Abstract
Background:
Reverse dynamization is a technology for enhancing the healing of osseous defects. With use of an external fixator, the axial stiffness across the defect is initially set low and subsequently increased. The purpose of the study described in this paper was to explore the efficacy of reverse dynamization under different conditions.
Methods:
Rat femoral defects were stabilized with external fixators that allowed the stiffness to be modulated on living animals. Recombinant human bone morphogenetic protein-2 (rhBMP-2) was implanted into the defects on a collagen sponge. Following a dose-response experiment, 5.5 μg of rhBMP-2 was placed into the defect under conditions of very low (25.4-N/mm), low (114-N/mm), medium (185-N/mm), or high (254-N/mm) stiffness. Reverse dynamization was evaluated with 2 different starting stiffnesses: low (114 N/mm) and very low (25.4 N/mm). In both cases, high stiffness (254 N/mm) was imposed after 2 weeks. Healing was assessed with radiographs, micro-computed tomography (μCT), histological analysis, and mechanical testing.
Results:
In the absence of dynamization, the medium-stiffness fixators provided the best healing. Reverse dynamization starting with very low stiffness was detrimental to healing. However, with low initial stiffness, reverse dynamization considerably improved healing with minimal residual cartilage, enhanced cortication, increased mechanical strength, and smaller callus. Histological analysis suggested that, in all cases, healing provoked by rhBMP-2 occurred by endochondral ossification.
Conclusions:
These data confirm the potential utility of reverse dynamization as a way of improving bone healing but indicate that the stiffness parameters need to be selected carefully.
Clinical Relevance:
Reverse dynamization may reduce the amount of rhBMP-2 needed to induce healing of recalcitrant osseous lesions, reduce the time to union, and decrease the need for prolonged external fixation.
Large segmental defects in long bones do not heal spontaneously and remain a clinical problem1. Recombinant human bone morphogenetic protein-2 (rhBMP-2), the active ingredient of Infuse (Medtronic)2, can be applied in such cases, but any benefit seems to be incremental3-6. Moreover, extremely large amounts of rhBMP-2 are used, which raises costs and contributes to adverse side effects, several of which are severe7.
We explored the possibility of improving the effectiveness of rhBMP-2 by mechanical manipulation of the defect site. It is well established that healing of long-bone fractures is strongly influenced by the ambient mechanical environment8-12. However, there is little information in the literature on its influence on the healing of critical-sized defects treated with rhBMP-2.
The mechanical environment is largely determined by the stiffness of the implant used to stabilize the fracture and by weight-bearing. The size and quality of the callus formed at the fracture site depend on the magnitude of interfragmentary movements occurring between the fractured ends during weight-bearing and muscle contraction. Accordingly, stiff fixation that minimizes interfragmentary movements results in limited callus formation, whereas flexible fixation that increases interfragmentary movements results in the formation of a larger callus. Moreover, interfragmentary movements produce local axial, shear, or torsional loading, depending on the type and stability of the fixation device used, and this affects the rate and quality of bone formation. For example, shear load is detrimental to fracture-healing12,13 whereas the same amount of axial load is beneficial12,14. The few papers on the influence of mechanics15-19 on the healing of large segmental defects largely addressed the strategy of dynamization, a modality in which the stiffness of fixation is initially high and is later reduced.
In a study of an 8-mm rat femoral defect model, Boerckel et al.15 found that the application of early mechanical loading with unstable fixation (stiffness of ∼8.4 N/mm) significantly inhibited vascular invasion and reduced bone formation compared with the healing following treatment with stiffer plates (∼214 N/mm and ∼350 N/mm). However, when full mechanical loading was delayed by switching from stiff (∼350 N/mm) to flexible (∼8.4 N/mm) fixation at 3 weeks, bone formation was enhanced15. A separate study using a 6-mm defect in rats confirmed the benefit of dynamization at 4 weeks16. Claes et al.17 found similar results using a 1-mm femoral osteotomy stabilized with an external fixator and dynamized after 3 to 4 weeks of healing. However, dynamization at 1 week impaired bone healing18.
In previous studies, we stabilized 5-mm critical-sized rat femoral defects with external fixators providing high, medium, or low axial stiffness19,20 in combination with application of rhBMP-2. Healing was improved by imposing “reverse dynamization,” a modality in which the stiffness of the fixator was switched from low to high after 2 weeks, a time when bone was forming within the defect20. In none of the histological samples was there evidence of an endochondral process, which was surprising given prior research suggesting that this is the route of healing in segmental defects exposed to rhBMP-221.
In the present study, we extended our evaluation of reverse dynamization in a rat model to a wider range of stiffnesses and rhBMP-2 concentrations to learn more about its scope and biology. The underlying hypothesis was that, with use of the appropriate stiffness parameters and timing, reverse dynamization can enhance the healing of large segmental defects in response to rhBMP-2.
Materials and Methods
Study Design
Rat femoral mid-diaphyseal 5-mm defects were treated with rhBMP-2 and stabilized with an external fixator with connection elements of different stiffnesses that can be interchanged in living animals as bone healing progresses (RISystem)19,21.
The first set of experiments determined a dose response for healing of segmental defects with rhBMP-2. Surgically created defects were treated with 3 different amounts of rhBMP-2 (1.1, 5.5, and 11 μg) delivered on a collagen sponge. An external fixator provided high (254-N/mm), medium (185-N/mm), low (114-N/mm), or very low (25.4-N/mm) stiffness (except for the 11-μg rhBMP group, which received only high and low-stiffness fixation), which remained unchanged during the 8-week experiment.
On the basis of the results of the first experiment, a dose of 5.5 μg of rhBMP-2 was selected for the second set of experiments, in which we evaluated 4 fixators providing high (254-N/mm), medium (185-N/mm), low (114-N/mm), or very low (25.4-N/mm) stiffness that remained unchanged during the 8-week experiment.
In the third series of experiments, reverse dynamization in which the initial fixation provided either low or very low stiffness was applied along with 5.5 μg of rhBMP-2. In both cases, high stiffness was imposed after 2 weeks.
Twelve animals per group were used in each of the experiments (Table I).
TABLE I.
Numbers of Animals Used in This Study*
| No. of Animals |
|||
| Dose response study | 1.1 μg of rhBMP-2 | 5.5 μg of rhBMP-2 | 11 μg of rhBMP-2 |
| Very low stiffness | 6 | 6 | |
| Low stiffness | 6 | 6 | 6 |
| Medium stiffness | 6 | 6 | |
| High stiffness | 6 | 6 | 6 |
| Main study | |||
| Very low stiffness | 6* | ||
| Low stiffness | 6* | ||
| Medium stiffness | 6* | ||
| High stiffness | 6* | ||
| RD† very low stiffness | 12 | ||
| RD† low stiffness | 12 | ||
| Total | 24 | 72 | 12 |
For the constant-stiffness experiments, rats from the dose response study using 5.5 μg of rhBMP-2 (n = 6 in each group) and the main study (n = 6 in each group) were combined to provide an aggregate number of 12 for statistical power, as described in the Materials and Methods section.
RD = reverse dynamization.
In each set of experiments, healing was monitored with weekly radiographs. Animals were killed after 8 weeks, and healing was further assessed with micro-computed tomography (μCT), mechanical testing, and histological analysis. In an additional series of experiments, animals were killed after 3 days for histological examination of early defects.
Surgery
A 5-mm critical-sized midfemoral defect was created in the right hindlimb of male Sprague-Dawley rats weighing 310 to 330 g. These defects do not heal spontaneously but heal in response to 11 μg of rhBMP-2 delivered on a collagen sponge, mimicking the product Infuse2.
Animal care and experimental protocols were followed in accordance with the local animal ethics committee. The rats were anesthetized with isoflurane (2% at 2 L/min). Before surgery, each rat received antibiotics (cefazolin, 20 mg/kg) and the analgesic buprenorphine (0.08 mg/kg) intramuscularly in the left leg. A 4-cm incision was made through the skin, and the diaphysis of the femur was exposed.
The external fixator bar was used as a guide for placement of 4 drill holes and to accommodate the screws. When the fixator was in place, a saw guide was used to create a 5-mm segmental defect with a 0.22-mm wire Gigli saw. The saw guide was then removed, and a collagen sponge impregnated with rhBMP-2 or phosphate-buffered saline solution (control) was inserted into the defect. For the first 3 postoperative days, the rats were given analgesic every 12 hours and antibiotic every 24 hours.
Radiographic Evaluation
Bone healing was evaluated with weekly radiographs for 8 weeks using a closed-cabin radiographic unit (Faxitron MX 20; Faxitron Bioptics).
Micro-CT
A desktop μCT imaging system (μCT 40; Scanco Medical) equipped with a 10-mm focal spot microfocus x-ray tube, at a 20-μm isotropic voxel size, 75-keV energy, and 200-ms integration time, was used to scan all femora (∼700 μCT slices per specimen). Evaluation was performed in the 4-mm central defect area to exclude preexisting cortical bone. We assessed the total volume of healed callus (in mm3), bone volume (in mm3), bone mineral density (in mg hydroxyapatite [HA]/cm3), and polar moment of inertia (in mm4). Images were thresholded using an adaptive-iterative algorithm, and morphometric variables were calculated from the binarized images using direct 3-dimensional techniques that do not rely on any prior assumptions about the underlying structure.
Ex Vivo Torsion Testing
After 8 weeks of healing and after all noninvasive imaging was completed, 9 specimens from each group of 12 rats were randomly selected and tested to failure using a torsional testing jig in conjunction with a materials testing system (Instron model-5848 MicroTester). Femora were tested to failure by rotating one axis of the femur at the continuous rate of 0.42°/s using a 50-N load cell. Angular deformation and applied load data were acquired at 10 Hz and used to calculate the torsional stiffness and strength of the healed defect.
Histological Analysis
Femora from 3 randomly selected animals per group were fixed using 4% paraformaldehyde for 3 days and decalcified for 4 weeks in 10% EDTA (ethylenediaminetetraacetic acid) solution at 4°C. Samples were then dehydrated in graded ethanol, cleared in xylene, embedded in paraffin, sectioned at a thickness of 5 μm, and stained with safranin O-fast green to detect cartilage. The evaluators were blinded to the groups.
Sample Size and Statistical Analysis
The numbers of animals were determined from historical data19,20, which showed 12 animals per group to be adequate. A power analysis demonstrated that the probability of detecting a significant difference with this number of animals per group was 95% for all of the imaging (12 animals) and 85% for mechanical testing data (9 animals). The sample-size study was designed to detect a relationship between the independent and dependent variables at a 2-sided 5% significance level. The power test confirmed that the animal numbers selected for each modality would be more than sufficient to detect significant differences. Comparisons of continuous variables between 2 treatment groups were performed with the use of a 2-tailed t test, and comparisons among 3 groups were done with the use of 1-way analysis of variance. If the difference between the ipsilateral and contralateral femora or between treatment groups was significant, a post hoc (Tukey) test was performed. A power analysis was done after the study to determine if we had a sufficient number of animals per group to detect a significant difference. The power levels for all of the data were found to be from 0.8 to 1. Thus, the numbers of animals per group used in these experiments were high enough to determine a 5% difference between the test groups. All tests were 2-tailed, with differences considered significant at p < 0.05. Data are presented as the mean and the standard error of the mean, unless otherwise noted.
Results
rhBMP-2 Dose Response
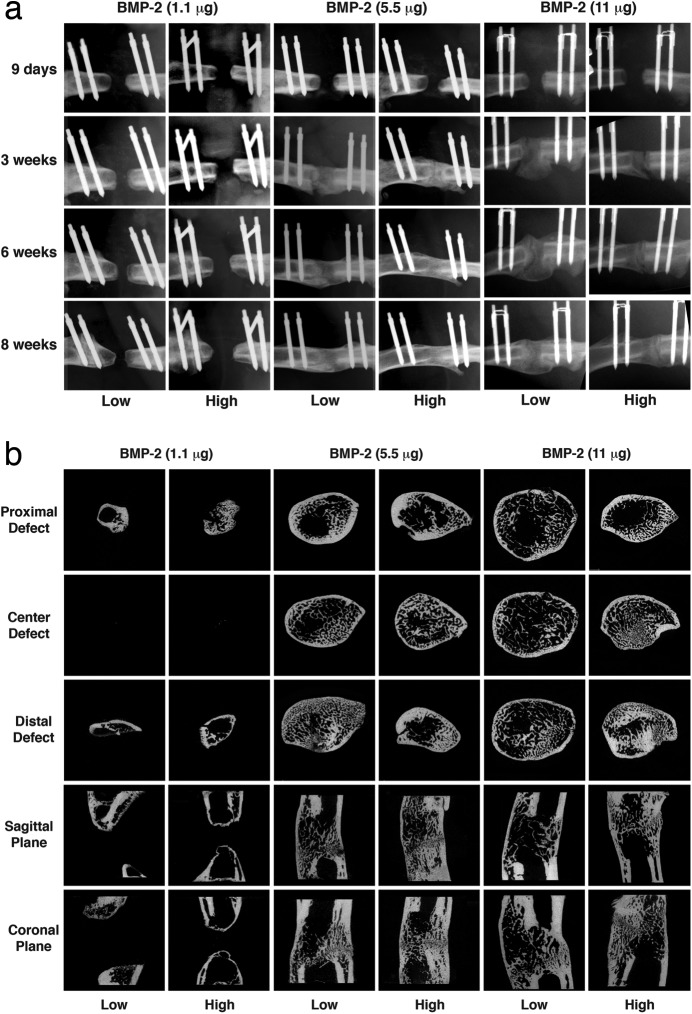
Segmental defects treated with 1.1 μg of rhBMP-2 failed to heal under any stiffness conditions, including low and high-stiffness fixation as shown in Figure 1. Complete healing occurred in all animals that received 5.5 or 11 μg of rhBMP-2 with low or high-stiffness fixation (Fig. 1); no major differences were apparent on radiographs or μCT except that a larger callus formed with 11 μg at low stiffness.
Fig. 1.
Healing of segmental defects with different doses of rhBMP-2 and external fixators with high or low stiffness. Defects were radiographed every week for 8 weeks (Fig. 1-A), at which time femora were harvested for analysis with μCT (Fig. 1-B). Low = low-stiffness fixator (114 N/mm), and High = high-stiffness fixator (254 N/mm).
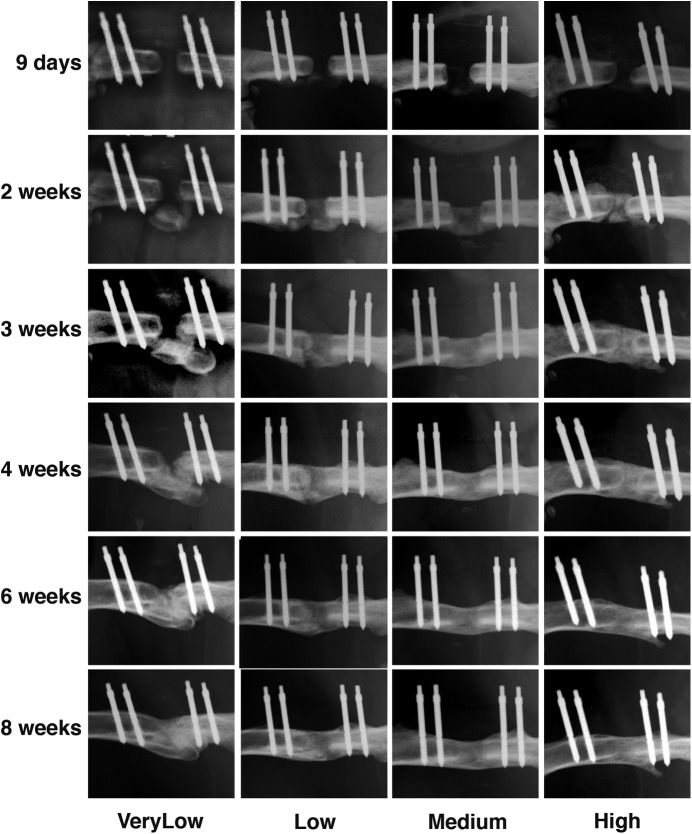
Effect of Fixator Stiffness
A dose of 5.5 μg of rhBMP-2 was used in conjunction with fixator stiffnesses ranging from very low (25.4 N/mm) to high (254 N/mm). As shown in Figures 2-A and 2-B, all defects healed but with different kinetics, depending on the stiffness of the external fixator. In particular, at a stiffness of 25.4 N/mm (very low) bridging was delayed. Whereas bridging of the defects treated with the higher-stiffness fixators was seen radiographically by 2 to 3 weeks, this did not occur until 3 to 4 weeks under conditions of very low stiffness. Moreover, when very low stiffness was used, bone was first deposited adjacent to the defect rather than within it. This may reflect displacement of the collagen sponge under conditions of greater flexibility.
Figs. 2-A and 2-B Healing of segmental defects treated with 5.5 μg of rhBMP-2 and external fixators of different stiffnesses. Defects were radiographed every week for 8 weeks (Fig. 2-A), at which time femora were harvested for analysis with μCT (Fig. 2-B). VeryLow = very-low-stiffness fixator (25.4 N/mm), Low = low-stiffness fixator (114 N/mm), Medium = medium-stiffness fixator (185 N/mm), and High = high-stiffness fixator (254 N/mm).
Fig. 2-A.
Fig. 2-B.
The radiographs (Fig. 2-A) showed medium-stiffness fixation to provide the best healing. However, by 8 weeks, the radiographs showed bridging of all defects under all stiffness conditions, with no major qualitative differences among them. The results of the analysis of the μCT images (Fig. 2-B) were in broad agreement with this assessment.
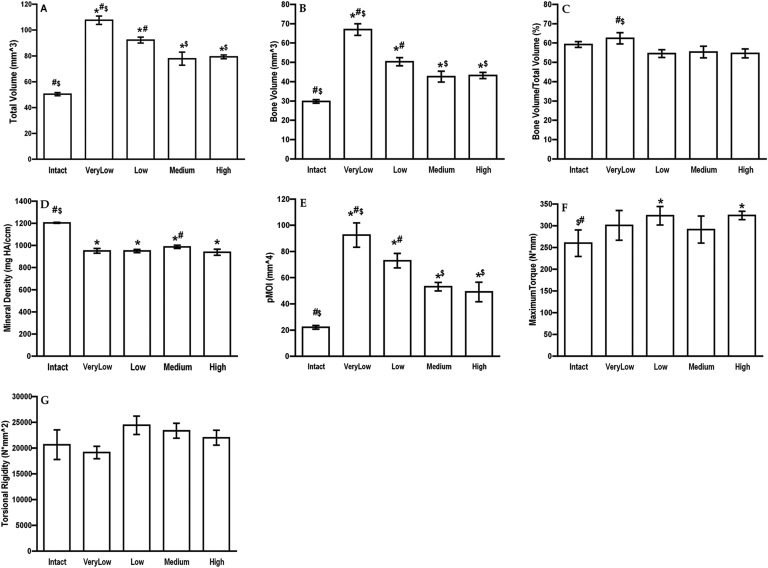
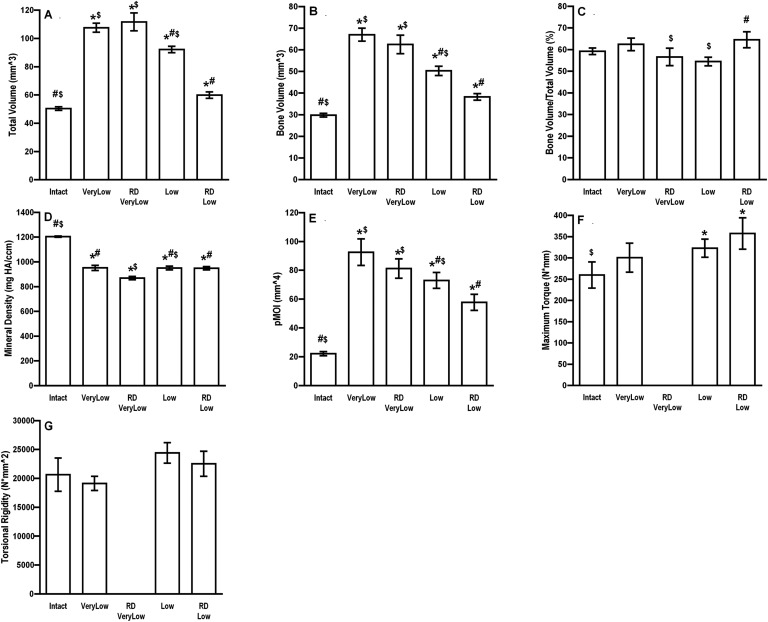
Quantitative assessment of the μCT data (Fig. 3) demonstrated larger total volume of callus under conditions of very low and low stiffness, but little difference in bone volume/total volume. This is reflected by the equivalent mechanical properties of the healed defects at 8 weeks (Fig. 3).
Fig. 3.
Physical properties of segmental defects treated with 5.5 μg of rhBMP-2 and external fixators of different stiffnesses. Rats were euthanized at 8 weeks, and the defects with analyzed with μCT (Figs. 3-A through 3-E) and mechanical testing (Figs. 3-F and 3-G). The error bars indicate the standard error of the mean. #Significantly different from the high-stiffness group. *Significantly different from the intact, contralateral femur. $Significantly different from the low-stiffness group. Intact = contralateral femur, VeryLow = very-low-stiffness fixator (25.4 N/mm), Low = low-stiffness fixator (114 N/mm), Medium = medium-stiffness fixator (185 N/mm), High = high-stiffness fixator (254 N/mm), HA = hydroxyapatite, and pMOI = polar moment of inertia.
The greatest differences between the groups were seen histologically (Fig. 4). In agreement with the radiographic data (Fig. 2-A), histological analysis showed that the defects that healed at medium stiffness (185 N/mm) contained no cartilage, showed cortication, and had the appearance of woven bone remodeling into lamellar bone with marrow elements forming. Under all other stiffness conditions, there was considerable residual cartilage and less new bone.
Fig. 4.
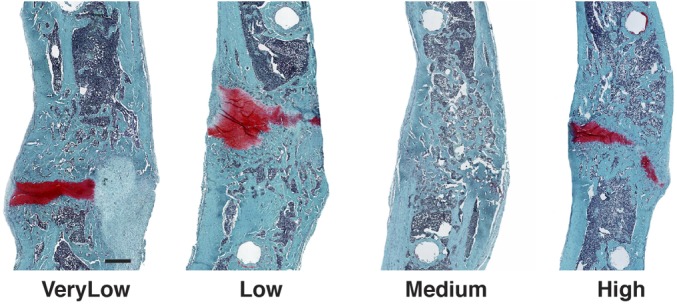
Histological sections of segmental defects treated with 5.5 μg of rhBMP-2 and external fixators of different stiffnesses. After 8 weeks, femora were harvested, decalcified, and embedded in paraffin. Sections were stained with safranin O-fast green. Scale bar = 1 mm. VeryLow = very-low-stiffness fixator (25.4 N/mm), Low = low-stiffness fixator (114 N/mm), Medium = medium-stiffness fixator (185 N/mm), and High = high-stiffness fixator (254 N/mm).
Effect of Reverse Dynamization
Reverse dynamization was applied under 2 different conditions, starting with very low or low stiffness. In both cases, high stiffness was imposed at 2 weeks.
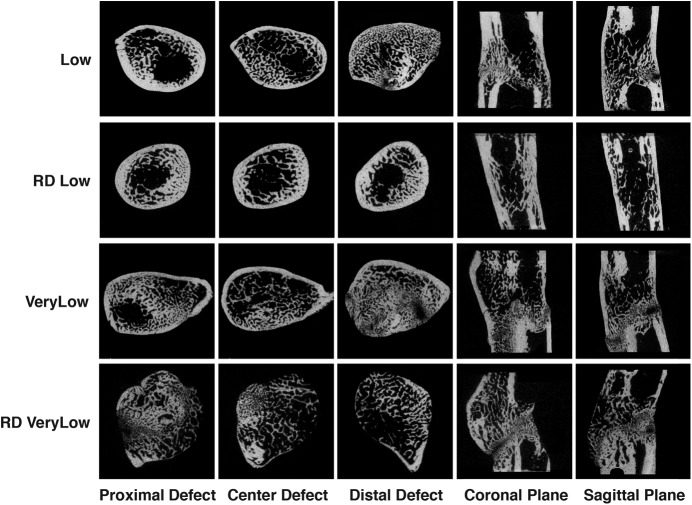
The radiographs (Fig. 5-A) suggested that, although reverse dynamization accelerated and smoothed the bridging of defects that had been initially treated at very low stiffness (25.4 N/mm), it introduced a discontinuity, seen as a radiolucent zone (Fig. 5-B). Histological examination (Fig. 6) revealed this to be a prominent band of cartilage. Indeed, the μCT analysis (Fig. 7) and histological images (Fig. 6) suggested that reverse dynamization was detrimental, resulting in less bone, reduced cortication, and increased cartilage within the defect indicating the possible formation of a pseudarthrosis. Moreover, these bones were too weak for mechanical testing.
Fig. 5.
Radiographs of defects treated with 5.5 μg of rhBMP-2 and reverse dynamization. The defects were stabilized initially with an external fixator of very low (25.4-N/mm) or low (114-N/mm) stiffness. After 2 weeks, the stiffness was increased to 254 N/mm (high stiffness) in the groups receiving reverse dynamization (RD). Defects were monitored by weekly radiographs (Fig. 5-A). Figure 5-B shows a close-up of the radiographs at 4 and 8 weeks, highlighting the radiolucent discontinuity that is present in all but the reverse dynamization group in which the initial fixation was of low stiffness (low stiffness RD).
Fig. 6.
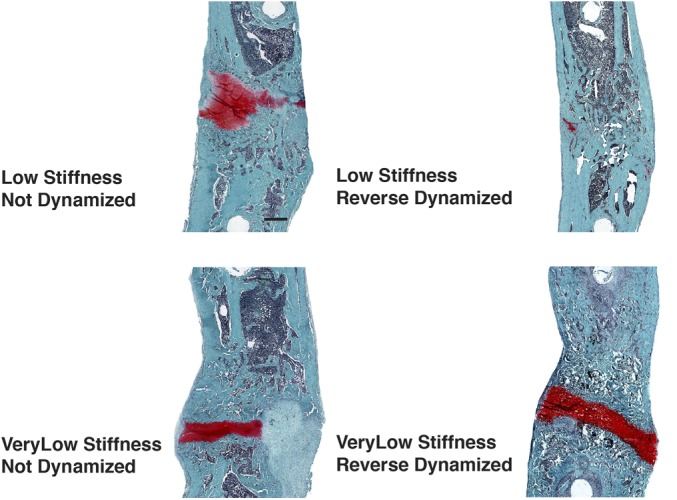
Histological sections of segmental defects treated with 5.5 μg of rhBMP-2 and reverse dynamization. The defects were stabilized initially with an external fixator of very low (25.4 N/mm) or low (114 N/mm) stiffness. After 2 weeks, the stiffness was increased to 254 N/mm (high stiffness) in the groups receiving reverse dynamization. The stiffness was not adjusted in the non-dynamized groups. After 8 weeks, the femora were harvested, decalcified, and embedded in paraffin. Sections were stained with safranin O-fast green. Scale bar = 1 mm.
Fig. 7.
Micro-CT images of defects treated with 5.5 μg of rhBMP-2 and reverse dynamization. The defects were stabilized initially with an external fixator of very low (25.4-N/mm) or low (114-N/mm) stiffness. After 2 weeks, the stiffness was increased to 254 N/mm (high stiffness) in the groups receiving reverse dynamization (RD Low and RD VeryLow). The animals treated with continuous (non-dynamized) fixation continued with low or very low-stiffness fixators (Low or VeryLow). After another 6 weeks, the femora were harvested and the defects were analyzed with μCT.
In contrast to the situation with very low stiffness, when the initial stiffness was low (114 N/mm), reverse dynamization improved bridging at all time points after the imposition of high stiffness at week 2 and eliminated the radiolucent zone (Figs. 5-A and 5-B) revealed by histological analysis to be a band of cartilage (Fig. 6). The histological findings were consistent with the μCT images (Fig. 7). Defects initially stabilized at low stiffness and then subjected to reverse dynamization had more uniform, thicker cortices; a more regular circumference; and less intramedullary bone than seen under all other conditions of healing, regardless of the stiffness. Quantitative analysis (Fig. 8) showed total volume and bone volume at nearly normal values, unlike the callus formed under other conditions. These bones were stronger than the contralateral femora but had normal stiffness (Fig. 8).
Fig. 8.
Physical properties of segmental defects treated with 5.5 μg of rhBMP-2 and reverse dynamization. Defects were stabilized initially with an external fixator of very low (25.4 N/mm) or low (114 N/mm) stiffness. After 2 weeks, the stiffness was increased to 254 N/mm (high stiffness) in the groups receiving reverse dynamization (RD Low and RDVeryLow). The animals treated with continuous (non-dynamized) fixation continued with low or very low-stiffness fixators (Low or VeryLow). After another 6 weeks, the femora were harvested and the defects were analyzed with μCT (Figs. 8-A through 8-E) and mechanical testing (Figs. 8-F and 8-G). The error bars indicate the standard error of the mean. #Significantly different from the RD very-low-stiffness group. *Significantly different from the intact, contralateral femur. $Significantly different from the RD low-stiffness group. Intact = contralateral femur, VeryLow = very-low-stiffness fixator (25.4 N/mm) throughout the entire 8 weeks, Low = low-stiffness fixator (114 N/mm) throughout the entire 8 weeks, RD VeryLow = very-low-stiffness fixator (25.4 N/mm) for the first 2 weeks followed by high-stiffness fixator (254 N/mm) for the remaining 6 weeks, RD Low = low-stiffness fixator (114 N/mm) for the first 2 weeks followed by high-stiffness fixator (254 N/mm) for the remaining 6 weeks, HA = hydroxyapatite, and pMOI = polar moment of inertia.
Early Histological Appearance of the Defect
Considerable amounts of cartilage formed under most of the healing regimens (Figs. 4 and 6). This is in marked contrast to the little or no cartilage that was detected in an earlier study in which a higher dose of rhBMP-2 was used20. To gain further insight, we performed histological analysis on defects 3 days after they had been treated with 5.5 μg of rhBMP-2 (Fig. 9). Cartilage was already being formed at the margins of the defects treated with medium-stiffness fixation. Analysis of those treated at very low stiffness showed more extensive chondrogenesis with some degree of cartilage deposition within the gap.
Fig. 9.

Histological sections of segmental defects 3 days after implantation of 5.5 μg of rhBMP-2. The defects were stabilized with an external fixator of very low (25.4-N/mm) or medium (185-N/mm) stiffness. After 3 days, the femora were harvested, decalcified, and embedded in paraffin. Sections were stained with safranin O-fast green. Scale bar = 1 mm.
Discussion
These data confirm the value of reverse dynamization as a means of enhancing the healing of segmental defects but indicate that the stiffness parameters, timing, and rhBMP-2 dose need to be selected carefully. In particular, if the initial stiffness is too low then early reverse dynamization is detrimental. Collectively, the data contribute to a growing appreciation that healing is particularly sensitive to the early biological and mechanical environment14,15. The data also raise the interesting possibility that the mechanical environment and the concentration of rhBMP-2 combine to determine whether healing is endochondral or intramembranous. This possibility is supported by recent in vitro data22.
The most effective healing occurred when the initial stiffness was 114 N/mm (low stiffness) and raised to 254 N/mm (high stiffness) at 2 weeks. This regimen was superior to constant stiffness at any one level (ranging from very low [25.4 N/mm] to high) or to reverse dynamization initiated at very low stiffness. The latter was detrimental to healing, but it is possible that high stiffness was imposed too early in this experiment.
The mechanism through which reverse dynamization mediates its improvement in healing is unknown. It was initially proposed as a means of enhancing the endochondral process, with the prediction that loose initial fixation would promote the formation of cartilage and subsequent rigid fixation would promote its endochondral ossification. However, when reverse dynamization was previously used in a 5-mm rat femoral defect treated with 11 μg of rhBMP-2, there was no evidence of cartilage formation during the early stages of healing under conditions of low, medium, or high stiffness20. In the present study, in which we used 5.5 μg of rhBMP-2, we noted early chondrogenesis with both medium-stiffness and very-low-stiffness fixation. This suggests that the dose of rhBMP-2 helps to determine whether progenitor cells differentiate into chondrocytes or osteoblasts.
Superimposed on the effects of rhBMP-2 are the effects of mechanical forces on differentiation, something appreciated since the time of Pauwels23 and understood now in terms of mechanotransduction24. These observations are consistent with the recent findings of Hagiwara et al.25 in their study of fracture-healing in mice. They noted that the stability of the fracture site greatly affected the cellular response during repair. In particular, an intramedullary pin promoted the formation of a large callus with endochondral healing. An external fixator provided greater rigidity, and healing occurred intramembranously with little callus formation.
The effects of reverse dynamization may also involve modulation of inflammation. Mechanical forces can inhibit or exacerbate responses to inflammatory mediators via effects on NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells)26,27. The relationship between bone healing and inflammation is complex28,29. Interleukin-1 and tumor necrosis factor-α, for instance, powerfully inhibit the differentiation of human MSCs (mesenchymal stem cells) into chondrocytes30 and might thus be expected to antagonize the early stages of differentiation that lead to endochondral bone formation. Their effects on the osteogenic differentiation of human MSCs are more complex. It is agreed that inflammatory mediators increase mineral deposition by human MSCs, but whether they become true osteoblasts is debated31-33.
There are two ways in which use of optimized reverse dynamization to aid healing of bones could provide marked clinical and economic benefit. First, lowering the dose of rhBMP-2 needed for bone healing will reduce costs and limit the risk of off-target adverse events. Second, external fixation has several advantages over plates or intramedullary nails for stabilizing fractures and segmental defects. It is less invasive and less expensive and does not require sophisticated tertiary care facilities. Application of external fixation, however, is constrained by the uncertain progress of bone healing and concern about pin-track infections. An optimized reverse dynamization protocol could provide reliability, and accelerate healing so that pins could be removed before infection becomes a problem.
Acknowledgments
Note: The authors thank Dr. Slobodan Tepic for reviewing an earlier version of this paper. They also thank Dr. Mirjam Neumann for help with the animal surgical procedure.
Footnotes
Investigation performed at the Queensland University of Technology, Brisbane, Australia
Disclosure: Funding was provided by the U.S. Department of Defense (Grant W81XWH-10-1-0888), the Vice-Chancellor’s Research Fellowship of Queensland University of Technology, and the Princess Alexandra Research Foundation, Brisbane, Australia. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article.
References
- 1.Verrier S, Alini M, Alsberg E, Buchman S, Kelly AJ, Laschke MW, Menger J, Stegemann JP, Schuetz MA, Miclau T, Stoddart M, Evans CH. Tissue engineering and regenerative approaches to improving the healing of large bone defects. Eur Cell Mater. [In press]. [DOI] [PubMed] [Google Scholar]
- 2.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft). Int Orthop. 2007. December;31(6):729-34. Epub 2007 Jul 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones AL, Bucholz RW, Bosse MJ, Mirza SK, Lyon TR, Webb LX, Pollak AN, Golden JD, Valentin-Opran A; BMP-2 Evaluation in Surgery for Tibial Trauma-Allgraft (BESTT-ALL) Study Group. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am. 2006. July;88(7):1431-41. [DOI] [PubMed] [Google Scholar]
- 4.Lissenberg-Thunnissen SN, de Gorter DJ, Sier CF, Schipper IB. Use and efficacy of bone morphogenetic proteins in fracture healing. Int Orthop. 2011. September;35(9):1271-80. Epub 2011 Jun 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swiontkowski MF, Aro HT, Donell S, Esterhai JL, Goulet J, Jones A, Kregor PJ, Nordsletten L, Paiement G, Patel A. Recombinant human bone morphogenetic protein-2 in open tibial fractures. A subgroup analysis of data combined from 2 prospective randomized studies. J Bone Joint Surg Am. 2006. June;88(6):1258-65. [DOI] [PubMed] [Google Scholar]
- 6.Wei S, Cai X, Huang J, Xu F, Liu X, Wang Q. Recombinant human BMP-2 for the treatment of open tibial fractures. Orthopedics. 2012. June;35(6):e847-54. [DOI] [PubMed] [Google Scholar]
- 7.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011. June;11(6):471-91. [DOI] [PubMed] [Google Scholar]
- 8.Duda GN, Mandruzzato F, Heller M, Goldhahn J, Moser R, Hehli M, Claes L, Haas NP. Mechanical boundary conditions of fracture healing: borderline indications in the treatment of unreamed tibial nailing. J Biomech. 2001. May;34(5):639-50. [DOI] [PubMed] [Google Scholar]
- 9.Goodship AE, Lawes TJ, Rubin CT. Low-magnitude high-frequency mechanical signals accelerate and augment endochondral bone repair: preliminary evidence of efficacy. J Orthop Res. 2009. July;27(7):922-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenwright J, Goodship AE. Controlled mechanical stimulation in the treatment of tibial fractures. Clin Orthop Relat Res. 1989. April;241:36-47. [PubMed] [Google Scholar]
- 11.Kenwright J, Richardson JB, Cunningham JL, White SH, Goodship AE, Adams MA, Magnussen PA, Newman JH. Axial movement and tibial fractures. A controlled randomised trial of treatment. J Bone Joint Surg Br. 1991. July;73(4):654-9. [DOI] [PubMed] [Google Scholar]
- 12.Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002. November;84(8):1093-110. [DOI] [PubMed] [Google Scholar]
- 13.Augat P, Burger J, Schorlemmer S, Henke T, Peraus M, Claes L. Shear movement at the fracture site delays healing in a diaphyseal fracture model. J Orthop Res. 2003. November;21(6):1011-7. [DOI] [PubMed] [Google Scholar]
- 14.Klein P, Schell H, Streitparth F, Heller M, Kassi JP, Kandziora F, Bragulla H, Haas NP, Duda GN. The initial phase of fracture healing is specifically sensitive to mechanical conditions. J Orthop Res. 2003. July;21(4):662-9. [DOI] [PubMed] [Google Scholar]
- 15.Boerckel JD, Uhrig BA, Willett NJ, Huebsch N, Guldberg RE. Mechanical regulation of vascular growth and tissue regeneration in vivo. Proc Natl Acad Sci U S A. 2011. September 13;108(37):E674-80. Epub 2011 Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boerckel JD, Kolambkar YM, Stevens HY, Lin AS, Dupont KM, Guldberg RE. Effects of in vivo mechanical loading on large bone defect regeneration. J Orthop Res. 2012. July;30(7):1067-75. Epub 2011 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claes L, Blakytny R, Besse J, Bausewein C, Ignatius A, Willie B. Late dynamization by reduced fixation stiffness enhances fracture healing in a rat femoral osteotomy model. J Orthop Trauma. 2011. March;25(3):169-74. [DOI] [PubMed] [Google Scholar]
- 18.Claes L, Blakytny R, Göckelmann M, Schoen M, Ignatius A, Willie B. Early dynamization by reduced fixation stiffness does not improve fracture healing in a rat femoral osteotomy model. J Orthop Res. 2009. January;27(1):22-7. [DOI] [PubMed] [Google Scholar]
- 19.Glatt V, Evans CH, Matthys R. Design, characterisation and in vivo testing of a new, adjustable stiffness, external fixator for the rat femur. Eur Cell Mater. 2012;23:289-98; discussion 299. Epub 2012 Apr 21. [DOI] [PubMed] [Google Scholar]
- 20.Glatt V, Miller M, Ivkovic A, Liu F, Parry N, Griffin D, Vrahas M, Evans C. Improved healing of large segmental defects in the rat femur by reverse dynamization in the presence of bone morphogenetic protein-2. J Bone Joint Surg Am. 2012. November 21;94(22):2063-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glatt V, Matthys R. Adjustable stiffness, external fixator for the rat femur osteotomy and segmental bone defect models. J Vis Exp. 2014;e51558(92). Epub 2014 Oct 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopf J, Petersen A, Duda GN, Knaus P. BMP2 and mechanical loading cooperatively regulate immediate early signalling events in the BMP pathway. BMC Biol. 2012;10:37 Epub 2012 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pauwels F. [A new theory on the influence of mechanical stimuli on the differentiation of supporting tissue. The tenth contribution to the functional anatomy and causal morphology of the supporting structure]. Z Anat Entwicklungsgesch. 1960;121:478-515. [German]. [PubMed] [Google Scholar]
- 24.Chen JC, Jacobs CR. Mechanically induced osteogenic lineage commitment of stem cells. Stem Cell Res Ther. 2013;4(5):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagiwara Y, Dyment NA, Jiang X, Jiang Ping H, Ackert-Bicknell C, Adams DJ, Rowe DW. Fixation stability dictates the differentiation pathway of periosteal progenitor cells in fracture repair. J Orthop Res. 2015. July;33(7):948-56. Epub 2015 May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal S, Deschner J, Long P, Verma A, Hofman C, Evans CH, Piesco N. Role of NF-kappaB transcription factors in antiinflammatory and proinflammatory actions of mechanical signals. Arthritis Rheum. 2004. November;50(11):3541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Z, Buckley MJ, Evans CH, Agarwal S. Cyclic tensile strain acts as an antagonist of IL-1 beta actions in chondrocytes. J Immunol. 2000. July 1;165(1):453-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012. March;8(3):133-43. Epub 2012 Jan 31. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt-Bleek K, Kwee BJ, Mooney DJ, Duda GN. Boon and bane of inflammation in bone tissue regeneration and its link with angiogenesis. Tissue Eng Part B Rev. 2015. August;21(4):354-64. Epub 2015 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wehling N, Palmer GD, Pilapil C, Liu F, Wells JW, Müller PE, Evans CH, Porter RM. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009. March;60(3):801-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding J, Ghali O, Lencel P, Broux O, Chauveau C, Devedjian JC, Hardouin P, Magne D. TNF-alpha and IL-1beta inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009. April 10;84(15-16):499-504. Epub 2009 Feb 3. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira E, Porter RM, Wehling N, O’Sullivan RP, Liu F, Boskey A, Estok DM, Harris MB, Vrahas MS, Evans CH, Wells JW. Inflammatory cytokines induce a unique mineralizing phenotype in mesenchymal stem cells derived from human bone marrow. J Biol Chem. 2013. October 11;288(41):29494-505. Epub 2013 Aug 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonomoto K, Yamaoka K, Oshita K, Fukuyo S, Zhang X, Nakano K, Okada Y, Tanaka Y. Interleukin-1β induces differentiation of human mesenchymal stem cells into osteoblasts via the Wnt-5a/receptor tyrosine kinase-like orphan receptor 2 pathway. Arthritis Rheum. 2012. October;64(10):3355-63. [DOI] [PubMed] [Google Scholar]