Key Points
The association between multiple BCR-ABL1 mutations and inferior response to nilotinib/dasatinib was not seen with ponatinib therapy.
However, chronic phase patients with T315I plus additional mutation(s) did have poorer responses to ponatinib than those with T315I only.
Abstract
The third-generation tyrosine kinase inhibitor (TKI) ponatinib shows activity against all common BCR-ABL1 single mutants, including the highly resistant BCR-ABL1-T315I mutant, improving outcome for patients with refractory chronic myeloid leukemia (CML). However, responses are variable, and causal baseline factors have not been well-studied. The type and number of low-level BCR-ABL1 mutations present after imatinib resistance has prognostic significance for subsequent treatment with nilotinib or dasatinib as second-line therapy. We therefore investigated the impact of low-level mutations detected by sensitive mass-spectrometry before ponatinib initiation (baseline) on treatment response in 363 TKI-resistant patients enrolled in the PONATINIB for Chronic Myeloid Leukemia Evaluation and Ph+ Acute Lymphoblastic Leukemia trial, including 231 patients in chronic phase (CP-CML). Low-level mutations were detected in 53 patients (15%, including low-level T315I in 14 patients); most, however, did not undergo clonal expansion during ponatinib treatment and, moreover, no specific individual mutations were associated with inferior outcome. We demonstrate however, that the number of mutations detectable by mass spectrometry after TKI resistance is associated with response to ponatinib treatment and could be used to refine the therapeutic approach. Although CP-CML patients with T315I (63/231, 27%) had superior responses overall, those with multiple mutations detectable by mass spectrometry (20, 32%) had substantially inferior responses compared with those with T315I as the sole mutation detected (43, 68%). In contrast, for CP-CML patients without T315I, the inferior responses previously observed with nilotinib/dasatinib therapy for imatinib-resistant patients with multiple mutations were not seen with ponatinib treatment, suggesting that ponatinib may prove to be particularly advantageous for patients with multiple mutations detectable by mass spectrometry after TKI resistance.
Introduction
Despite the extraordinary success of imatinib for the treatment of chronic myeloid leukemia (CML), up to 40% of patients experience intolerance or resistance. The most common mechanism of treatment failure is the acquisition of mutations within the kinase domain (KD) of BCR-ABL1 that impair drug binding. Although more potent second-generation tyrosine kinase inhibitors (TKIs) were designed to target most imatinib-resistant mutations, some mutations confer resistance to either nilotinib or dasatinib or both (T315I).1,2 Therefore, mutation analysis using direct Sanger sequencing is recommended to guide rational therapy selection after imatinib failure.3 We have developed a sensitive multiplexed mass spectrometry (MS)-based mutation detection assay that detects the most common mutations that confer clinical resistance to imatinib, nilotinib, and/or dasatinib (detection limit, 0.05%-0.5%). Using this assay, we showed that detection of low-level resistant mutations after imatinib failure predicts second-line therapy failure because of rapid expansion of the drug-resistant mutant clones, when an inappropriate TKI is used.4 Consequently, the same recommendations for TKI selection should apply to these particular mutations, regardless of their abundance within a sample. We further showed that ∼25% of imatinib-resistant patients had >1 BCR-ABL1 KD mutation detectable using sensitive analysis, and demonstrated that the presence of multiple mutations defines a poor-risk subgroup of chronic phase (CP) CML patients who had a higher likelihood of acquiring new resistant mutations and inferior response to treatment with second-generation TKIs, irrespective of the resistance profile of the mutations.5
The highly resistant T315I mutation is the most commonly detected mutation6 and confers resistance to all first- and second-generation TKIs.7 Consequently, patients with T315I have historically had very poor outcome.8 Emerging data suggest that all common individual mutants, including T315I, are sensitive to the third-generation TKI ponatinib,9-12 and, importantly these in vitro sensitivity profiles13 have translated into therapeutic successes in patients.9,14 Treatment responses among patients are variable, however, and baseline factors that may lead to differential responses have not been well-studied. We aimed to determine if our previous findings demonstrating the prognostic significance of multiple BCR-ABL1 KD mutations for response to second-line therapy with second-generation TKIs holds true for patients treated with ponatinib, or whether the potency of this third-generation TKI is able to circumvent the poor outcome for this subgroup of patients.
Methods
Patients
We studied refractory Philadelphia chromosome-positive (Ph+) patients enrolled in the PONATINIB for Chronic Myeloid Leukemia Evaluation and Ph+ Acute Lymphoblastic Leukemia (ALL) (PACE) study. This phase II trial studied the outcome of ponatinib therapy in CML and Ph+ ALL patients who were resistant or intolerant to other TKIs, the design and results of which have been reported previously.9 Among the 381 patients who consented for sensitive mutation analysis, samples collected before initiation of ponatinib therapy (baseline) of 363 patients were assessable by both Sanger sequencing and MS (CP-CML n = 231; accelerated phase [AP-CML] CML, n = 76; blastic phase [BP-CML] ALL, n = 40; Ph+ ALL, n = 16). Minimum follow-up was 22 months.
Mutation analysis
The BCR-ABL1 mutation analyses performed in this study are represented diagrammatically in supplemental Figure 1, available on the Blood Web site. Mutation analysis using Sanger sequencing (detection limit ∼10% mutation allele frequency) was performed at a central laboratory on peripheral blood samples collected at baseline from all patients. RNA was sent to the Adelaide laboratory where complementary DNA was prepared and sensitive multiplexed MS-based mutation analysis was performed retrospectively on duplicate complementary DNA samples as previously described (Agena MassARRAY, custom BCR-ABL1 assay detects 31 mutations with detection limit between 0.05% and 0.5% mutation allele frequency; supplemental Table 1).4 MS analysis was performed without knowledge of the Sanger sequencing results, and mutations were considered present if detected in both replicate samples.
Mutation analysis was also attempted at the central laboratory using Sanger sequencing for all patients who discontinued ponatinib therapy or failed to achieve or lost milestone responses (CP-CML, major cytogenetic response [MCyR] by 12 months; AP-CML, BP-CML, Ph+ ALL, major hematological response by 6 months; 242 patients: CP-CML, n = 134; AP-CML, n = 57; BP-CML, n = 36; Ph+ ALL, n = 15). The median duration between ponatinib discontinuation and sample collection for end-of-treatment mutation analysis was 4 days (range, 288 days before to 244 days after discontinuation; samples were collected >2 weeks after discontinuation for 51 patients). Evaluable postbaseline Sanger sequencing data were obtained for 189 patients (CP-CML, n = 108; AP-CML, n = 47; BP-CML, n = 25, Ph+ ALL, n = 9). These correlative studies were conducted in accordance with the Declaration of Helsinki, and ethics clearance was obtained from the institutional review boards of all participating institutions.
Statistical analyses
We studied the correlation between our sensitive mutation analysis results and the end points of the study. Cumulative incidence of MCyR, complete CyR (CCyR), and major molecular response (MMR) were estimated and tested with Gray’s K-sample test. Fine and Gray regression models were used to assess the effect of baseline variables on cumulative response over time, with treatment discontinuation for any reason as a competing risk.15 Phase-appropriate definitions of progression-free survival (PFS) were adopted from existing literature:16-18 for CP-CML, progression was defined as death, progression to AP-CML or BP-CML, loss of complete hematological response in the absence of CyR, loss of MCyR, and increasing white blood cell count without complete hematological response.16 Failure-free survival (FFS) was defined according to Guilhot et al,19 and included failure of European LeukemiaNet milestone responses and loss of response. Survival was estimated by Kaplan-Meier and differences were examined by log-rank. Cox proportional hazards models were used to assess the significance of baseline variables on survival outcome. Variables significantly correlated (P < .05) with ≥1 outcome measure were included in the multivariate models. Frequencies were compared using the χ2 and Fisher exact tests. Data cutoff was January 2014.
Results
Mutation analysis of samples collected before commencing ponatinib therapy
Mutation analysis of the BCR-ABL1 KD was performed retrospectively using direct Sanger sequencing and sensitive MS for 363 patients before starting ponatinib therapy (baseline). Patient baseline characteristics are shown in supplemental Table 2. Using Sanger sequencing, 243 mutations were detected in 196 patients (54%; CP-CML, n = 114 [49%]; AP-CML, n = 39 [51%]; BP-CML, n = 29 [73%]; Ph+ ALL, n = 14 [88%]). A total of 28 different mutations were detected (Figure 1).
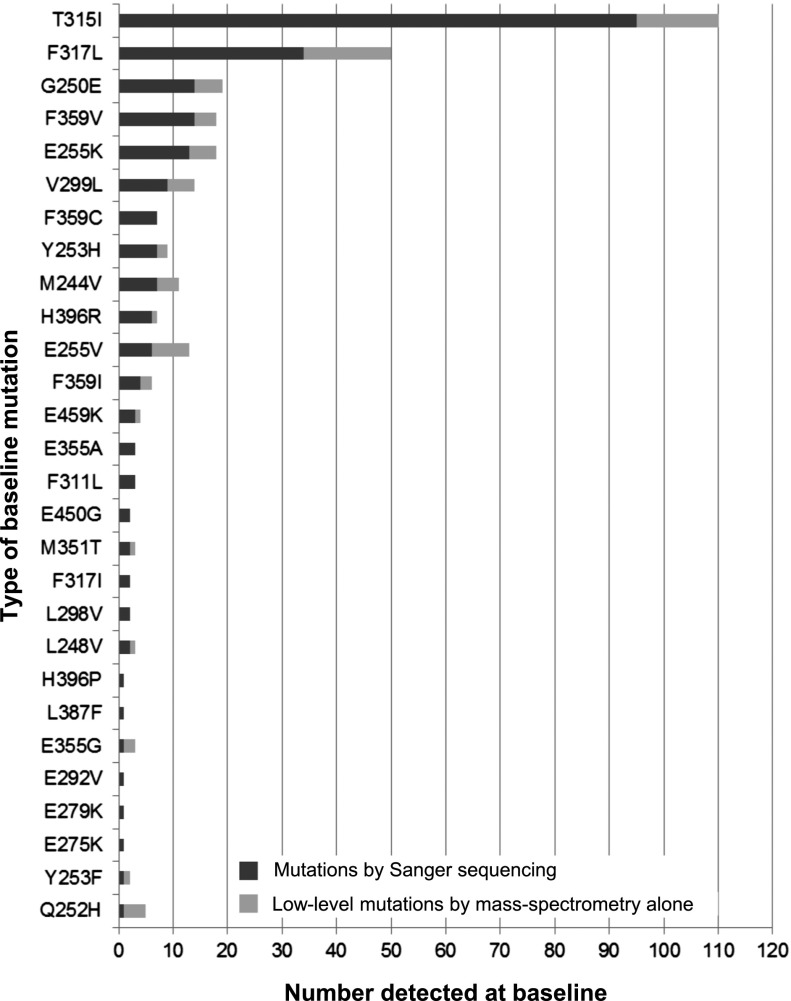
Figure 1.
Type of mutations detected at baseline. Mutations detected before ponatinib initiation (baseline) in 363 patients enrolled in the PACE trial, arranged from the top according to the most frequently detected by Sanger sequencing. Low-level mutations were detected by MS but not by Sanger sequencing, and therefore represent between 0.05% and 10% of the BCR-ABL1 species within the sample.
Of the 243 mutations detected by Sanger sequencing, 233 (96%) were included in the MS assay design and thus potentially detectable.4 All except 6 (2.6%; M244V, L248V, T315I, E355A, F359V, F359I) were detected by MS. We have confirmed retrospectively that the RNA quality in these 6 samples was suboptimal,20 suggesting that the detection discordance is most likely the result of sample degradation during transport from the central laboratory.
In addition to the mutations detected by Sanger sequencing, using our sensitive MS assay,4 with detection limit between 0.05% and 0.2% mutant, we detected 76 mutations present below the detection limit of direct Sanger sequencing (∼10% mutation allele frequency). These mutations are defined as low-level mutations and were detected in 53 patients (15%; CP-CML, n = 28; AP-CML, n = 12; BP-CML, n = 8; Ph+ ALL, n = 5; Table 1 and Figure 1). A total of 17 different low-level mutations were detected. Of these 76 low-level mutations, 20 (26%) had also been detected by Sanger sequencing in historical samples of the same patients.
Table 1.
Longitudinal mutation status of patients with low-level mutations detected by MS at baseline
| Pt ID | Disease phase at study entry | Number of prior TKIs | Mutation history* | Mutation status by Sanger sequencing at baseline | Low-level mutations at baseline† | Months on ponatinib | Outcome | Mutation status by Sanger sequencing at treatment failure or discontinuation (%)‡ |
|---|---|---|---|---|---|---|---|---|
| 109 | CP-CML | 3 | No mutation detected | No mutation detected | E459K | 4.2 | Discontinued therapy§ | Not evaluable |
| 210 | CP-CML | 3 | E255K | E255K | G250E | 10.7 | Discontinued therapy§ | Not evaluable |
| 239 | CP-CML | 3 | M244V, E255K, T315I | E255K, T315I | M244V | 17.6 | Discontinued therapy‖ | Not evaluable |
| 288 | CP-CML | 2 | T315I | No mutation detected | T315I | 21.9 | Discontinued therapy‖ | T315I (20) |
| 35 | CP-CML | 2 | No mutation detected | No mutation detected | F359V | 31.0 | Maintained MCyR | No mutation detected |
| 45 | CP-CML | 2 | E255K, T315I | E255K | E255V, T315I | 24.8 | Maintained MCyR | |
| 215 | CP-CML | 3 | T240M, E255V, T315I | T315I | E255V | 30.7 | Maintained MCyR | |
| 271 | CP-CML | 1 | G250E, T315I | G250E, E255V | M244V, Y253H | 26.3 | Maintained MCyR | |
| 253 | CP-CML | 2 | F317L | F317L | F317L¶ | 27.8 | Maintained MMR | |
| 314 | CP-CML | 1 | T315I | No mutation detected | M244V, G250E, F359I | 28.3 | Maintained MMR | |
| 358 | CP-CML | 3 | Y253F, D276G | Y253F | Q252H, T315I | 28.5 | Maintained MMR | |
| 361 | CP-CML | 3 | M244V, F359V | F359V | E355G | 28.2 | Maintained MMR | |
| 365 | CP-CML | 2 | T315I, F359V | F359V | T315I | 28.4 | Maintained MMR | |
| 255 | CP-CML | 1 | T315I | T315I | F317L | 22.1 | Lost MMR | |
| 381 | CP-CML | 1 | No mutation detected | No mutation detected | E255K | 25.8 | Lost MMR | |
| 216 | CP-CML | 2 | E355A, T315I | E355A | T315I | 30.7 | No MCyR | T315I, E355A (10, 10) |
| 19 | CP-CML | 4 | M351T, F359V | F317L, E450G | F359V | 29.6 | Progressive disease | F317L, E450G (10, 10) |
| 26 | CP-CML | 3 | V299L | V299L | E255K, F317L | 32.2 | Progressive disease | E255K (70) |
| 47 | CP-CML | 2 | T315I, F317L | T315I | F317L | 3.1 | Progressive disease | T315I (100) |
| 96 | CP-CML | 3 | T315I, F359C | T315I, F359C | V299L | 0.9 | Progressive disease | Not evaluable |
| 97 | CP-CML | 3 | No mutation detected | No mutation detected | F317L | 0.9 | Progressive disease | Not evaluable |
| 153 | CP-CML | 3 | No mutation detected | No mutation detected | T315I | 8.2 | Progressive disease | No mutation detected |
| 168 | CP-CML | 3 | T315I | T315I | F317L | 3.9 | Progressive disease | T315I (100) |
| 228 | CP-CML | 2 | T315I, F317L | T315I, F317L | F317L¶ | 8.0 | Progressive disease | T315I (100) |
| 258 | CP-CML | 4 | M244V, F359V | F359V | M244V | 7.3 | Progressive disease | Y253H, F359V (100, 100) |
| 281 | CP-CML | 3 | G250E | No mutation detected | G250E | 18.9 | Progressive disease | G250E (20) |
| 348 | CP-CML | 2 | Y253H, F317L, H396R | F317L, H396R | G250E, T315I | 2.7 | Progressive disease | F317L, H396R (60, 20) |
| 389 | CP-CML | 3 | T315I, F359V | F359V | E255K, T315I | 7.4 | Progressive disease | T315I (100) |
| 83 | AP-CML | 3 | Y253F, E255K, T315I | T315I | Y253F, E255K | 2.1 | Discontinued therapy‖ | T315I (50) |
| 150 | AP-CML | 3 | E355G, L248V | L248V | E355G | 27.5 | Maintained MMR | |
| 292 | AP-CML | 1 | M244V, T315I | M244V, T315I | F359I | 29.2 | Lost MMR | T315I (50) |
| 331 | AP-CML | 3 | T315I, F317L, H396R | H396R | F317L | 28.4 | No MCyR | H396R (50) |
| 127 | AP-CML | 2 | T315I | T315I | E255V | 24.8 | Progressive disease | Not evaluable |
| 130 | AP-CML | 2 | T315I | No mutation detected | T315I | 2.4 | Progressive disease | Not evaluable |
| 143 | AP-CML | 2 | T315I | No mutation detected | F317L | 20.0 | Progressive disease | Not evaluable |
| 208 | AP-CML | 2 | T315I | T315I | E255V, H396R | 6.2 | Progressive disease | T315I (100) |
| 278 | AP-CML | 2 | T315I, F359C | F359C | T315I | 31.0 | Progressive disease | Not evaluable |
| 290 | AP-CML | 3 | T277A, F317L, M351T, L387F | L387F | F317L | 30.9 | Progressive disease | Not evaluable |
| 301 | AP-CML | 2 | T315I | No mutation detected | T315I | 28.6 | Progressive disease | Not evaluable |
| 332 | AP-CML | 3 | No mutation detected | No mutation detected | T315I | 28.4 | Progressive disease | Not evaluable |
| 37 | BP-CML | 2 | T315I | E255K | 3.2 | Discontinued therapy# | T315I (100) | |
| 4 | BP-CML | 2 | T315I | T315I | V299L, F317L | 1.8 | Progressive disease | T315I (100) |
| 163 | BP-CML | 2 | T315I, F359V | T315I, F359V | F317L | 1.2 | Progressive disease | T315I, F359V (100, 100) |
| 1 | BP-CML | 3 | No mutation detected | L248V, G250E, Q252H, F317L, F359V | 0.2 | Death | No mutation detected | |
| 66 | BP-CML | 2 | T315I | E255K, T315I | E255V | 0.7 | Death | Not evaluable |
| 77 | BP-CML | 3 | No mutation detected | E255K | E255V, T315I | 0.0 | Death | Not evaluable |
| 88 | BP-CML | 3 | E255K | E255K | E255V | 2.9 | Death | E255K, E255V (20, 20) |
| 252 | BP-CML | 2 | M244V, F317L | M244V, F317L | F317L¶ | 12.5 | Death | Not evaluable |
| 3 | Ph+ ALL | 2 | F317I | V299L | 5.6 | Progressive disease | E255V, F317I (100, 100) | |
| 93 | Ph+ ALL | 3 | T315I | T315I | M351T | 3.8 | Progressive disease | Not evaluable |
| 144 | Ph+ ALL | 2 | No mutation detected | E255V, T315I | Q252H ×2, V299L ×2, F317L ×2 | 1.0 | Progressive disease | E255V, T315I (100, 60) |
| 171 | Ph+ ALL | 3 | G250E, F317L | G250E, F317L | T315I, F359V | 7.5 | Progressive disease | G250E, Y253H, E255V, T315I, F317L (10, 40, 10, 30, 100) |
| 167 | Ph+ ALL | 2 | T315I | No mutation detected | Y253H, T315I | 15.5 | Death | Not evaluable |
Mutation analysis performed by Sanger sequencing. Mutations detected at low levels at baseline are underlined.
Low-level mutations detected by MS alone (present below the detection limit of Sanger sequencing, ∼10%). Of the mutations detected by Sanger sequencing and included in the MS assay design, all except 6 were detected.
Percent mutant at therapy failure/discontinuation estimated by Sanger sequencing, mutations detected at low levels at baseline are underlined; mutations not detected at baseline by either method (“new mutations”) are bold.
Reason for discontinuation: withdrawal by subject (n = 2).
Reason for discontinuation: adverse event (n = 3).
MS detected 2 different nucleotide substitutions that both resulted in an F317L mutation in these samples, only 1 of which was also detected by Sanger sequencing.
Reason for discontinuation: other (n = 1).
T315I was the most common mutation detected at baseline, and was present at substantially higher frequency than in other cohorts of TKI-resistant patients (an expected finding because the PACE trial stratified patients with T315I detected by Sanger sequencing as a separate enrollment cohort).9 Using MS, we detected T315I in an additional 14 patients (CP-CML, n = 7; AP-CML, n = 4; BP-CML, n = 1; Ph+ ALL, n = 2) compared with Sanger sequencing (T315I was detected in 109 patients [30%] vs 95 [25%]).
As expected from our previous studies of TKI-resistant patients,4 a higher mutation burden was revealed by MS compared with Sanger sequencing: >1 mutation was detected at baseline in 66 patients (18%) vs 43 patients (12%), respectively, with the 2 techniques (Figure 2A). Up to 8 mutations were detected per patient by MS, whereas up to 3 mutations per patient were detected using Sanger sequencing. For the CP-CML patients with >1 mutation detected by MS at baseline (n = 37), compound mutations identified by next-generation sequencing (Ion Torrent) of BCR-ABL1 amplicons11,21,22 are shown in supplemental Table 3. A total of 17 compound mutants were reported in 13 patients, 3 of which involved the T315I mutation.
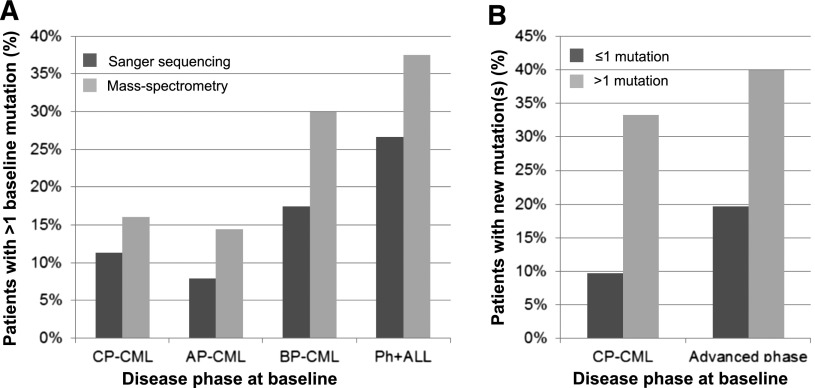
Figure 2.
The association between multiple mutations and acquisition of new mutations during ponatinib therapy. (A) The frequency of patients with multiple BCR-ABL1 KD mutations detected by Sanger sequencing (dark gray bars) or MS (light gray bars) at baseline, according to disease phase at study entry. (B) Rate of new mutations detected by Sanger sequencing after ponatinib failure/discontinuation, according to the disease phase and number of mutations detected by MS at baseline (≤1 mutation, dark gray bars; >1 mutation, light gray bars; advanced phase: AP-CML, BP-CML, and Ph+ ALL combined).
Response to ponatinib in CP-CML patients stratified by the type of BCR-ABL1 mutation detected by MS at baseline
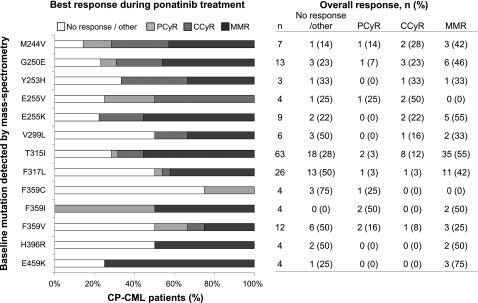
The efficacy of ponatinib for the subset of patients included in this study was similar to that previously reported for the PACE trial as a whole.9 Of 231 CP-CML patients included in our analysis, 137 (59%) achieved MCyR and 109 (47%) experienced treatment failure.19 Response rates did not differ substantially between CP-CML patients with different BCR-ABL1 mutation types (Figure 3).
Figure 3.
Best response achieved in each patient and overall rates of response after ponatinib treatment of CP-CML patients with different BCR-ABL1 KD mutations. Individual mutations detected by MS in at least 2 patients are shown.
Mutational status after ponatinib treatment
To examine the emergence of new BCR-ABL1 mutations during treatment with ponatinib, Sanger sequencing of samples collected after/during ponatinib treatment was attempted for all patients in whom milestone responses were not maintained. Mutation results were obtained for 189 patients. Forty-four new mutations were detected in 34 patients (34/189, 18%) when comparing baseline and treatment failure/discontinuation (postbaseline) Sanger sequencing results (T315I, n = 13; E255K, n = 11; E255V, n = 7; Y253H, n = 4; Q252H, n = 2; and G250E, E275K, L298V, F317L, M351T, F359V, and F359I, n = 1 each). Of these 44 new mutations, 7 were detected at low level by MS at baseline (Table 1; T315I, n = 4; G250E, E255K, and E255V, n = 1 each). Of the remaining 37 mutations, 6 had previously been detected by Sanger sequencing in samples from the same patients collected during prior therapy (T315I, n = 2; E255V, n = 2; Y253H and E275K, n = 1 each); the other 31 mutations had not been previously detected (T315I, n = 7; E255K, n = 10; E255V, n = 4; Y253H, n = 3; Q252H, n = 2; and L298V, F317L, M351T, F359V, and F359I, n = 1 each). This indicates the enrichment or expansion of preexisting mutant clones present below the detection sensitivity of MS23 or the expansion of clones with newly acquired mutations. Clonal enrichment and/or expansion of mutant clones during ponatinib treatment was more frequent in patients with advanced phase disease at ponatinib initiation (CP-CML, 14/108 [13%]; AP-CML, 5/47 [11%]; BP-CML, 9/25 [36%]; Ph+ ALL, 6/9 [67%]). Multiple new mutations (up to 4 per patient) were observed in 7 patients.
Since we demonstrated previously that CP-CML patients with >1 mutation detectable by MS after imatinib resistance were significantly more likely to acquire new mutations during second-line therapy with nilotinib or dasatinib than patients with ≤1 mutation,5 we investigated whether this was also the case for patients treated with ponatinib. Segregating patients according to the number of baseline mutations detected by MS, we found that those with >1 mutation at baseline were also more likely to gain new mutations during ponatinib therapy than those with ≤1 mutation: 33% (5/15) of CP-CML patients with >1 mutation gained new mutations compared with only 10% (9/93) of CP-CML patients with ≤1 mutation (P = .05). This was also observed for patients with advanced phase disease (AP-CML, BP-CML, and Ph+ ALL combined): 40% (8/20) vs 20% (12/61), respectively (P = .18), Figure 2B.
Response to ponatinib in CP-CML patients stratified by the number of BCR-ABL1 mutations detected by MS at baseline
Because we have previously demonstrated that the number of mutations detected by MS after imatinib failure is predictive of response to treatment with nilotinib or dasatinib as second-line therapy in CP-CML patients, we examined whether the number of baseline mutations was also associated with ponatinib treatment responses and outcome. Because of the differences in study inclusion criteria for patients with and without the T315I mutation leading to confounding baseline risk factors,9 we analyzed patients with this mutation separately. Patients within each disease phase were subdivided into the following 5 subgroups based on the presence of (1) no mutation, (2) 1 mutation (not T315I), (3) >1 mutation (not T315I), (4) T315I alone, or (5) T315I plus ≥1 additional mutation(s) at baseline (supplemental Table 4). The cumulative incidence of MCyR, CCyR, and MMR, and the probability of PFS and FFS were compared between subgroups.
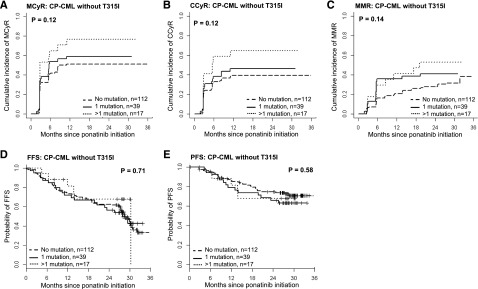
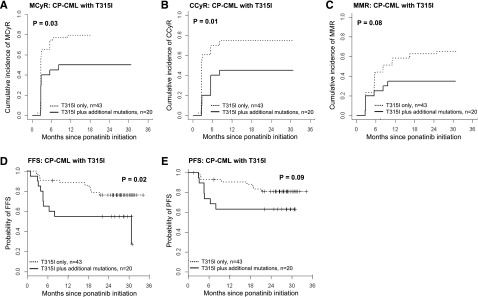
For patients with advanced phase disease, there were no significant differences in outcome according to baseline mutational status (supplemental Figures 2 and 3). Among CP-CML patients, however, significant differences in outcome were observed (Figures 4 and 5; supplemental Figure 4). Among the 231 CP-CML patients, T315I was not detectable at baseline by MS in 168 patients (73%): 112 (48%) had no detectable mutation at baseline, 39 (17%) had 1 mutation, and 17 (7%) had >1 mutation. Outcomes and responses, stratified by number of baseline mutations, are shown in Figure 4; no significant differences between subgroups were observed. T315I was detectable by MS at baseline in 63/231 CP-CML patients (27%). Those with T315I as the sole mutation detected (n = 43, 19%) had significantly better responses and outcome compared with those with T315I plus additional mutation(s) (n = 20, 9%; Figure 5). The cumulative incidence of MCyR by 12 months and CCyR and MMR by 18 months for CP-CML patients with T315I only was 79%, 74%, and 63%, respectively, whereas it was only 50%, 45%, and 35%, respectively, for those with T315I plus additional mutation(s) (P = .03, P = .01, and P = .07, respectively). The probability of FFS and PFS at 18 months was 86% and 88% for CP-CML patients with T315I only, compared with 55% and 63% for those with T315I plus additional mutation(s), respectively (P = .02 and P = .09, respectively).
Figure 4.
The impact on outcome of the number of mutations detected by MS at baseline for CP-CML patients without the T315I mutation. CP-CML patients without T315I by MS were grouped according to the number of mutations detected by MS before commencing ponatinib therapy: no mutation, n = 112; 1 mutation, n = 39; >1 mutation, n = 17. (A) MCyR. (B) CCyR. (C) MMR. (D) FFS. (E) PFS.
Figure 5.
The impact on outcome of the number of mutations detected by MS at baseline for CP-CML patients with the T315I mutation. CP-CML patients with T315I by MS were grouped according to the number of mutations detected by MS before commencing ponatinib therapy: T315I alone, n = 43; T315I plus additional mutation(s), n = 20. (A) MCyR. (B) CCyR. (C) MMR. (D) FFS. (E) PFS.
Response to ponatinib in CP-CML patients stratified by the number of BCR-ABL1 mutations detected by Sanger sequencing at baseline
For CP-CML patients, we also assessed the association between treatment response and the number of mutations detectable at baseline by Sanger sequencing. The same trends were observed as described previously when mutation analysis was performed by MS(supplemental Figure 4). However, the differences in treatment responses between subgroups were not statistically significant because fewer patients were identified by Sanger sequencing as having T315I plus additional mutation(s) (subgroup 5, n = 14, when mutation analysis performed by Sanger sequencing, compared with n = 20, when MS was used).
Correlation between baseline clinical and molecular factors and treatment response for CP-CML patients with the T315I mutation
The baseline clinical and molecular factors that may influence treatment response for CP-CML patients with T315I by MS were examined by univariate regression analyses. These results are summarized in Table 2. Baseline mutational status by MS was the only variable with a statistically significant correlation with the achievement of MCyR, CCyR, and FFS: CP-CML patients with T315I as the sole mutation detected by MS had significantly superior probability of achieving these treatment outcomes than those with T315I plus additional mutation(s). Furthermore, CP-CML patients with T315I plus additional mutation(s) also had inferior PFS and MMR, although these differences were not statistically significant because of the small number of events. Trends were also seen for superior CCyR with younger age at study entry, and for inferior FFS with a higher number of prior TKI therapies. However, for all treatment outcome analyses, the correlation was strongest with respect to mutational status by MS. Conclusions and inference on the presence of T315I alone vs T315I plus additional mutation(s) did not change when tested using a multivariate model comprising the factors used in the univariate analyses (data not shown).
Table 2.
Prognostic significance of baseline molecular and clinical factors for therapeutic outcome prediction for CP-CML patients with T315I by univariate regression analysis
| Clinical and molecular factors | MCyR | CCyR | MMR | FFS | PFS | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCyR by 12 mo (%)* | No. of events | HR† (95% CI) | P | CCyR by 18 mo (%)* | No. of events | HR† (95% CI) | P | MMR by 18 mo (%)* | No. of events | HR† (95% CI) | P | FFS at 18 mo (%)* | No. of events | HR† (95% CI) | P | PFS at 18 mo (%)* | No. of events | HR† (95% CI) | P | |
| Age at study entry, y | .18 | .07 | .12 | .13 | .21 | |||||||||||||||
| <54 (median), n = 31 | 77 | 24 | 1 | 77 | 24 | 1 | 65 | 20 | 1 | 84 | 7 | 1 | 90 | 5 | 1 | |||||
| ≥54 (median), n = 32 | 63 | 20 | 0.69 (0.40-1.19) | 53 | 17 | 0.58 (0.32-1.04) | 44 | 15 | 0.6 (0.31-1.15) | 69 | 13 | 2.02 (0.80-5.12) | 72 | 10 | 2 (0.68-5.86) | |||||
| No. of prior TKI therapies | .16 | .25 | .2 | .07 | .37 | |||||||||||||||
| 1, n = 11 | 91 | 10 | 1 | 82 | 9 | 1 | 73 | 9 | 1 | 100 | 1 | 1 | 100 | 1 | 1 | |||||
| 2, n = 29 | 76 | 22 | 0.86 (0.51-1.46) | 72 | 21 | 0.95 (0.51-1.75) | 48% | 14 | 0.54 (0.26-1.09) | 79 | 8 | 3.46 (0.43-27.72) | 79 | 7 | 3.11 (0.38-25.30) | |||||
| 3, n = 23 | 52 | 12 | 0.51 (0.25-1.02) | 48 | 11 | 0.54 (0.25-1.18) | 52 | 12 | 0.62 (0.29-1.34) | 61 | 11 | 6.8 (0.88-52.82) | 72 | 7 | 4.11 (0.50-33.41) | |||||
| Years since diagnosis | .55 | .54 | .18 | .32 | .42 | |||||||||||||||
| <5 (median), n = 31 | 77 | 24 | 1 | 70 | 22 | 1 | 65 | 21 | 1 | 84 | 8 | 1 | 87 | 6 | 1 | |||||
| ≥5 (median), n = 32 | 63 | 20 | 0.85 (0.49-1.47) | 59 | 19 | 0.84 (0.47-1.48) | 44 | 14 | 0.63 (0.32-1.24) | 69 | 12 | 1.58 (0.64-3.86) | 75 | 9 | 1.52 (0.54-4.28) | |||||
| Average daily ponatinib dose | .52 | .8 | .3 | .59 | .89 | |||||||||||||||
| ≤15 mg/day, n = 3 | 33 | 1 | 1 | 33 | 1 | 1 | 33 | 1 | 1 | 67 | 2 | 1 | 67 | 1 | 1 | |||||
| 15-30 mg/day, n = 20 | 75 | 16 | 2.77 (0.26-29.9) | 70 | 14 | 2.21 (0.21-23.8) | 45 | 9 | 1.32 (0.15-11.6) | 75 | 5 | 0.429 (0.083-2.22) | 80 | 4 | 0.63 (0.070-5.61) | |||||
| >30 mg/day, n = 40 | 68 | 27 | 2.16 (0.20-23.1) | 65 | 26 | 2.05 (0.19-21.7) | 60 | 25 | 2.23 (0.27-18.4) | 77 | 13 | 0.551 (0.12-2.45) | 82 | 10 | 0.79 (0.10-6.17) | |||||
| Mutation status by MS | .03 | .01 | .08 | .02 | .09 | |||||||||||||||
| T315I alone, n = 43 | 79 | 34 | 1 | 74 | 32 | 1 | 63 | 28 | 1 | 86 | 10 | 1 | 88 | 7 | 1 | |||||
| T315I plus additional mutation(s), n = 20 | 50 | 10 | 0.48 (0.25-0.94) | 45 | 9 | 0.42 (0.21-0.82) | 35 | 7 | 0.46 (0.20-1.09) | 55 | 10 | 2.72 (1.13-6.55) | 63 | 8 | 2.35 (0.85-6.50) | |||||
Point estimates of response by 12 (MCyR) or 18 (CCyR, MMR, FFS, PFS) mo.
The 95% confidence interval (95% CI) is shown for the hazard ratio (HR).
Discussion
Ponatinib has efficacy in Ph+ leukemia patients who are resistant to other TKIs,9 and currently represents the only TKI-based therapeutic option for patients with T315I-mutant BCR-ABL1. However, treatment responses are variable, and causal baseline factors are not well-understood. We have developed an MS-based assay that can sensitively detect BCR-ABL1 KD mutations present at levels between 10- and 100-fold below the detection limit of conventional Sanger sequencing.4 Using this method, we have previously demonstrated that the type and number of mutations detectable after imatinib resistance correlated with subsequent response to nilotinib/dasatinib therapy, regardless of the abundance of the mutants within a sample.5 Importantly, we observed that compared with patients with ≤1 mutation, patients with >1 mutation that was considered clinically sensitive6 to the second-line TKI that they received had substantially inferior treatment responses and were more likely to acquire new resistant mutations during nilotinib/dasatinib therapy.5 This suggests that the presence of multiple BCR-ABL1 KD mutations detectable by MS may indicate the presence of vast intratumoral genetic heterogeneity among leukemic subclones. The coexistence of multiple genetically diverse leukemic subclones may then serve as a reservoir for selection of drug-resistance subclones. Intratumoral heterogeneity has been linked to disease progression and treatment outcome in hematological and solid tumors24,25 and may be associated with longer disease duration or aggressiveness.
Because of the significant association observed between the number of mutations detectable before second-line treatment with second-generation TKIs and subsequent treatment response, we examined the clinical significance of low-level mutations present before initiation of ponatinib therapy (baseline) in patients enrolled in the PACE study. Although the relationship between baseline mutational status and response to ponatinib treatment is complex, we demonstrate that the number of mutations detected at baseline by MS is associated with treatment response and outcome, and could be used to refine the therapeutic approach.
In contrast to first- and second-generation TKIs, no specific individual BCR-ABL1 KD mutation was associated with inferior outcome with ponatinib therapy. Treatment responses were achieved in CP-CML patients with and without mutations (Figures 3-5), and mutational status at ponatinib discontinuation or treatment failure largely reflected mutations present at study entry. Interestingly, CP-CML patients with T315I had the highest response rates (Figure 3; 45/63, 71%, achieved MCyR), but this was also the most common new mutation detected after ponatinib treatment (n = 13; CP-CML, n = 7). Conversely, although CP-CML patients with the F359C mutation had the lowest response rates (1/4, 25%, achieved MCyR), no new F359C mutations were detected. Moreover, F359C was detected at baseline in 3 advanced phase patients (1 AP-CML, 2 BP-CML), 2 of whom achieved and maintained responses (MMR: AP-CML; MCyR: BP-CML). This suggests that the mutation does not inherently confer poor response, but may merely be present within a clone with an alternative mechanism of resistance. Indeed, in 2 of the CP-CML patients with F359C who did not achieve MCyR, T315I was also detected at baseline. This is consistent with our finding that the presence of T315I plus additional mutation(s) is the most significant prognostic baseline factor of the factors tested for poor outcome in CP-CML patients.
Low-level mutations were detected at baseline in 15% of patients. Comparing the low-level mutants with the mutations detected after ponatinib therapy by Sanger sequencing, we found that the majority of low-level mutants did not undergo clonal expansion. Among the 23 patients with low-level mutants for whom Sanger sequencing was performed at ponatinib failure/discontinuation (Table 1), 7/38 (18%) low-level mutants clonally expanded during ponatinib therapy. Interestingly, in our previous study, low-level nilotinib/dasatinib sensitive mutants that were detected after imatinib resistance expanded during subsequent treatment with nilotinib/dasatinib at a similar rate (12/64, 19%).5 Although the expanded low-level BCR-ABL1 mutants do have slightly lower in vitro sensitivity compared with unmutated BCR-ABL1, other resistance mechanisms are likely to have contributed to nilotinib/dasatinib failure in these instances (possibly including BCR-ABL1–independent mechanisms).5 For 4 of the 7 patients in the current study with low-level mutants that clonally expanded during ponatinib treatment, the existence of these mutants had been demonstrated to predate ponatinib initiation. Oscillating selection, deselection, and reselection of mutant clones with changing or stopping TKI therapy has been reported previously,23,26,27 and is in accordance with lower in vitro ponatinib sensitivity of these mutants compared with unmutated BCR-ABL1.12,13 In the other 3/7 patients in whom a low-level mutant expanded during ponatinib therapy (Table 1: patients 216, 88, 172) more than 1 BCR-ABL1 KD mutation was detected at ponatinib failure/discontinuation. Although the methods used to detect these mutations cannot definitively determine if they are in the same (compound) or different cells, the frequency of these mutations within the sample (inferred by peak heights of Sanger sequencing chromatograms) suggest that the low-level mutations may have been selected within the context of a compound mutation. Certain compound mutants, most of which include T315I or E255K/V, have been shown to confer potent ponatinib resistance and are clinically associated with treatment failure in patients with advanced phase disease.12 However, next-generation sequencing of BCR-ABL1 amplicons at baseline11 for CP-CML patient 216 did not identify compound mutations (supplemental Table 3).
At baseline, 18% of patients had >1 mutation detected using MS. Similar to our previous study of imatinib-resistant patients,4,5 multiple mutations were more common in patients with advanced phase disease (Figure 2A). The frequency of patients with multiple mutations in our current study, however, was lower than in our earlier study4 (CP-CML, 16% vs 22%; AP-CML, 14% vs 34%; BP-CML/Ph+ ALL, 32% vs 36% of patients had >1 mutation in the current vs previous study, respectively). The patients examined in our current study had been previously treated with up to 4 TKIs (median, 3), whereas the patients examined in our earlier study had only received 1 TKI (imatinib). Thus, a possible explanation for the difference in frequency of multiple mutations in these 2 cohorts is that because the potent second-generation TKIs are vulnerable to a more narrow range of BCR-ABL1 KD mutations compared with imatinib, fewer BCR-ABL1-KD mutant clones coexist at levels detectable using our mutation analysis assay after treatment with second-generation TKIs.
New mutations were detected after ponatinib treatment in 34 patients. Similar to our previous studies,5 we found that patients with >1 baseline mutation by MS were significantly more likely to gain new mutations than patients with ≤1 mutation. Patients with BP-CML/Ph+ ALL and >1 mutation at baseline were therefore at greatest risk of acquiring new mutations. For CP-CML patients with ≤1 mutation at baseline, it appears that ponatinib may be associated with a lower incidence of new mutation acquisition when compared with our earlier study of nilotinib/dasatinib-treated patients. For all other patient subgroups, however, the incidence of new mutation acquisition was similar in both studies. For instance, 21% of CP-CML patients with ≤1 mutation at baseline acquired new resistant mutations during treatment with nilotinib/dasatinib,5 whereas in the PACE study, new mutations were detected in only 10% of CP-CML patients with ≤1 mutation. However, mutation analysis was performed at ≥1 time point after commencing nilotinib/dasatinib for all patients in our earlier study,5 regardless of their outcome, whereas mutational status after ponatinib treatment was available for only 93/194 CP-CML patients with ≤1 mutation (48%). Moreover, for 31/93 patients (33%), samples for analysis were collected >2 weeks after ponatinib discontinuation and previous studies have shown that mutations can rapidly become undetectable after stopping TKI therapy.23,26 Therefore, the number of mutations gained during ponatinib therapy may have been underestimated.
The relationship between baseline factors and the ultimate success of ponatinib therapy in the salvage setting is complex, and therefore definitive identification of the major determinants of variation in treatment response is difficult for this heavily pretreated patient population. This task is complicated by the high correlation between clinical variables, such as the number of previous TKI therapies and time since diagnosis. Nevertheless, our data allowed for some firm conclusions to be made. CP-CML patients with T315I tended to have better treatment responses. However, patients in the T315I cohort were also more likely to have other favorable prognostic characteristics, such as younger age, higher dose intensity, and fewer prior TKI therapies (supplemental Table 2).9,28 This is likely a result of the study design, resulting in different demographics of the T315I and non-T315I patients. To reduce confounding variables, we segregated our analyses based on the presence of T315I at baseline. For CP-CML patients with T315I, our previous findings demonstrating that patients with multiple mutations have inferior treatment responses held true. In this patient group, our multivariate analyses showed that the number of mutations detectable by MS at baseline is the most significant prognostic factor for treatment outcome of the factors tested. This suggests that ponatinib may be more effective in cases in which T315I-mutant BCR-ABL1 is the only identified cause of resistance to prior therapy; multiple mutations in this context may indicate greater underlying clonal diversity as a result of greater genomic instability or longer duration since diagnosis, both of which are important factors in development of treatment resistance. As yet unknown cooperative relationships may also exist between different BCR-ABL1 mutants, which could contribute toward treatment resistance.
For CP-CML patients without T315I, the relationship between baseline mutational status and treatment response is more complex, perhaps from confounding interactions between clinical and molecular factors for this heavily pretreated cohort. The MMR rate and the probability of FFS and PFS for the subgroups of CP-CML patients without T315I were similar; baseline BCR-ABL1 KD mutational status appears to have no impact on response. Therefore, for CP-CML patients without T315I, the association between multiple mutations and inferior response previously observed for nilotinib or dasatinib therapy5 is not seen for ponatinib therapy (MMR by 18 months for CP-CML patients with >1 baseline mutations treated with nilotinib/dasatinib vs ponatinib: 2/15 [13%] vs 8/17 [47%]). This suggests that the poor response conferred by multiple mutations may be overcome by ponatinib therapy. When comparing treatment responses for CP-CML patients in the current analysis with our previous analyses of nilotinib/dasatinib treated patients, responses to ponatinib compared favorably for all CP-CML patient subgroups.5 As shown previously,9 this is particularly significant for patients with T315I, present at either a clonal or low level (low-level T315I was detectable in 3.5% of the CP-CML patients in this study). Additionally, although responses to ponatinib for patients with T315I plus additional mutation(s) are inferior to those with T315I alone, they are encouraging when compared with other treatments currently available for patients with this mutation.29 Moreover, they compare favorably to responses to second-line therapy with nilotinib/dasatinib for CP-CML patients with multiple sensitive mutations (CCyR by 18 months: 9/20 [45%] vs 5/15 [33%]; MMR by 18 months: 7/20 [35%] vs 2/15 [13%], with ponatinib vs nilotinib/dasatinib treatment, respectively).5
In summary, although CP-CML patients with the T315I mutation tended to have better responses to ponatinib treatment overall, those with T315I plus additional mutation(s) (32% of CP-CML patients with the T315I mutation) had significantly inferior responses and outcome compared with those with T315I as the sole mutation detected by MS at baseline. Consequently, these patients may benefit from close monitoring, experimental approaches, or stem cell transplantation to reduce the risk of TKI failure. In contrast, for CP-CML patients without T315I, the poor responses to nilotinib or dasatinib therapy previously observed for patients with >1 baseline BCR-ABL1 mutation were not seen with ponatinib therapy. This suggests that ponatinib may be a particularly effective option for patients with multiple mutations detected by MS at baseline. This demonstrates the utility of sensitive mutation analysis to offer information to guide therapy adjustment after TKI failure, particularly in identifying patients with either low-level T315I or multiple BCR-ABL1 KD mutations for whom the high risk of treatment failure with second-generation TKIs may be overcome by treatment with more potent TKIs such as ponatinib.
Acknowledgments
We thank the patients who contributed samples for this study. The Centre for Cancer Biology is an alliance between SA Pathology and the University of South Australia.
This work was supported by ARIAD Pharmaceuticals, including research support (S.B. and T.P.H.); a scholarship from the Leukaemia Foundation of Australia and the AR Clarkson Foundation (D.T.Y.); and a postdoctoral fellowship from the Leukaemia Foundation of Australia/Cure Cancer Australia (W.T.P.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: W.T.P. contributed to experimental design, data generation and analysis, and wrote the manuscript; A.L.Y., H.K.A., B.A.J., and C.R.F. contributed to data generation; J.G.H. and V.M.R. provided clinical data and Sanger and next-generation sequencing results; S.L. contributed to data analysis; and D.T.O.Y., T.P.H., and S.B. contributed to experimental design, data analysis, and manuscript preparation.
Conflict-of-interest disclosure: S.B. and T.P.H. are advisory board members and have received research funding and honoraria from Novartis, Bristol-Myers Squibb, and ARIAD Pharmaceuticals. D.T.O.Y. has received research funding from Novartis and Bristol-Myers Squibb and is an advisory board member for ARIAD Pharmaceuticals. J.G.H., S.L., and V.M.R. are employees of ARIAD Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Wendy Parker, ACRF Cancer Genomics Facility, Centre for Cancer Biology, SA Pathology, PO Box 14 Rundle Mall, Adelaide, SA 5000, Australia; e-mail: wendy.parker@sa.gov.au.
References
- 1.Hughes T, Saglio G, Branford S, et al. Impact of baseline BCR-ABL mutations on response to nilotinib in patients with chronic myeloid leukemia in chronic phase. J Clin Oncol. 2009;27(25):4204–4210. doi: 10.1200/JCO.2009.21.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Müller MC, Cortes JE, Kim D-W, et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: analysis of responses according to preexisting BCR-ABL mutations. Blood. 2009;114(24):4944–4953. doi: 10.1182/blood-2009-04-214221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soverini S, Hochhaus A, Nicolini FE, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118(5):1208–1215. doi: 10.1182/blood-2010-12-326405. [DOI] [PubMed] [Google Scholar]
- 4.Parker WT, Lawrence RM, Ho M, et al. Sensitive detection of BCR-ABL1 mutations in patients with chronic myeloid leukemia after imatinib resistance is predictive of outcome during subsequent therapy. J Clin Oncol. 2011;29(32):4250–4259. doi: 10.1200/JCO.2011.35.0934. [DOI] [PubMed] [Google Scholar]
- 5.Parker WT, Ho M, Scott HS, Hughes TP, Branford S. Poor response to second-line kinase inhibitors in chronic myeloid leukemia patients with multiple low-level mutations, irrespective of their resistance profile. Blood. 2012;119(10):2234–2238. doi: 10.1182/blood-2011-08-375535. [DOI] [PubMed] [Google Scholar]
- 6.Branford S, Melo JV, Hughes TP. Selecting optimal second-line tyrosine kinase inhibitor therapy for chronic myeloid leukemia patients after imatinib failure: does the BCR-ABL mutation status really matter? Blood. 2009;114(27):5426–5435. doi: 10.1182/blood-2009-08-215939. [DOI] [PubMed] [Google Scholar]
- 7.Apperley JF. Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007;8(11):1018–1029. doi: 10.1016/S1470-2045(07)70342-X. [DOI] [PubMed] [Google Scholar]
- 8.Nicolini FE, Ibrahim AR, Soverini S, et al. The BCR-ABLT315I mutation compromises survival in chronic phase chronic myelogenous leukemia patients resistant to tyrosine kinase inhibitors, in a matched pair analysis. Haematologica. 2013;98(10):1510–1516. doi: 10.3324/haematol.2012.080234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. PACE Investigators. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbons DL, Pricl S, Posocco P, et al. Molecular dynamics reveal BCR-ABL1 polymutants as a unique mechanism of resistance to PAN-BCR-ABL1 kinase inhibitor therapy. Proc Natl Acad Sci USA. 2014;111(9):3550–3555. doi: 10.1073/pnas.1321173111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deininger MW, Shah NP, Cortes JE, et al. Impact of baseline (BL) mutations, including low-level and compound mutations, on ponatinib response and end of treatment (EOT) mutation analysis in patients (pts) with chronic phase chronic myeloid leukemia (CP-CML) [abstract]. Blood. 2013;122(21) Abstract 652. [Google Scholar]
- 12.Zabriskie MS, Eide CA, Tantravahi SK, et al. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell. 2014;26(3):428–442. doi: 10.1016/j.ccr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16(5):401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367(22):2075–2088. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. 2010;45(9):1388–1395. doi: 10.1038/bmt.2009.359. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien SG, Guilhot F, Larson RA, et al. IRIS Investigators. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 17.Apperley JF, Cortes JE, Kim DW, et al. Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: the START a trial. J Clin Oncol. 2009;27(21):3472–3479. doi: 10.1200/JCO.2007.14.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354(24):2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 19.Guilhot J, Baccarani M, Clark RE, et al. Definitions, methodological and statistical issues for phase 3 clinical trials in chronic myeloid leukemia: a proposal by the European LeukemiaNet. Blood. 2012;119(25):5963–5971. doi: 10.1182/blood-2011-10-383711. [DOI] [PubMed] [Google Scholar]
- 20.Branford S, Hughes T. Detection of BCR-ABL mutations and resistance to imatinib mesylate. Methods Mol Med. 2006;125:93–106. doi: 10.1385/1-59745-017-0:93. [DOI] [PubMed] [Google Scholar]
- 21.Deininger M, Cortes J, Kim D, et al. Impact of baseline mutations on response to ponatinib and end of treatment mutation analysis in patients with chronic myeloid leukemia [abstract]. J Clin Oncol. 2013;31(suppl) Abstract 7001. [Google Scholar]
- 22.Deininger MW, Hodgson JG, Shah NP, et al. Compound mutations in BCR-ABL1 are not major drivers of primary or secondary resistance to ponatinib in CP-CML patients. Blood. 2016;127(6):703–712. doi: 10.1182/blood-2015-08-660977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker WT, Yeoman AL, Jamison BA, et al. BCR-ABL1 kinase domain mutations may persist at very low levels for many years and lead to subsequent TKI resistance. Br J Cancer. 2013;109(6):1593–1598. doi: 10.1038/bjc.2013.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mroz EA, Rocco JW. MATH, a novel measure of intratumor genetic heterogeneity, is high in poor-outcome classes of head and neck squamous cell carcinoma. Oral Oncol. 2013;49(3):211–215. doi: 10.1016/j.oraloncology.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SY, Gönen M, Kim HJ, Michor F, Polyak K. Cellular and genetic diversity in the progression of in situ human breast carcinomas to an invasive phenotype. J Clin Invest. 2010;120(2):636–644. doi: 10.1172/JCI40724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanfstein B, Müller MC, Kreil S, et al. Dynamics of mutant BCR-ABL-positive clones after cessation of tyrosine kinase inhibitor therapy. Haematologica. 2011;96(3):360–366. doi: 10.3324/haematol.2010.030999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gruber FXE, Lamark T, Anonli A, et al. Selecting and deselecting imatinib-resistant clones: observations made by longitudinal, quantitative monitoring of mutated BCR-ABL. Leukemia. 2005;19(12):2159–2165. doi: 10.1038/sj.leu.2403983. [DOI] [PubMed] [Google Scholar]
- 28.Mauro MJ, Cortes JE, Kim D-W, et al. Multivariate analyses of the clinical and molecular parameters associated with efficacy and safety in patients with chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) treated with ponatinib in the PACE trial [abstract]. Blood. 2012;120(21) Abstract 3747. [Google Scholar]
- 29.Nicolini FE, Mauro MJ, Martinelli G, et al. Epidemiologic study on survival of chronic myeloid leukemia and Ph(+) acute lymphoblastic leukemia patients with BCR-ABL T315I mutation. Blood. 2009;114(26):5271–5278. doi: 10.1182/blood-2009-04-219410. [DOI] [PMC free article] [PubMed] [Google Scholar]