4 | 4.5 (1.2-11.5) | 3/53.3 | 5.6 (1.2-16.5) | 1/36.1 | 2.8 (0.1-15.4) |
Table 4.
Uterine bleeding leading to transfusion during the at-risk period in women with and without concomitant hormonal therapy
| Characteristic | No use | All hormonal therapies | Estrogen-containing therapy | Progestin-only therapy | ||||
|---|---|---|---|---|---|---|---|---|
| Events/patient-years | %/year (95% CI) | Events/patient years | %/year (95% CI) | Events/patient-years | %/year (95% CI) | Events/patient-years | %/year (95% CI) | |
| All patients | 19/749.1 | 2.5 (1.5-4.0) | 3/182.0 | 1.6 (0.3-4.8) | 2/104.7 | 1.9 (0.2-6.9) | 1/77.2 | 1.3 (0.0-7.2) |
| Age | ||||||||
| <40 years | 7/285.1 | 2.5 (1.0-5.1) | 0/105.7 | 0.0 (0.0-3.5) | 0/55.3 | 0.0 (0.0-6.7) | 0/50.4 | 0.0 (0.0-7.3) |
| ≥40 years | 12/464.0 | 2.6 (1.3-4.5) | 3/76.2 | 3.9 (0.8-11.5) | 2/49.4 | 4.0 (0.5-14.6) | 1/26.8 | 3.7 (0.1-20.8) |
| Time period after randomization | ||||||||
| Days 1-30 | 10/112.0 | 8.9 (4.3,16.4) | 3/26.4 | 11.4 (2.4,33.2) | 2/19.5 | 10.3 (1.2-37.1) | 1/6.9 | 14.5 (0.4-80.9) |
| Days 31-90 | 2/210.8 | 0.9 (0.1-3.4) | 0/54.8 | 0.0 (0.0-6.7) | 0/33.1 | 0.0 (0.0-11.1) | 0/21.6 | 0.0 (0.0-17.1) |
| Days 91-180 | 6/275.4 | 2.2 (0.8-4.7) | 0/71.3 | 0.0 (0.0-5.2) | 0/37.3 | 0.0 (0.0-9.9) | 0/34.0 | 0.0 (0.0-10.9) |
| Days 181-end | 1/150.9 | 0.7 (0.0-3.7) | 0/29.6 | 0.0 (0.0-12.5) | 0/14.8 | 0.0 (0.0-24.9) | 0/14.7 | 0.0 (0.0-25.1) |
| History of DVT/PE before index event | ||||||||
| Yes | 2/145.9 | 1.4 (0.2-5.0) | 0/18.0 | 0.0 (0.0-20.5) | 0/5.7 | 0.0 (0.0-64.5) | 0/12.3 | 0.0 (0.0-30.1) |
| No | 17/603.1 | 2.8 (1.6-4.5) | 3/164.0 | 1.8 (0.4-5.4) | 2/99.0 | 2.0 (0.2-7.3) | 1/65.0 | 1.5 (0.0-8.6) |
| Index event | ||||||||
| DVT | 10/320.0 | 3.1 (1.5-5.8) | 2/68.8 | 2.9 (0.4-10.5) | 2/47.8 | 4.2 (0.5-15.1) | 0/21.1 | 0.0 (0.0-17.5) |
| PE ± DVT | 9/429.1 | 2.1 (0.9-4.0) | 1/113.1 | 0.9 (0.0-4.9) | 0/57.0 | 0.0 (0.0-6.5) | 1/56.2 | 1.8 (0.1-9.9) |
| Prior use of hormonal therapy | ||||||||
| Yes | 4/200.1 | 2.0 (0.5-5.1) | 3/158.4 | 1.9 (0.4-5.5) | 2/99.1 | 2.0 (0.2-7.3) | 1/59.3 | 1.7 (0.0-9.4) |
| No | 15/549.0 | 2.7 (1.5-4.5) | 0/23.6 | 0.0 (0.0-15.6) | 0/5.7 | 0.0 (0.0-65.1) | 0/17.9 | 0.0 (0.0-20.6) |
| Randomized treatment | ||||||||
| Rivaroxaban | 17/356.0 | 4.8 (2.8-7.7) | 2/93.5 | 2.1 (0.3-7.7) | 1/52.6 | 1.9 (0.1-10.6) | 1/40.9 | 2.4 (0.1-13.6) |
| Enoxaparin/VKA | 2/393.1 | 0.5 (0.1-1.8) | 1/88.5 | 1.1 (0.0-6.3) | 1/52.2 | 1.9 (0.1-10.7) | 0/36.3 | 0.0 (0.0-10.2) |
| Anemia* | ||||||||
| Yes | 18/186.2 | 9.7 (5.7-15.3) | 2/35.8 | 5.6 (0.7-20.2) | 2/22.0 | 9.1 (1.1-32.8) | 0/13.7 | 0.0 (0.0-26.9) |
| No | 1/560.5 | 0.2 (0.0-1.0) | 1/145.7 | 0.7 (0.0-3.8) | 0/82.2 | 0.0 (0.0-4.5) | 1/63.5 | 1.6 (0.0-8.8) |
| Gynecologic disorders*,† | ||||||||
| Yes | 7/35.7 | 19.6 (7.9-40.4) | 3/4.3 | 69.0 (14.2-201.3) | 2/3.7 | 53.8 (6.5-193.9) | 1/0.6 | 158.8 (4.0-876.1) |
| No | 12/713.4 | 1.7 (0.9-2.9) | 0/177.6 | 0.0 (0.0-2.1) | 0/101.0 | 0.0 (0.0-3.7) | 0/76.6 | 0.0 (0.0-4.8) |
As reported at baseline.
Defined as presence at baseline of uterine fibroids/adenomyosis, abnormal uterine bleeding events, and/or gynecologic cancer.
Recurrent VTE
A total of 7 recurrent VTE events occurred during the use of hormonal therapy, whereas 38 events occurred during the period without use, giving crude incidence densities of 3.7%/year on hormonal therapy and 4.7%/year off such therapy, for an adjusted HR of 0.56 (95% CI, 0.23-1.39). Sensitivity analyses using lag periods after stopping hormonal therapy of 14, 21, and 28 days did not create any important changes in the results. The crude incidence densities for estrogen-containing and progestin-only therapy were 3.7%/year and 3.8%/year, respectively. Recurrent VTE occurred at approximately the same rate for rivaroxaban- and enoxaparin/VKA-treated patients (Pinteraction = .40). Incidence densities for important subgroups are given in Table 2. Considering ethinylestradiol-containing therapy only, 4 recurrent VTE events occurred during 100.3 patient-years (4.0%/year; 95% CI, 1.1-10.2).
Abnormal uterine bleeding
Excluding women who had undergone a hysterectomy, a total of 1737 women were evaluated, of whom 463 used hormonal therapy. A total of 37 (28 receiving estrogen and 9 progestin-only) events of abnormal uterine bleeding occurred during hormonal therapy, whereas 148 events occurred during the period without use, giving crude incidence densities of 22.5%/year on hormonal therapy and 21.4%/year off such therapy (Table 3), for an adjusted HR of 1.02 (95% CI, 0.66-1.57). The crude incidence densities for estrogen-containing and progestin-only therapy were 28.9%/year and 13.3%/year, respectively. Abnormal uterine bleeding occurred more often in rivaroxaban-treated women compared with enoxaparin/VKA-treated women (HR, 2.13; 95% CI, 1.57-2.89). The incidence density of abnormal uterine bleeding during hormonal therapy was 29.8%/year for rivaroxaban recipients and 15.5%/year for enoxaparin/VKA recipients, whereas these values were 30.7%/year and 13.4%/year, respectively, without hormonal therapy (Pinteraction = .68). Incidence densities for important subgroups are given in Table 3. Abnormal uterine bleeding resulted in interruption or permanent discontinuation of study anticoagulation in 3 (8.1%) of the 37 events that occurred during hormonal therapy and 19 (12.8%) of the 148 events that occurred without hormonal therapy.
Table 3.
All abnormal uterine bleeding during the at-risk period in women with and without concomitant hormonal therapy
| Characteristic | No hormone use | All hormonal therapies | Estrogen-containing therapy | Progestin-only therapy | ||||
|---|---|---|---|---|---|---|---|---|
| Events/patient-years | %/year (95% CI) | Events/patient-years | %/year (95% CI) | Events/patient-years | %/year (95% CI) | Events/patient-years | %/ year (95% CI) | |
| All patients | 148/693.0 | 21.4 (18.1-25.1) | 37/164.8 | 22.5 (15.8-31.0) | 28/96.8 | 28.9 (19.2-41.8) | 9/67.9 | 13.3 (6.1-25.1) |
| Age | ||||||||
| <40 years | 52/266.2 | 19.5 (14.6-25.6) | 19/97.2 | 19.5 (11.8-30.5) | 14/52.3 | 26.8 (14.6-44.9) | 5/44.8 | 11.2 (3.6-26.0) |
| ≥40 years | 96/426.8 | 22.5 (18.2-26.6) | 18/67.6 | 26.6 (15.8-42.1) | 14/44.5 | 31.5 (17.2-52.8) | 4/23.1 | 17.3 (4.7-44.3) |
| Time period after randomization | ||||||||
| Days 1-30 | 89/108.2 | 82.3 (66.1-101.2) | 23/25.1 | 91.5 (58.0-137.2) | 19/18.6 | 102.0 (61.4-159.2) | 4/6.5 | 61.5 (16.8-157.3) |
| Days 31-90 | 31/196.3 | 15.8 (10.7-22.4) | 10/50.2 | 19.9 (9.6-36.7) | 7/30.6 | 22.9 (9.2-47.2) | 3/19.6 | 15.3 (3.2-44.7) |
| Days 91-180 | 24/251.0 | 9.6 (6.1-14.2) | 3/64.5 | 4.7 (1.0-13.6) | 1/34.4 | 2.9 (0.1-16.2) | 2/30.1 | 6.6 (0.8-24.0) |
| Days 181-end | 4/137.5 | 2.9 (0.8-7.5) | 1/24.9 | 4.0 (0.1-22.3) | 1/13.2 | 7.6 (0.2-42.0) | 0/11.7 | 0.0 (0.0-31.5) |
| History of DVT/PE before index event | ||||||||
| Yes | 25/135.2 | 18.5 (12.0-27.3) | 1/16.2 | 6.2 (0.2-34.4) | 1/4.9 | 20.4 (0.5-113.4) | 0/11.3 | 0.0 (0.0-32.7) |
| No | 123/557.7 | 22.1 (18.3-26.3) | 36/148.6 | 24.2 (17.0-33.5) | 27/91.9 | 29.4 (19.4-42.7) | 9/56.7 | 15.9 (7.3-30.1) |
| Presentation of index event | ||||||||
| DVT | 54/299.2 | 18.0 (13.6-21.8) | 14/64.3 | 21.8 (11.9-36.5) | 13/45.6 | 28.5 (15.2-48.7) | 1/18.7 | 5.3 (0.1-29.8) |
| PE ± DVT | 94/393.7 | 23.9 (19.3-29.2) | 23/100.5 | 22.9 (14.5-34.3) | 15/51.2 | 29.3 (16.45.8-48.3) | 8/49.3 | 16.2 (7.0-32.0) |
| Prior use of hormonal therapy | ||||||||
| Yes | 41/186.7 | 22.0 (15.8-29.8) | 34/145.2 | 23.4 (16.2-32.7) | 27/91.9 | 29.4 (19.4-42.7) | 7/53.3 | 13.1 (5.3-27.1) |
| No | 107/506.3 | 21.1 (17.3-25.5) | 3/19.6 | 15.3 (3.2-44.7) | 1/4.9 | 20.3 (0.5-112.9) | 2/14.7 | 13.6 (1.7-49.2) |
| Randomized treatment | ||||||||
| Rivaroxaban | 98/319.1 | 30.7 (24.9-37.4) | 24/80.6 | 29.8 (19.1-44.3) | 19/46.9 | 40.5 (24.4-63.3) | 5/33.8 | 14.8 (4.8-34.5) |
| Enoxaparin/VKA | 50/373.8 | 13.4 (9.9-17.6) | 13/84.1 | 15.5 (8.2-26.4) | 9/50.0 | 18.0 (8.2-34.2) | 4/34.2 | 11.7 (3.2-30.0) |
| Anemia* | ||||||||
| Yes | 45/172.5 | 26.1 (19.0-34.9) | 8/32.7 | 24.5 (10.6-48.2) | 6/20.4 | 29.4 (10.8-63.9) | 2/12.2 | 16.3 (2.0-59.0) |
| No | 103/518.2 | 19.9 (16.2-24.1) | 29/131.6 | 22.0 (14.8-31.7) | 22/75.9 | 29.0 (18.2-43.9) | 7/55.7 | 12.6 (5.1-25.9) |
| Gynecologic disorders (%)† | ||||||||
| Yes | 10/30.6 | 32.7 (15.7-60.1) | 2/5.4 | 37.0 (4.5-133.4) | 2/4.5 | 44.6 (5.4-160.8) | 0/0.9 | 0.0 (0.0-397.6) |
| No | 138/662.4 | 20.8 (17.5-24.6) | 35/159.4 | 22.0 (15.3-30.5) | 26/92.3 | 28.1 (18.4-41.3) | 9/67.0 | 13.4 (6.1-25.5) |
As reported at baseline.
Defined as presence at baseline of uterine fibroids/adenomyosis, abnormal uterine bleeding, and/or gynecologic cancer.
Uterine bleeding leading to transfusion
A total of 3 uterine bleeding events leading to transfusion occurred during use of hormonal therapy, whereas 19 events occurred during the period without use, for crude incidence densities of 1.6%/year on hormonal therapy and 2.5%/year off such therapy (Table 4; Pinteraction = .28).The HR could not be calculated owing to the low number of events. The crude incidence densities for estrogen-containing and progestin-only therapy were 1.9%/year and 1.3%/year, respectively. Uterine bleeding that led to transfusion occurred more often in rivaroxaban-treated women (n = 19) compared with enoxaparin/VKA-treated women (n = 3). Twenty of the 22 women with uterine bleeding leading to transfusion had anemia (hemoglobin <12 g/L) at baseline, and 9 had uterine fibroids and/or adenomyosis. Incidence densities for important subgroups are shown in Table 4.
Results in subgroups according to hormonal therapy
In women using ethinylestradiol-containing hormonal therapy, 4 recurrent VTE events occurred during 100.3 patient-years (4.0%/year; 95% CI, 1.1-10.2), 28 abnormal uterine bleeding events occurred during 89.9 patient-years (31.3%/year; 95% CI, 20.7-45.0), and 2 abnormal uterine bleeding events leading to transfusion occurred during 97.8 patient-years (2.1%/year; 95% CI, 0.3-7.4). In women using a levonorgestrel-releasing intrauterine system, no recurrent venous thromboembolic events occurred during 15.3 patient-years (0%/year; 95% CI, 0.0-24.0), 2 abnormal uterine bleeding events occurred during 14.0 patient-years (14.3%/year; 95% CI, 1.7-51.5), and no abnormal uterine bleeding events leading to transfusion occurred during 15.4 patient-years (0%/year; 95% CI, 0.0-24.1). In the 4 women using transdermal estradiol-containing therapy, no recurrent venous thromboembolism, abnormal uterine bleeding, or abnormal uterine bleeding events leading to transfusion occurred.
Discussion
This analysis of women treated with anticoagulants for acute VTE showed a similar rate of recurrent VTE in those who did and did not receive hormonal therapy. Moreover, the upper limit of the 95% CI around the observed point estimate of the HR excludes a clinically important difference in recurrent VTE. Consistent results were observed for estrogen-containing and progestin-only therapies. These results challenge the WHO guidelines16 and instead support the International Society on Thrombosis and Haemostasis recommendations.17
Remarkably, rates of all abnormal uterine bleeding and uterine bleeding leading to blood transfusion were not different between users and nonusers of hormonal therapy. However, this comparison has a high risk of confounding by indication because hormonal therapy, including the levonorgestrel intrauterine system, is often prescribed to prevent or treat menorrhagia. Almost all women who had uterine bleeding leading to transfusion had anemia at baseline. The incidence densities of all abnormal uterine bleeding and those leading to transfusion were more frequent among rivaroxaban recipients compared with women treated with enoxaparin/VKA.
The observed increased risk of abnormal uterine bleeding with rivaroxaban compared with enoxaparin/VKA treatment is compatible with the earlier observation of increased rates of bleeding in women receiving treatment with rivaroxaban, apixaban, or ximelagatran.19,22 The randomized assignment to rivaroxaban and enoxaparin/VKA and the large observed effect size, especially for uterine bleeding leading to transfusion, makes it likely that the observation is valid, despite the potential for bias due to the open-label design. A detailed analysis of recent trials on the other anti-Factor Xa inhibitors (apixaban, edoxaban) and direct thrombin inhibitor (dabigatran) is warranted. Indeed, in the apixaban studies in which women with anemia at baseline were excluded, menorrhagia was numerically more frequent in the apixaban recipients compared with standard therapy.23
Because uterine bleeding leading to transfusion was mostly confined to women who had anemia at the time of starting anticoagulation, improved management of this anemia (eg, iron supplements) may have the potential to prevent the need for transfusion. For clinical practice, the enhanced risk of abnormal uterine bleeding with the use of rivaroxaban should be considered against the greater convenience of its use and the lower risk of acute major bleeding compared with enoxaparin/VKA therapy.24-26 However, the large observed effect size warrants counseling of women aged <60 years at the beginning of anticoagulation, addressing both the risk for abnormal uterine bleeding and its management options. If menorrhagia occurs and anticoagulant treatment is deemed necessary, a trial of hormonal contraceptives appears to be a sensible option in premenopausal women.27-29 Additionally, antifibrinolytic agents could be considered, because in general these agents have not been shown to increase the risk of thrombosis.30,31
Some limitations of our study should be noted. First, the EINSTEIN DVT and PE studies used an open-label design that could have biased assessment of outcomes. Nevertheless, efforts were made to limit investigator bias, including the requirement to use objective and validated tests to confirm suspected recurrent VTE and the use of an independent adjudication committee that was blinded to treatment assignment and use of concomitant medication. Second, our analyses were not prespecified in the protocol or statistical analysis plan. However, data on concomitant medication were collected and monitored prospectively, and the protocol required only adequate contraceptive measures without further specification. Therefore, it cannot be excluded that women who were perceived to be at low risk of recurrent VTE were more likely to receive hormonal therapy. However, it is unlikely that such selection of patients played an important role, because the incidence of recurrent VTE was low and is hard to predict during anticoagulation. Third, the screening and definition for uterine bleeding did not include qualitative or quantitative measures and therefore was potentially subject to reporting bias. However, following the National Institute for Health and Care Excellence guidance recommendations on heavy menstrual bleeding,32 we believe that the subjectivity of the assessment of menstrual bleeding can even be regarded as a strength of our study. Although only the most severe cases of abnormal uterine bleeding may result in hospitalization, transfusion, or even hysterectomy, it is also clear that the much more common bleeding complications of lesser intensity may have a significant impact on the patent’s quality of life. In 2007, the National Institute for Health and Care Excellence guidance paper stated that “heavy menstrual bleeding should be recognized as having a major impact on a woman's quality of life, and any intervention should aim to improve this rather than focusing on menstrual blood loss.” Therefore, the inclusion of patient-reported events that may not have fulfilled a standardized menstrual bleeding scale may have resulted in a much more balanced view on the overall impact of abnormal uterine bleeding in patients receiving oral anticoagulation. Fourth, analyses were not adjusted for the use of antiplatelet therapy or nonsteroidal anti-inflammatory drugs because their use could be influenced by the experienced severity of (normal) menstrual bleeding.33 Fifth, in the rivaroxaban arm, most uterine bleeding occurred during the first month of treatment when the dose of rivaroxaban was highest (30 vs 20 mg daily). However, a similar pattern over time for bleeding in the comparator arm and for recurrent VTE in both treatment arms was observed, congruent with observation with virtually all studies on the treatment of acute VTE. Finally, because this was a post hoc analysis, the pre- or postmenopausal status of women and their indication for hormonal therapy were not specifically documented. The reported average age of menopause is ∼50 years, with a range between 40 and 60 years. Because we wanted to document the hormonal therapy-related risk of recurrent VTE in premenopausal women, we chose the cutoff age of 40 years for the subgroup analysis to ensure that virtually all women included in that analysis would be premenopausal. An increase of this age limit would inherently lead to the inclusion of an incremental number of postmenopausal women, potentially confounding the analysis. Moreover, it is important to realize that during anticoagulant treatment of VTE, age has never been identified as a risk factor for recurrent VTE. Hence, our analyses in women <40 years of age can be regarded as the best estimate currently available on the risk of recurrent VTE during anticoagulation and hormonal therapy.
During the conduct of our study, the WHO guidelines on the use of hormonal therapy during anticoagulation were issued, which might have impacted physicians’ choices.16 Although all our calculations of the relative effects of hormonal therapy were adjusted for age, prerandomization use of hormonal therapy, presence of cancer, and, additionally, for anemia at baseline and gynecologic disorders for the analyses of abnormal uterine bleeding, residual confounding cannot be excluded. Furthermore, incidence densities were used, although it is known that the risk of recurrent VTE is highest during the first months. However, this pattern did not differ between women who did or did not use hormonal therapy, and Cox models with time-dependent variables were used to calculate relative effects. A final limitation was the absence of a formal sample size calculation. However, the upper limit of the 95% CI of the observed HR of recurrent VTE in women using hormonal therapy was 1.39, which compared favorably with the a priori specified threshold for noninferiority (1.75) for the primary analysis of the pooled EINSTEIN DVT and PE studies.34
During oral anticoagulant therapy, adequate contraceptive measures in premenopausal women are indicated to prevent pregnancy and therefore avoid serious toxic effects on a fetus.2-4 Among the hormonal birth control regimens, those containing a combination of estrogen and progestogen are by far the most frequently used.35,36 However, the confirmed increased risk of VTE with estrogen-containing contraceptives makes many physicians (and women) reluctant to continue their use when VTE is diagnosed, even when anticoagulant treatment is used. Indeed, the product labels of combined oral contraceptives around the world state that active or prior VTE is a contraindication for their use, although no specific reference is made to the potential for use concomitantly with anticoagulation. Progestin-only therapy has been recommended in women receiving anticoagulants for established VTE,16 based on population-based studies that did not demonstrate an increased risk of first VTE with progestin-only contraceptive pills37,38 and progestin-releasing intrauterine devices.37,39 However, progestin-only oral contraceptive pills are infrequently used because they are less forgiving of nonadherence to the dosing schedule and are therefore associated with a higher contraceptive failure rate40 and because all progestin-only regimens are associated with irregular menstrual bleeding, especially during the first 18 months of use.41,42 Hence, our finding of similar risks of recurrent VTE for women who did or did not receive hormonal therapy, whether progestin-only or estrogen-containing therapy, supports a treatment selection that incorporates patient preference, including the choice of estrogen-containing contraception. It is important that hormonal therapy is stopped and alternative contraception is considered before discontinuation of anticoagulation, albeit that any increased risk of recurrent VTE with hormonal therapy after stopping anticoagulation is not well defined.
In conclusion, there is no indication that the use of estrogen-containing or progestin-only hormonal therapy is associated with an increased risk of recurrent VTE in pre- or postmenopausal women on anticoagulant treatment. The finding that use of rivaroxaban was associated with an increased rate of abnormal uterine bleeding needs further mechanistic exploration. Physicians should pay special attention to women with anemia or those with uterine fibroids or adenomyosis who start anticoagulant therapy.
Acknowledgments
The authors thank the patients and investigators who participated in the EINSTEIN DVT and PE studies. The investigators also thank Hayley Dawson for editorial assistance in the preparation of the manuscript, with funding from Bayer HealthCare Pharmaceuticals.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: I.M., A.W.A.L., M.G., and M.H.P. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; I.M., A.W.A.L., J.B.-W., M.T., P.L., and M.H.P. conceived and designed the study; all authors were involved with acquisition, analysis, or interpretation of data; I.M., A.W.A.L., S.M., M.L., J.B.-W., H.B., T.A.B., A.T.C., P.S.W., and M.H.P. drafted the manuscript; I.M., A.W.A.L., S.M, M.L., H.B., T.A.B., A.T.C., P.S.W., and M.H.P. performed critical revision of the manuscript for important intellectual content; A.W.A.L., M.G., and M.H.P. performed statistical analysis; I.M., A.W.A.L., M.T., and M.H.P. provided administrative, technical, or material support; and I.M., A.W.A.L., and M.H.P. supervised the study.
Conflict-of-interest disclosure: I.M. reports no conflicts of interest; S.M. reports grant support from Sanquin, grant support and fees paid to her institution from GlaxoSmithKline and Bristol-Myers Squibb/Pfizer, and fees paid to her institution from Bayer, Boehringer Ingelheim, and Daiichi Sankyo; M.L. reports no conflicts of interest; J.B.-W. reports grant support and personal fees from Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Pfizer; B.v.B. reports personal fees from Bayer and Daiichi Sankyo; H.B. reports personal fees from Bayer; and A.T.C. reports receiving personal fees from Bayer, Boehringer-Ingelheim, BMS, Daiichi, Johnson and Johnson, Mitsubishi Pharma, Pfizer, Portola, Sanofi, Schering Plough, and XO1. A.T.C. is an advisor to the UK Government Health Select Committee, the all-party working group on thrombosis, the Department of Health, and the National Health Service, on the prevention of VTE. A.T.C. is also an advisor to Lifeblood: the thrombosis charity and is the founder of the European educational charity the Coalition to Prevent Venous Thromboembolism. P.S.W. has received grant support from BMS/Pfizer and honoraria from Bayer HealthCare Pharmaceuticals. A.W.A.L, M.T., M.G., and P.L. are employees of Bayer HealthCare Pharmaceuticals; and M.H.P. reports personal fees from Bayer, Pfizer, Daiichi Sankyo, and Boehringer Ingelheim.
Correspondence: Ida Martinelli, A Bianchi Bonomi Hemophilia and Thrombosis Center, Fondazione IRCCS Ca’ Granda-Ospedale Maggiore Policlinico, Via Pace 9, 20122 Milan, Italy; e-mail: martin@policlinico.mi.it.
References
- 1.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e419S–e494S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall JG, Pauli RM, Wilson KM. Maternal and fetal sequelae of anticoagulation during pregnancy. Am J Med. 1980;68(1):122–140. doi: 10.1016/0002-9343(80)90181-3. [DOI] [PubMed] [Google Scholar]
- 3.Bates SM, Greer IA, Hirsh J, Ginsberg JS. Use of antithrombotic agents during pregnancy: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):627S–644S. doi: 10.1378/chest.126.3_suppl.627S. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg JS, Hirsh J, Turner DC, Levine MN, Burrows R. Risks to the fetus of anticoagulant therapy during pregnancy. Thromb Haemost. 1989;61(2):197–203. [PubMed] [Google Scholar]
- 5.van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12(3):320–328. doi: 10.1111/jth.12485. [DOI] [PubMed] [Google Scholar]
- 6.Jick H, Jick SS, Gurewich V, Myers MW, Vasilakis C. Risk of idiopathic cardiovascular death and nonfatal venous thromboembolism in women using oral contraceptives with differing progestagen components. Lancet. 1995;346(8990):1589–1593. doi: 10.1016/s0140-6736(95)91928-7. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Venous thromboembolic disease and combined oral contraceptives: results of international multicentre case-control study. Lancet. 1995;346(8990):1575–1582. [PubMed] [Google Scholar]
- 8.Spitzer WO, Lewis MA, Heinemann LA, Thorogood M, MacRae KD Transnational Research Group on Oral Contraceptives and the Health of Young Women. Third generation oral contraceptives and risk of venous thromboembolic disorders: an international case-control study. BMJ. 1996;312(7023):83–88. doi: 10.1136/bmj.312.7023.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farmer RD, Lawrenson RA, Thompson CR, Kennedy JG, Hambleton IR. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet. 1997;349(9045):83–88. doi: 10.1016/s0140-6736(96)07496-x. [DOI] [PubMed] [Google Scholar]
- 10.Todd J, Lawrenson R, Farmer RD, Williams TJ, Leydon GM. Venous thromboembolic disease and combined oral contraceptives: A re-analysis of the MediPlus database. Hum Reprod. 1999;14(6):1500–1505. doi: 10.1093/humrep/14.6.1500. [DOI] [PubMed] [Google Scholar]
- 11.Parkin L, Skegg DC, Wilson M, Herbison GP, Paul C. Oral contraceptives and fatal pulmonary embolism. Lancet. 2000;355(9221):2133–2134. doi: 10.1016/S0140-6736(00)02382-5. [DOI] [PubMed] [Google Scholar]
- 12.Lidegaard Ø, Edström B, Kreiner S. Oral contraceptives and venous thromboembolism: a five-year national case-control study. Contraception. 2002;65(3):187–196. doi: 10.1016/s0010-7824(01)00307-9. [DOI] [PubMed] [Google Scholar]
- 13.Pearce HM, Layton D, Wilton LV, Shakir SA. Deep vein thrombosis and pulmonary embolism reported in the Prescription Event Monitoring Study of Yasmin. Br J Clin Pharmacol. 2005;60(1):98–102. doi: 10.1111/j.1365-2125.2005.02373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Hylckama Vlieg A, Helmerhorst FM, Vandenbroucke JP, Doggen CJ, Rosendaal FR. The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case-control study. BMJ. 2009;339:b2921. doi: 10.1136/bmj.b2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lidegaard Ø, Løkkegaard E, Svendsen AL, Agger C. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890. doi: 10.1136/bmj.b2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Medical Eligibility Criteria for Contraceptive Use. Geneva: World Health Organization; 2010. [Google Scholar]
- 17.Baglin T, Bauer K, Douketis J, Buller H, Srivastava A, Johnson G SSC of the ISTH. Duration of anticoagulant therapy after a first episode of an unprovoked pulmonary embolus or deep vein thrombosis: guidance from the SSC of the ISTH. J Thromb Haemost. 2012;10(4):698–702. doi: 10.1111/j.1538-7836.2012.04662.x. [DOI] [PubMed] [Google Scholar]
- 18.Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190(4 Suppl):S5–S22. doi: 10.1016/j.ajog.2004.01.061. [DOI] [PubMed] [Google Scholar]
- 19.Alotaibi GS, Almodaimegh H, McMurtry MS, Wu C. Do women bleed more than men when prescribed novel oral anticoagulants for venous thromboembolism? A sex-based meta-analysis. Thromb Res. 2013;132(2):185–189. doi: 10.1016/j.thromres.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Büller HR, Prins MH, Lensin AW, et al. EINSTEIN–PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 21.Bauersachs R, Berkowitz SD, Brenner B, et al. EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 22.De Crem N, Peerlinck K, Vanassche T, et al. Abnormal uterine bleeding in VTE patients treated with rivaroxaban compared to vitamin K antagonists. Thromb Res. 2015;136(4):749–753. doi: 10.1016/j.thromres.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 23. Pfizer Canada Inc., Bristol-Myers Squibb Canada. Eliquis (apixaban tablets 2.5 mg and 5 mg) Canadian Product Monograph. Available at: http://www.pfizer.ca/sites/g/files/g10017036/f/201505/Marketed_ELIQUIS_178226_PM_200215.pdf. Accessed July 13, 2015.
- 24.Prins MH, Lensing AWA, Bauersachs R, et al. EINSTEIN Investigators. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J. 2013;11(1):21. doi: 10.1186/1477-9560-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bamber L, Wang MY, Prins MH, et al. Patient-reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of acute symptomatic deep-vein thrombosis. Thromb Haemost. 2013;110(4):732–741. doi: 10.1160/TH13-03-0243. [DOI] [PubMed] [Google Scholar]
- 26.Prins MH, Bamber L, Cano SJ, et al. Patient-reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of pulmonary embolism; results from the EINSTEIN PE trial. Thromb Res. 2015;135(2):281–288. doi: 10.1016/j.thromres.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Andersson JK, Rybo G. Levonorgestrel-releasing intrauterine device in the treatment of menorrhagia. Br J Obstet Gynaecol. 1990;97(8):690–694. doi: 10.1111/j.1471-0528.1990.tb16240.x. [DOI] [PubMed] [Google Scholar]
- 28.Apgar BS, Kaufman AH, George-Nwogu U, Kittendorf A. Treatment of menorrhagia. Am Fam Physician. 2007;75(12):1813–1819. [PubMed] [Google Scholar]
- 29.Lethaby A, Irvine G, Cameron I. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(2):CD001016. doi: 10.1002/14651858.CD001016. [DOI] [PubMed] [Google Scholar]
- 30.Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;(1):CD001886. doi: 10.1002/14651858.CD001886. [DOI] [PubMed] [Google Scholar]
- 31.Mannucci PM, Levi M. Prevention and treatment of major blood loss. N Engl J Med. 2007;356(22):2301–2311. doi: 10.1056/NEJMra067742. [DOI] [PubMed] [Google Scholar]
- 32.National Institute for Heath and Clinical Excellence. Heavy menstrual bleeding. NICE guidelines [CG44]. Available at: https://www.nice.org.uk/guidance/cg44. Accessed October 12, 2015.
- 33.Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947–953. doi: 10.1001/jamainternmed.2014.946. [DOI] [PubMed] [Google Scholar]
- 34.Prins MH, Lensing AWA. Derivation of the non-inferiority margin for the evaluation of direct oral anticoagulants in the treatment of venous thromboembolism. Thromb J. 2013;11(1):13. doi: 10.1186/1477-9560-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall KS, Trussell J, Schwarz EB. Progestin-only contraceptive pill use among women in the United States. Contraception. 2012;86(6):653–658. doi: 10.1016/j.contraception.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall KS, Trussell J. Types of combined oral contraceptives used by US women. Contraception. 2012;86(6):659–665. doi: 10.1016/j.contraception.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lidegaard Ø, Nielsen LH, Skovlund CW, Skjeldestad FE, Løkkegaard E. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ. 2011;343:d6423. doi: 10.1136/bmj.d6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantha S, Karp R, Raghavan V, Terrin N, Bauer KA, Zwicker JI. Assessing the risk of venous thromboembolic events in women taking progestin-only contraception: a meta-analysis. BMJ. 2012;345:e4944. doi: 10.1136/bmj.e4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Hylckama Vlieg A, Helmerhorst FM, Rosendaal FR. The risk of deep venous thrombosis associated with injectable depot-medroxyprogesterone acetate contraceptives or a levonorgestrel intrauterine device. Arterioscler Thromb Vasc Biol. 2010;30(11):2297–2300. doi: 10.1161/ATVBAHA.110.211482. [DOI] [PubMed] [Google Scholar]
- 40.Apgar BS, Greenberg G. Using progestins in clinical practice. Am Fam Physician. 2000;62(8):1839–1846. 1849-1850. [PubMed] [Google Scholar]
- 41.Grimes DA, Lopez LM, O’Brien PA, Raymond EG. Progestin-only pills for contraception. Cochrane Database Syst Rev. 2013;11:CD007541. doi: 10.1002/14651858.CD007541.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdel-Aleem H, d’Arcangues C, Vogelsong KM, Gülmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin only contraceptives. Cochrane Database Syst Rev. 2007;(4):CD003449. doi: 10.1002/14651858.CD003449.pub3. [DOI] [PubMed] [Google Scholar]