Abstract
Background
Rich in genetic information and cost-effective to genotype, the Insertion-Deletion (InDel) molecular marker system is an important tool for studies in genetics, genomics and for marker-assisted breeding. Advent of next-generation sequencing (NGS) revolutionized the speed and throughput of sequence data generation, and enabled genome-wide identification of insertion and deletion variation. However, current NGS-based InDel mining tools, such as Samtools, GATK and Atlas2, all rely on a reference genome for variant calling which hinders their application on unsequenced organisms and the output of short InDels compromised their use on gel-based genotyping platforms. To address these issues, an enhanced platform is needed to identify longer InDels and develop markers in absence of a reference genome.
Results
Here we present mInDel (multiple InDel), a next-generation variant calling tool specifically designed for InDel marker discovery. By taking in raw sequence reads and assembling them into contigs de novo, this software identifies InDel polymorphisms using a sliding window alignment from assembled contigs, rendering a unique advantage when a reference genome is unavailable. By providing an option of combining multiple discovered InDels as output, mInDel is amiable to gel-based genotyping platforms where markers with large polymorphisms are preferred. We demonstrated the usability and performance of this software through a case study using a set of maize NGS data, and experimentally validated the accuracy of markers generated from mInDel.
Conclusions
mInDel is a novel and practical tool that enables rapid genome-wide InDel marker discovery. The features of being independent from a reference genome and the flexibility with downstream genotyping platforms will allow a broad range of applications across genetics research and plant breeding. The mInDel pipeline is freely available at www.github.com/lyd0527/mInDel.
Keywords: Next-generation sequencing (NGS), Insertions and deletions (InDels), Genome-wide marker discovery, Marker platform, Marker-assisted breeding
Background
A robust system of molecular markers is instrumental to the quality and efficiency of various genetic and genomic research as well as marker-assisted breeding [1–3]. Compared to other markers, Insertion-Deletion (InDel) markers provide a number of advantages: InDels are an important and abundant form of genome variation, often more prevalent than other structural variants such as SSRs; InDels are potentially multi-allelic and co-dominant, offering more genomic information than the usually bi-allelic SNPs; Genotyping of InDel markers is technologically less demanding, whereas SNP detection usually requires expensive chemistries and equipment. The last advantage is especially appealing when a low-cost genotyping platform is preferred in any laboratory.
Recent advances of next-generation sequencing (NGS) technologies have significantly increased the speed and scale of sequence information generation, and greatly accelerated the discovery process for genetic markers [4]. The massive amount of data together with the short read nature of NGS nonetheless, created a hurdle for effective InDel variation mining. Even though software such as SAMtools [5], GATK [6] and Atlas2 [7] offer tools for variant discovery, their algorithms were not tailor for InDel discovery and therefore are limited to identifying short InDels (usually <10 bp). This prevented their use with traditional gel electrophoresis-based genotyping, where small polymorphisms are hard to score accurately. In addition, all current variant callers rely on the presence of a reference genome for read mapping, which significantly hampered their broad application on non-model organisms whose reference genomes are usually unavailable.
To address these issues, we developed the mInDel suite that is specialized in calling multiple contiguous InDels independent of a reference genome. Built upon de novo assembled scaffolds/contigs from short sequence reads, mInDel identifies multiple contiguous InDels and uses the combined cumulative variation for marker development. We confirmed the computing efficiency of our pipeline by testing on a set of maize sequencing data, and experimentally verified the high accuracy of the developed InDel markers. Flexible, easy to use and highly practical, this modular software takes in raw sequence reads and outputs InDel markers with PCR primer sequences designed. Together with its independence from a reference genome, mInDel is a useful resource to various genetic and genomic researches across species.
Implementation
In general, mInDel takes in raw sequence reads, pre-processes for quality control, and creates de novo assembled contigs. It then generates a pool of random primer pairs on the contigs and uses the inferred in silico amplicons to screen for InDel polymorphisms as candidate markers. Compared to direct polymorphism screening from contigs, the in silico amplicon design enables outputting developed InDel markers with PCR primer information ready, which further increases the practicality and efficiency of the marker development process. mInDel is implemented in five steps, with each step carried out by an independent module. The modularity allows users to run certain steps only based on their needs. A flow chart of the implementation is shown in Fig. 1.
Fig. 1.
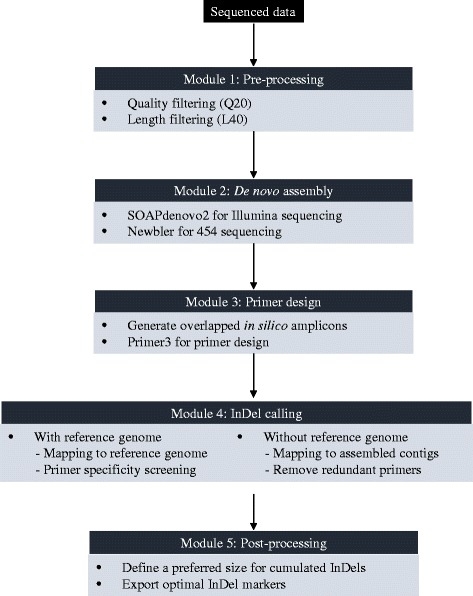
Overview of mInDel. mInDel is designed in five steps and implemented by five independent modules, which forms a pipeline of sequence analysis from raw sequences to InDel marker development. Modular design allows flexible choices of analysis steps based on users’ needs
Module 1: Pre-processing analysis
Raw sequence files were taken in FASTQ format. Sequence coverage depths greater than 1x has been seen successfully run using mInDel. For quality control of sequence data, script trim_fastq takes in files in FASTQ format and filters against low quality, short reads and low complex regions by calling the SolexaQA++ program [8]. Single- and paired-end reads are both acceptable. In case that a sequence from a sequence-pair was removed, the remaining one is put into a separate file and used as a singlet during de novo assembly.
Module 2: De novo assembly
The de novo assembly module provides two different assembly strategies for two commonly used platforms, Illumina (de_novo_assembly_illu script) and Sanger/454 (de_novo_assembly_454 script). For the Illumina platform with relatively short reads, the SOAPdenovo2 [9] is adopted, which utilizes de-Bruijn graphs algorithm and accurately assembles sequences from short reads [10–12]. For Sanger or 454 platforms with relatively long reads, Newbler [13] incorporates the overlap algorithm to efficiently generating high quality assemblies from long reads. De novo assembly is necessary for samples that lack a reference genome.
Module 3: Overlap primers design
This module generates a large pool of primer pairs, with inferred in silico amplicons in overlap spanning the entire input region. Overlapped amplicons are first generated using a sliding window method by over_fragments script, and then passed onto the Primer3 [14] program for primer design by primers_design script. The default window size is 300 bp, but can be readily changed to reflect users’ needs.
Module 4: Paired-end mapping and specific screening
The mapping module is a module for inferring amplicon sizes between samples. The primer pool undergoes a paired-end mapping analysis using the Bowtie aligner [15, 16] with maximum insertion size < =1000 bp. Here, the value 1000 is set based on the consideration of single-pass PCR amplification ability. Users can increase the value for detecting longer InDels. Inferred amplicon sizes are then used to mine potential InDels among homologous loci between input entries. InDels with polymorphisms above the user-set threshold can be mapped to the reference genome (if available) for specific screening.
Module 5: InDel screening and marker development
In the post-processing module, mInDel finalizes results and generates tab-delimited and Excel-compatible files by InDel_screening script. Accrued InDels facilitate genotyping from standard gel electrophoresis, but are also compatible with high-throughput fluorescent melting curve based genotyping assays. The output compiles information on optimum InDel markers including forward and reverse primers, product sizes, InDel sizes and chromosome locations ranked by a value score.
A set of test data is provided with mInDel for feature testing and performance check, which consists of simulated Illumina 100 bp*2 paired-end reads and raw reference sequences. Users are strongly encouraged to run this test with the command ‘sh test.sh’ after installation for runtime and performance check purposes, which usually takes about 5 minutes to run on a 8-core 2.5 GHz computer. The test data also serves as the foundation for example analyses described in the tutorial file.
Results & Discussion
Comparison with other InDel callers
We compared our mInDel pipeline with other InDel callers including SAMtools, GATK and Atlas2 (summarized in Table 1). By de novo assembling short reads into contigs, mInDel does not rely on a reference genome like other software do, which makes it feasible to identify InDel variants on samples with a partial or missing reference genome. mInDel also applies separate assembly algorithms for short and long sequencing reads, an approach that have been reported to help increase variant discovery accuracy [13]. Furthermore, mInDel can accumulate InDels into larger polymorphisms that are not only high-throughput genotyping compatible, but also PCR and gel electrophoresis friendly. This is especially appealing in scenarios where a low-cost genotyping approach is preferred.
Table 1.
Comparison between mInDel with other InDel calling tools
| Method | mInDel | SAMtools | GATK | Atlas2 |
|---|---|---|---|---|
| De novo assembly | Yes | No | No | No |
| Reference genome-independent | Yes | No | No | No |
| Different aligners for different read lengths | Yes | No | No | No |
| InDel sized called | Short, Long | Short | Short | Short |
| High-throughput genotyping compatibility | Yes | Yes | Yes | Yes |
| PCR/electrophoresis genotyping amiability | High | Low | Low | Low |
InDel marker discovery in maize: a case study using mInDel
To demonstrate the functionality of mInDel, we obtained and analyzed a publicly available sequence data on four maize inbred lines. Due to its relatively large complex genome and the high percentage of repetitive sequences, maize would be a fitting organism for evaluating mInDel’s computational performance and processing proficiency. A total of 24 Gb, 36.9 Gb and 2.7 Gb of Illumina sequences from three maize inbred lines Mo17, Zheng58 and Qi319 were obtained from NCBI Sequence Read Archive (SRA) database. After pre-processing and de novo assembly, contigs for Mo17, Qi319 and Zheng58 were generated, which accounted for approximately 75 %, 19.5 % and 8.8 % of the B73 reference genome (Table 2).
Table 2.
Summary of three genotypes genome data
| Inbred Line | B73 genome | Mo17 | Zheng58 | Qi319 |
|---|---|---|---|---|
| Raw data | / | 24 Gb | 36.9 Gb | 2.7 Gb |
| Sequencing platform | / | Illumina | Illumina | Illumina |
| Cleaned data | / | 21.2Gb | 32.6Gb | 2.1Gb |
| Assembled size | 2.1G | 1.5G | 408.9 M | 183.6 M |
| No. of Contigs | / | 1,351,472 | 476,470 | 395,075 |
| Longest contig | / | 33,817 bp | 18,068 bp | 7,286 bp |
| N50 size | / | 2,012 bp | 1,157 bp | 457 bp |
We conducted two experiments as a comparison to show the distinctive feature of the InDel discovery independent of a reference genome sequence. Mo17 vs. B73 was an experiment to map Mo17 sequence reads to the maize B73 reference genome [17]; Zheng58 vs. Qi319 was the other experiment where Zheng58 sequence reads were mapped to the assembled Qi319 contigs, mimicking the scenario where a reference genome is unavailable. After primers design and paired-end mapping analysis, 4,205,672 primer pairs for Mo17 were generated and aligned against the B73 reference genome, and 610,091 Zheng58 primer pairs were generated and aligned against the de novo assembled Qi319 contigs. With a threshold of less than 3 mismatched bases, a total of 739,250 and 223,084 InDel loci from these two comparisons were successfully identified using mInDel (Table 3).
Table 3.
Summary of InDel markers developed from two experiments
| Types | Mo17 vs. B73 (with reference genome) | Zheng58 vs. Qi319 (without reference genome) |
|---|---|---|
| Total InDels | 739,250 | 223,084 |
| Mean size | 71 bp | 66 bp |
| 1 ~ 10 bp | 76,376 | 25,144 |
| 11 ~ 20 bp | 63,714 | 21,291 |
| 21 ~ 100 bp | 374,847 | 123,788 |
| >100 bp | 213,698 | 52,861 |
| Discovery accuracy | 86.8 % (276 out of 318) | 72.6 % (437 out of 602) |
The InDel discovery results from the aforementioned experiments demonstrated the unique feature of accrued polymorphism output 18.1 % and 19.9 % markers in these two experiments have an InDel of 1–20 bp, while 80.4 % and 80.1 % of markers have an InDel greater than 20 bp. The average length of the InDels was 71 bp and 66 bp, with the maximum InDels up to 387 bp and 371 bp respectively. Single-copied in silico amplicons with InDel size larger than 20 bp were selected and considered as optimum InDel markers for the ease of PCR detection and screening with agarose gel electrophoresis.
This pipeline was executed on a Dell PowerEdge 11G R910 (E7-4800 CPU) with 512 GB RAM under a Linux CentOS. The run time was 2 h 12 min for the Mo17 vs. B73 experiment with a fully assembled B73 genome, and 3 h 33 min for the Zheng58 vs. Qi319 experiment using assembled Qi319 contigs.
Experimental validation of mInDel's discovery accuracy
To assess the accuracy of mInDel marker discovery, we conducted the following experimental validation. Primer pairs generated from mInDel were randomly sampled across the genome from the two aforementioned assigned groups and synthesized. PCRs were conducted in 20 μl reactions containing 10 μl HotStar Taq Master Mix (Qiagen, Germany), 1 μl of 10 μM primers, 2 μl DNA (50 ng/μl) and 7 μl water at 95 °C, 5 min; 34 cycles of (95 °C, 30 s; 58 °C, 30 s; 72 °C, 30 s) and 10 min extension at 72 °C. PCR products were electrophoresed on 2 % agarose gel and visualized with ethidium bromide. A high discovery accuracy rate was observed (Table 3), where 86.8 % (276 in 318 primer pairs) showed expected amplicon and polymorphism sizes from Mo17 vs. B73, and 72.6 % (437 in 602 primer pairs) in Zheng58 vs. Qi319.
Computational Performance
mInDel is designed to allow processing NGS data on a standard desktop computer or a small server in a reasonable amount of time. Run time hinges on whether a reference genome is used to; for example, the run time was 2.5 hours the Mo17 vs. B73 experiment where B73 is a fully assembled genome, and 3.5 hours for the Qi319 vs. Zheng58 experiment where Zheng58 has assembled contigs. mInDel has been extensively tested using large datasets from various organisms. In general, Using 128 CPU threads with 2.00 GHz Intel Xeon E7-4850 Processor on a computational cluster, we were able to process 92 genomic libraries in ~11 hours, which demonstrated that mInDel is also well suited for population-scale analyses.
Conclusions
To address the needs for a reliable and efficient InDel marker discovery tool, we developed this mInDel package that is able to output long InDel variants in absence of a fully assembled genome. This freely available and fully integrated suite offers demonstrated data processing performance, and provides great discovery accuracy. With its highly applied features, mInDel is a useful resource for various studies in genetics, genomics and marker-assisted breeding.
Availability and requirements
Project home page: www.github.com/lyd0527/mInDel
Operating system(s): Linux, UNIX and OS X
Programming language: Perl and BASH
Other requirements: Bowtie, SOAPdenovo2 and Primer3.
License: GNU GPLv2
Any restrictions to use by non-academics: None
Availability of data and materials
The data set supporting the results of this article is available in the NCBI SRA (Zheng58 from SRX120186; Qi319 from SRX121748; Mo17 from SRX026939).
Funding and acknowledgements
This project was supported by the Natural Science Foundation of China (NSFC) under grant 31401393 and the Jiangsu Agriculture Science and Technology Innovation Fund (JASTIF) under grant CX (14) 2009.
Abbreviations
- NGS
next-generation sequencing
- InDel
insertion and deletion
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HZ and YDL conceived and designed the pipeline. YDL and YHL wrote the manuscript. HZ revised the manuscript and provided substantial advice and guidance during all phases of the project. All authors read and approved the final manuscript.
Contributor Information
Yuanda Lv, Email: lyd0527@jaas.ac.cn.
Yuhe Liu, Email: yuheliu1@illinois.edu.
Han Zhao, Email: zhaohan@jaas.ac.cn.
References
- 1.Eathington SR, Crosbie TM, Edwards MD, Reiter RS, Bull JK. Molecular markers in a commercial breeding program. Crop Sci. 2007;47:154–163. doi: 10.2135/cropsci2007.04.0015IPBS. [DOI] [Google Scholar]
- 2.Väli Ü, Brandström M, Johansson M, Ellegren H. Insertion-deletion polymorphisms (indels) as genetic markers in natural populations. BMC Genet. 2008;9(1):1. doi: 10.1186/1471-2156-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekblom R, Galindo J. Applications of next generation sequencing in molecular ecology of non-model organisms. Heredity. 2011;107(1):1–15. doi: 10.1038/hdy.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudson ME. Sequencing breakthroughs for genomic ecology and evolutionary biology. Mol Ecol Resour. 2008;8(1):3–17. doi: 10.1111/j.1471-8286.2007.02019.x. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J. The Sequence Alignment-Map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Challis D, Yu J, Evani US, Jackson AR, Paithankar S, Coarfa C, Milosavljevic A, Gibbs RA, Yu F. An integrative variant analysis suite for whole exome next-generation sequencing data. BMC Bioinforma. 2012;13(1):1–12. doi: 10.1186/1471-2105-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox MP, Peterson DA, Biggs PJ. SolexaQA: At-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinforma. 2010;11(1):1–6. doi: 10.1186/1471-2105-11-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, He G, Chen Y, Pan Q, Liu Y. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. GigaScience. 2012;1(1):1–6. doi: 10.1186/2047-217X-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt M, Newbold C, Berriman M, Otto TD. A comprehensive evaluation of assembly scaffolding tools. Genome Biol. 2014;15(3):1–15. doi: 10.1186/gb-2014-15-3-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salzberg SL, Phillippy AM, Zimin A, Puiu D, Magoc T, Koren S, Treangen TJ, Schatz MC, Delcher AL, Roberts M. GAGE: A critical evaluation of genome assemblies and assembly algorithms. Genome Res. 2012;22(3):557–567. doi: 10.1101/gr.131383.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zamin I, Mario C, Isaac T, Paul F, Gil MV. De novo assembly and genotyping of variants using colored de Bruijn graphs. Nat Genet. 2012;44(2):226–232. doi: 10.1038/ng.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiGuistini S, Liao NY, Platt D, Robertson G, Seidel M, Chan SK, Docking TR, Birol I, Holt RA, Hirst M. De novo genome sequence assembly of a filamentous fungus using Sanger, 454 and Illumina sequence data. Genome Biol. 2009;10(9):1–12. doi: 10.1186/gb-2009-10-9-r94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics. 2007;23(10):1289–1291. doi: 10.1093/bioinformatics/btm091. [DOI] [PubMed] [Google Scholar]
- 15.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biol. 2009;10(3):1–10. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, Pasternak S, Liang C, Zhang J, Fulton L, Graves TA. The B73 maize genome: complexity, diversity, and dynamics. Science. 2009;326(5956):1112–1115. doi: 10.1126/science.1178534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set supporting the results of this article is available in the NCBI SRA (Zheng58 from SRX120186; Qi319 from SRX121748; Mo17 from SRX026939).


