Abstract
Mercury (Hg) is a global contaminant whose presence in the biosphere has been increased by human activity, particularly coal burning/energy production, mining, especially artisanal scale gold mining, and other industrial activities. Mercury input to the surface ocean has doubled over the past century leading governments and organizations to take actions to protect humans from the harmful effects of this toxic element. Recently, the UN Environmental Program led 128 countries to negotiate and sign a legally binding agreement, the 2013 Minimata Convention, to control Hg emissions and releases to land and water globally. In an effort to communicate science to this emerging international policy, the Dartmouth Superfund Research Program formed the Coastal and Marine Mercury Ecosystem Research Collaborative (C-MERC) in 2010 that brought together more than 70 scientists and policy experts to analyze and synthesize the science on Hg pollution in the marine environment from Hg sources to MeHg in seafood. The synthesis of the science revealed that the sources and inputs of Hg and their pathways to human exposure are largely determined by ecosystem spatial scales and that these spatial scales determine the organizational level of policies. The paper summarizes the four major findings of the report.
Keywords: mercury pollution, Minimata Treaty, seafood contamination, mercury policy
Introduction
Mercury (Hg) is a global contaminant whose presence in the biosphere has been increased by human activity, particularly coal burning/energy production, mining, especially artisanal scale gold mining, and other industrial activities (1–3). In the US, Hg is the third most important contaminant on the CERCLA Priority List of Hazardous Substances (4). It strongly bioaccumulates in its organic form, methylmercury (MeHg), driving exposure of this potent toxin with neurological, immunological, and cardiovascular effects (5–8). Consumption of MeHg contaminated fish is a serious public health concern and 50 US states have MeHg consumption advisories, including advisories for coastal waters where subpopulations of recreational and subsistence fishermen are at high risk for MeHg exposure. Exposure to Hg through fish consumption is a concern even in relatively pristine environments, as Hg is readily transported through the atmosphere and can be deposited both nearby and far from source areas (1,9–11). Marine ecosystems are critical environments for Hg contamination because human exposure to MeHg primarily occurs through the consumption of seafood. Approximately 92% of the global fish harvest for human consumption consists of marine fish with the majority coming from the coastal fisheries.
Mercury input to the surface ocean has doubled over the past century leading governments and organizations to take actions to protect humans from the harmful effects of this toxic element. Most of the legacy Hg in the atmosphere and oceans has come from historic industrial development in North America and Europe (12). However, 40% of the current Hg emissions to the atmosphere now originate from East and Southeast Asia largely by coal combustion and artisanal scale gold mining (12). In the US, President Obama signed the Hg and Air Toxics Standard (MATS) in 2011 for controlling atmospheric emissions predominantly from coal fired power plants. Internationally, the UN Environmental Program led 128 countries to negotiate and sign a legally binding agreement, the 2013 Minimata Convention, to control Hg emissions and releases to land and water globally. Regulations on Hg emissions will undoubtedly decrease new inputs of Hg into aquatic ecosystems, yet legacy Hg as well as continuing atmospheric inputs will still pose risks to human health through bioaccumulation and biomagnification for decades into the future. Rates of change of Hg in food webs may not reflect the same rate of decrease in primary anthropogenic emissions due to local-scale processes, recycling of Hg between the atmosphere and the ocean and terrestrial environments, and the impact of influential variables other than emission or deposition rates (13–19). Understanding linkages between environmental processes and human health is critical to understanding risk and predicting how and to what magnitude both small- and large-scale changes in environment variables will influence human exposure, particularly to vulnerable populations.
Methods
In 2010, the Dartmouth Superfund Research Program formed the Coastal and Marine Mercury Ecosystem Research Collaborative (C-MERC) that brought together more than 70 scientists and policy experts to analyze and synthesize the current science on Hg pollution in the marine environment from Hg sources to MeHg in seafood. In 2012, C-MERC authors published a series of 11 papers in the peer reviewed journals (Environmental Health Perspectives and Environmental Research) and produced a synthesis report, “Sources to Seafood: Mercury Pollution in the Marine Environment” (20), based on these papers and the literature in order to inform policies and management actions under consideration at the local, national and international level to limit Hg exposure and safeguard human health.
Findings and Conclusions
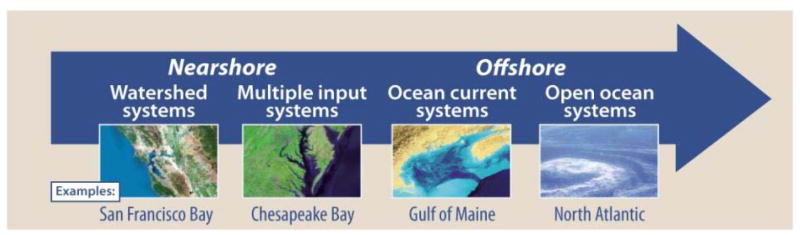
The synthesis of the science on Hg pollution in marine systems revealed that the sources and inputs of Hg and their pathways to human exposure are largely determined by ecosystem spatial scales. Those scales ranging from small marine ecosystems to large determine the main sources and endpoints of Hg pollution, and MeHg bioaccumulation. The inorganic Hg inputs to systems ranging from small embayments to the open ocean include river inputs, internal inputs (sediments), ocean currents, and the atmosphere (Figure 1). Human exposure to Hg pollution via MeHg exposure from fish consumption is also determined by ecosystem scale because marine fisheries range from local fisheries in small bays and estuaries to the global open ocean fisheries. The smaller ecosystems are strongly linked to local releases of Hg from watersheds, and the potential for external inputs of MeHg, whereas the larger open oceans mostly receive atmospheric inputs of Hg, and most MeHg is produced in situ. Lastly, the management of Hg sources that ultimately lead to human exposure to MeHg is also determined by ecosystem scale: local scale sources and inputs need to be controlled by local or regional policies, while atmospheric sources and inputs must be managed with national policies and international treaties like the Minamata Convention (Figure 2).
Figure 1.
Scales of mercury inputs to marine ecosystems from estuaries to the open ocean. Reproduced from Sources to Seafood: Mercury Pollution in the Marine Environment, p. 10.
Figure 2.
Scales of mercury policies from the local to the global. Reproduced from Sources to Seafood: Mercury Pollution in the Marine Environment, p. 19.
Four major findings emerged from the C-MERC synthesis involving the different pathways between Hg inputs, types of marine ecosystems, and consumers of seafood:
Mercury pollution is ubiquitous in the world’s oceans and coastal waters. Therefore, MeHg contaminates most fish and other seafoods that are important sources of protein and nutrition for people worldwide. Despite improvements in some regions, MeHg in commonly consumed marine fish continues to exceed human health guidelines, and Hg pollution is increasing.
Mercury pollution enters the marine environment along distinct pathways that are linked to different Hg sources. Atmospheric inputs from global sources of Hg emissions dominate the “open ocean” and “ocean current” (large coastal ocean) systems. Riverine Hg inputs dominate coastal waters that are “watershed systems.” Some coastal waters are “multiple input” systems that reflect both atmospheric and riverine inputs.
Many seafood consumers are “general consumers” whose MeHg exposure comes from fish typically harvested from the open oceans that receive high atmospheric inputs from global Hg emission sources. “Local consumers” generally eat seafood from nearby coastal waters that are contaminated by riverine inputs from local, regional, and global sources of Hg.
We anticipate that MeHg concentrations in marine fish will decline roughly in proportion to decreases in Hg inputs, though the timing of the response will vary. Specifically, MeHg in open ocean fish will likely begin to decrease within many years to decades after emissions controls because production of MeHg mostly occurs in the upper ocean where most fish feed. In contrast, MeHg in fish from coastal systems contaminated by legacy Hg may take many decades, or even centuries, to fully reflect the declines in inputs, because of the slower rate of burial in coastal sediments and the continued inputs from the watershed.
The Minimata Convention is currently being ratified by signatory countries. To date, 12 of the 128 signatories have ratified the Convention with 50 countries needed for the convention to come into force22. In the meantime, Hg scientists around the world are continuing to investigate and understand the spatial and temporal patterns of Hg sources, inputs, concentrations in the oceans and its food webs, as well as the extent and degree of human exposure. Moreover, the future implementation of the Convention will require effectiveness evaluation and Hg scientists are working to build a framework for a global monitoring system which will be based on current monitoring programs and will be expanded to include new monitoring sites and additional endpoints. The local to global scale of the Hg pollution problem requires policy solutions at local to international levels for decreasing Hg pollution in the environment.
Acknowledgments
This publication was made possible by NIH Grant Number P42 ES007373 to B. Stanton from the National Institute of Environmental Health Sciences (www.niehs.nih.gov/).
Contributor Information
Celia Y. Chen, Email: Celia.Y.Chen@Dartmouth.edu.
Charles T. Driscoll, Email: ctdrisco@syr.edu.
Kathleen F. Lambert, Email: klambert01@fas.harvard.edu.
Robert P. Mason, Email: robert.mason@uconn.edu.
Elsie M. Sunderland, Email: elsie_sunderland@harvard.edu.
Literature Cited
- 1.Driscoll CT, Han YJ, Chen CY, Evers DC, Lambert KF, Holsen TM, Kamman NC, Munson RK. Mercury contamination in forest and freshwater ecosystems in the Northeastern United States. Bioscience. 2007;57:17–28. [Google Scholar]
- 2.Mason RP, Fitzgerald WF, Morel FMM. The biogeochemical cycling of elemental mercury - anthropogenic influences. Geochimica Et Cosmochimica Acta. 1994;58:3191–3198. [Google Scholar]
- 3.Selin NE, Jacob DJ, Yantosca RM, Strode S, Jaegle L, Sunderland EM. Global 3-D land-ocean-atmosphere model for mercury: Present-day versus preindustrial cycles and anthropogenic enrichment factors for deposition. Global Biogeochemical Cycles. 2008;22 [Google Scholar]
- 4.ATSDR. Priority list of hazardous substances. Agency for Toxic Substances and Disease Registry; 2011. [Google Scholar]
- 5.Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, Cowell W, Grandjean P, Korrick S. Evidence on the human health effects of low level methylmercury exposure. Environmental Health Perspectives. 2012 doi: 10.1289/ehp.1104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahaffey KR, Sunderland EM, Chan HM, Choi AL, Grandjean P, Marien K, Oken E, Sakamoto M, Schoeny R, Weihe P, Yan CH, Yasutake A. Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption. Nutrition Reviews. 2011;69:493–508. doi: 10.1111/j.1753-4887.2011.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mergler D, Anderson HA, Chan LHM, Mahaffey KR, Murray M, Sakamoto M, Stern AH. Methylmercury exposure and health effects in humans: A worldwide concern. Ambio. 2007;36:3–11. doi: 10.1579/0044-7447(2007)36[3:meahei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Nyland JF, Wang SB, Shirley DL, Santos EO, Ventura AM, de Souza JM, Silbergeld EK. Fetal and maternal immune responses to methylmercury exposure: A cross-sectional study. Environmental Research. 2011;111:584–589. doi: 10.1016/j.envres.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CY, Stemberger RS, Kamman NC, Mayes BM, Folt CL. Patterns of Hg bioaccumulation and transfer in aquatic food webs across multi-lake studies in the northeast US. Ecotoxicology. 2005;14:135–147. doi: 10.1007/s10646-004-6265-y. [DOI] [PubMed] [Google Scholar]
- 10.Kirk JL, Louis VLS, Hintelmann H, Lehnherr I, Else B, Poissant L. Methylated Mercury Species in Marine Waters of the Canadian High and Sub Arctic. Environmental Science & Technology. 2008;42:8367–8373. doi: 10.1021/es801635m. [DOI] [PubMed] [Google Scholar]
- 11.Riget F, Braune B, Bignert A, Wilson S, Aars J, Born E, Dam M, Dietz R, Evans M, Evans T, Gamberg M, Gantner N, Green N, Gunnlaugsdottir H, Kannan K, Letcher R, Muir D, Roach P, Sonne C, Stern G, Wiig O. Temporal trends of Hg in Arctic biota, an update. Science of the Total Environment. 2011;409:3520–3526. doi: 10.1016/j.scitotenv.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 12.UNEP. Global Mercury Assessment 2013L Sources, Emissions, Releases and Environmental Transport. UNEP Chemicals Branch; Geneva, Switzerland: 2013. [Google Scholar]
- 13.Bhavsar SP, Gewurtz SB, McGoldrick DJ, Keir MJ, Backus SM. Changes in mercury levels in Great Lakes fish between 1970s and 2007. Environmental Science & Technology. 2010;44:3273–3279. doi: 10.1021/es903874x. [DOI] [PubMed] [Google Scholar]
- 14.Carrie J, Wang F, Sanei H, Macdonald RW, Outridge PM, Stern GA. Increasing contaminant burdens in an Arctic fish, Burbot (Lota lota), in a warming climate. Environmental Science & Technology. 2010;44:316–322. doi: 10.1021/es902582y. [DOI] [PubMed] [Google Scholar]
- 15.Conaway CH, Ross JRM, Looker R, Mason RP, Flegal AR. Decadal mercury trends in San Francisco Estuary sediments. Environmental Research. 2007;105:53–66. doi: 10.1016/j.envres.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Gratz LE, Keeler GJ, Miller EK. Long-term relationships between mercury wet deposition and meteorology. Atmospheric Environment. 2009;43:6218–6229. [Google Scholar]
- 17.Greenfield BK, Davis JA, Fairey R, Roberts C, Crane D, Ichikawa G. Seasonal, interannual, and long-term variation in sport fish contamination, San Francisco Bay. Science of the Total Environment. 2005;336:25–43. doi: 10.1016/j.scitotenv.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 18.Macdonald RW, Harner T, Fyfe J. Recent climate change in the Arctic and its impact on contaminant pathways and interpretation of temporal trend data. Science of the Total Environment. 2005;342:5–86. doi: 10.1016/j.scitotenv.2004.12.059. [DOI] [PubMed] [Google Scholar]
- 19.Sunderland EM, Dalziel J, Heyes A, Branfireun BA, Krabbenhoft DP, Gobas F. Response of a macrotidal estuary to changes in anthropogenic mercury loading between 1850 and 2000. Environmental Science & Technology. 2010;44:1698–1704. doi: 10.1021/es9032524. [DOI] [PubMed] [Google Scholar]
- 20.Chen CY, Driscoll CT, Lambert KF, Mason RP, Rardin LR, Schmitt CV, Serrell NS, Sunderland EM. Sources to Seafood: Mercury Pollution in the Marine Environment. Hanover, NH: Toxic Metals Superfund Research Program, Dartmouth College; 2012. http://www.dartmouth.edu/~toxmetal/C-MERC/ [Google Scholar]
- 21.Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N. Mercury as a global pollutant: sources, pathways, and effects. Environmental Science & Technology. 2013;47:4967–4983. doi: 10.1021/es305071v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minimata Convention on Mercury: Texts and Annexes. United Nation Environment Programme GE.14-00280 UNEP/CHEMICALS/2014/1. Publishing Service; United Nations, Geneva Switzerland: 2014. [Google Scholar]