Abstract
Background
Streptococcus pneumoniae (SP) is a major cause of morbidity and mortality worldwide in all age groups. Serious diseases often caused by pneumococci include pneumonia, meningitis and bacteremia.
Objective
The aim of this work was to estimate the hospitalization rate for SP in the Veneto region by investigating pneumococcal-related discharges.
Material and methods
This was a retrospective study based on hospital discharge data collected from 2008 to 2012 in the Veneto Region (north-east Italy). All hospitalizations for diseases potentially associated with SP were identified by searching the hospital discharge records, then the proportions of hospital admissions for pneumonia, meningitis and septicemia attributable to the infection were calculated. Comorbidities were also graded according to the Charlson Comorbidity Index (CCI). Data were analyzed using the chi square test and Student's t-test for unpaired data, as appropriate. Significant trends over the years considered were examined in terms of average annual percent changes (AAPC). A p value < 0.05 was considered significant.
Results
We identified 62,946 hospital discharge records concerning diseases potentially associated with SP. Among them, the proportion of SP-related hospital admissions (SP-HA) was estimated to be 23,089 (37.2%). The estimated incidence of SP-HA was 94.0/100,000 population (102.8/100,000 in males and 85.6/100,000 in females; p < 0.01): 89.0 for pneumonia, 0.9 for meningitis, and 4.1 for septicemia. The incidence of SP-HA was higher in children and the elderly, and the overall fatality rate was 11.0%. The overall economic burden of SP-HA during the period considered was around €14.8 million a year, with an average cost of €3120 per hospitalization.
Conclusion
This study shows that hospitalization for SP-related disease has a considerable impact on the health services, especially as far as children and the elderly are concerned.
Keywords: Streptococcus pneumoniae, Hospitalization, Epidemiology
Highlights
-
•
Estimated hospitalization rate was higher in children and the elderly
-
•
Overall fatality rate was 11.0%.
-
•
Economic burden of SP-HA was around €14.8 million a year in Veneto Region.
Introduction
Streptococcus pneumoniae (SP) is a major cause of morbidity and mortality worldwide in all age groups. It can cause a number of diseases of variable severity. After colonizing the nasopharyngeal mucosa, pneumococci may spread contiguously and non-invasively to other sites in the respiratory tract. Invasive pneumococcal disease (IPD) is defined as any condition in which SP is present in the blood, cerebrospinal fluid or other normally sterile body sites ( W.H.O., 2003 ). Pneumococcal pneumonia is associated with bacteremia in 10–30% of cases ( W.H.O., 2012a ), in which case it is classified as invasive. There are two other invasive forms of pneumococcal disease, i.e. septicemia and meningitis. Septicemia is severe infection in the bloodstream, which can rapidly become life-threatening. Patients are usually treated in intensive care units, but septicemia is often fatal even with the most expensive treatments. Survivors of severe sepsis are likely to have permanent organ damage, cognitive impairments, and physical disabilities. The organisms that cause bacterial meningitis differ somewhat by geographical region and age, and they relate to different vaccination programs. In the USA, where Haemophilus influenza vaccine was introduced in 1996, the epidemiological features of bacterial meningitis have changed dramatically in recent years, and nowadays its most common cause among adults young and old is SP ( Bhimraj, 2012 ).
Most of the burden of pneumococcal infections in adults relates to pneumonia. Community-acquired pneumonia (CAP) is a respiratory disease highly prevalent in the general population that has clinically heterogeneous features and may be more or less severe ( Blasi et al., 2012 ). The most common cause of CAP in adults in industrialized countries is SP, and this pathogen is identified in a significant proportion of patients hospitalized with CAP ( Fine et al., 1996, Woo et al., 2001, W.H.O., 2012b, Rodrigo et al., 2014). The methods used to identify a pathogen responsible for pneumonia vary, depending on the equipment available at a hospital's laboratory and on the design of studies on the topic; and different pathogens are found in different seasons of the year and geographical regions. Pneumococcal pneumonia has been associated with a more severe clinical course, demanding more medical resources than non-pneumococcal pneumonia ( Pletz et al., 2012 ). Pneumococcus is also an important cause of pneumonia in residents at long-term care facilities ( Loeb, 2014 ), and patients with pneumococcal pneumonia are also more frequently admitted to hospital, especially if they are elderly ( Pletz et al., 2012, Bechini et al., 2014).
The aim of this work was to estimate the burden of hospitalization for SP in the Veneto region by examining the SP-related hospital admissions (SP-HA) to obtain epidemiological data and calculate the fatality rate among these patients.
Material and methods
This was a retrospective study based on data collected from 2008 to 2012 in the National Surveillance System hospital database for the Veneto Region (north-east Italy). This region has a total of 81 hospitals and similar healthcare facilities, all of which were included in our analysis. In general, the first-listed diagnosis concerns the main condition identified during the patient's hospital stay, while other diagnoses indicate associated or contributing conditions (comorbidities and/or complications).
All hospital discharge records containing one of the following codes in the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) as a primary diagnosis were selected: (i) pneumonia group: 481: “Pneumococcal pneumonia”; 485: “Bronchopneumonia, organism unspecified”; 486: “Pneumonia, organism unspecified”, 482.30 “Pneumonia due to Streptococcus, unspecified”, 482.9 “Bacterial pneumonia, unspecified”; (ii) meningitis group: 320.1: “Pneumococcal meningitis”; 322.9: “Meningitis of unspecified cause”; and (iii) septicemia group: 038.2: “pneumococcal septicemia”; 038.9 “Unspecified septicemia”.
In the event of patients being readmitted to hospital within 30 days, only their first hospitalization was included in the analysis.
The proportion of SP-HA was calculated on the assumption that all discharge records mentioning this pathogen were SP-HA, and that this was also true for 36%, 58% and 20%, respectively, of the cases of pneumonia, meningitis and septicemia for which no pathogen was specified ( Welte et al., 2010, Thigpen et al., 2011, Rüggeberg et al., 2004, Pere Domingo et al., 2013).
Age was categorized in five groups (0–4 years; 5–14 years, 15–64 years, 65–79 years and 80 + years). Length of stay (LOS) was defined as the time from admission to discharge from the hospital or in-hospital death. Comorbidities were considered, based on the other diagnostic codes recorded, identifying the following medical conditions: diabetes mellitus (ICD9-CM code: 250), heart diseases (ICD9-CM codes: 410–414; 402.01, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91,404.93, 452, 428), cerebrovascular diseases (ICD9-CM codes: 430–434, 436–438), respiratory diseases (ICD-9-CM codes: 491.1–491.8; 492–496), malignant neoplasms (ICD-9-CM codes: 140–209), renal diseases (ICD-9-CM codes: 580–588, 591) and dementia (ICD-9-CM codes: 290, 331). Comorbidities were also graded according to the Charlson Comorbidity Index (CCI) ( Charlson et al., 1987 ), calculated from the sum of the scores for the severity of each comorbid condition, and then categorized as 0, 1, and 2 or more.
The cost to the health care system of the hospitalizations associated with SP was calculated by considering the diagnostic cost groups and the number of discharges. The diagnostic cost grouping is based on diagnosis-related groups (DRG) of hospitalized patients according to their ICD-9 code(s) at discharge, age, sex, and resource consumption. The DRG calculations were completed using 3 M with Core Grouping System Software.
The study was conducted on data routinely collected by the health services and linked to anonymized records that make it impossible to identify the individuals concerned. The data analysis was performed on anonymized aggregated data. Data in the Local Health Authority registries are recorded with the patient's consent and can be used as aggregate data for scientific studies without further authorization ( Garante per la protezione dei dati personal, 2012 ). This study complies with the Declaration of Helsinki and with Italian privacy law (Decree n. 196/2003 on the protection of personal data).
Statistical analysis
The SP-HA rates (by gender, age group, type of disease, and comorbidities) were obtained by dividing the estimated annual number of hospitalizations for pneumonia by the annual population, according to the figures supplied by the Veneto Region Statistics Office, with the rates expressed as hospitalizations per 100,000 population. The Veneto population during the study period was stable, and the data analyzed only concerned the resident population, excluding people living outside the region.
The mean days of hospital stay and case-mortality rates (%) were also calculated.
The data were analyzed using the chi square test and Student's t-test for unpaired data, as appropriate. A p value < 0.05 was considered significant. The analyses were performed using the Statistical Package for the Social Sciences (SPSS 16.0; SPSS Inc., Chicago, IL, USA). Significant trends over the years considered were assessed in terms of average annual percent changes (AAPC), a summary measure of the trend over a given fixed interval. It is computed as a weighted average of the annual percent change (APC) emerging from the join-point model, using weights equating to the length of the APC interval. If an AAPC lies entirely within a single join-point segment, the AAPC is the same as the APC for that segment ( Boehmer et al., 2011 ).
Results
We identified 62,946 hospital discharge records concerning diseases potentially associated with SP between January 1, 2008 and December 31, 2012; and 935 (1.5%) were readmissions within 30 days. A specific diagnosis of SP-related disease was mentioned in 2.9%, 6.5% and 58.0% of patients admitted for pneumonia, septicemia and meningitis, respectively.
The proportion of SP-HA was estimated at 23,089 of all the discharge records selected (37.2%) and 21,848 (94.6%) were coded as cases of pneumonia, 1016 (4.4%) as septicemia, and 225 (1.0%) as meningitis. Overall, the highest proportion of hospitalizations involved elderly patients > 65 years old (71.9%). Among all the discharge records with a SP-related diagnosis, 36.2% concerned patients with at least one comorbidity. The characteristics of the sample are shown in Table 1 .
Table 1.
SP-related hospital admissions in the Veneto Region by patients' features (2008–2012).
| Total |
Pneumonia |
Meningitis |
Septicemia |
|||||
|---|---|---|---|---|---|---|---|---|
| Total |
23089 |
21848 |
225 |
1016 |
||||
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Gender | ||||||||
| Females | 10748 | (46.6) | 10163 | (46.5) | 114 | (50.7) | 471 | (46.4) |
| Males | 12341 | (53.4) | 11685 | (53.5) | 111 | (49.3) | 545 | (53.6) |
| Age group (years) | ||||||||
| 0–4 | 1776 | (7.7) | 1685 | (7.7) | 20 | (8.9) | 71 | (7.0) |
| 5–14 | 1223 | (5.3) | 1160 | (5.3) | 9 | (4.0) | 54 | (5.3) |
| 15–64 | 3490 | (15.1) | 3173 | (14.5) | 96 | (42.7) | 221 | (21.8) |
| 65–79 | 5491 | (23.8) | 5142 | (23.5) | 76 | (33.8) | 273 | (26.9) |
| 80 + | 11109 | (48.1) | 10688 | (48.9) | 24 | (10.7) | 397 | (39.1) |
| Nationality | ||||||||
| Italian | 22023 | (95.4) | 20856 | (95.5) | 208 | (92.4) | 959 | (94.4) |
| Foreign | 1066 | (4.6) | 992 | (4.5) | 17 | (7.6) | 57 | (5.6) |
| Length of stay | ||||||||
| 0–7 | 7064 | (30.6) | 6743 | (30.9) | 48 | (21.3) | 273 | (26.9) |
| 8–15 | 10219 | (44.3) | 9787 | (44.8) | 64 | (28.4) | 368 | (36.2) |
| 16–30 | 4730 | (20.5) | 4381 | (20.1) | 83 | (36.9) | 266 | (26.2) |
| > 30 | 1076 | (4.7) | 937 | (4.3) | 30 | (13.3) | 109 | (10.7) |
| Charlson comorbidity index | ||||||||
| 0 | 14130 | (61.2) | 13372 | (61.2) | 193 | (85.8) | 565 | (55.6) |
| 1 | 5482 | (23.7) | 5295 | (24.2) | 17 | (7.6) | 170 | (16.7) |
| ≥ 2 | 3477 | (15.1) | 3181 | (14.6) | 15 | (6.7) | 281 | (27.7) |
| Comorbidities | ||||||||
| Cerebrovascular diseases | 1434 | (6.2) | 1368 | (6.3) | 8 | (3.6) | 58 | (5.7) |
| Heart diseases | 1770 | (7.7) | 1697 | (7.8) | 6 | (2.7) | 67 | (6.6) |
| Respiratory diseases | 365 | (1.6) | 361 | (1.7) | 1 | (0.4) | 3 | (0.3) |
| Diabetes mellitus | 2140 | (9.3) | 2033 | (9.3) | 13 | (5.8) | 94 | (9.3) |
| Cancer | 1251 | (5.4) | 1063 | (4.9) | 10 | (4.4) | 178 | (17.5) |
| Renal diseases | 1545 | (6.7) | 1423 | (6.5) | 8 | (3.6) | 114 | (11.2) |
| Died during hospital stay | 2542 | (11.0) | 2296 | (10.5) | 29 | (12.9) | 217 | (21.4) |
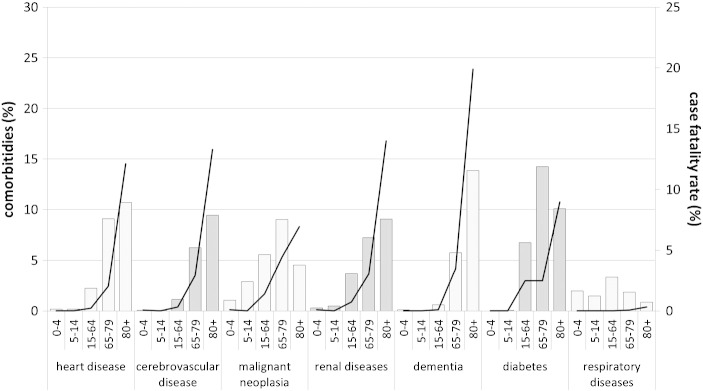
For the sample of SP-HA as a whole, the length of stay (LOS) was a mean 11.6 ± 10.2 days, and it was significantly higher in females (11.8 ± 10.1 vs 11.5 ± 10.3 in males p < 0.01). Divided by diagnostic group, the mean hospital stay was 14.2 ± 13.0, 16.9 ± 16.2 and 11.4 ± 9.1 for septicemia, meningitis and pneumonia, respectively. Patients who had comorbidities had a longer mean hospital stay than those who did not (CCI = 2 + LOS: 14.0 days; CCI = 1, LOS 13.4 days; CCI = 0, LOS 10.3 days; CCI = 0 versus both other groups p < 0.05). The number of chronic comorbidities generally rose with age. Their frequency and case fatality rate by type is shown in Fig. 1 .
Fig. 1.
Percentages of chronic comorbidities (bars) and case fatality rate (lines) of SP-related hospital admissions according to age group and type of comorbidity (2008–2012).
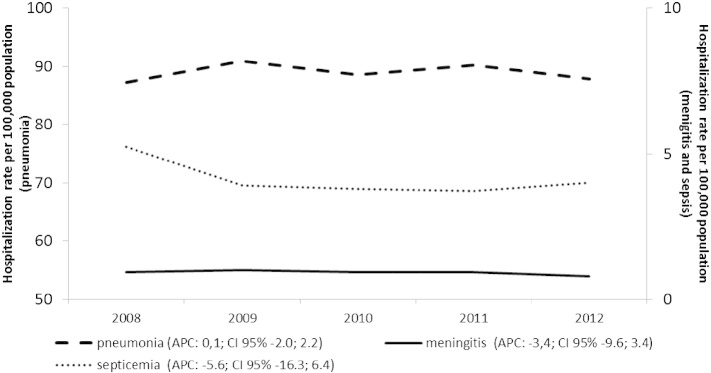
During the period considered, the estimated SP-HA rate was 94.0/100,000 population (89.0, 0.9 and 4.1 per 100,000 for pneumonia, meningitis and septicemia, respectively). Fig. 2 shows the trend of the hospitalization rates year by year, by studied group.
Fig. 2.
Annual SP-related hospital admissions per 100,000 population in the Veneto Region, by type of disease (2008–2012).
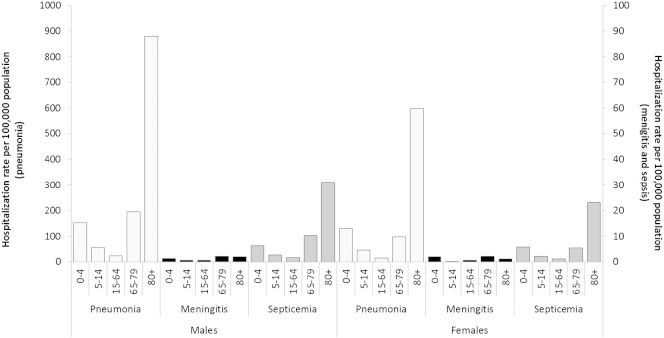
The estimated hospitalization rates suggest a greater burden of disease for males (102.8/100,000 as opposed to 85.6/100,000 females; p < 0.01) with no significant changes seen over the years considered (females AAPC: − 0.0, CI 95%: − 1.5; 1.5; males AAPC: − 0.4 CI 95%: − 2.2;1.3). In both genders, the SP-HA rate showed two age peaks, one in children and the other in elderly adults ( Fig. 3 ).
Fig. 3.
SP-HA rates (× 100,000 population) in the Veneto Region by gender, type of disease, and age group (2008–2012).
The overall expenditure for SP-HA during the period of observation was around €14.8 million a year, with an average cost of €3120 per patient (€2927 for cases of pneumonia, €8239 for meningitis, and €5520 for septicemia).
Discussion
The present study estimated the trends of the hospital admission rates for SP-related invasive disease in a cohort of 5 million citizens, and the results indicate that the heavy burden of these conditions on hospital resources is an important public health issue.
It is not easy to estimate the morbidity of invasive SP-related diseases in the general population. This is partly because the currently-available epidemiological picture is still hazy due to differences in study designs and population profiles, in access to health care and recording methods, but hospital discharge data can prove useful for assessing the cases severe enough to warrant hospitalization ( Boehmer et al., 2011 ).
The overall estimated SP-HA rate was 94.0/100,000 population during the years 2008–2012, and it was 1.2 times higher for males than for females. The age groups considered in our sample also revealed differences in the hospitalization rates, with children and older people being more affected in all the diagnostic groups considered.
One of the difficulties encountered in studies in this setting concerns the limited availability of microbiological data and the dubious reliability of the figure identified for all SP-HA due to coding errors in hospital discharge records. In our data set, there were very few diagnoses specifying that SP was involved, and the figures varied by type of disease — low for pneumonia and high for meningitis (2.9% vs 58%). A recent review analyzing the burden of CAP among adults in Europe reported that Italy had the lowest pneumococcus identification rate, and recommended that diagnostic assays be improved to detect pneumococcal pneumonia more effectively and thus provide a more accurate epidemiological picture ( Welte et al., 2010 ).
Using hospital discharges records makes it difficult to distinguish community-acquired infections, but our analyses were conducted on the first diagnosis prompting admission to hospital, so our cases are unlikely to have included hospital-acquired infections.
Despite the relatively short period considered, our data indicated a higher SP-HA rate among elderly people, and particularly among those with comorbidities. Our figures also showed a slight (but not statistically significant) downward trend in the Veneto's SP-HA rate.
The rate of SP-HA found associated with pneumonia (89.0/100,000 population) is bound to underestimate the true burden of this disease in the region considered because most patients with pneumonia are not hospitalized. Our figure probably only concerns the more severe cases. CAP is quite a common condition, for which only about 30% of patients require admission to hospital ( Guest and Morris, 1997 ).
A recent paper indicated that the overall pneumonia hospitalization rate in the Veneto region had increased slightly, but dropped significantly among infants aged 0–4 years. This could be thanks to a greater PCV vaccination coverage in this age group since 2008 ( Baldo et al., 2014 ). The same trend has been seen in other regions too ( Durando et al., 2009, Martinelli et al., 2014).
The incidence of sepsis has recently increased in industrialized countries, and it is an important cause of death. It is commonly caused by Gram-positive bacterial and fungal organisms ( Martin et al., 2003, Alberti et al., 2003). Meanwhile, the rate of bacterial meningitis has been declining, although the disease is still often fatal. Patients with meningitis are more likely to be older adults ( Thigpen et al., 2011 ). An important aspect of this trend relates to the vaccine-preventable bacterial diseases in areas where vaccine coverage is high, so the use of effective vaccines can be expected to have a substantial impact on these infectious diseases. The benefits have been most notable in children with the advent of effective conjugate vaccines since the 1990s ( Martin et al., 2014 ). Our data confirm that the elderly and children are at much higher risk of developing meningitis and sepsis than younger adults.
Regarding outcome, the overall fatality rate for patients involved in SP-HA was 10.4% and, divided by disease group, it was 21.5%, 10.5% and 9.9% for septicemia, meningitis and pneumonia, respectively. The worldwide morbidity and mortality rates associated with pneumococcal pneumonia are still high, with the mortality rate ranging from 6.4% to > 40% in different outpatient, inpatient and intensive-care patient settings ( Carr et al., 1991 ). There are clearly many factors that can contribute to mortality among patients requiring hospitalization, which can be grouped into patient-related factors, care process factors, physician-related factors, and others. In our analysis, the factors associated with a higher mortality risk among hospitalized cases were older age, male gender, and a higher score on the Charlson Co-morbidity Index. Many studies have confirmed that age and comorbidities are predictors of outcome ( Thomas and Wu, 2005, Martin et al., 2006).
The economic burden of pneumonia for the health system depends on a patient's age, children and the elderly having the highest incidence of SP-HA and incurring the greatest economic burden. The mean hospital stay and the in-hospital mortality rates were found higher for patients with comorbidities, suggesting a more severe disease on admission.
Our study shows that SP-HA has a considerable impact on the health services in the Veneto region. Vaccination strategies can be used for the primary prevention of the infection, and could reduce the burden of SP-related disease. Prevention remains the most valuable tool in efforts to contain the phenomenon. The development of pneumococcal conjugate vaccines has had a significant impact on the burden of the related diseases in countries where they have been introduced ( Albrich et al., 2007 ). Vaccination can naturally only protect against CAP, not hospital-acquired pneumonia, which is caused by different types of pathogens ( Micek et al., 2011 ).
Conclusion
Our study shows that hospitalizations for patients with conditions caused by S. pneumoniae have a considerable impact on the health services, especially when children and the elderly are involved. Judging from our hospital discharge records, it would seem important to conduct prospective epidemiological studies with the cooperation of specialists in this field, with a view to obtaining a more precise picture of the epidemiological situation regarding these diseases.
Conflict of interest
The authors declare that there are no conflicts of interest.
Contributor Information
Vincenzo Baldo, Email: vincenzo.baldo@unipd.it.
Silvia Cocchio, Email: silvia.cocchio@unipd.it.
Roberta Lazzari, Email: roberta.lazzari.1@unipd.it.
Patrizia Furlan, Email: patrizia.furlan.73@alice.it.
Chiara Bertoncello, Email: chiara.bertoncello@unipd.it.
Francesca Russo, Email: Francesca.Russo@regione.veneto.it.
Mario Saia, Email: Mario.Saia@regione.veneto.it.
Tatjana Baldovin, Email: tatjana.baldovin@unipd.it.
References
- Alberti C., Brun-Buisson C., Goodman S.V., Guidici D., Granton J., Moreno R., Smithies M., Thomas O., Artigas A., Le Gall J.R. Influence of systemic inflammatory response syndrome and sepsis on outcome of critically ill infected patients. Am. J. Respir. Crit. Care Med. 2003;168:77–84. doi: 10.1164/rccm.200208-785OC. [DOI] [PubMed] [Google Scholar]
- Albrich W.C., Baughman W., Schmotzer B., Farley M.M. Changing characteristics of invasive pneumococcal disease in Metropolitan Atlanta, Georgia, after introduction of a 7-valent pneumococcal conjugate vaccine (1997–2004) Clin. Infect. Dis. 2007;44:1569–1576. doi: 10.1086/518149. [DOI] [PubMed] [Google Scholar]
- Baldo V., Cocchio S., Baldovin T., Furlan P., Buja A., Bertoncello C., Russo F., Saia M. A population-based study on the impact of hospitalization for pneumonia in different age groups. BMC Infect. Dis. 2014;14:485. doi: 10.1186/1471-2334-14-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechini A., Taddei C., Barchielli A., Levi M., Tiscione E., Santini M.G., Niccolini F., Mechi M.T., Panatto D., Amicizia D., Azzari C., Bonanni P., Boccalini S. A retrospective analysis of hospital discharge records for S. pneumoniae diseases in the elderly population of Florence, Italy, 2010–2012: implications for immunization policies. Hum. Vaccin. Immunother. 2014;11:1–9. doi: 10.4161/hv.34418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhimraj A. Acute community-acquired bacterial meningitis in adults: an evidence-based review. Cleve. Clin. J. Med. 2012;79(6):393–400. doi: 10.3949/ccjm.79gr.12003. [DOI] [PubMed] [Google Scholar]
- Blasi F., Mantero M., Santus P.A., Tarsia P. Understanding the burden of pneumococcal disease in adults. Clin. Microbiol. Infect. 2012;18(Suppl. 5):7–14. doi: 10.1111/j.1469-0691.2012.03937.x. [DOI] [PubMed] [Google Scholar]
- Boehmer T.K., Patnaik J.L., Burnite S.J., Ghosh T.S., Gershman K., Vogt R.L. Use of hospital discharge data to evaluate notifiable disease reporting to Colorado's electronic disease reporting system. Public Health Rep. 2011;126:100–106. doi: 10.1177/003335491112600114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr B., Walsh J.B., Coakley D., Mulvihill E., Keane C. Prospective hospital study of community acquired lower respiratory tract infection in the elderly. Respir. Med. 1991;85:185–187. doi: 10.1016/s0954-6111(06)80077-6. [DOI] [PubMed] [Google Scholar]
- Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chron. Dis. 1987;140:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Durando P., Crovari P., Ansaldi F., Sticchi L., Sticchi C., Turello V., Marensi L., Giacchino R., Timitilli A., Carloni R., Azzari C., Icardi G. Universal childhood immunisation against Streptococcus pneumoniae: the five-year experience of Liguria Region, Italy. Vaccine. 2009;27:3459–3462. doi: 10.1016/j.vaccine.2009.01.052. [DOI] [PubMed] [Google Scholar]
- Fine M.J., Smith M.A., Carson C.A., Mutha S.S., Sankey S.S., Weissfeld L.A., Kapoor W.N. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275(2):134–141. [PubMed] [Google Scholar]
- Garante per la protezione dei dati personal . Vol. 72. Gazzetta Ufficiale della Repubblica Italiana; 2012. Autorizzazione generale al trattamento dei dati personali effettuato per scopi di ricerca scientifica — 1° marzo 2012; pp. 47–52. [Google Scholar]
- Guest J.F., Morris A. Community-acquired pneumonia: the annual cost to the National Health Service in the UK. Eur. Respir. J. 1997;10:1530–1534. doi: 10.1183/09031936.97.10071530. [DOI] [PubMed] [Google Scholar]
- Loeb M. Pneumonia in the elderly. Curr. Opin. Infect. Dis. 2014;17:127–130. doi: 10.1097/00001432-200404000-00010. [DOI] [PubMed] [Google Scholar]
- Martin G.S., Mannino D.M., Eaton S., Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Martin G.S., Mannino D.M., Moss M. The effect of age on the development and outcome of adult sepsis. Crit. Care Med. 2006;34(1):15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- Martin N.G., Sadarangani M., Pollard A.J., Goldacre M.J. Hospital admission rates for meningitis and septicaemia caused by Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae in children in England over five decades: a population-based observational study. Lancet Infect. Dis. 2014;14(5):397–405. doi: 10.1016/S1473-3099(14)70027-1. [DOI] [PubMed] [Google Scholar]
- Martinelli D., Pedalino B., Cappelli M.G., Caputi G., Sallustio A., Fortunato F., Tafuri S., Cozza V., Germinario C., Chironna M., Prato R. Towards the 13-valent pneumococcal conjugate universal vaccination: effectiveness in the transition era between PCV7 and PCV13 in Italy, 2010–2013. Hum. Vaccin Immunother. 2014;10:33–39. doi: 10.4161/hv.26650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micek S.T., Welch E.C., Khan J., Pervez M., Doherty J.A., Reichley R.M., Hoppe-Bauer J., Dunne W.M., Kollef M.H. Resistance to empiric antimicrobial treatment predicts outcome in severe sepsis associated with Gram-negative bacteremia. J. Hosp. Med. 2011;6(7):405–410. doi: 10.1002/jhm.899. [DOI] [PubMed] [Google Scholar]
- Pere Domingo P., Pomar V., de Benito N., Coll P. The spectrum of acute bacterial meningitis in elderly patients. BMC Infect. Dis. 2013;13:108. doi: 10.1186/1471-2334-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletz M.W., von Baum H., van der Linden M., Rohde G., Schütte H., Suttorp N., Welte T. The burden of pneumococcal pneumonia — experience of the German competence network CAPNETZ. Pneumologie. 2012;66:470–475. doi: 10.1055/s-0032-1310103. [DOI] [PubMed] [Google Scholar]
- Rodrigo C., Bewick T., Sheppard C., Greenwood S., Trotter C., Slack M., George R., Lim W.S. Clinical features of adults with seven-valent-conjugated-vaccine-serotype pneumococcal pneumonia. Vaccine. 2014;32:1460–1465. doi: 10.1016/j.vaccine.2014.01.035. [DOI] [PubMed] [Google Scholar]
- Rüggeberg J.U., Ketteler K., MacKenzie C.R., Von Kries R., Reinert R.R., Schroten H. Blood culture sampling rates at a German pediatric university hospital and incidence of invasive pneumococcal disease. Infection. 2004;32(2):78–81. doi: 10.1007/s15010-004-3104-2. [DOI] [PubMed] [Google Scholar]
- Thigpen M.C., Whitney C.G., Messonnier N.E., Zell E.R., Lynfield R., Hadler J.L., Harrison L.H., Farley M.M., Reingold A., Bennett N.M., Craig A.S., Schaffner W., Thomas A., Lewis M.M., Scallan E., Schuchat A. Emerging infections programs network. Bacterial meningitis in the United States, 1998–2007. N. Engl. J. Med. 2011;364(21):2016–2025. doi: 10.1056/NEJMoa1005384. [DOI] [PubMed] [Google Scholar]
- Thomas J.M., Wu L. Factors influencing in-hospital mortality in community-acquired pneumonia: a prospective study of patients not initially admitted to the ICU. Chest. 2005;127(4):1260–1270. doi: 10.1016/S0012-3692(15)34475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- W.H.O. Pneumococcal vaccines, WHO position paper — 2012. Wkly. Epidemiol. Rec. 2012;87:129–144. [PubMed] [Google Scholar]
- W.H.O. Biologicals: Pneumococcal Disease. 2003. http://www.who.int/biologicals/vaccines/pneumococcal/en/ Available from. (accessed 05.11.14)
- W.H.O. International Travel and Health: Pneumococcal Disease. 2012. http://www.who.int/ith/diseases/pneumococcal/en/ Available from. (accessed 05.11.14)
- Welte T., Torres A., Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2010;67(1):71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- Woo J.H., Kang J.M., Kim Y.S., Shin W.S., Ryu J.H., Choi J.H., Kim Y.R., Cheong H.J., Uh S.T., Park C.S., Chung M.H., Chung K.S., Lee C.J., Ryu J. A prospective multicenter study of community-acquired pneumonia in adults with emphasis on bacterial etiology. Korean J. Infect. Dis. 2001;33:1–7. [Google Scholar]