Abstract
Whereas the role of sphingosine 1-phosphate receptor 1 (S1PR1) in T cell egress and the regulation of S1P gradients between lymphoid organs and circulatory fluids in homeostasis are increasingly well-understood, much remains to be learned about S1P signaling and distribution during an immune response. Recent data suggest that the role of S1PR1 in directing cells from tissues into circulatory fluids is reprised again and again, particularly in guiding activated T cells from non-lymphoid tissues into lymphatics. Conversely, S1P receptor 2 (S1PR2), which antagonizes migration towards chemokines, confines cells within tissues. Here we review the current understanding of the roles of S1P signaling in activated T cell migration. In this context, we outline open questions, particularly regarding the shape of S1P gradients in different tissues in homeostasis and inflammation, and discuss recent strategies towards measuring S1P.
Establishing tissue residence
A basic question in immune cell migration is how a cell decides whether to stay in a tissue – surveying for antigen, becoming activated, or performing effector functions – or to move on. This decision fundamentally shapes the course of the immune response.
A series of elegant experiments, which has been previously reviewed, established that S1PR1 guides lymphocytes out of lymphoid organs into blood and lymph in response to the high concentration of S1P at exit sites[1–3]. Briefly, a natural products screen for immune suppressive compounds that inhibited the mixed lymphocyte reaction yielded the fungal metabolite myriocin. To reduce toxicity, myriocin was modified to FTY720, which effectively blocked transplant rejection in vivo at concentrations that were ineffective in the mixed lymphocyte reaction[4, 5]. Two observations that held the key to the mechanism were (1) that FTY720 depletes lymphocytes from efferent lymphatics in the lymph nodes, suggesting that FTY720 blocks lymph node exit, and (2) that FTY720 is phosphorylated in vivo and phospho-FTY720 binds four of five S1P receptors[6, 7]. Fetal liver chimeras, conditional knockouts, and adoptive transfer experiments revealed that cell-intrinsic S1PR1 expression is required for T cell egress from the thymus and secondary lymphoid organs[8, 9]. Subsequent studies showed that S1PR1 guides T cells from the low-S1P environment of the lymphoid organs towards high S1P concentrations at the sites of exit into blood and lymph. An investigation of why high doses of caramel food coloring cause lymphopenia revealed that maintenance of low concentrations of interstitial S1P within lymphoid organs by S1P lyase is required for lymphocyte egress[10], while conditional knockouts of the two sphingosine kinases that make S1P revealed that high levels of S1P in circulatory fluids are also required for exit[11].
Most of this early work was in homeostasis, but S1P signaling also regulates activated T cell exit from lymph nodes. FTY720 is FDA-approved for treatment of multiple sclerosis, and next-generation drugs targeting S1P receptors have shown promise in a range of diseases including colitis and psoriasis[12]. One important reason for these drugs’ efficacy is that they prevent activated T cells from exiting the lymphoid organs and accessing inflamed tissues. Yet much remains to be learned about how S1P signaling and S1P gradients are regulated during an immune response. How does the distribution of cues that promote egress or tissue retention change? Do different T cell subsets integrate these cues differently? How does the role of S1P in egress differ between lymphoid and non-lymphoid tissues? This review will discuss recent discoveries about the role of S1P signaling in activated T cell migration and will highlight some important outstanding questions, particularly about the location and regulation of S1P gradients that guide T cells.
S1P signaling and T cell exit from lymphoid organs
One key issue is how both naïve and activated T cells balance cues that retain them within lymph nodes versus S1P signals that guide them out of lymph nodes into circulation. In lymph nodes, naïve T cells can continue to survey for antigen, T cells that have encountered antigen can continue their activation, and some subsets of T cells such as follicular T helper cells can exert effector function. In circulation, naïve T cells can travel elsewhere in search of antigen, and activated T cells can migrate to the site of infection.
A classic study showed that signaling through the chemokine receptor CCR7 counteracts S1PR1’s role in promoting egress [13]. Ccr7+/− naïve T cells exit the lymph nodes more quickly than WT, and the failure of S1PR1-deficient T cells to exit lymph nodes can be partially rescued by treatment of the cells with pertussis toxin, which inhibits all Gi protein coupled receptor signaling. In line with these findings, treatment of chronic lymphoblastic leukemia patients with the Btk inhibitor ibrutinib induces lymph node shrinkage and concomitant transient lymphocytosis, which is associated with upregulation of S1PR1 and downregulation of CCR7 on peripheral blood B cells[14]. Recently, it has been demonstrated that the chemokine receptor CXCR4 acts with CCR7 to retain T cells within lymph nodes [15]. Notably, while CXCR4 retains both T and B cells in lymph nodes, in Peyer’s patches CXCR4 brings B cells in proximity to the efferent lymphatics and promotes B cell exit; this result highlights the importance of considering differences in the anatomy of each lymphoid tissue [16]. The integrin LFA-1 appears to favor response to lymph node chemokines over S1P, as Itgal−/− T cells exit lymph nodes faster than WT and Itgal−/− T cells probing lymphatic vessels more frequently cross into lymph than their WT counterparts [17]. While dynamin 2, a component of the endocytic pathway, regulates the response of both S1PR1 and CXCR4, the dominant effect of dynamin 2 deficiency in T cells is an exit block[18].
Much ongoing work addresses how the balance of cues shifts in inflammation, either because of changes in expression of homing receptors or of their ligands. A canonical feature of T cell activation is altered expression of chemokine receptors, which enable T cells to perform their effector function in the correct location [19]. Loss of CCR7 upon activation likely contributes to accelerated exit from lymph nodes, although this effect may be mitigated in some settings. For example, recent work showed that β2 adrenergic receptors physically couple with both CCR7 and CXCR4, enhance signaling through these receptors, and restrain both naïve and activated T cell exit [15]. IFNγ in infected lymph nodes reduces expression of CCL21 (one of two CCR7 ligands) and CXCL13 (the CXCR5 ligand); this results in reduced homing of naïve lymphocytes to infected nodes, and likely increased lymphocyte egress [20]. It is less clear how acquisition of receptors for inflammatory, non-homeostatic chemokines may affect exit rates, which will likely vary depending on the distribution of the corresponding chemokines relative to exit sites. An important study showed that regulatory T cell depletion after herpes simplex virus infection caused substantial elevation of pro-inflammatory chemokines in the draining lymph nodes. This was associated with delayed arrival of lymphocytes to the mucosal site of infection and impaired viral clearance [21]. CXCL10 production by dendritic cells in the hepatic LN has been implicated in retaining Th1 cells within LN and reducing liver injury upon infection with Propionibacterium acnes [22].
Like the chemokine receptors, S1PR1 is regulated both post-translationally and transcriptionally during inflammation. In response to type I interferons and other stimuli, lymphocytes up-regulate the early activation marker CD69. CD69 binds surface S1PR1 and induces its internalization, trapping naïve lymphocytes in inflamed lymph nodes and increasing the time they have to survey for cognate antigen [23, 24]. Upon T cell receptor stimulation, S1pr1 is down-regulated transcriptionally [1]. Although these two factors are clearly important, much more remains to be learned about regulation of S1PR1 signaling in the context of infection, and how pathogens may manipulate S1P sensing. T cells are sequestered in lymph nodes of HIV patients, and T cells from lymph nodes of viremic patients have blunted S1PR1 signaling without a parallel defect in signaling through CXCR4; the defect cannot be explained either by CD69 expression or transcriptional down-regulation of S1PR1[25]. Intriguingly, the mycobacterial cell wall glycophospholipid mannose-capped lipoarabinomannan (ManLAM) inhibits Th1 cell migration towards S1P[26]. By contrast, CD4−CD8− thymocytes from mice infected with Trypanosoma cruzi have enhanced chemotactic responses to S1P, although it is not clear to what extent this represents an effect on developing T cells or infiltration of the thymus by another CD4−CD8− cell type[27].
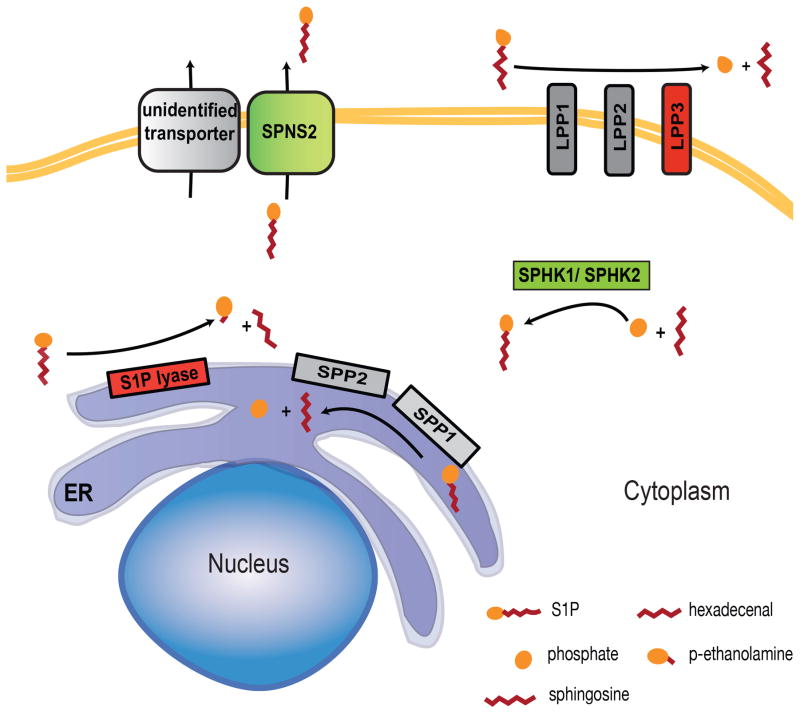
Recent studies have further improved our understanding of how S1P gradients are regulated in homeostasis (Figure 1), but we know little about how these gradients are regulated in inflammation. In homeostasis, lipid phosphate phosphatase 3 acts with S1P lyase to maintain low parenchymal S1P within lymphoid organs [28](Ramos-Perez et al., in press). The high level of lymph S1P is supplied by lymphatic endothelial cells using the transporter Spns2, while blood S1P is largely supplied by red blood cells using an unidentified transporter[29–33]. There is a small drop in blood S1P concentration in the absence of Spns2 (roughly 25%), which is likely due to loss of S1P export by vascular endothelial cells[29–33]. Interestingly, loss of S1P production or export by endothelial cells has a more dramatic effect on T cell exit from the thymus than loss of S1P production by red blood cells, although red blood cells contribute the bulk of plasma S1P[11, 29–33]. This observation suggests that local production of S1P at the exit site may be the most important determinant of lymphocyte egress. The majority of blood S1P is bound to ApoM/HDL, with most of the remaining S1P bound to albumin; the carriers of S1P in tissues remain to be defined[34, 35]. It will be very important to address whether altered expression of S1P metabolic enzymes, transporters, and carriers shifts S1P gradients in disease.
Figure 1.
A provocative series of related studies addresses whether the principles that regulate T cell exit extend to other cell types in lymph nodes. Echoing reports that S1P receptor expression by dendritic cells regulates dendritic cell egress from non-lymphoid tissues, a study examining how Yersinia pestis spreads suggests that expression of S1PR1 by monocytes enables these cells to carry Y. pestis from lymph node to lymph node via efferent lymphatics. Targeting S1PR1 with the small molecule SEW2871 upon footpad injection of Y. pestis had little effect on the number of infected cells or bacterial burden in the primary draining popliteal lymph node, but there was a reduction in the downstream iliac lymph node and prolonged survival of infected mice. Deletion of S1PR1 in mononuclear phagocytes with CX3CR1-Cre had a similar effect [36]. Dendritic cells entering lymph nodes from afferent lymph travel first into the T zone, then move to the medullary regions, and some can be seen in efferent lymphatics [37, 38]. This observation raises the question of whether dendritic cell exit also requires S1PR1 – if so, it would be possible to test how dendritic cell movement among lymph nodes affects the immune response. It will be particularly important to address whether cancer cells use S1PR1 to metastasize beyond lymph nodes.
Positioning T cells within lymphoid organs
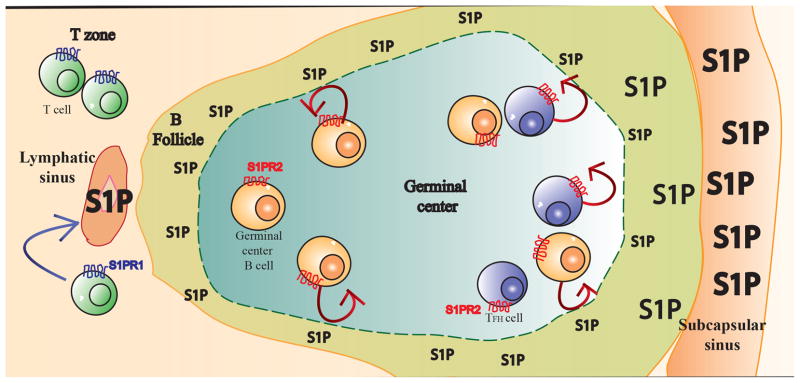
In addition to guiding egress from lymphoid organs, S1P signaling is also increasingly recognized to position lymphocytes within lymphoid organs. S1P receptor 2 (S1PR2) expression by germinal center B cells confines these cells to the germinal center [39, 40]. A recent elegant study demonstrates that S1PR2 also positions follicular T helper cells (Tfh) in the germinal center. The subset of Tfh that localizes in germinal centers expresses high levels of S1PR2, and S1PR2-deficiency reduces the number of Tfh cells in the germinal center at least in part because these cells fail to return to the germinal center upon migration towards the boundary between the germinal center and the follicle mantle [41]. Reduced S1PR2 expression by germinal center Tfh cells in a memory response may facilitate more rapid exchange of Tfh among follicles at that stage[42]. How S1PR2 confines T and B cells to the germinal centers remains an outstanding question. While S1PR1 couples to Gαi, activates Rac, and induces migration towards S1P, S1PR2 couples to Gα12/13, activates Rho, and suppresses Rac-mediated migration towards chemokines [43]. An attractive model that has been proposed to explain these findings is that S1P levels are lower in the germinal center than the surrounding tissue, and that as germinal B cells reach the boundary of the germinal center S1PR2 senses elevated S1P and causes the cells to reverse direction (Figure 2)[39, 43].
Figure 2.
Interestingly, while S1PR2 signaling is not required for Tfh development [41], loss of S1PR1 signaling is necessary for Tfh differentiation [44]. Tfh cells have low levels of S1PR1 and KLF2, a transcription factor that promotes S1PR1 expression, and forced expression of S1PR1 or KLF2 prevents differentiation of Tfh cells[44–46]. S1PR1 expression may inhibit Tfh differentiation by preventing Tfh from localizing in the B follicle and pulling them prematurely out of the draining lymph node. However, KLF2 downregulation is required for efficient Tfh formation even in the absence of S1PR1; this is in large part due to effects on Blimp-1, and may also be due to effects on other homing receptors [44, 45].
S1P signaling and T cell exit from non-lymphoid organs
In the last 3 years, findings about the role of S1P signaling in lymphocyte exit from lymphoid organs have been substantially extended with the discovery of a role of S1P signaling in immune cell migration through non-lymphoid organs, particularly in positioning of resident memory T cells (TRM). TRM are retained at a site of infection after pathogen clearance and guard against re-infection, and different subsets sit in different anatomical locations within tissues – many CD8 TRM lodge in epithelial layers, and both CD8 and CD4 TRM reside in non-epithelial locations, often in organized memory lymphocyte clusters [47–49]. One hallmark of TRM in many tissues is the transcriptional loss of S1pr1 and surface expression of CD69 [50–53]. But although it had been speculated that S1PR1 down-regulation retained TRM in tissues, until recently it was unclear whether these changes were functionally meaningful. It was possible that S1pr1 loss was simply secondary to down-regulation of pleotropic transcriptional regulators such as Klf2, and that the up-regulation of CD69 was simply secondary to loss of S1PR1, as CD69 and S1PR1 negatively regulate each other’s surface expression.
Recent work has demonstrated that loss of S1pr1 is required for establishment of resident memory T cells in many tissues, likely by preventing TRM from exiting tissues via afferent lymphatics. In a critical study, Skon et al. found that forced expression of S1pr1 prevented “settling” of TRM in kidney, salivary gland, intestine, and skin [54]. Mackay et al. further found that Cd69−/− T cells failed to persist in the skin after herpes simplex virus infection [50]. This failure was associated with a transcriptional up-regulation of Cd69 that preceded full transcriptional down-regulation of S1pr1. Moreover, it was linked to the ability of CD69 to repress S1PR1 expression, because when S1PR1 internalization was induced by FTY720 treatment, Cd69−/− T cells remained in skin similar to WT T cells early after infection [55]. Whether CD69 plays any additional roles later in TRM function, after transcriptional down-regulation of S1pr1, remains unknown.
Many interesting questions remain, and they parallel the outstanding questions about S1P signaling and T cell exit from and positioning within lymphoid organs. One issue is how additional chemokine/chemokine receptor pairs complement or compete with S1P/S1PR1 signaling. Like S1PR1, CCR7 guides T cells out of peripheral tissues [56–58], and Ccr7−/− T cells that have been activated in vitro and intradermally transferred into WT recipients are more efficient in forming TRM than WT controls [50]. In physiological conditions, it is not clear when CCR7 acts in concert with S1PR1 to guide tissue exit, and whether the two receptors can fully compensate for each other. Interestingly, S1pr5, which directs NK cell exit from lymph nodes and is not down-modulated by CD69, is also transcriptionally down-regulated in resident memory T cells, but the functional significance of this decreased expression is unknown [50, 60, 61]. Analogous to lymph nodes, egress cues from non-lymphoid tissues are countered by retention cues. For example, CXCR3 facilitates establishment of CD8 TRM in the epidermis, CXCR3 ligands are necessary and sufficient to establish and retain CD8 TRM in the vaginal mucosa, and CCL5 retains both CD8 and CD4 TRM in the vaginal mucosa [50, 53, 62]. More remains to be learned about retention cues in different tissues, and where gradients of these chemokines intersect with S1P gradients.
A second unanswered question is how S1P gradients in non-lymphoid tissues are regulated in infection. Increases in tissue S1P would be predicted to slow exit, and indeed drugs targeting S1PR1 can retain circulating cells in peripheral tissues, likely by blinding them to endogenous gradients that would guide the cells out. FTY720-treated T cells injected into the footpad fail to migrate to the draining popliteal lymph node [59], and FTY720 similarly traps circulating hematopoietic stem cells surveying non-lymphoid tissues [63]. Whether extracellular S1P gradients are altered in inflammation is unknown, although studies using mass spectrometry have found that S1P increases in several diseases, including experimental autoimmune encephalomyelitis, multiple sclerosis, asthma, rheumatoid arthritis, and adenovirus-induced skin inflammation [59, 64–67]. While these findings are tantalizing, extrapolating increases in total tissue S1P to signaling-available extracellular S1P is problematic, as discussed below.
Finally, the differences in the S1PR1 requirement for exit from different tissues and different structures within tissues, and for exit during different phases of the immune response, remain to be fully described and explained. During chronic inflammation, neither loss of CCR7 nor FTY720 treatment (nor the combination) fully blocks exit of T cells from CFA-inflamed skin [68]. Interestingly, over-expression of S1PR1 had less effect on seeding of TRM in the small intestinal epithelium than the kidney, salivary gland, or small intestine lamina propria [54]. The integrin CD103 retains CD8 TRM cells in some epithelial tissues [50, 69–71], and one might predict that CD103 compensates for S1PR1 over-expression in the small intestinal epithelium. However, the S1PR1-transduced cells that established residence in the small intestine and salivary gland were not enriched in CD103+ cells[54], and CD103−CD8+ TRM in the lamina propria do not downregulate Klf2 or S1pr1 transcripts to the same extent as CD103+ TRM in the intestinal lamina propria or the epithelium [72]. A landmark study of the distribution of LCMV-specific CD8 TRM showed that CD69, which represses surface S1PR1 expression, is only an imperfect marker of residence; in the pancreas, only 25% of TRM were CD69+, and only 65%–75% of TRM in the female reproductive tract and salivary gland were CD69+ [73]. Whether these CD69− TRM remain responsive to S1P remains to be determined. The recent discovery of efferent lymphatics in the brain begs the question of whether S1P signaling regulates T cell trafficking through the central nervous system [74, 75]. One study has shown that migration of macrophages and dendritic cells from the central nervous system into the cervical lymph nodes is blocked by FTY720 [76], and this work should be revisited with the new understanding of the anatomy of CNS drainage.
Measurement of S1P gradients
A common theme in the above discussion is that we do not know the shape of S1P gradients in tissues. It has been challenging to measure the distribution of any signaling lipid, including S1P. Much has been learned about the location of protein chemokines by knocking fluorescent reporters into the chemokine-coding locus [52, 77, 78]. But lipids are not encoded genetically, and instead sit amid complex metabolic pathways in which the expression of a single enzyme or transporter is not predictive of extracellular lipid concentrations. In the case of S1P, this pathway is not even completely defined; most notably we do not know which transporter supplies S1P to blood. Mass spectrometry has been widely used to measure bioactive molecules, including S1P. But many signaling lipids including S1P have dual roles inside and outside cells, making mass spectrometry measurements of total tissue concentrations misleading. While extracellular S1P is a ligand for 5 G protein-coupled receptors, all cells make S1P intracellularly as an intermediate in sphingolipid metabolism and in some cases as a protein co-factor, and total concentrations of tissue S1P have been reported to be very high[1, 79]. Although some bioactive molecules have been selectively extracted from interstitial fluid by insertion of a probe, the inflammatory response to the probe may in itself alter S1P levels. Moreover, many lipids, including S1P, are only sparingly soluble and are largely protein bound; we do not have a full picture of which molecules carry S1P in different tissues, and how these carriers either sequester S1P from or present S1P to receptors [80, 81].
Our knowledge of S1P distribution to date has been based on two types of measurement. First, mass spectrometry measurements of S1P in blood and lymph plasma have revealed high concentrations of lipid – in the 100nM-1 μM range for blood plasma, and approximately 6-fold lower in lymph[82]. While the issue of carriers complicates interpretation of the biological significance of these numbers, these concentrations are much higher than those required for signaling through S1PR1[7, 83]. Second, we have learned much by taking advantage of the observation that S1PR1 is internalized upon binding S1P[10, 84]. Assuming no transcriptional, translational, or post-translational modifications of S1PR1, a cell with high levels of surface S1PR1 is sensing little S1P, while a cell with low surface S1PR1 is sensing abundant S1P. This inference has been validated in many ways for naïve T cells circulating among lymphoid organs[1]. Consistent with the high concentrations of circulatory S1P measured by mass spectrometry, naïve T cells in blood and lymph have little surface S1PR1. By contrast, T cells in the lymph nodes and white pulp of the spleen have high S1PR1, and are seeing little S1P[85]. While these experiments demonstrate the existence of the gradient that guides lymphocyte exit from lymphoid organs in homeostasis, in inflammation and non-lymphoid tissues there are many transcriptional and post-translational modifications to S1PR1 that complicate interpretation of S1P levels. Furthermore, we have had little insight into S1P gradients within tissues.
To address these problems, we have recently developed a mouse that expresses an S1P reporter (Ramos-Perez et al., in press). The core of the reporter is GFP-tagged S1PR1, which sits on the plasma membrane in the absence of S1P and in endosomes in the presence of S1P. Cells also express an RFP-tagged mutant S1PR1 with a single amino acid substitution that prevents S1P binding; this marks the plasma membrane, allows calculation of ratiometric measure of surface S1PR1, and flags situations in which there is ligand-independent receptor internalization. The two receptors are separated by a 2A ribosomal skip sequence. The construct is knocked into the Rosa26 locus, driven by the CAG promoter, and preceded by a floxed transcriptional stop.
Thus far, we have used the S1P reporter mouse to measure S1P gradients in the spleen in homeostasis, and the clearest message is that assumptions about S1P distribution based on common inferences are unreliable. We had expected the splenic red pulp to have abundant S1P, because red blood cells are the main source of plasma S1P, and the red pulp has a hematocrit even higher than circulating blood[11, 86]. Yet many areas of the red pulp are S1P-low. We had expected the white pulp to have low S1P, based on the high expression of S1PR1 by naïve lymphocytes in the spleen[85]. Yet macrophages in the white pulp near the marginal sinus are clearly detecting S1P, an observation which is consistent with the hypothesis that S1P gradients regulate Tfh confinement to germinal centers. We hypothesize that much regulation of S1P will be local, depending on the balance of synthetic and degrading enzymes in individual cells.
A complementary reporter has been recently developed to measure signaling through endogenous S1PR1 by adapting the TANGO system [87]. In this system, genomic S1PR1 is replaced with S1PR1 linked at the C-terminus to the tetracycline-controlled transactivator. The linkage is a TEV protease cleavage site, and the mice also express a β-arrestin/TEV protease fusion protein. Activated S1PR1 recruits the β-arrestin/protease fusion, which releases the transactivator, and in turn stimulates expression of a stable GFP reporter gene. This elegant reporter reflects a combination of S1P levels and S1PR1 receptor expression and function, and used with the ligand reporter should provide a rich picture of the anatomy of S1P signaling.
As in the case of the S1P ligand reporter, results from the S1PR1 signaling reporter suggest that the regulation of S1P signaling is more complex than previously predicted. Blood has a high concentration of S1P that is secreted in part by vascular endothelial cells themselves, and blood vessel endothelial cells express abundant S1PR1; nonetheless, few endothelial cells have detectable S1PR1 signaling in the absence of inflammation[81, 87]. Consistent with these observations, the S1P ligand reporter also suggests that endothelial cells, unlike circulating lymphocytes, may not be exposed to high levels of S1P (WDRP and SRS, unpublished data). Much work will be required to understand these results. One possibility is that both reporters, which rely on β-arrestin recruitment to S1PR1 upon ligand binding, fail to detect S1P bound to certain carriers. A second possibility is that local degradation of S1P limits S1P’s access to endothelial S1P receptors. The latter possibility is consistent with two additional findings suggesting that endothelial S1PR1, unlike S1PR1 on T cells in blood, is not saturated in homeostasis: the observation that S1PR1 agonists alter endothelial permeability in vivo, and that inflammation increases endothelial S1PR1 signaling[87, 88]. Tonic endothelial S1PR1 signaling is important to maintain vascular integrity, but limiting this signal is likely important to prevent receptor desensitization and to enable endothelial cells to respond to inflammatory challenges[89]. Defining S1P gradients around the endothelium will be important for understanding both regulation of vascular integrity and immune cell migration into vessels.
Concluding remarks
S1P signaling is a key regulator of T cell exit from both lymphoid and non-lymphoid tissues, as well as T cell retention in tissue microenvironments such as the germinal center. Many questions remain outstanding, particularly about how S1P distribution is regulated in inflammation in both lymphoid and non-lymphoid tissues, and how different T cell subsets integrate S1P signaling and other chemotactic cues (Outstanding Questions). Imaging gradients of S1P and chemokines that regulate tissue retention may provide part of the answers, and we now have tools to accomplish this. In addition to the implications for the basic biology of the immune system, manipulation of S1P signaling is of intense interest for the development of immune suppressive drugs, and this work will inform the most promising applications of these therapies.
Outstanding Questions.
What is the shape of S1P gradients within lymphoid and non-lymphoid organs? How do these differ among tissues and disease state?
How do microbes manipulate S1P gradients or S1P signaling?
How does S1PR1 signaling interact with signaling through other receptors such as CCR7 and S1PR5, which in some cases also promote tissue egress, or CXCR3, which promotes tissue retention?
Do different T cell subsets have different dependence on S1P signaling?
Trends.
S1PR1 signaling guides T cells out of lymph nodes, and T cells balance the pull of S1P with retention cues from chemokines within the lymph nodes. The relative weight of these signals changes over the course of an immune response.
S1PR2 signaling, by contrast, retains follicular T helper (Tfh) cells in germinal centers (GCs). S1PR2 is expressed in high amounts in Tfh cells that localize to GCs, and genetic deletion of S1pr2 leads to lower numbers of Tfh cells in GC.
S1PR1 signaling regulates effector T cell residence time in non-lymphoid tissues, with many parallels to S1PR1 function in lymphoid organs. S1PR1 is downregulated in CD8+ resident memory T (TRM) cells in many tissues, and forced expression leads to failed establishment of TRM cells. Cytokines in the tissue environment induce downregulation of KLF2, a transcription factor that induces expression of S1PR1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annual review of immunology. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- 2.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8(12):1295–301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann V, Lynch KR. FTY720: targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunity. Curr Opin Immunol. 2002;14(5):569–75. doi: 10.1016/s0952-7915(02)00374-6. [DOI] [PubMed] [Google Scholar]
- 4.Adachi K, et al. Design, synthesis, and structure-activity relationships of 2-substituted-2-amino-1,3-propanediols: Discovery of a novel immunosuppresant, FTY720. Bioorganic and Medicinal Chemistry Letters. 1995;5(8):853–856. [Google Scholar]
- 5.Chiba K, et al. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol. 1998;160(10):5037–44. [PubMed] [Google Scholar]
- 6.Brinkmann V, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277(24):21453–7. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 7.Mandala S, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296(5566):346–9. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 8.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 9.Allende ML, et al. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279(15):15396–401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 10.Schwab SR, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309(5741):1735–9. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 11.Pappu R, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316(5822):295–8. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 12.Bigaud M, et al. Second generation S1P pathway modulators: research strategies and clinical developments. Biochim Biophys Acta. 2014;1841(5):745–58. doi: 10.1016/j.bbalip.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Pham TH, et al. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 2008;28(1):122–33. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patrussi L, et al. Enhanced chemokine receptor recycling and impaired S1P1 expression promote leukemic cell infiltration of lymph nodes in chronic lymphocytic leukemia (CLL) Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-15-0986. [DOI] [PubMed] [Google Scholar]
- 15.Nakai A, et al. Control of lymphocyte egress from lymph nodes through beta2-adrenergic receptors. J Exp Med. 2014;211(13):2583–98. doi: 10.1084/jem.20141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt TH, et al. CXCR4 promotes B cell egress from Peyer’s patches. J Exp Med. 2013;210(6):1099–107. doi: 10.1084/jem.20122574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reichardt P, et al. A role for LFA-1 in delaying T-lymphocyte egress from lymph nodes. EMBO J. 2013;32(6):829–43. doi: 10.1038/emboj.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willinger T, et al. Dynamin 2-dependent endocytosis is required for sustained S1PR1 signaling. J Exp Med. 2014;211(4):685–700. doi: 10.1084/jem.20131343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 20.Mueller SN, et al. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317(5838):670–4. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- 21.Lund JM, et al. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320(5880):1220–4. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoneyama H, et al. Pivotal role of dendritic cell-derived CXCL10 in the retention of T helper cell 1 lymphocytes in secondary lymph nodes. J Exp Med. 2002;195(10):1257–66. doi: 10.1084/jem.20011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J Biol Chem. 2010;285(29):22328–37. doi: 10.1074/jbc.M110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiow LR, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–4. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 25.Mudd JC, et al. Impaired T-cell responses to sphingosine-1-phosphate in HIV-1 infected lymph nodes. Blood. 2013;121(15):2914–22. doi: 10.1182/blood-2012-07-445783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richmond JM, et al. Mannose-capped lipoarabinomannan from Mycobacterium tuberculosis preferentially inhibits sphingosine-1-phosphate-induced migration of Th1 cells. J Immunol. 2012;189(12):5886–95. doi: 10.4049/jimmunol.1103092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lepletier A, et al. Early double-negative thymocyte export in Trypanosoma cruzi infection is restricted by sphingosine receptors and associated with human chagas disease. PLoS Negl Trop Dis. 2014;8(10):e3203. doi: 10.1371/journal.pntd.0003203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breart B, et al. Lipid phosphate phosphatase 3 enables efficient thymic egress. J Exp Med. 2011;208(6):1267–78. doi: 10.1084/jem.20102551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuhara S, et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. The Journal of clinical investigation. 2012;122(4):1416–26. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hisano Y, et al. Mouse SPNS2 Functions as a Sphingosine-1-Phosphate Transporter in Vascular Endothelial Cells. PloS one. 2012;7(6):e38941. doi: 10.1371/journal.pone.0038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendoza A, et al. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell reports. 2012;2(5):1104–10. doi: 10.1016/j.celrep.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nijnik A, et al. The role of sphingosine-1-phosphate transporter spns2 in immune system function. Journal of immunology. 2012;189(1):102–11. doi: 10.4049/jimmunol.1200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagahashi M, et al. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 2013;27(3):1001–11. doi: 10.1096/fj.12-219618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christoffersen C, et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci U S A. 2011;108(23):9613–8. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murata N, et al. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem J. 2000;352(Pt 3):809–15. [PMC free article] [PubMed] [Google Scholar]
- 36.St John AL, et al. S1P-Dependent trafficking of intracellular yersinia pestis through lymph nodes establishes Buboes and systemic infection. Immunity. 2014;41(3):440–50. doi: 10.1016/j.immuni.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hor JL, et al. Spatiotemporally Distinct Interactions with Dendritic Cell Subsets Facilitates CD4 and CD8 T Cell Activation to Localized Viral Infection. Immunity. 2015 doi: 10.1016/j.immuni.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 38.Braun A, et al. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat Immunol. 2011;12(9):879–87. doi: 10.1038/ni.2085. [DOI] [PubMed] [Google Scholar]
- 39.Green JA, et al. The sphingosine 1-phosphate receptor S1P(2) maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat Immunol. 2011;12(7):672–80. doi: 10.1038/ni.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muppidi JR, et al. Loss of signalling via Galpha13 in germinal centre B-cell-derived lymphoma. Nature. 2014;516(7530):254–8. doi: 10.1038/nature13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moriyama S, et al. Sphingosine-1-phosphate receptor 2 is critical for follicular helper T cell retention in germinal centers. J Exp Med. 2014;211(7):1297–305. doi: 10.1084/jem.20131666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suan D, et al. T follicular helper cells have distinct modes of migration and molecular signatures in naive and memory immune responses. Immunity. 2015;42(4):704–18. doi: 10.1016/j.immuni.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Green JA, Cyster JG. S1PR2 links germinal center confinement and growth regulation. Immunological reviews. 2012;247(1):36–51. doi: 10.1111/j.1600-065X.2012.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JY, et al. The transcription factor KLF2 restrains CD4(+) T follicular helper cell differentiation. Immunity. 2015;42(2):252–64. doi: 10.1016/j.immuni.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber JP, et al. ICOS maintains the T follicular helper cell phenotype by down-regulating Kruppel-like factor 2. J Exp Med. 2015;212(2):217–33. doi: 10.1084/jem.20141432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kitano M, et al. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34(6):961–72. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 47.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41(6):886–97. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carbone FR. Tissue-Resident Memory T Cells and Fixed Immune Surveillance in Nonlymphoid Organs. J Immunol. 2015;195(1):17–22. doi: 10.4049/jimmunol.1500515. [DOI] [PubMed] [Google Scholar]
- 49.Iijima N, Iwasaki A. Tissue instruction for migration and retention of T cells. Trends Immunol. 2015 doi: 10.1016/j.it.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackay LK, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nature immunology. 2013;14(12):1294–301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 51.Wakim LM, et al. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol. 2012;189(7):3462–71. doi: 10.4049/jimmunol.1201305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sathaliyawala T, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38(1):187–97. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346(6205):93–8. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skon CN, et al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nature immunology. 2013;14(12):1285–93. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mackay LK, et al. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol. 2015;194(5):2059–63. doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- 56.Debes GF, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6(9):889–94. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6(9):895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 58.Gomez D, et al. Effector T Cell Egress via Afferent Lymph Modulates Local Tissue Inflammation. J Immunol. 2015 doi: 10.4049/jimmunol.1500626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ledgerwood LG, et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2008;9(1):42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- 60.Jenne CN, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206(11):2469–81. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walzer T, et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8(12):1337–44. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 62.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491(7424):463–7. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Massberg S, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131(5):994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi JW, et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(2):751–6. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ammit AJ, et al. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J. 2001;15(7):1212–4. doi: 10.1096/fj.00-0742fje. [DOI] [PubMed] [Google Scholar]
- 66.Kulakowska A, et al. Intrathecal increase of sphingosine 1-phosphate at early stage multiple sclerosis. Neuroscience letters. 2010;477(3):149–52. doi: 10.1016/j.neulet.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 67.Lai WQ, et al. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J Immunol. 2008;181(11):8010–7. doi: 10.4049/jimmunol.181.11.8010. [DOI] [PubMed] [Google Scholar]
- 68.Brown MN, et al. Chemoattractant receptors and lymphocyte egress from extralymphoid tissue: changing requirements during the course of inflammation. J Immunol. 2010;185(8):4873–82. doi: 10.4049/jimmunol.1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Casey KA, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. 2012;188(10):4866–75. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A. 2010;107(42):17872–9. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schon MP, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162(11):6641–9. [PubMed] [Google Scholar]
- 72.Bergsbaken T, Bevan MJ. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8(+) T cells responding to infection. Nat Immunol. 2015;16(4):406–14. doi: 10.1038/ni.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steinert EM, et al. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell. 2015;161(4):737–49. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aspelund A, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–9. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Louveau A, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–41. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohammad MG, et al. Immune cell trafficking from the brain maintains CNS immune tolerance. J Clin Invest. 2014;124(3):1228–41. doi: 10.1172/JCI71544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Groom JR, et al. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity. 2012;37(6):1091–103. doi: 10.1016/j.immuni.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blaho VA, et al. HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature. 2015;523(7560):342–6. doi: 10.1038/nature14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galvani S, et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci Signal. 2015;8(389):ra79. doi: 10.1126/scisignal.aaa2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee YM, et al. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC) Prostaglandins Other Lipid Mediat. 2007;84(3–4):154–62. doi: 10.1016/j.prostaglandins.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee MJ, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279(5356):1552–5. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 84.Liu CH, et al. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol Biol Cell. 1999;10(4):1179–90. doi: 10.1091/mbc.10.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lo CG, et al. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J Exp Med. 2005;201(2):291–301. doi: 10.1084/jem.20041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacDonald IC, Schmidt EE, Groom AC. The high splenic hematocrit: a rheological consequence of red cell flow through the reticular meshwork. Microvasc Res. 1991;42(1):60–76. doi: 10.1016/0026-2862(91)90075-m. [DOI] [PubMed] [Google Scholar]
- 87.Kono M, et al. Sphingosine-1-phosphate receptor 1 reporter mice reveal receptor activation sites in vivo. J Clin Invest. 2014;124(5):2076–86. doi: 10.1172/JCI71194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanna MG, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2(8):434–41. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 89.Camerer E, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119(7):1871–9. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]