Abstract
Introduction
A detailed study of reports on the immunomodulatory properties of vitamin A and select flavonoids may pave the way for using these natural compounds or compounds with similar structures in novel drug and vaccine designs against infectious and autoimmune diseases and cancers.
Areas Covered
Intracellular transduction pathways, cellular differentiation and functional immunomodulatory responses have been reviewed. The reported studies encompass in vitro, in vivo preclinical and clinical studies that address the role of Vitamin A and select flavonoids in induction of innate and adaptive B and T cell responses, including TH1, TH2 and Treg.
Expert Opinion
While the immunomodulatory role of vitamin A, and related compounds, is well-established in many preclinical studies, its role in humans has begun to gain wider acceptance. In contrast, the role of flavonoids is mostly controversial in clinical trials, due to the diversity of the various classes of these compounds, and possibly due to the purity and the selected doses of the compounds. However, current preclinical and clinical studies warrant further detailed studies of these promising immuno-modulatory compounds.
Keywords: vitamin A, flavonoid, catechin, immunomodulation, antiviral
1. Introduction
Vitamins and flavonoids are two separate classes of naturally occurring small chemical compounds. One of the most widely studied vitamins is vitamin A. Vitamin A and related forms are found as pro-vitamin A, pre-formed vitamin A, carotenes, all trans retinoic acid (ATRA) and retinoic acid (RA). While emphasis of the use of vitamin A in the general population currently is for healthy vision, the immunomodulatory roles of vitamin A have been suggested for many decades. Recently, ATRA and RA have been shown to directly induce changes in innate and adaptive immune responses and their uses have been suggested as vaccine adjuvants.
Flavonoids encompass a large number of structurally related small chemical molecules, naturally occurring in vegetables, herbs and fruits. These compounds include catechins in black and green tea, and curcumin as a component of the food spice turmeric. While originally they gained interest for their antioxidant properties, accumulating evidence suggests that these compounds can exert immunomodulating effects on various cells of the immune system. Importantly, certain flavonoids possess direct anti-bacterial and antiviral properties, independent of their immunomodulating activity.
Recently, the surprising synergistic immune-enhancing properties of mixing a flavonoid (e.g. catechins) and a vitamin (e.g. vitamin A or E) in a pharmaceutically accepted carrier (e.g. a vegetable oil) were reported [1,2]. Although the mechanism of the synergistic immune-enhancing action of these two naturally occurring class of small molecules remains to be determined, the immunomodulating properties of each class of these molecules will be separately discussed in this review.
Thus, in this review, we examine reports on the intra-cellular signal transduction pathways and cellular differentiation and functional immune responses induced by vitamin A and various flavonoids in vitro, as well as in preclinical and in clinical studies. The cited studies cover the period 1946–2015 as found in the Medline/Pubmed search engine.
2.1 Vitamin A Definition and Classifications
Preformed Vitamin A is a fat soluble essential vitamin found mainly in animal products such as meat, fish, poultry and dairy foods, whereas pro-vitamin A (carotenoids) is found in fruits and vegetables [3,4]. Carotenoids are organic pigment compounds found primarily in the chloroplast of plants, giving them their unique color. Structurally, carotenoids can be found in two major forms of polyene hydrocarbon chains terminated by rings with oxygen, i.e. xanthophylls, or without oxygen, i.e. carotenes. Carotenes refer to the oxygen free carotenoids such as α- and β-carotenes and lycopene, of which β-carotene, a precursor of vitamin A, is the most commonly consumed carotenoid [3,4]. Although vitamin A is a necessary dietary supplement for human health, it does not have any biological effects in the human body on its own. It functions in the body through mediators identified as all trans retinoic acid (ATRA), which control many vital biological functions such as vision, reproduction, development, growth and immunity [3,4]. ATRA is an active metabolite of vitamin A, which is also synthesized by dendritic cells expressing retinalaldehyde dehydrogenases (RALDH). Retinoic acid (RA) is one of the important metabolites of vitamin A that regulates the expression of target genes through receptor mediated activities [5,6]. Stromal cells from intestinal mesenteric lymph nodes (MLN) produce RA, which induced the expression of the gut mucosal homing receptors, α4β7 and CCR9, on local T cells and this effect was further enhanced by the presence of bone marrow derived DC, in vitro [7].
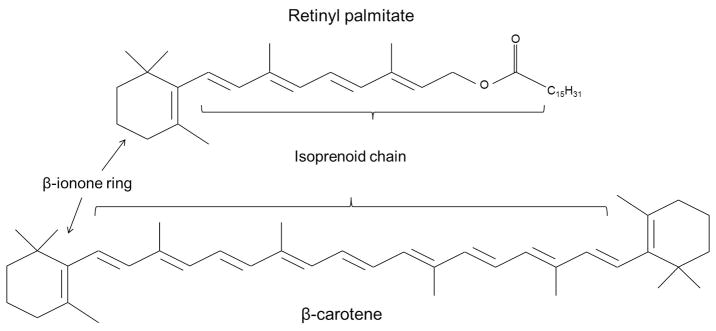
There are two modes through which vitamin A can be absorbed into the body, namely in the form of retinyl palmitate (Figure 1) from animal products or as a natural byproduct of carotenoids (Figure 1) referred to as retinoids. Carotenoids undergo irreversible oxidative cleavage to produce retinal as the final product [8]. In the small intestine, carotenes containing retinyl groups are broken down into retinal in the presence of bile salts and an enzyme called β-carotene dioxygenase. Retinal is also a precursor for other forms of vitamin A [8]. These two metabolites of vitamin A, retinol and retinal, are inter-convertible (Figure 2) in the body as needed and are catalyzed by retinol dehydrogenases (RDHs) or alcohol dehydrogenases. In the presence of the enzymes retinaldehyde dehydrogenase (RALDHs) or retinol oxidase, retinals are catalyzed into retinoic acid (RA) via transfer of one or more hydride ions to electron acceptor molecules [8].
Figure 1.
Basic structure of retinyl palmitate and β-carotene showing important structural features essential for vitamin activity.
Figure 2.
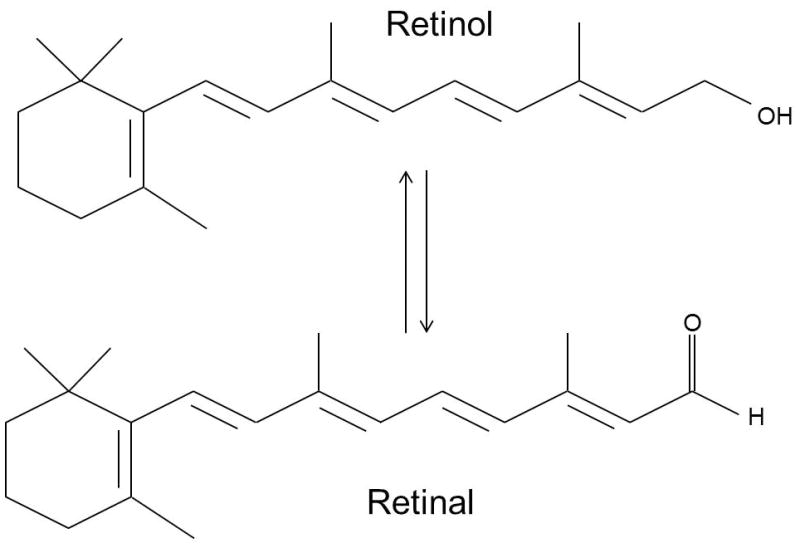
Reversible reaction of Retinal and Retinol.
Regardless of its dietary origin, preformed vitamin A and carotenoids are mainly released from proteins during proteolysis in the stomach [9,10]. These fragments in turn combine with lipids to form globules, which are then transported to the small intestine. Absorption of vitamin A occurs in the upper intestinal mucosa with the help of pancreatic lipase enzymes where it is enzymatically re-esterified (retinyl esters). Once absorbed into the circulatory system, retinyl esters are hydrolyzed into retinols, which bind to the retinol binding protein (RBP) for transportation [9,10]. The formation of this retinol-RBP complex is the principal form of intracellular transport of retinols. Once this complex is taken up by liver, retinol is converted back to retinyl ester by lecithin retinol acyltransferase (LRAT) for storage or it binds to cellular retinol-binding protein (CRBP) to carry out activation of target genes [9,10]. In the presence of retinol dehydrogenase enzyme retinol is metabolized to retinal, which is further metabolized to RA in the presence of RALDH. Furthermore, RA binds to cellular RA binding protein which allows the RA molecule to enter the nucleus where it can serve as a ligand and bind to RA receptors (RARs) or retinoid X receptors (RXRs) causing them to heterodimerize and mediate genomic and non-genomic events [11,12].
Although liver is a primary storage site of retinoids, the mechanisms by which RA exerts its effects in the liver are poorly understood. Under normal physiological conditions, stellate cells, also referred to as fat storing cells, are responsible for storing 80 – 90 % of vitamin A and hepatocytes store 10 – 20 % [11,12]. It has been shown that liver bile-derived retinol supported small intestinal, but not colonic, lamina propria CD103+ DC to be imprinted with the ability to metabolize retinol, in a RA-dependent fashion, and this in turn was important in causing the expression of gut mucosal homing receptors, α4β7 and CCR9, on local T cells [13]. A more recent study by Mamoon et al. reported several novel RA responsive genes in murine hepatocytes [14]. The study also showed how small heterodimer partners (SHP) are involved in RA induced signaling in a non-transformed hepatic cell line AML12 [14]. To further provide evidence of their findings, an animal study in 2014 by the same group reported that RA inhibits several crucial genes important for bile acid metabolism via up-regulation of SHP [15].
2.2 Induction of Intra-cellular Signal Transduction Pathways by Vitamin A
RA has been reported to play an important role in maintaining cell cycle, proliferation, differentiation and apoptosis of activated T cells [16,17]. Although the importance of ATRA signaling in T cells is poorly understood, several studies have attempted to identify the enzymes that play a major role in maintaining proper T cell function. In this regard, a recent study identified that in the presence of RA, expression of cytochrome P450 family 26, subfamily b, polypeptide 1 (Cyp26b1) was greatly induced in naïve CD4+ T cells, while transforming growth factor (TGF-β) inhibited the ATRA-induced RA expression of Cyp26b1 [18]. In accordance with these findings, a recent in vivo study using Cyp26b1 knockout (KO) mice reported significantly less intestinal inflammation during T cell dependent colitis when CD4+ T cells from these mice were transferred to Rag1 KO mice [19]. This in turn underlined a mechanism by which Cyp26b1 enzyme plays an important role in regulating RA-dependent T cell immune responses in the intestine.
Along with playing a major role in cell cycle and apoptosis, ATRA has been reported to influence different cytokine specific transcription factors such as signal transducers and activators of transcription (STAT), interferon regulatory factors (IRF) and GATA-3, which are responsible for facilitating differentiation and anti-proliferative effects in cells [20–22]. In this regard, an earlier study on the regulation of IRF and STAT gene expression by ATRA reported that ATRA activates IRF-1 in several different leukemia cell lines, which responded to ATRA by growth inhibition [21]. The study also reported up-regulated gene expression of STAT1, STAT2 and p48 during ATRA induced myeloid differentiation, leading to the suggestion that ATRA-induced expression of these transcription factors may be one of the molecular mechanisms that facilitate cellular growth inhibition by ATRA [21].
GATA-3 is one of the transcription factors that regulate epithelial cell differentiation in mammary glands [22]. Under GATA-3 deficient conditions, ATRA could inhibit a TH1 response while simultaneously promoting a TH2 response in vitro [23]. The results of this study suggested vitamin A may be used as a potential effective treatment for certain diseases caused by TH1/TH2 imbalances [23]. In accordance with these data, results of another study showed increased expression of IL-4, IL-5 and IL-13 in the presence of ATRA or 9-cis-RA, but not IFN-γ, TNF-α, IL-2 and IL-12p70, all of which are TH1 cytokines [20]. In vitro expression of the TH2 cytokine, IL-4, and the TH2 transcription factors, GATA-3, c-MAF and STAT6 were enhanced and the TH1 transcription factor, T-bet, was decreased following ATRA treatment of human T cells over various time intervals. Data from this study provided strong evidence that ATRA plays multiple roles in development of TH2 responses [20].
RA analogues have been reported to play important roles in various cancers. A second generation retinoid, acitretin, was reported to induce apoptosis in squamous cell carcinoma (SCL-1) cells via the CD95/CD95L apoptotic signaling pathway [24]. ATRA treatment of SCL-1 cells resulted in increased cell population at G1 phase along with decrease in cell populations in the S and G2 phases [25]. Protein complexes cyclin D1/CDK4 (cyclin dependent kinase) and cyclin E/CDK2 are required to facilitate transition of cells from G1 to S phase during differentiation. After 24 hours of treatment with ATRA, expression of cyclin D1, CDK2 and CDK4 was greatly down-regulated resulting in a mechanism through which ATRA induced apoptosis in SCL-1 cells [25]. The study also provided further evidence that ATRA inhibits the activation of the extracellular signal-regulated kinases 1/2 (ERK1/2) and c-Jun N-terminal kinases 1/2 (JNK1/2) pathways via inhibition of the transcription activity of AP-1 in SCL-1 cells [25]. This in turn provided further evidence that ATRA induces cell cycle arrest in human SCL-1 cells by inhibiting the mitogen activated protein kinase (MAPK)-AP1 pathway [25]. A recent in vitro study showed that in addition to activating the retinoic acid receptor (RAR), RA also mediates activation of another nuclear receptor PPARβ/δ, which plays an important role in regulation of metabolism, cellular differentiation and development. While CRABP-II delivered RA to RAR, FABP5 transported RA to PPARβ/δ. The study also showed that mammary carcinoma MCF-7 cells display a high CRABP-II/FABP5 ratio and reversing the effects resulted in conversion of RA from pro-apoptotic to anti-apoptotic agent suggesting its growth inhibitory activities and a survival response [26]. Another ATRA signaling pathway was revealed by demonstrating that the cellular retinoic acid binding protein 2 (CRABP2) suppressed tumor growth in carcinomas by binding to RAR in the presence of RA and increased expression of the pro-apoptotic genes, i.e. Apoptotic protease activating factor 1 (Apaf-1), Caspase-7, apoptosis-related cysteine peptidase (Casp7) and the human antigen receptor (HuR) [27].
AKT/PKB is another pathway that has been reported to play an important role in maintaining many cellular functions. A recent study using the human acute promyelocytic leukemia cell line, NB4, identified two substrates, the Serine/arginine-rich splicing factor 3 (SRSF3) and the prohibitin 2 (PHB2), which are associated with AKT/PBK pathway and their phosphorylation increased during in vitro activation with ATRA [28]. This, in turn, led to activation of AKT/PBK resulting in promotion of cell survival and differentiation vs. apoptosis [28].
One of the more recent studies reported down-regulatory effects of ATRA on Down Regulated Adenoma (DRA) expression, an important luminal membrane transporter responsible for NaCl absorption [29]. DRA has been shown to be suppression in intestinal inflammation. In vitro cultures of Caco-2 cells with 10 μM of ATRA resulted in ~ 3.5 fold increase of DRA expression at 24 hour time point [29]. These inhibitory effects of ATRA on DRA expression was reported to be mediated via RAR-β receptor subtype. The study also reported significantly enhanced levels of HNF-1β with ATRA treatment, which resulted in inhibition of DRA expression by ATRA providing further evidence that ATRA has down-regulatory effects on specific genes responsible for inflammation [29].
2.3 Induction of Cellular Immunomodulation by Vitamin A
Vitamin A is an essential trace nutrient for the human immune system, which functions in regulation of RA. In addition to vitamin A’s association with immunity, it is also important for pre and post development and reproduction processes [30]. In an important recent study, retinoic acid exposure in utero was reported to be important for development of full sized lymph nodes and subsequent ability to mount effective immune responses in the offspring [31]. The mechanism of action of retinoic acid occurred through the transcription factor RORγt, whose expression was important for proper development of differentiation of lymphoid tissue inducer cells. Retinoic acid regulated RORyt expression, and lack of retinoic acid signaling within the cells could be rescued by overexpression of RORyt [31]. Due to constant breakdown of dietary vitamin A by gut epithelial cells, high concentrations of RA are present in the small intestine as opposed to the low concentration found throughout the body [32]. Studies using various animal models revealed the importance of RA in intestinal mucosal immunity. Results of several in vivo and in vitro studies have demonstrated the modulating effects of RA on innate immunity and differentiation of dendritic cells [33–35]. Importantly, RA primed intestinal lamina propria DCs to express RALDH2 and become CD103+ DCs, which resulted in production of more RA [36,37]. In addition, a number of studies have reported the induction of mucosal DCs in the presence of ATRA. A recent study by Saurer et al. demonstrated the mechanism by which RA plays an important role in mucosal immunomodulation through targeting DCs, and that DCs can act as efficient carriers of RA in vitro [35]. In accordance with these results, another study reported the mechanisms by which mucosal factors TGF-β and RA induce functionally and phenotypically distinct DC types with non-inflammatory properties [38]. Zhu et al. suggested a mechanism by which pro-inflammatory properties of inflammatory monocytes (IMCs) and inflammatory dendritic cells (IDCs) are reduced converting them into immunoregulatory DCs [34]. The results showed that during co-treatment of GM-CSF-differentiated IDCs with IL-4 and RA, expression of ALDH was induced, and expression of CD103 was up-regulated, which in turn reduced the production of pro-inflammatory cytokines [34]. The data also showed this co-treatment of IL-4 and RA strongly induced CD4+ Foxp3 Treg cell differentiation and suppressed TH1/TH17 differentiationsuggesting a synergistic effect of IL-4 and RA on IDCs, and providing evidence that the use of RA may be a possible treatment option for autoimmune disorders [34].
Using the murine embryonic stem cell-derived embryoid bodies (EBs), a recent in vitro study provided evidence that RA up-regulates the lymphatic markers LYVE-1 and Prox1, as regulators of lymphatic development. The study showed incubation of EBs with VEGF-C, growth hormone, IGF-1 and IL-7 significantly promoted the expression of LYVE-1 in CD31+ structures. The study also showed development of CD31+/LYVE-1+/Prox1+ cell clusters in the presence of RA and cAMP [39]. In a report of both in vitro and in vivo studies, it was shown that 9-cis RA plays an important role in the molecular mechanisms underlying the pro-lymphangiogenic effects. Hence, in vitro, addition of RA to lymphatic endothelial cells supported their proliferation and tube formation, through the action of fibroblast growth factor, and further RA influenced expression of cell-cycle regulators through both nongenomic and genomic mechanisms. In support of the in vitro findings, 9-cis RA promoted lymphangiogenesis in trachea and ameliorated tail lymphedema by increased lymphatic vessel generation [40]. A recent study investigated the involvement of neural crest cells and RA in specific steps in lymphatic endothelial cell (LEC) differentiation and nuchal edema, a condition describing a swollen fetal cervical region. This study showed enlarged jugular lymph sacs and nuchal edema in the absence of RA synthesis and in utero inhibition of RA signaling resulting in nuchal edema. These data suggested that neural crest cells are important for lymphatic development and RA is required to mediate the differentiation of LECs, emphasizing the importance of RA signaling in mouse embryos in normal lymphatic development and nuchal edema [41].
Several preclinical studies indicated that vitamin A or retinoids are potent modifiers of TH1 (IFN-γ, IL-2 and TNF-β) and TH2 (IL-4, IL-13 and IL-5) responses [42,43]. An earlier study by Carmen and Hayes reported overproduction of IFN-γ by vitamin A deficient CD4+ T cells [42]. On the other hand, secretion of IL-2 and IL-4 were equivalent in both vitamin A deficient and vitamin A sufficient mice leading to a conclusion that vitamin A is important for induction of IFN-γ production [42]. Other studies reported decreased IL-4 production during vitamin A deficiency [43], and induction of IL-4 synthesis during in vitro murine T cell activation by retinoids [44–46]. Contrary to these results, a study by Mehta et al. reported down regulatory effects of ATRA on TNF-α production by peritoneal macrophages activated by endotoxin and IFN-γ [47]. The study also reported suppression of nitric oxide (NO) by ATRA, which has been previously reported to be a mediator of inflammatory responses [47]. While the above studies indicated that vitamin A or RA are TH2 mediators, other studies have suggested a role of vitamin A and RA in the Treg induction. In this regard, an in vitro study showed that in the presence of ATRA, DC induced CD4+ cells to secrete IL-10 (a regulatory cytokine), while reducing the production of IL-4 and IL-13 (TH2 cytokines) [48]. Because it may be argued that in general IFN-γ is a good indicator of a TH1 type response, the above data suggest that RA and vitamin A may suppress TH1 (IFN-γ) in favor of TH2 (IL-4) responses [48].
Many synthetic retinoids have been reported to play an important role in T cell responses [24,49]. It was reported that tretinoin and etretinate, first and second generation retinoids respectively, have direct inhibitory effects on alloresponsive T cells, which could be the result of induction of immunosuppressive effects by regulatory T cells (Treg) [49].
Natural regulatory T cells (nTregs) are important for prevention of autoimmune diseases. Lu et al. showed that ATRA prevented human nTregs from converting to TH1 and TH17 cells upon stimulation with IL-1 and IL-6 cytokines, and thus allowing them to sustain their suppressive properties during inflammatory conditions [50]. This study also reported following inflammatory stimulations, ATRA suppressed IL-1 receptor up-regulation and accelerated IL-6 receptor down-regulation and affected epigenetic modifications in the Foxp3 locus in nTregs. The authors suggested nTregs primed with ATRA may function as a novel treatment for chronic immune mediated diseases [50]. These data suggest that RA may promote CD4+Foxp3+ Treg function in the form of IL-10 secretion and suppression of proinflammatory innate IL-6 and IL-1 as well as TH1 and TH17 responses.
Several studies have addressed the regulatory role of ATRA in the intestinal mucosal immune system. The association of Foxp3+ Treg cell development with synergistic effects of RA derived from CD103+ DCs and TGF-β have been reported in many studies [51–54]. The mechanism described in these studies emphasized T cell tolerance to antigens derived from commensal flora or dietary origin [51–54]. Some of the earlier studies have also reported IL-10 as another essential immune regulator for mucosal tolerance [55,56]. In IL-10 deficient mice raised under germ-free conditions, T cell activation and colitis were induced leading to a conclusion that IL-10 is important in maintenance of tolerance to commensal bacterial antigens [55,56]. However, it has been shown that RA induces the IL-10 producing Treg cells, thus suggesting a mechanism by which RA and IL-10 maintain tolerance to intestinal commensal flora [57]. Further evidence for this notion was provided by showing that IL-22, a member of the IL-10 family, is important in maintaining epithelial integrity and is also induced by RA. The study reported the expression of IL-22 and IL-22 binding protein (IL-22BP) in a subset of conventional DCs in lymphoid and non-lymphoid tissues in both rats and mice, and expression of IL-22BP in murine intestinal lamina propria DCs [58]. To provide further understanding of the RA role, in vitro cultures of monocyte-derived dendritic cells (MDDCs) from peripheral blood of healthy donors were primed with various molecules present in the gut which showed no effect on the expression of IL-22BP. However, IL-22BP expression was up-regulated by addition of retinal during differentiation of DCs, suggesting a mechanism by which retinal is metabolized by human MDDCs allowing production of RA, which in turn results in IL-22BP expression [58]. In addition, a recent study reported down-regulation of RAR-α, increased number of DC cells, increased IL-12 secretion and decreased IL-10 and IFN-γ secretion in the intestinal mucosa of vitamin A deficient rats [33]. In vitro ATRA effects were reported as well, such as maturation of DCs, up-regulation of RAR-α, reduced IL-12 and IFN-γ but not IL-10. However, these effects were reversed in the presence of Ro 41–5253 (specific antagonist of RAR-α) [33]. This study also provided a link between the effects of vitamin A on DCs and altered mucosal immunity and proposed vitamin A can be used as an anti-inflammatory treatment in mucosal tissues and to restore impaired antibody responses under vitamin A deficient conditions [33].
An association of vitamin A deficiency and decreased mucosal immunity in the intestine has been shown in rats in that [59]. correcting vitamin A deficiency early in the development process lead to improved intestinal mucosal immune responses [59] A significant increase in intestinal levels of secretory immunoglobulin A was observed following vitamin A supplementation postnatal on day 1 [59]. These rats also had higher numbers of CD8+ intestinal intraepithelial lymphocytes and CD4+CD25+ T cells in the spleen compared with the vitamin A-deficient rats [59]. Taken together, the above studies demonstrate that vitamin A and ATRA play important roles in development of mucosal immunity and tolerance in the intestine.
Germ-free mice and pigs have provided important tools to determine the role of vitamin A and retinoic acid in the development of innate and adaptive immune responses. In one study, while intestinal lamina propria DC and stromal cells (SC) readily produced RA in conventionally reared mice and the SC promote the production of RA in the DC in an RA- and GM-CSF-dependent fashion, these two co-localized cells were unable to constitutively produce RA in germ-free mice [60]. Furthermore, in another study, the interactions of intestinal stromal cells with DC played an important role in the maintenance intestinal Foxp3+ Treg, while stromal cells from germ-free or vitamin A deficient sources did not support the activity of Foxp3+ Treg [61]. More support of the role of RA in intestinal Treg regulation was provided by a study in which CD4+ cells transferred from germ-free and conventionally reared wild-type and IL10−/− mice to IL-10−/−, or Rag2−/− or IL-10 repleted Rag2−/− mice. In this model, more severe colitis was observed in both IL10−/− and Rag2−/− compared to the IL-10 repleted Rag2−/− mice in an RA-dependent manner [62]. These murine studies delineated the role of the microenvironment of the intestine and RA in the regulation of local inflammation.
The role of the gut microbiota and vitamin A in the control of innate and adaptive immune responses have been elegantly shown by using gnotobiotic (Gn) piglets. To recreate a similar environment as malnourished children in developing countries, Vlasova et al. vaccinated vitamin A deficient (VAD) and vitamin A sufficient (VAS) Gn piglets, with attenuated human Rotavirus (HRV) followed by challenge with virulent HRV. Although compared to VAS pigs, the number of conventional and plasmacytoid DCs were higher in VAD piglet before challenge, a significant decrease was observed after challenge. Furthermore, significantly higher frequency of CD103 expressing DCs were observed in VAS piglets compared to VAD piglets after challenge. In keeping with the murine data on the role of RA in reducing inflammation, significantly higher circulating IFN-α was found in VAD piglets 2 days after challenge, a finding that was consistent with higher virulent HRV replication titers and prominent intestinal inflammation [63]. Using a similar VAD/VAS Gn piglet model, the protective efficacy of pentavalent rotavirus vaccine, RotaTeq(®) against HRV challenge was measured [64]. In this model, significantly lower levels of hepatic vitamin A were detected in in VAD compared to that of VAS piglets. Vaccinated VAD piglets showed a 350 fold higher fecal virus shedding titers compared to VAS piglets post challenge. A 100% protection rate was observed in vaccinated non-supplemented VAS piglets compared to 25% protection in VAD piglets. Along with elevated levels of IL-8 and lower IL-10 responses, the data also showed 11 fold lower intestinal HRV-specific IgA antibody tiers in vaccinated VAD compared to VAS piglets post challenge. Overall, the study provided further evidence that VAD impaired immune responses to RotaTeq(®) vaccination and additional oral vitamin A supplementation did not increase the protective efficacy of the vaccine in VAD piglets, which in turn underlined the important role vitamin A plays in development of immune system [64].
Overall, these preclinical studies using various genetically modified, wild-type germ-free and conventionally reared animal models clearly establish a strong role for vitamin A and its metabolites and derivatives as modulators of innate and adaptive mucosal and systemic responses by cells intimately involved in the generation of innate and adaptive immune responses. Thus, while a significant body of evidence suggests a role of vitamin A and its derivatives in the development of intestinal mucosal Treg, other data strongly argue for dependence of protective immunity following vaccination against infectious agents on vitamin A and its derivatives.
2.4 Clinical Evidence of Induction of Immunomodulation by Vitamin A
Over the past decade, association of vitamin A and mucosal immunity has been a topic of interest and several in vitro and in vivo animal studies have set the foundation for small scale clinical trials. A recent clinical trial in Zambian men showed that administration of ATRA before and after oral typhoid vaccination increased intestinal mucosal immunity [65]. The study measured the effect of 10 mg ATRA given 1 hour before and for 7 days following oral typhoid vaccination. Significantly increased levels of IgA responses against lipopolysaccharides and proteins were observed in whole gut lavage fluid [65]. However, no difference was seen in serum IgA or IgG levels suggesting that ATRA plays a major role in developing mucosal immunity without exerting any effects on systemic IgA and IgG levels. Absorption of ATRA was well tolerated in men with no adverse events, suggesting the use of ATRA as a potential oral therapy before and after oral vaccinations [65].
In addition to maintaining tolerance to intestinal mucosa, vitamin A supplementation modified associations between gut cytokine immune responses and resolution of diarrheal infections [66,67]. A recent randomized placebo control study in Brazilian children showed vitamin A supplementation resulted in significantly reduced Giardia spp parasitic infections [66]. Another recent randomized trial also showed vitamin A supplementation with zinc improved G. lamblia infection in Mexican children, whereas supplementation of zinc alone increased A. lumbricoides incidences but decreased E. histolytica-associated diarrhea [67]. Similarly, another clinical trial reported the effect vitamin A supplementation had on the production of monocyte chemoattractant protein 1 (MCP-1), which is an essential chemokine involved in pathogen-specific mucosal immune response [68]. Compared to the placebo group, the study reported lower fecal concentration of MCP-1 in vitamin A supplemented group in Mexican children infected with enteropathogenic E. coli (EPEC). Thus, reduction of MCP-1 concentration in gastrointestinal tract resulted in an anti-inflammatory effect of vitamin A [68]. In agreement with these findings, a clinical study in Mexican children between the ages of 5 to 15 months reported vitamin A supplementation lead to significantly higher fecal concentration of IL-10 and less than detectable concentrations of IL-8 and MCP-1 resulting in longer duration of EPEC infection [69]. However, the scientists also reported the presence of lower fecal concentrations of TNF-α and IL-6 resulted in shorter duration of enterotoxigenic E. coli (ETEC) infection, whereas higher fecal concentration of MCP-1 was associated with longer duration of infection. The data showed down-regulation of the pro-inflammatory response and up-regulation of the Treg regulatory response in the vitamin A supplemented group [69]. They also reported in the supplemented group at least median detectable levels of IL-4, IL-5 and IFN-γ concentrations resulted in shorter duration of G. lamblia infections, which could be the result of TH1 and TH2 cytokine responses [69].
Other randomized clinical trials on vitamin A suggested that correcting vitamin A deficiency in an at risk population could lead to better outcomes in terms of reduced mortality and morbidity from measles, malaria, and certain forms of diarrhea in developing countries [70–75]. A clinical trial conducted in a population from Haryana, India, where vitamin A deficiency is a moderate public health problem, showed that vitamin A supplementation in children aged 6 months to 5 years reduced the mortality rate between the vitamin A supplementation group compared to the placebo group [76]. In conjunction with these findings, a similar recent clinical trial conducted in Gambia reported that vitamin A supplementation in infants between the ages of 6 to 59 months improved infant survival [77].
A randomized placebo-control trial investigated the effects of vitamin A and beta-carotene supplementation on vitamin A deficiency due to pregnancy in Napalese women [78]. The data provided in this study established a relationship between serum retinol levels, insulin-like growth factor -1 (IGF-1) and haemoglobin (Hb) during pregnancy [78]. IGF-1 was also reported to increase Hb concentration and decrease risk of anemia and iron deficiency, which was the result of increased erythropoietic activity. Thus, in turn the analysis provided evidence suggesting a mechanism by which retinol improved circulating Hb in pregnant women by increasing IGF-1 [78]. Because IGF-I has been implicated in regulating immune function [79], taken together, these results provide clinical evidence that vitamin A and beta-carotene directly impact the immune system.
3.1 Flavonoids Definition and Classifications
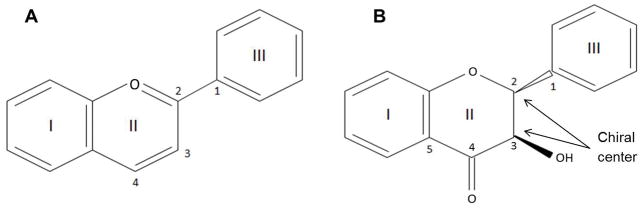
Flavonoids are natural ketone containing chemical compounds found in plants as their secondary metabolites [80]. They are natural phytochemical antioxidants that have been proposed to possess anti-inflammatory, antiviral, anti-proliferative and anti-carcinogenic properties [80]. The basic structure of flavonoids includes a 15 carbon compound in the form of 2 benzene rings connected by a 3 carbon chain, which forms a pyran ring containing oxygen (Figure 3). The degree of polymerization based on the flavan nucleus, the number, positions and types of substitutions, which influences the free radical reaction, is important for the mechanism by which they may exert their immunomodulatory and anti-inflammatory activities [80]. The structural features of ring III (Figure 3A) and patterns of glycosylation and hydroxylation of the three rings together make them one of the largest and most diversified naturally occurring family of chemical compounds in plants.
Figure 3.
Basic chemical structure of all flavonoids (A). Basic structure of catechins representing the two chiral centers (B).
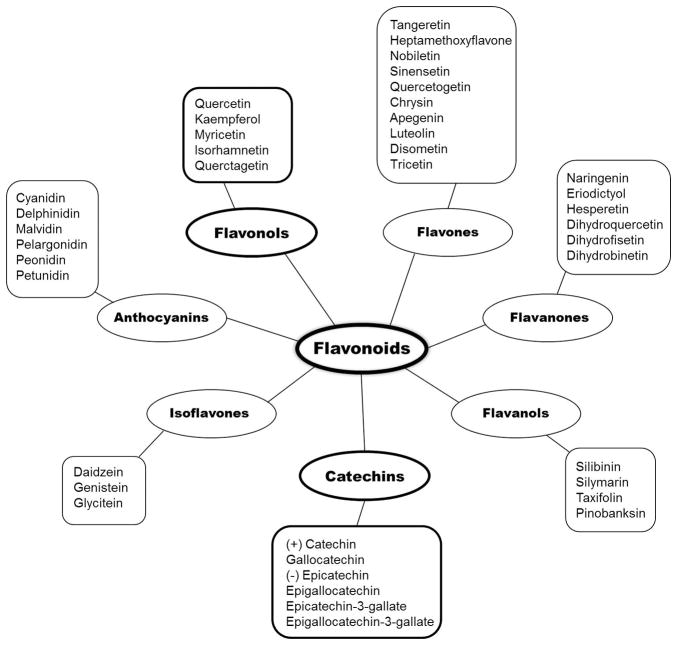
Flavonoids are divided into subgroups based on the location of ring III and the degree of unsaturation and oxidation of ring II, which refers to the number of hydrogens and oxygens present respectively [80]. For example, flavonoids where III ring is attached to position 3 of the ring II are referred to as isoflavones, whereas flavonoids with III ring on position 2 of the II ring are further divided into subgroups which include, flavones, flavonols, flavanones, flavanonols, anthocyanins and catechins (Figure 4) [81,82]. Flavonols are considered to be one of the largest subgroups of flavonoids present in fruits and vegetables, whereas catechins are found mainly in edible plants. Flavonols exhibit a hydroxyl group in position 3 of the II ring, which allows them to have diverse glycosylation, methylation and hydroxylation patterns [80]. Catechins also share the same hydroxyl group in position 3 of the II ring as flavonols. There is no double bond between position 2 and 3 and position 4 has a keto group, a structural feature that allows the catechins to have two chiral centers in the molecule at position 2 and 3 resulting in cis- (epicatechin) and trans- (catechin) isomers of the compounds (Figure 3B). One of the key features of these compounds is that the structural changes allow them to form polymers called proanthocyanidins, in turn providing further stability to the molecule in order to carry out free radical reactions [80]. (−)Epigallocatechin-3-gallate (EGCG), a major active ingredient of green tea, is one of the most widely studied flavonoids. Another widely studied compound is quercetin in the flavonol subgroup, which is an integral component of the human diet and occurs mainly in onions, apples and red wine.
Figure 4.
Classification of various flavonoids.
3.2 Induction of Intracellular Signal Transduction Pathways by Flavonoids
Intra-cellular signal transduction following activation of various cell types with flavonoids may shed light on their mechanism of immunomodulation. Several in vitro studies on activation of select signal transduction pathways have shed some light on what may occur in vivo. These cells have been as diverse as macrophage, smooth muscle, carcinoma and adenocarcinoma, pancreatic beta, ovarian cancer cell lines and Jurkat T cells. EGCG activation of the macrophage cell line Raw 264.7 upregulated cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) production [83]. Treatment of RAW 264.7 cells with 25–100 mM of EGCG for 12 hours caused a concentration dependent increase in the release of PGE2 and the expression of a 70 kDa COX-2 protein [83]. The underlying mechanism was activation of ERK (Extracellular signal related Kinases), MAPK (Mitogen Activated Protein Kinase) signaling pathways, that in turn plays a major role in COX-2 gene expression [83]. In a recent study, EGCG upregulated the expression of Mitofusin 2 (Mfn-2), thereby suppressing the proliferation of vascular smooth muscle cells through modulation of the Ras-Raf (Rat sarcoma-Rapidly Accelerated Fibrosarcoma) ERK/MAPK pathway exhibiting its cardio-protective roles [84]. In human anaplastic thyroid carcinoma (ATC) cells, EGCG suppressed EGFR-ERK phosphorylation and inhibited apoptosis [85]. A combined therapeutic effect of EGCG, theaflavin-3-30-digallate (TF3) and Vitamin C was observed in human lung adenocarcinoma SPC-A-1 cells and esophageal carcinoma Eca-109 cells [86]. In the presence of MAPK inhibitors, a combined treatment with EGCG and Vitamin C as well as TF3 and Vitamin C was found to enhance the apoptosis through modulation of the ERK, JNK and p38 pathways and activation of Caspase-3 and 9 [86].
Quercetin inhibited LPS-induced expression of TNF-α, IL-1β and IL-6 by suppressing the activation of ERK and p38 MAP kinases in macrophages [87]. Quercetin at a concentration of 1–100 mM inhibited the IL-1 induced IL-6 secretion, as well as p38 and protein kinase C-theta (PKC-θ) phosphorylation in a dose dependent manner [88]. In human Caco-2 cells, procyanidin B2 helped the expression of glutathione S-transferase P1 (GSTP1) via the activation of ERK and p38 MAPK and thus protected against colonic oxidative stress [89]. The suppression of MEK/ERK phosphorylation by flavonoids was found to be an important mechanism for the regulation of ligand-activated transcription factor aryl hydrocarbon receptor (AhR) in mouse hepatoma Hepa-1c1c7 cells [90]. AhR mediates toxic cancerous effects by binding to polycyclic aromatic hydrocarbons [91]. Catechin, epicatechin (EC), and EGCG inhibited Angiotensin II, a peptide hormone that causes vasoconstriction in rat aortic vascular smooth muscle cells [91]. This protective action was mediated through suppression of Angiotensin II mediated phosphorylation of ERK 1/2, JNK1/2, and p38 MAP kinases [91]. Based on these findings, quercetin mediates its effects on macrophages in response to oxidative stress via the ERK and MAPK pathways.
Several different cell lines have been used to investigate how EGCG mediates NF-κB activity. EGCG protected cytokine induced pancreatic beta-cell damage, which is partially mediated by suppression of NF-κB activity [92]. An in vitro study using the RINm5F pancreatic beta cell line, showed pretreatment of these cells with EGCG resulted in inhibition of apoptosis, whereas treatment with IL-1β in the presence of IFN-γ resulted in apoptosis by almost 50% [92]. The study also showed induced NO production in the presence of EGCG, which was the result of decreased inducible form of NO synthase (iNOS) protein levels through inhibition of the NF-κB signaling pathway in the RINm5F pancreatic beta-cells [92]. NF-κB in cooperation with other transcription factors coordinated the expression of genes encoding both iNOS and TNF-α [93]. It also activated lymphocytes by up regulating the expression of many cytokines, mainly IL-1, IL-6, TNF-α, lymphotoxin and IFN-γ. EGCG has also been reported to inhibit the phenotypic and functional maturation of murine DCs by suppressing the LPS-induced activation of ERK, JNK and p38 MAPK [93]. The study also showed pretreatment of DCs with EGCG resulted in suppression of NF-κB p65 signaling pathway [93]. Results of the study suggested this to be an important potential mechanism by which EGCG may exert protective effects against autoimmune diseases, including arthritis, allergy and diabetes.
In Jurkat T-cells monomers of catechins, (−) catechin and (−) epicatechin and their dimers, procyanidin B2, exerted immunomodulatory effects through inhibition of PMA induced NF-κB activation [94]. This inhibition was found to occur at the early stages of the NF-κB activation cascade as well as by direct binding of the monomeric and dimeric catechins to the NF-κB protein as suggested by molecular modeling. Through this pathway, a significant inhibition of PMA-induced IL-2 secretion was found, which may be a consequence of NF-κB inhibition [94]. EGCG exerted a neuro-protective role in auto-immune encephalomyelitis by suppressing the proteolipid protein and exerted a neuro-protective role by inhibiting the formation of reactive oxygen species in the neurons [95]. Grape seed procyanidin reversed the multi-drug resistance to the anticancer drug paclitaxel in ovarian cancer cells by blocking the expression of the p-glycoprotein [96]. Procyanidin down regulated the NF-κB and MAPK-ERK pathway by suppressing the nuclear translocation of YB-1, an oncogenic translation factor commonly over-expressed in cancer cells [96]. These data suggested that the various forms of catechins play an important role in inhibition of NF-κB signaling pathway, and thus the downstream immunomodulation in the form of inhibition of secretion of select cytokines.
EGCG has been reported to protect cell damage from Helicobacter pylori infection by blocking the Toll-like receptor 4 (TLR-4) glycosylation, which in-turn resulted in inactivation of ERK and NF-κB [97]. One of the recent studies showed down-regulation of Toll-like receptor (TLR) signaling in DCs by EGCG. The study also showed expression of molecules essential for antigen presentation by DCs, i.e. CD80, CD86 and MHC Class I and II were inhibited by EGCG [98]. In addition to these findings, another study showed EGCG-treated DCs inhibited lipopolysaccharide (LPS) induced pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) and activation of MAPK. EGCG elevated the expression of Tollip protein, a negative regulator of TLR signaling, by binding to 67LR (67 kDa Laminin receptor), which is a cell-surface EGCG receptor [99]. Dimeric procyanidin B2 down-regulated TLR-4 signal transduction in macrophages by expressing IL-1 receptor associated kinase (IRAK-M) protein which is a negative regulator for TLR-signaling. It also reduced LPS-induced expression of CD80, CD86 and MHC II and pro-inflammatory cytokines TNF-α, IL-1β, IL-6, and IL-12p70 via activation of MAPK [100]. A very recent study explored the importance of regioselective methylation in five different classes of flavonoids [101]. Many flavonoids are converted to their methylated forms in epithelial cells of the small intestine as well as in intestinal lumen. In the presence of the Toll-like receptor 2 (TLR-2) agonist Pam3CSK4, the methylated flavonoids, 3-methoxy flavonol casticin, quercetin-3-methylether and quercetin-3,4-dimethylether showed an enhanced IL-1β production [101]. Findings from these studies underlined mechanisms by which EGCG down-regulated TLR signaling, thus resulting in reduced pro-inflammatory cytokines.
3.3 Antimicrobial Properties of Flavonoids
Flavonoids have also been shown to possess antiviral and anti-bacterial properties, either directly through membrane-bound mechanisms or through immune responses. Anti-viral activities of several flavonoids have been demonstrated against several viruses, including hepatitis B (HBV) and C (HCV), influenza and adenovirus, and several bacteria, including Bacillus subtilis and Listeria monocytogenes. The antiviral activity of Hyperoside, a 3-O-galactoside of quercetin, was shown against HBV through strong inhibition of HBeAg and HBeAg secretion in 2.2.15 cells [102]. The effect of the treatment with flavonols, quercetin and kaempferol was studied on HCV replication efficiency in an in vitro model [103]. Among the two flavonols used in the study, quercetin was the most effective inhibitor of HCV replication in HCV-G1 cells by inhibiting HCV induced ROS/ RNS (reactive oxygen and nitrogen species) formation [103]. A combination treatment of 5-FluroUracil with quercetin and luteolin was studied in two MSI human colon cancer cell lines CO115, wild type for p53 and HCT15 that has a p53 mutation [104]. Knockdown of p53 in the wild type cells by siRNA and p53 knockout cells completely abolished apoptosis induction, suggesting modulation of p53 by quercetin [104]. EGCG was also found to bind Retinoic acid inducible gene-1 (RIG-1) and inhibit its signaling at low molecular concentrations in HEK293T cells and thus may be a potential immune-modulator of RIG-I mediated immune response [105]. RIG-I is a receptor that recognizes cytoplasmic RNA’s and has a role in viral defense mechanisms against several viruses, including influenza and HCV. Though RIG-I plays an active role in antiviral responses, a higher response could result in pathological effects and autoimmune disorders [105].
Several studies have shown that EGCG has antiviral and antibacterial activity, many of which were recently reviewed [106]. A recent study by Kim et al. has reported the suppressive effects of EGCG on influenza infections, by blocking an early step in the influenza viral life cycle, although it did not have any effect on viral adsorption and viral RNA replication [107]. EGCG was shown to inhibit hemifusion events between viral particles and cellular membrane by reducing the viral membrane integrity suggesting that EGCG may have antiviral effects against enveloped viruses [107]. In accordance to this study, reports of another resent study showed EGCG to act directly on the virions, without having any effects on fluidity or integrity of the envelopes [108]. EGCG was shown to interact with virion surface proteins, resulting in inhibition of attachment of several different virions. These data all together underlined a specific mechanism by which EGCG can inhibit attachment of unrelated viruses [108]. The antiviral and inhibitory effects of various tea polyphenols on influenza A and B virus were further supported in another study, in which dimeric polyphenols, theaflavin and procyanidin B-2 were shown to have the more potent antiviral activity compared to the catechin monomers [109]. The antiviral activity of EGCG against adenovirus was suggested to occur through both intracellular and extracellular mechanisms [110].
In addition to possessing strong antiviral properties, there is also sufficient evidence that flavonoids exert anti-bacterial effects as well. EGCG was shown to inhibit the growth of Bacillus subtilis through binding to several surface membrane proteins [111]. EGCG was also shown to inhibit Listeria monocytogenes growth in macrophages derived from murine peritoneal cavity, through mechanisms that involved inhibition of disruption of the phagosomal membrane, which is important during the escaping phase of the bacteria [112]. Although most of these studies have been performed in vitro, there is sufficient evidence to evoke interest in development of select classes of flavonoids as alternatives to antiviral and antibiotics.
3.4 Induction of Cellular immunomodulation by Flavonoids
Many flavonoids exert their immunomodulatory effects by either inducing or decreasing the expression of pro-inflammatory cytokines such as IFN-γ, IL-1β, IL-6, IL-12 and TNF-α as well as expression of activation surface molecules such as CD80, CD86 and MHC Class I and II [113]. This response of the innate immune system forms the first line of defense against pathogens and also plays a critical role in initiating the adaptive immune response.
EGCG has been reported to have a variety of physiological activities which include anti-oxidant, anti-angiogenic, anti-proliferative, antiviral and anti-inflammatory properties [113]. Over the past two decades, many in vitro and a few in vivo studies have been conducted to examine these properties and suggest a possible mechanism of action. In one of the early studies, EGCG was shown to inhibit the growth of Legionella pneumophila in macrophages and selectively up-regulate the secretion of IL-12, IFN-γ and TNF-α while down-regulating IL-10 secretion in a dose dependent manner [114]. EGCG at physiologically relevant concentrations of 0.5–10 μM inhibited T cell proliferation by inducing cell cycle arrest and failure to divide [115]. EGCG also induced a reduction in IL-2R expression which may hinder IL-2 signaling pathways and thus contribute to T-cell proliferation [115]. In Jurkat T-cells, EGCG was reported to significantly up-regulate the mRNA expression of TH1 and TH2 cytokines (IL-2, IFN-γ, IL-5 and IL-13) [116]. EGCG epigenetically modified Foxp3 methylation by reducing the DNMT expression and DNA methylation and by promoting regulatory T-cell (Treg) activation and expansion [117].
In one of the early studies, quercetin inhibited over-production of TNF-α and nitric oxide (NO) in LPS stimulated murine macrophage RAW 264.7 cells [118]. In T-helper cells, quercetin suppressed the secretion of IFN-γ and IL-2 by blocking the gene transcription of IL-2Rα, which is required for high affinity binding [119]. Another study showed compared to stimulation with 10 U/ml IFN-γ, unstimulated RAW 264.7 macrophages significantly increased TNF-α secretion in the presence of procyanidin C2 (PCA C2) or Pycnogenol (PYC); whereas pretreatment with the dimeric and monomeric catechins did not have any effect on TNF-α secretion [120]. Immuno-suppressive effects of quercetin were studied in dendritic cells (DCs) where it inhibited LPS induced production of pro-inflammatory cytokines/chemokines as well as the expression levels of MHC Class II molecules. It also blocked endocytosis by DCs and decreased LPS-induced DC migration [121]. The trimeric form of catechin, procyanidin C1, was found to exert a TH1-mediated response by expression of TH1 mediated cytokines like IFN-γ, IL-12p70, and IL-2 in murine splenocytes. It also resulted in activation of macrophages through expression of cell surface molecules CD80 and CD86 [122]. Results from these findings suggested that flavonoids may exert both immuno-suppressive and immune-enhancing properties, depending on their chemical structures and the ability to mediate free radical reactions.
Another flavonoid with proposed anticancer activity is morin, which is present in mulberry twigs. In vitro data showed Morin to up-regulate Fas receptor and activate caspase-8, -9 and -3 in human colon cancer HCT 116 cells [123]. The data also showed induction of ROS generation and the loss of mitochondrial membrane potential with Bax protein activation and cytochrome C release [123]. The results also provided evidence that Morin suppressed the anti-apoptotic proteins Bcl-2 and cIAP-1, thus contributing to augmentation of Morin-triggered apoptosis. The data provided in this study suggested that Morin-induced apoptosis occurs through upregulating Fas receptor, as well as by controlling production of anti-apoptotic proteins [123].
A number of in vivo studies also correlated well with published in vitro reports. Topical application of green tea extract rich in EGCG before a UV-B exposure reduced the infiltration of leukocytes, antigen-presenting cells and oxidative stress due to its immunosuppressive properties [124]. In another in vivo study in IL-2 deficient mice, EGCG was used for treating chronic inflammatory conditions [125]. Oral intake of green tea polyphenols in mice reduced the chances of succumbing to arthritis by diminishing the production of COX-2, IFN-γ and TNF-α in arthritic joint [126]. EGCG was used in combination with a DNA vaccine for HPV to investigate its role in immunotherapy with chemotherapy [127]. EGCG was found to act synergistically with the DNA anti-cancer vaccine by inhibiting tumor growth. The study showed oral administration of EGCG (0.5mg/ml) on the day of vaccination and continuation for the next 14 days resulted in increased antigen specific CD8+ and CD4+ T-cell mediated immune responses [127]. Effects of dietary supplementation of procyanidins on immunomodulation were studied in vivo [128]. The study reported that the secretion of LPS induced pro-inflammatory cytokines TNF-α, IL-1β and IL-6 were reduced in pigs fed a diet of 0.02% and 0.04% of procyanidins for 4 weeks [128].
Most of the in vitro studies on EGCG have focused on the effects of higher concentrations of EGCG. However; due to its poor bioavailability, similar to other polyphenolic compounds, its effects in vivo could be varied. For instance, a recent in vivo study using C57BL/6 mice reported the effects of dietary supplementation with different doses of EGCG on inflammatory responses [129]. Results of the study reported mice with a 1% EGCG diet produced more pro-inflammatory cytokines such as TNF-α, IL-6 and IL-1β as well as prostaglandin E2 in their splenocytes and macrophages compared to the groups fed with a 0.15% w/w and 0.3% w/w EGCG diet [129]. Results of the study also showed increased proportions of T cells, natural killer (NK) cells and natural killer T (NKT) cells in mice fed with 1% EGCG diet, whereas lower concentration of EGCG at 0.15% and 0.3% w/w had no effect on the pro-inflammatory responses [129].
Curcumin is a component of turmeric; the powdered rhizome of the plant Curcuma longa that is used as a spice in Asian cuisine. It has shown to have both anti-inflammatory and chemoprotective properties particularly against colon cancer in rats [130]. Another study reported curcumin supplementation increased numbers of CD4+ T-cells in intestinal mucosa of mice in a colorectal cancer model. Curcumin was also found to increase the intestinal immune function in high fat fed animals through elevation of luminal IgA by increasing IgA production or suppressing IgA degradation [131]. Tannic acid and quercetin were shown to have a therapeutic effect on atopic dermatitis (AD) through suppression of angiogenesis and TH2 related cytokine expression in an AD like NC/Nga mouse model. These mice are hairless, and of albino background and exhibit AD symptoms spontaneously with age [132].
3.5 Clinical Evidence of Induction of Immunomodulation by Flavonoids
Animal studies over the past several decades have reported flavonoids to have immunomodulatory roles through their anti-oxidant and anti-inflammatory activities. Such studies have prompted clinical evaluation of select flavonoids in treatment of various cancers, including prostate, ovarian, breast and colon cancers. Several prospective and case control studies using various flavonoids have been reported to have positive effects on prostate cancer. A clinical study showed the positive effect polyphenols and their bioactive metabolites from green tea have on prostate cancer prevention [133]. Patients with clinically localized prostate cancer were given six cups of green tea for 3 to 6 weeks before undergoing radical prostatectomy. Compared to the control group, 50 to 60 % of both EGCG and ECG in methylated form were present in the prostate tissue and urine in the group treated with green tea [133]. Relevant to this study, compared to EGCG, in vitro cultures of LNCaP prostate cancer cells showed methylated form of EGCG (4′-MeEGCG) decreased the NF-κB activation and induced apoptosis. These data suggested methylated forms of EGCG could exert anti-cancer effects on prostate cancer [133]. Another study also showed treatment with EGCG significantly reduced serum levels of PSA, HGF and VEGF in men with prostate cancer [134]. A recent study investigated the effects of oligonol, a low molecular weight polyphenol of lychee fruit extract supplementation on leukocyte and immune cell counts after heat loading in healthy males [135]. Heat loading is a method in which subjects were conditioned to thermoneutral climate chamber (26 ± 0.5 °C; 60% ± 3% relative humidity; air velocity < 1 m/s) for 1 hour prior to applying heat load for 30 minutes with half of the body immersed in hot water maintained at 42 ± 0.5 °C [135]. Along with significantly increasing the number of leukocytes and lymphocytes, the study also showed daily administration of 200 mg oligonol for 1 week decreased serum concentrations of IL-6 and IL-1β 1 hour after heating the subjects compared to placebo group [135]. A similar study by Shin et al. reported decreased serum concentration of PGE2 and COX-2 and body temperature after passive heating in oligonol supplemented group compared to the placebo [136]. Results from these findings provide some evidence on the antipyretic, analgesic and anti-inflammatory effects of oligonols as well as shed some light on the possible view that oligonol could be used to suppress the elevation of body temperature under heat stress.
Flavonoids may have positive effects against cancer development though various biochemical pathways due to their ability to mediate free radical reactions. A recent prospective study in women investigated the effects of 5 different flavonoids, myricetin, kaempferol, quercetin, luteolin and apigenin on ovarian cancer [137]. Although, the study was not able to provide a clear link between the effects of each of these flavonoids on ovarian cancer; some results showed significant decrease in ovarian cancer incidences following kaempferol and luteolin intakes [137]. On the other hand, daily dose of an EGCG enriched tea drink was shown to have no effect on advanced stage ovarian cancer [138]. Another case control study in Japanese women provided further evidence of consumption of polyphenols from green tea did not reduce the risk of breast cancer [139]. In contrast to the data reported in several preclinical studies, a recent clinical study in healthy individuals showed daily intake of hesperidin from orange juice did not induce immunomodulation [140]. Another clinical study reported positive effects of daily dose of apigenin and EGCG on recurrence rate of colon neoplasia in patients with resected colon cancer [141]. In contrast, several case-control studies have reported inverse associations of site-specific cancer risk with intake of kaempferol [142,143], quercetin [143–145], catechin [145], epicatechin [143,145].
Several clinical trials have reported curcumin to have anti-inflammatory and protective effects against various cancer treatments. In accordance with the animal studies reported previously, a study in patients with colorectal cancer reported a mechanism by which curcumin supplementation resulted in increased p53 expression in tumor cells resulting in increased body weight, decreased serum TNF-α levels, increased apoptotic tumor cells, and thus, a modulation of the p53 tumor cell apoptotic pathway [146]. In a related in vitro study, it was reported that curcurmin exerted inhibitory effects on human endometrial carcinoma cells in down regulating their androgen receptor (AR) expression through the Wnt signaling pathway [147]. Another recent clinical trial showed oral daily supplementation of 6 g of curcumin during radiotherapy reduced the severity of radiation dermatitis in breast cancer patients [148]. These results, suggested a mechanism by which curcumin may exert its protective effects during treatments of certain chronic illnesses. A recent randomized clinical trial reported the chemopreventive effects of green tea (GT) and black tea (BT) in patients with prostate cancer [149]. Patients in this trial consumed 6 cups of GT, BT or water (control) daily prior to radical prostatectomy. The study reported significantly decreased NF-κB in RP tissue of men consuming GT compared to control. Data also provided evidence of systemic antioxidant effect with GT group compared to BT or control suggesting the use of GT as a supplementation for prostate cancer prevention and treatment [149]. Based on these contrasting clinical findings, further understanding of the effect of flavonoids on cancer development and immunomodulation in general is required. This may be accomplished through more extensive and detailed analysis of the effects of flavonoids on intracellular and extracellular events in well-controlled clinical trials using highly purified and well-characterized compounds.
4. Expert Opinion
It is logical to consider that cells need nutrition to perform their functions. It is, however, surprising that until relatively recently the role of nutritional compounds in cells of the immune system was not seriously considered. Similar to various pathogenic stimuli that dictate the functions of various cells of the immune system, presence or absence of certain nutrient components, also can direct cells in various differentiation and effector function pathways. Conversely, metabolic pathways in leukocytes can provide guidance on intracellular events that lead to various gene expression and effector functions. Thus, it is not surprising that recent accumulation of reports on metabolic pathways and the role of various nutrients have shed light on this relatively ignored field of Nutritional Immunology.
There is now irrefutable evidence that several nutritional compounds, including vitamin A and flavonoids trigger immune-enhancing or immune-suppressing effects, through various intra-cellular signal transduction mechanism(s), including that of NF-κB. It may be argued that while much focus has been placed on the role of TLR and other compounds that cause innate pro-inflammatory responses, and hence their role in vaccine and drug design, there is sufficient evidence to suggest that vitamin A and flavonoids may induce independent modes of signaling that may lead to immune-enhancing or immunosuppressive functions. However, the clear advantage of using nutritional compounds such as vitamin A and flavonoids is that of their enhanced safety compared to TLR or similar molecules, since these naturally occurring compounds have been safely consumed, or even injected as is the case in vitamin A deficiency, for decades. Signal transduction pathways for vitamins A, flavonoids, and similar structures, hence suggest a bidirectional link between these nutritional components and immune response elements. It is interesting that ATRA and EGCG treatments in different cells each induce the same ERK signal transduction pathway. These data strongly suggest that common gene clusters, involving immune response elements, are affected by vitamin A and flavonoids. Ultimately, it is the safety and efficacy of these compounds in clinical trials that will dictate future interest in them as immune-enhancers or immuno-suppressors. The use of vitamin E, which has a completely different chemical structure than vitamin A, in an influenza vaccine used in Europe, which also contained squalene oil, caused narcolepsy in children. Hence, although vitamins and flavonoids may be safe if used on their own, formulating them with other compounds will have to be carefully examined for safety. Since it appears vitamin A and at least one class of flavonoids may share a similar signal transduction pathway, one important unexplored area is how and whycombinations of vitamins, including vitamin A, and flavonoids, would induce synergistic immunomodulation when formulated appropriately. This is because, there is surprising preclinical evidence that indeed combinations of vitamins, including vitamin A and some flavonoids, synergistically enhance B and T cell responses, and can act as vaccine adjuvants [1,150]. It is also noteworthy that while strong antimicrobial properties of flavonoids have been reported, most such effects have been demonstrated in vitro, and in vivo preclinical studies are scant. Because most flavonoids have limited in vivo bioavailability, the focus on practical pharmaceutical use of these compounds against viruses and bacteria in future clinical settings will need to include delivery systems that enhance and sustain their in vivo bioavailability at concentrations that are sufficient for their exertion of microbial activity.
Article Highlights.
Vitamin A derivatives and metabolites and select flavonoids are defined and Classified
Induction of Intra-cellular Signal Transduction Pathways by Vitamin A and Flavonoids as they relate to their immunomodulation activities
Cellular Immunomodulation by Vitamin A and Flavonoids and production of effector B and T cell molecules against infectious diseases and cancers
Antibacterial and antiviral Properties of select Flavonoids
Clinical Evidence of Immunomodulation by Vitamin A and Flavonoids in the context of cancer treatment or vaccinations against infectious diseases
Acknowledgments
The authors would like to acknowledge Deepa Pednekar, a former employee of EpitoGenesis, for providing a limited literature search and related text on select flavonoids.
Footnotes
Financial and competing interests disclosure
This work was supported by grants from the NIAIR 1R43AJ084690-01, NIAID 1R41AI096706-01A1, the Treasury Department’s Therapeutic Discovery Award and the Sate of Connecticut’s Department of Economic Development. M Vajdy owns shares of EpitoGenesis, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- 1.Yu M, Vajdy M. A novel retinoic acid, catechin hydrate and mustard oil-based emulsion for enhanced cytokine and antibody responses against multiple strains of HIV-1 following mucosal and systemic vaccinations. Vaccine. 2011;29:2429–36. doi: 10.1016/j.vaccine.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vajdy M. Immunomodulatory properties of vitamins, flavonoids and plant oils and their potential as vaccine adjuvants and delivery systems. Expert Opin Biol Ther. 2011;11:1501–13. doi: 10.1517/14712598.2011.623695. [DOI] [PubMed] [Google Scholar]
- 3.D’Ambrosio DN, Clugston RD, Blaner WS. Vitamin A metabolism: an update. Nutrients. 2011;3:63–103. doi: 10.3390/nu3010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Napoli JL. Biochemical pathways of retinoid transport, metabolism, and signal transduction. Clin Immunol Immunopathol. 1996;80:S52–62. doi: 10.1006/clin.1996.0142. [DOI] [PubMed] [Google Scholar]
- 5.Kurokawa R, Söderström M, Hörlein A, et al. Polarity-specific activities of retinoic acid receptors determined by a co-repressor. Nature. 1995;377:451–4. doi: 10.1038/377451a0. [DOI] [PubMed] [Google Scholar]
- 6.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–7. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 7.Molenaar R, Greuter M, van der Marel APJ, et al. Lymph node stromal cells support dendritic cell-induced gut-homing of T cells. J Immunol. 2009;183:6395–402. doi: 10.4049/jimmunol.0900311. [DOI] [PubMed] [Google Scholar]
- 8.Woggon W-D. Oxidative cleavage of carotenoids catalyzed by enzyme models and beta-carotene 15,15′-monooxygenase. Pure Appl Chem International Union of Pure and Applied Chemistry. 2002;74:1397–408. [Google Scholar]
- 9.MacDonald PN, Ong DE. Evidence for a lecithin-retinol acyltransferase activity in the rat small intestine. J Biol Chem. 1988;263:12478–82. [PubMed] [Google Scholar]
- 10.Harrison EH, Hussain MM. Mechanisms involved in the intestinal digestion and absorption of dietary vitamin A. J Nutr. 2001;131:1405–8. doi: 10.1093/jn/131.5.1405. [DOI] [PubMed] [Google Scholar]
- 11.Penzes P, Napoli JL. Holo-cellular retinol-binding protein: distinction of ligand-binding affinity from efficiency as substrate in retinal biosynthesis. Biochemistry. 1999;38:2088–93. doi: 10.1021/bi982228t. [DOI] [PubMed] [Google Scholar]
- 12.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–30. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 13.Jaensson-Gyllenbäck E, Kotarsky K, Zapata F, et al. Bile retinoids imprint intestinal CD103+ dendritic cells with the ability to generate gut-tropic T cells. Mucosal Immunol. 2011;4:438–47. doi: 10.1038/mi.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mamoon A, Ventura-Holman T, Maher JF, et al. Retinoic acid responsive genes in the murine hepatocyte cell line AML 12. Gene. 2008;408:95–103. doi: 10.1016/j.gene.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Mamoon A, Subauste A, Subauste MC, et al. Retinoic acid regulates several genes in bile acid and lipid metabolism via upregulation of small heterodimer partner in hepatocytes. Gene Elsevier BV. 2014;550:165–70. doi: 10.1016/j.gene.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–92. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 17.Engedal N, Gjevik T, Blomhoff R, et al. All-trans retinoic acid stimulates IL-2-mediated proliferation of human T lymphocytes: early induction of cyclin D3. J Immunol. 2006;177:2851–61. doi: 10.4049/jimmunol.177.5.2851. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi H, Yokota A, Ohoka Y, et al. Cyp26b1 regulates retinoic acid-dependent signals in T cells and its expression is inhibited by transforming growth factor-β. PLoS One. 2011;6:e16089. doi: 10.1371/journal.pone.0016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chenery A, Burrows K, Antignano F, et al. The retinoic acid-metabolizing enzyme Cyp26b1 regulates CD4 T cell differentiation and function. PLoS One. 2013;8:e72308. doi: 10.1371/journal.pone.0072308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawson HD, Collins G, Pyle R, et al. Direct and indirect effects of retinoic acid on human Th2 cytokine and chemokine expression by human T lymphocytes. BMC Immunol. 2006;7:27. doi: 10.1186/1471-2172-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matikainen S, Lehtonen a, Sareneva T, et al. Regulation of IRF and STAT gene expression by retinoic acid. Leuk Lymphoma. 1998;30:63–71. doi: 10.3109/10428199809050930. [DOI] [PubMed] [Google Scholar]
- 22.Kouros-Mehr H, Slorach EM, Sternlicht MD, et al. GATA-3 Maintains the Differentiation of the Luminal Cell Fate in the Mammary Gland. Cell. 2006;127:1041–55. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Zhu YF, Hu JZ, Zhao PN, et al. All-transretinoic acid regulates Th1/Th2 balance in CD4+ T cells when GATA-3 is deficient. Biomed Environ Sci. 2013;26:774–7. doi: 10.3967/0895-3988.2013.09.010. Important in vivo study showing the mechanism by which GATA-3 mediates TH1 and TH2 responses in the presence of VA. [DOI] [PubMed] [Google Scholar]
- 24.Lin X-Y, He C-D, Xiao T, et al. Acitretin induces apoptosis through CD95 signalling pathway in human cutaneous squamous cell carcinoma cell line SCL-1. J Cell Mol Med. 2009;13:2888–98. doi: 10.1111/j.1582-4934.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Zhang M-L, Tao Y, Zhou W-Q, et al. All-trans retinoic acid induces cell-cycle arrest in human cutaneous squamous carcinoma cells by inhibiting the mitogen-activated protein kinase-activated protein 1 pathway. Clin Exp Dermatol. 2014;39:354–60. doi: 10.1111/ced.12227. Very well written paper demonstrating the in vitro effects of ATRA on MAPK pathway. [DOI] [PubMed] [Google Scholar]
- 26.Schug TT, Berry DC, Shaw NS, et al. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–33. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vreeland AC, Levi L, Zhang W, et al. Cellular retinoic acid-binding protein 2 inhibits tumor growth by two distinct mechanisms. J Biol Chem. 2014;289:34065–73. doi: 10.1074/jbc.M114.604041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bavelloni A, Piazzi M, Faenza I, et al. Prohibitin 2 represents a novel nuclear AKT substrate during all-trans retinoic acid-induced differentiation of acute promyelocytic leukemia cells. FASEB J. 2014;28:2009–19. doi: 10.1096/fj.13-244368. [DOI] [PubMed] [Google Scholar]
- 29.Priyamvada S, Anbazhagan AN, Gujral T, et al. All-Trans-Retinoic Acid Increases SLC26A3 (DRA) Expression In Intestinal Epithelial Cells Via HNF-1β. J Biol Chem. 2015 doi: 10.1074/jbc.M114.566356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warkany J, Schraffenberger E. Congenital malformations induced in rats by maternal vitamin A deficiency; defects of the eye. Arch Ophthalmol (Chicago, Ill 1929) 1946;35:150–69. doi: 10.1001/archopht.1946.00890200155008. [DOI] [PubMed] [Google Scholar]
- 31**.Van de Pavert Sa, Ferreira M, Domingues RG, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–7. doi: 10.1038/nature13158. One of the recent in vivo studies demonstrating the effects VA deficiency on development of offspring immunity. The study underlines a mechanism by which RA signaling affects secondary lymphoid organ formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lampen A, Meyer S, Arnhold T, et al. Metabolism of vitamin A and its active metabolite all-trans-retinoic acid in small intestinal enterocytes. J Pharmacol Exp Ther. 2000;295:979–85. [PubMed] [Google Scholar]
- 33.Dong P, Tao Y, Yang Y, et al. Expression of retinoic acid receptors in intestinal mucosa and the effect of vitamin A on mucosal immunity. Nutrition Elsevier Ltd. 2010;26:740–5. doi: 10.1016/j.nut.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Zhu B, Buttrick T, Bassil R, et al. IL-4 and Retinoic Acid Synergistically Induce Regulatory Dendritic Cells Expressing Aldh1a2. J Immunol. 2013;191:3139–51. doi: 10.4049/jimmunol.1300329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saurer L, McCullough KC, Summerfield A. In Vitro Induction of Mucosa-Type Dendritic Cells by All-Trans Retinoic Acid. J Immunol American Association of Immunologists. 2007;179:3504–14. doi: 10.4049/jimmunol.179.6.3504. [DOI] [PubMed] [Google Scholar]
- 36.Edele F, Molenaar R, Gütle D, et al. Cutting edge: instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J Immunol. 2008;181:3745–9. doi: 10.4049/jimmunol.181.6.3745. [DOI] [PubMed] [Google Scholar]
- 37.Iliev ID, Spadoni I, Mileti E, et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–9. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 38.Den Hartog G, Van Altena C, Savelkoul HFJ, et al. The mucosal factors retinoic acid and TGF-β1 induce phenotypically and functionally distinct dendritic cell types. Int Arch Allergy Immunol. 2013;162:225–36. doi: 10.1159/000353243. [DOI] [PubMed] [Google Scholar]
- 39.Marino D, Dabouras V, Brändli AW, et al. A role for all-trans-retinoic acid in the early steps of lymphatic vasculature development. J Vasc Res. 2011;48:236–51. doi: 10.1159/000320620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi I, Lee S, Kyoung Chung H, et al. 9-cis retinoic acid promotes lymphangiogenesis and enhances lymphatic vessel regeneration: therapeutic implications of 9-cis retinoic acid for secondary lymphedema. Circulation. 2012;125:872–82. doi: 10.1161/CIRCULATIONAHA.111.030296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burger NB, Stuurman KE, Kok E, et al. Involvement of neurons and retinoic acid in lymphatic development: new insights in increased nuchal translucency. Prenat Diagn. 2014;34:1312–9. doi: 10.1002/pd.4473. [DOI] [PubMed] [Google Scholar]
- 42.Carman Ja, Hayes CE. Abnormal regulation of IFN-gamma secretion in vitamin A deficiency. J Immunol. 1991;147:1247–52. [PubMed] [Google Scholar]
- 43.Cantorna MT, Nashold FE, Hayes CE. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J Immunol. 1994;152:1515–22. [PubMed] [Google Scholar]
- 44.Racke MK, Burnett D, Pak SH, et al. Retinoid treatment of experimental allergic encephalomyelitis. IL-4 production correlates with improved disease course. J Immunol. 1995;154:450–8. [PubMed] [Google Scholar]
- 45*.Stephensen CB, Rasooly R, Jiang X, et al. Vitamin A Enhances in Vitro Th2 Development Via Retinoid X Receptor Pathway. J Immunol American Association of Immunologists. 2002;168:4495–503. doi: 10.4049/jimmunol.168.9.4495. One of the earlier studies showing how VA enhances in vitro TH2 responses via RXR pathway. [DOI] [PubMed] [Google Scholar]
- 46.Stephensen CB, Jiang X, Freytag T. Vitamin A Deficiency Increases the In Vivo Development of IL-10-Positive Th2 Cells and Decreases Development of Th1 Cells in Mice. J Nutr. 2004;134:2660–6. doi: 10.1093/jn/134.10.2660. [DOI] [PubMed] [Google Scholar]
- 47.Mehta K, McQueen T, Tucker S, et al. Inhibition by all-trans-retinoic acid of tumor necrosis factor and nitric oxide production by peritoneal macrophages. J Leukoc Biol. 1994;55:336–42. doi: 10.1002/jlb.55.3.336. [DOI] [PubMed] [Google Scholar]
- 48.Jones LH, Cook PC, Ivens AC, et al. Modulation of dendritic cell alternative activation and function by the vitamin A metabolite retinoic acid. Int Immunol. 2015 doi: 10.1093/intimm/dxv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stosi-Grujici S, Ejdus L. Modulation of in vitro T cell alloreactivity by synthetic retinoids. Immunopharmacology. 1994;27:87–92. doi: 10.1016/0162-3109(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 50*.Lu L, Lan Q, Li Z, et al. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci. 2014;111:E3432–E3440. doi: 10.1073/pnas.1408780111. A very well written paper demonstrating the critical role ATRA plays in stabilizing human Treg cells under inflammatory conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benson MJ, Pino-Lagos K, Rosemblatt M, et al. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–74. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun C-M, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 55.Kühn R, Löhler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 56.Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57*.Bakdash G, Vogelpoel LT, van Capel TM, et al. Mucosal Immunol. Nature Publishing Group; 2014. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells; pp. 1–14. One of the more recent studies providing evidence on how RA together with IL-10 maintains the intestinal mucosal tolerance. [DOI] [PubMed] [Google Scholar]
- 58.Martin JCJ, Bériou G, Heslan M, et al. Interleukin-22 binding protein (IL-22BP) is constitutively expressed by a subset of conventional dendritic cells and is strongly induced by retinoic acid. Mucosal Immunol. 2014;7:101–13. doi: 10.1038/mi.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59**.Liu X, Cui T, Li Y, et al. Vitamin A supplementation in early life enhances the intestinal immune response of rats with gestational vitamin A deficiency by increasing the number of immune cells. PLoS One. 2014;9:e114934. doi: 10.1371/journal.pone.0114934. Another important preclinical study providing further evidence on importance of VA supplementation for enhancing mucosal immune responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vicente-Suarez I, Larange A, Reardon C, et al. Unique lamina propria stromal cells imprint the functional phenotype of mucosal dendritic cells. Mucosal Immunol. 2015;8:141–51. doi: 10.1038/mi.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cording S, Wahl B, Kulkarni D, et al. The intestinal micro-environment imprints stromal cells to promote efficient Treg induction in gut-draining lymph nodes. Mucosal Immunol. 2014;7:359–68. doi: 10.1038/mi.2013.54. [DOI] [PubMed] [Google Scholar]
- 62.Liu B, Tonkonogy SL, Sartor RB. Antigen-presenting cell production of IL-10 inhibits T-helper 1 and 17 cell responses and suppresses colitis in mice. Gastroenterology. 2011;141:653–62. 662.e1–4. doi: 10.1053/j.gastro.2011.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63**.Vlasova AN, Chattha KS, Kandasamy S, et al. Prenatally acquired vitamin A deficiency alters innate immune responses to human rotavirus in a gnotobiotic pig model. J Immunol. 2013;190:4742–53. doi: 10.4049/jimmunol.1203575. An important study on the role of vitmain A in innate immunity against a prevalent mucosal viral infection using an elegant gnotobiotic pigelt model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64**.Kandasamy S, Chattha KS, Vlasova AN, et al. Prenatal vitamin A deficiency impairs adaptive immune responses to pentavalent rotavirus vaccine (RotaTeq®) in a neonatal gnotobiotic pig model. Vaccine. 2014;32:816–24. doi: 10.1016/j.vaccine.2013.12.039. A significant milestone in providing preclinical evidence on the importance of vitamin A in mounting protective immunity against an important mucosl pathogen, Rotavirus. [DOI] [PubMed] [Google Scholar]
- 65.Lisulo MM, Kapulu MC, Banda R, et al. Adjuvant potential of low dose all-trans retinoic acid during oral typhoid vaccination in Zambian men. Clin Exp Immunol. 2014;175:468–75. doi: 10.1111/cei.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lima AAM, Soares AM, Lima NL, et al. Effects of vitamin A supplementation on intestinal barrier function, growth, total parasitic, and specific Giardia spp infections in Brazilian children: a prospective randomized, double-blind, placebo-controlled trial. J Pediatr Gastroenterol Nutr. 2010;50:309–15. doi: 10.1097/MPG.0b013e3181a96489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Long KZ, Rosado JL, Montoya Y, et al. Effect of vitamin A and zinc supplementation on gastrointestinal parasitic infections among Mexican children. Pediatrics. 2007;120:e846–55. doi: 10.1542/peds.2006-2187. [DOI] [PubMed] [Google Scholar]
- 68.Long KZ, Santos JI, Estrada Garcia T, et al. Vitamin A Supplementation Reduces the Monocyte Chemoattractant Protein-1 Intestinal Immune Response of Mexican Children. J Nutr. 2006;136:2600–5. doi: 10.1093/jn/136.10.2600. [DOI] [PubMed] [Google Scholar]
- 69.Long KZ, Santos JI, Rosado JL, et al. Vitamin A supplementation modifies the association between mucosal innate and adaptive immune responses and resolution of enteric pathogen infections. Am J Clin Nutr. 2011;93:578–85. doi: 10.3945/ajcn.110.003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Semba RD. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc Nutr Soc. 1999;58:719–27. doi: 10.1017/s0029665199000944. [DOI] [PubMed] [Google Scholar]
- 71.Villamor E, Fawzi WW. Vitamin A supplementation: implications for morbidity and mortality in children. J Infect Dis. 2000;182(Suppl):S122–33. doi: 10.1086/315921. [DOI] [PubMed] [Google Scholar]
- 72.Bhutta ZA, Ahmed T, Black RE, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371:417–40. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 73.Sommer A, Vyas KS. A global clinical view on vitamin A and carotenoids. Am J Clin Nutr. 2012;96:1204S– 6S. doi: 10.3945/ajcn.112.034868. [DOI] [PubMed] [Google Scholar]
- 74.Semba RD. The vitamin A and mortality paradigm: past present and future. Scand J Nutr. 2015;45 [Google Scholar]
- 75.Beaton George H, Martorell Reynaldo, Aronson Kristan A, Edmonston Barry, George McCabe A, Catharine Ross BH. Vitamin A supplementation and child morbidity and mortality in developing countries [Internet] Food Nutr Bull. 1994:282–9. cited 2015 Jun 10. [Google Scholar]
- 76*.Mazumder S, Taneja S, Bhatia K, et al. Efficacy of early neonatal supplementation with vitamin A to reduce mortality in infancy in Haryana, India (Neovita): a randomised, double-blind, placebo-controlled trial. Lancet. 2014;385:1333–42. doi: 10.1016/S0140-6736(14)60891-6. Recent clinical evidence on the importance of neonatal VA supplementation for improved immune responses resulting in reduced mortality and morbidity in developing counties where VA deficiency is more prominent. [DOI] [PubMed] [Google Scholar]
- 77.McDonald SL, Savy M, Fulford AJ, et al. A double blind randomized controlled trial in neonates to determine the effect of vitamin A supplementation on immune responses: The Gambia protocol. BMC Pediatr BMC Pediatrics. 2014;14:92. doi: 10.1186/1471-2431-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arguello MA, Schulze KJ, Wu LS, et al. Circulating IGF-1 may mediate improvements in haemoglobin associated with vitamin A status during pregnancy in rural Nepalese women. Asia Pac J Clin Nutr. 2015;24:128–37. doi: 10.6133/apjcn.2015.24.1.12. [DOI] [PubMed] [Google Scholar]
- 79.Smith TJ. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacol Rev. 2010;62:199–236. doi: 10.1124/pr.109.002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Castellano G, González-Santander JL, Lara A, et al. Classification of flavonoid compounds by using entropy of information theory. Phytochemistry. 2013;93:182–91. doi: 10.1016/j.phytochem.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 81.Scalbert A, Williamson G. Dietary Intake and Bioavailability of Polyphenols. J Nutr. 2000;130:2073S– 2085. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 82.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park JW, Choi YJ, Suh SI, et al. Involvement of ERK and protein tyrosine phosphatase signaling pathways in EGCG-induced cyclooxygenase-2 expression in Raw 264.7 cells. Biochem Biophys Res Commun. 2001;286:721–5. doi: 10.1006/bbrc.2001.5415. [DOI] [PubMed] [Google Scholar]
- 84.Shu Z, Yu M, Zeng G, et al. Epigallocatechin-3-gallate inhibits proliferation of human aortic smooth muscle cells via up-regulating expression of mitofusin 2. Eur J Cell Biol. 2014;93:137–44. doi: 10.1016/j.ejcb.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 85.Lim YC, Cha YY. Epigallocatechin-3-gallate induces growth inhibition and apoptosis of human anaplastic thyroid carcinoma cells through suppression of EGFR/ERK pathway and cyclin B1/CDK1 complex. J Surg Oncol. 2011;104:776–80. doi: 10.1002/jso.21999. [DOI] [PubMed] [Google Scholar]
- 86.Gao Y, Li W, Jia L, et al. Enhancement of (−)-epigallocatechin-3-gallate and theaflavin-3-3′-digallate induced apoptosis by ascorbic acid in human lung adenocarcinoma SPC-A-1 cells and esophageal carcinoma Eca-109 cells via MAPK pathways. Biochem Biophys Res Commun. 2013;438:370–4. doi: 10.1016/j.bbrc.2013.07.078. [DOI] [PubMed] [Google Scholar]
- 87.Cho S-Y, Park S-J, Kwon M-J, et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases andNF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol Cell Biochem. 2003;243:153–60. doi: 10.1023/a:1021624520740. [DOI] [PubMed] [Google Scholar]
- 88.Kandere-Grzybowska K, Kempuraj D, Cao J, et al. Regulation of IL-1-induced selective IL-6 release from human mast cells and inhibition by quercetin. Br J Pharmacol. 2006;148:208–15. doi: 10.1038/sj.bjp.0706695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodríguez-Ramiro I, Ramos S, Bravo L, et al. Procyanidin B2 induces Nrf2 translocation and glutathione S-transferase P1 expression via ERKs and p38-MAPK pathways and protect human colonic cells against oxidative stress. Eur J Nutr. 2012;51:881–92. doi: 10.1007/s00394-011-0269-1. [DOI] [PubMed] [Google Scholar]
- 90*.Mukai R, Shirai Y, Saito N, et al. Suppression mechanisms of flavonoids on aryl hydrocarbon receptor-mediated signal transduction. Arch Biochem Biophys. 2010;501:134–41. doi: 10.1016/j.abb.2010.05.002. A well carried out study pointing out the suppression of the Aryl hydrocarbon receptor through the ERK-MAPK pathway. [DOI] [PubMed] [Google Scholar]
- 91.Won S-M, Park Y-H, Kim H-J, et al. Catechins inhibit angiotensin II-induced vascular smooth muscle cell proliferation via mitogen-activated protein kinase pathway. Exp Mol Med. 2006;38:525–34. doi: 10.1038/emm.2006.62. [DOI] [PubMed] [Google Scholar]
- 92.Han M-K. Epigallocatechin gallate, a constituent of green tea, suppresses cytokine-induced pancreatic beta-cell damage. Exp Mol Med. 2003;35:136–9. doi: 10.1038/emm.2003.19. [DOI] [PubMed] [Google Scholar]
- 93.Ahn S-C, Kim G-Y, Kim J-H, et al. Epigallocatechin-3-gallate, constituent of green tea, suppresses the LPS-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and NF-kappaB. Biochem Biophys Res Commun. 2004;313:148–55. doi: 10.1016/j.bbrc.2003.11.108. [DOI] [PubMed] [Google Scholar]
- 94*.Mackenzie GG, Carrasquedo F, Delfino JM, et al. Epicatechin, catechin, and dimeric procyanidins inhibit PMA-induced NF-kappaB activation at multiple steps in Jurkat T cells. FASEB J. 2004;18:167–9. doi: 10.1096/fj.03-0402fje. Well studied paper focusing on the activation of NF-κB signaling pathway by dimeric procyanidins suggesting a possible model for binding between dimeric procyanidins and NF-κB proteins. [DOI] [PubMed] [Google Scholar]
- 95.Aktas O, Prozorovski T, Smorodchenko A, et al. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J Immunol. 2004;173:5794–800. doi: 10.4049/jimmunol.173.9.5794. [DOI] [PubMed] [Google Scholar]
- 96.Zhao B, Sun Y, Wang S, et al. Grape seed procyanidin reversal of p-glycoprotein associated multi-drug resistance via down-regulation of NF-κB and MAPK/ERK mediated YB-1 activity in A2780/T cells. PLoS One. 2013;8:e71071. doi: 10.1371/journal.pone.0071071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee K-M, Yeo M, Choue J-S, et al. Protective mechanism of epigallocatechin-3-gallate against Helicobacter pylori-induced gastric epithelial cytotoxicity via the blockage of TLR-4 signaling. Helicobacter. 2004;9:632–42. doi: 10.1111/j.1083-4389.2004.00281.x. [DOI] [PubMed] [Google Scholar]
- 98.Byun E-B, Choi H-G, Sung N-Y, et al. Green tea polyphenol epigallocatechin-3-gallate inhibits TLR4 signaling through the 67-kDa laminin receptor on lipopolysaccharide-stimulated dendritic cells. Biochem Biophys Res Commun. 2012;426:480–5. doi: 10.1016/j.bbrc.2012.08.096. [DOI] [PubMed] [Google Scholar]
- 99**.Hong Byun E, Fujimura Y, Yamada K, et al. TLR4 signaling inhibitory pathway induced by green tea polyphenol epigallocatechin-3-gallate through 67-kDa laminin receptor. J Immunol. 2010;185:33–45. doi: 10.4049/jimmunol.0903742. A good paper demonstrating role of EGCG in expressing Tollip protein which is a negative regulator of TLR2 signaling. The paper discusses the role of laminin receptor in TLR-signaling. [DOI] [PubMed] [Google Scholar]
- 100.Sung N-Y, Yang M-S, Song D-S, et al. Procyanidin dimer B2-mediated IRAK-M induction negatively regulates TLR4 signaling in macrophages. Biochem Biophys Res Commun. 2013;438:122–8. doi: 10.1016/j.bbrc.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 101.Lim E-K, Mitchell PJ, Brown N, et al. Regiospecific methylation of a dietary flavonoid scaffold selectively enhances IL-1β production following Toll-like receptor 2 stimulation in THP-1 monocytes. J Biol Chem. 2013;288:21126–35. doi: 10.1074/jbc.M113.453514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu L-L, Yang X-B, Huang Z-M, et al. In vivo and in vitro antiviral activity of hyperoside extracted from Abelmoschus manihot (L) medik. Acta Pharmacol Sin. 2007;28:404–9. doi: 10.1111/j.1745-7254.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 103.Pisonero-Vaquero S, García-Mediavilla MV, Jorquera F, et al. Modulation of PI3K-LXRα-dependent lipogenesis mediated by oxidative/nitrosative stress contributes to inhibition of HCV replication by quercetin. Lab Invest. 2014;94:262–74. doi: 10.1038/labinvest.2013.156. [DOI] [PubMed] [Google Scholar]
- 104.Xavier CPR, Lima CF, Rohde M, et al. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother Pharmacol. 2011;68:1449–57. doi: 10.1007/s00280-011-1641-9. [DOI] [PubMed] [Google Scholar]
- 105*.Ranjith-Kumar CT, Lai Y, Sarisky RT, et al. Green tea catechin, epigallocatechin gallate, suppresses signaling by the dsRNA innate immune receptor RIG-I. PLoS One. 2010;5:e12878. doi: 10.1371/journal.pone.0012878. A very well characterized study describing the possible role EGCG in RIG-I modulation and its anti-viral potential. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106**.Steinmann J, Buer J, Pietschmann T, et al. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol. 2013;168:1059–73. doi: 10.1111/bph.12009. A very well written recent review focusing on the antimicrobial and antiviral activities of EGCG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim M, Kim S-Y, Lee HW, et al. Inhibition of influenza virus internalization by (−)-epigallocatechin-3-gallate. Antiviral Res. 2013;100:460–72. doi: 10.1016/j.antiviral.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 108.Colpitts CC, Schang LM. A small molecule inhibits virion attachment to heparan sulfate- or sialic acid-containing glycans. J Virol. 2014;88:7806–17. doi: 10.1128/JVI.00896-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Z-F, Bai L-P, Huang W, et al. Comparison of in vitro antiviral activity of tea polyphenols against influenza A and B viruses and structure-activity relationship analysis. Fitoterapia. 2014;93:47–53. doi: 10.1016/j.fitote.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 110.Weber JM, Ruzindana-Umunyana A, Imbeault L, et al. Inhibition of adenovirus infection and adenain by green tea catechins. Antiviral Res. 2003;58:167–73. doi: 10.1016/s0166-3542(02)00212-7. [DOI] [PubMed] [Google Scholar]
- 111.Nakayama M, Shimatani K, Ozawa T, et al. Mechanism for the antibacterial action of epigallocatechin gallate (EGCg) on Bacillus subtilis. Biosci Biotechnol Biochem. 2015;79:845–54. doi: 10.1080/09168451.2014.993356. [DOI] [PubMed] [Google Scholar]
- 112.Kohda C, Yanagawa Y, Shimamura T. Epigallocatechin gallate inhibits intracellular survival of Listeria monocytogenes in macrophages. Biochem Biophys Res Commun. 2008;365:310–5. doi: 10.1016/j.bbrc.2007.10.190. [DOI] [PubMed] [Google Scholar]
- 113.Rosa FT, Zulet MÁ, Marchini JS, et al. Bioactive compounds with effects on inflammation markers in humans. Int J Food Sci Nutr. 2012;63:749–65. doi: 10.3109/09637486.2011.649250. [DOI] [PubMed] [Google Scholar]
- 114.Matsunaga K, Klein TW, Friedman H, et al. Legionella pneumophila replication in macrophages inhibited by selective immunomodulatory effects on cytokine formation by epigallocatechin gallate, a major form of tea catechins. Infect Immun. 2001;69:3947–53. doi: 10.1128/IAI.69.6.3947-3953.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu D, Guo Z, Ren Z, et al. Green tea EGCG suppresses T cell proliferation through impairment of IL-2/IL-2 receptor signaling. Free Radic Biol Med. 2009;47:636–43. doi: 10.1016/j.freeradbiomed.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 116.Wu H, Zhu B, Shimoishi Y, et al. (−)-Epigallocatechin-3-gallate induces up-regulation of Th1 and Th2 cytokine genes in Jurkat T cells. Arch Biochem Biophys. 2009;483:99–105. doi: 10.1016/j.abb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 117.Wong CP, Nguyen LP, Noh SK, et al. Induction of regulatory T cells by green tea polyphenol EGCG. Immunol Lett. 2011;139:7–13. doi: 10.1016/j.imlet.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Manjeet KR, Ghosh B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-alpha production in murine macrophages. Int J Immunopharmacol. 1999;21:435–43. doi: 10.1016/s0192-0561(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 119.Yu ES, Min HJ, An SY, et al. Regulatory mechanisms of IL-2 and IFNgamma suppression by quercetin in T helper cells. Biochem Pharmacol. 2008;76:70–8. doi: 10.1016/j.bcp.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 120*.Park YC, Rimbach G, Saliou C, et al. Activity of monomeric, dimeric, and trimeric flavonoids on NO production, TNF-alpha secretion, and NF-kappaB-dependent gene expression in RAW 264.7 macrophages. FEBS Lett. 2000;465:93–7. doi: 10.1016/s0014-5793(99)01735-4. A very well written paper that established the role of polymerization of catechins to the NO production, TNFα secretion and NF-κB activity in macrophages. [DOI] [PubMed] [Google Scholar]
- 121.Huang R-Y, Yu Y-L, Cheng W-C, et al. Immunosuppressive effect of quercetin on dendritic cell activation and function. J Immunol. 2010;184:6815–21. doi: 10.4049/jimmunol.0903991. [DOI] [PubMed] [Google Scholar]
- 122.Sung N-Y, Yang M-S, Song D-S, et al. The procyanidin trimer C1 induces macrophage activation via NF-κB and MAPK pathways, leading to Th1 polarization in murine splenocytes. Eur J Pharmacol. 2013;714:218–28. doi: 10.1016/j.ejphar.2013.02.059. [DOI] [PubMed] [Google Scholar]
- 123.Hyun H-B, Lee WS, Go S-I, et al. The flavonoid morin from Moraceae induces apoptosis by modulation of Bcl-2 family members and Fas receptor in HCT 116 cells. Int J Oncol. 2015;46:2670–8. doi: 10.3892/ijo.2015.2967. [DOI] [PubMed] [Google Scholar]
- 124.Katiyar SK, Mukhtar H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen-presenting cells, and oxidative stress. J Leukoc Biol. 2001;69:719–26. [PubMed] [Google Scholar]
- 125.Varilek GW, Yang F, Lee EY, et al. Green tea polyphenol extract attenuates inflammation in interleukin-2-deficient mice, a model of autoimmunity. J Nutr. 2001;131:2034–9. doi: 10.1093/jn/131.7.2034. [DOI] [PubMed] [Google Scholar]
- 126*.Haqqi TM, Anthony DD, Gupta S, et al. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc Natl Acad Sci U S A. 1999;96:4524–9. doi: 10.1073/pnas.96.8.4524. An important study showing the anti-inflammatory role of flavonoids in arthritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kang TH, Lee JH, Song CK, et al. Epigallocatechin-3-gallate enhances CD8+ T cell-mediated antitumor immunity induced by DNA vaccination. Cancer Res. 2007;67:802–11. doi: 10.1158/0008-5472.CAN-06-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Park JC, Lee SH, Hong JK, et al. Effect of dietary supplementation of procyanidin on growth performance and immune response in pigs. Asian-Australasian J Anim Sci. 2014;27:131–9. doi: 10.5713/ajas.2013.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pae M, Ren Z, Meydani M, et al. Dietary supplementation with high dose of epigallocatechin-3-gallate promotes inflammatory response in mice. J Nutr Biochem. 2012;23:526–31. doi: 10.1016/j.jnutbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 130*.Huang MT, Lou YR, Ma W, et al. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994;54:5841–7. One of the early studies demonstrating anti-cancer properties of curcumin in colon cancer. [PubMed] [Google Scholar]
- 131.Okazaki Y, Han Y, Kayahara M, et al. Consumption of curcumin elevates fecal immunoglobulin A, an index of intestinal immune function, in rats fed a high-fat diet. J Nutr Sci Vitaminol (Tokyo) 2010;56:68–71. doi: 10.3177/jnsv.56.68. [DOI] [PubMed] [Google Scholar]
- 132.Jung MK, Hur DY, Song SB, et al. J Invest Dermatol. Vol. 130. The Society for Investigative Dermatology, Inc; 2010. Tannic acid and quercetin display a therapeutic effect in atopic dermatitis via suppression of angiogenesis and TARC expression in Nc/Nga mice; pp. 1459–63. [DOI] [PubMed] [Google Scholar]
- 133.Wang P, Aronson WJ, Huang M, et al. Green tea polyphenols and metabolites in prostatectomy tissue: implications for cancer prevention. Cancer Prev Res (Phila) 2010;3:985–93. doi: 10.1158/1940-6207.CAPR-09-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McLarty J, Bigelow RLH, Smith M, et al. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in v. Cancer Prev Res (Phila) 2009;2:673–82. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 135.Lee JB, Shin YO. Oligonol supplementation affects leukocyte and immune cell counts after heat loading in humans. Nutrients. 2014;6:2466–77. doi: 10.3390/nu6062466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shin YO, Lee JB, Song YJ, et al. Oligonol supplementation attenuates body temperature and the circulating levels of prostaglandin E2 and cyclooxygenase-2 after heat stress in humans. J Med Food. 2013;16:318–23. doi: 10.1089/jmf.2012.2543. [DOI] [PubMed] [Google Scholar]
- 137.Gates MA, Tworoger SS, Hecht JL, et al. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int J Cancer. 2007;121:2225–32. doi: 10.1002/ijc.22790. [DOI] [PubMed] [Google Scholar]
- 138.Trudel D, Labbé DP, Araya-Farias M, et al. A two-stage, single-arm, phase II study of EGCG-enriched green tea drink as a maintenance therapy in women with advanced stage ovarian cancer. Gynecol Oncol. 2013;131:357–61. doi: 10.1016/j.ygyno.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 139.Iwasaki M, Mizusawa J, Kasuga Y, et al. Green tea consumption and breast cancer risk in Japanese women: a case-control study. Nutr Cancer. 2014;66:57–67. doi: 10.1080/01635581.2014.847963. [DOI] [PubMed] [Google Scholar]
- 140.Perche O, Vergnaud-Gauduchon J, Morand C, et al. Orange juice and its major polyphenol hesperidin consumption do not induce immunomodulation in healthy well-nourished humans. Clin Nutr. 2014;33:130–5. doi: 10.1016/j.clnu.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 141.Hoensch H, Groh B, Edler L, et al. Prospective cohort comparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence. World J Gastroenterol. 2008;14:2187–93. doi: 10.3748/wjg.14.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Garcia-Closas R, Agudo A, Gonzalez CA, et al. Intake of specific carotenoids and flavonoids and the risk of lung cancer in women in Barcelona, Spain. Nutr Cancer. 1998;32:154–8. doi: 10.1080/01635589809514734. [DOI] [PubMed] [Google Scholar]
- 143.Cui Y, Morgenstern H, Greenland S, et al. Dietary flavonoid intake and lung cancer--a population-based case-control study. Cancer. 2008;112:2241–8. doi: 10.1002/cncr.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Le Marchand L, Murphy SP, Hankin JH, et al. Intake of flavonoids and lung cancer. J Natl Cancer Inst. 2000;92:154–60. doi: 10.1093/jnci/92.2.154. [DOI] [PubMed] [Google Scholar]
- 145.Theodoratou E, Kyle J, Cetnarskyj R, et al. Dietary flavonoids and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:684–93. doi: 10.1158/1055-9965.EPI-06-0785. [DOI] [PubMed] [Google Scholar]
- 146.He Z-Y, Shi C-B, Wen H, et al. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest. 2011;29:208–13. doi: 10.3109/07357907.2010.550592. [DOI] [PubMed] [Google Scholar]
- 147.Feng W, Yang CX, Zhang L, et al. Curcumin promotes the apoptosis of human endometrial carcinoma cells by downregulating the expression of androgen receptor through Wnt signal pathway. Eur J Gynaecol Oncol. 2014;35:718–23. [PubMed] [Google Scholar]
- 148.Ryan JL, Heckler CE, Ling M, et al. Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat Res. 2013;180:34–43. doi: 10.1667/RR3255.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Henning SM, Wang P, Said JW, et al. Randomized clinical trial of brewed green and black tea in men with prostate cancer prior to prostatectomy. Prostate. 2015;75:550–9. doi: 10.1002/pros.22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vajdy M, Padrah S. Justia Patents Database [Internet] PHARMACEUTICAL COMPOSITIONS, COMPRISING A COMBINATION OF SELECT CARRIERS, VITAMINS, TANNINS AND FLAVONOIDS AS ANTIGEN-SPECIFIC IMMUNO-MODULATORS Patent Application (Application #20130028961 issued January 31, 2013) cited 2015 Jun 10. [Google Scholar]