Abstract
Objective
Low density lipoprotein (LDL) particles are heterogeneous in terms of size, density, chemical composition and electric charge with certain particle of LDL being more atherogenic than the others. The present study aimed to look at the LDL particle heterogeneity, particle size and association with other cardiovascular disease (CVD) risk factors in young Indian industrial population.
Methodology
600 employees of an industry of Delhi, aged 20-39 years were selected for the study. Data on demographics, individual characteristics associated with major risk factors of CVD, past medical history, clinical and anthropometric profile was collected. Fasting glucose, lipid profile, apolipoprotein (A1, B, and E), lipoprotein (a), high sensitive C-reactive protein (hsCRP) and insulin were estimated. LDL particle size was determined in ethylenediamminetetraacetate (EDTA) plasma by 3% polyacrylamide gel electrophoresis.
Result
We found a prevalence of small dense LDL phenotype (LDL size ≤ 26.3) in 27.4% of males and 24.0% of females. The mean waist circumference, blood pressure, triglycerides (TAG), cholesterol, hsCRP, apolipoprotein (A1, B and E) and insulin were higher in males whereas mean high density lipoprotein was higher in females. Females also had a significantly higher mean LDL particle diameter as compared to males.
Conclusion
TAG, physical activity and lipoprotein (a) correlated with small dense LDL in this young Indian population.
Keywords: Coronary Artery Disease, Lipids
Introduction
Low density lipoprotein (LDL) is recognised as an important risk factor for coronary artery disease and is the main target for lipid lowering therapy.1 LDL particles are heterogeneous in terms of size, density, chemical composition and electric charge,2 with certain particles of LDL being more atherogenic than the others. A number of case control and prospective studies3–5 from different parts of the world have found association between small, dense, LDL particles and increased risk of coronary heart disease (CHD). Small, dense, LDL occurs along with other well-recognised risk factors, such as increased plasma triglycerides and apolipoprotein (Apo) B, as well as decreased high density lipoprotein (HDL) cholesterol levels.
The greater atherogenecity of small, dense, LDL is due to a number of factors. The small, dense, LDL particle shows much greater susceptibility to oxidation,6 has a decreased affinity for LDL receptor,7 binds to the arterial wall with more affinity,8 and crosses the arterial wall more readily.9 Furthermore, the small, dense, LDL particle also has a negative effect on endothelial function.10
Lipid lowering trials also support the role of small, dense, LDL in CHD progression.11 However, evidence in the literature is conflicting with a few prospective and case control studies showing that small, dense, LDLs are not risk factors for CHD.12–15 Some of these studies have in fact suggested that large LDL particles may also be associated with CHD.12
About 30%–60% of the variance in LDL particle size is attributable to genetic factors, and the remainder due to non-genetic influences. A number of environmental factors, like food, physical activity, abdominal obesity, insulin resistance and hyperinsulinemia affect the LDL particle size. LDL particle size is under the influence of a number of genes, which include: Apo E, hepatic lipase, cholesterol ster transfer protein (CETP), LPL and the ApoA1/C3/A4/A5 cluster.
Reports from India and abroad have demonstrated that Indians have a high propensity to coronary artery disease (CAD) compared with other ethnic groups despite lower prevalence of conventional risk factors. To the best of our knowledge, no study has evaluated LDL heterogeneity in the resident Indian population and its association with other established cardiovascular risk factors. Study of LDL heterogeneity may be particularly important as Indians have an atherogenic/metabolic profile, and small, dense, LDL may be part of this multifaceted phenotype.
The present study aimed to look at the (a) LDL particle heterogeneity in a young industrial population from India. (The industrial setting may not be representative of the general population. The participants in this study formed a stable surrogate community for surveillance, unlike the general population where loss to follow-up is high, and continuous monitoring is difficult); (b) determinants of LDL particle diameter in Indians and (c) association between LDL particle size and other cardiovascular disease (CVD) risk factors in this population.
Materials and methods
All employees and family members of Bharat Electronics Limited, an industry located in Ghaziabad, New Delhi, who were below the age of 40 years, were eligible for the study. Of the total subjects available, 600 individuals, aged 20–39 years were selected by stratified random subsampling for adequate representation of the two age deciles and sex groups. Data on demographics, individual characteristics associated with major risk factors of CVD, past medical history, clinical and anthropometric profile were collected. Information on tobacco and alcohol use was obtained from all subjects. Subjects were asked to describe their daily physical activity in the past 5 years as very light, light, moderate or heavy. Dietary information with respect to consumption of egg, meat, chicken and fish was collected.
For biochemical investigations, blood was collected after an overnight fast of 10–12 h in one vacutainer with no additive, a second vacutainer with EDTA as additive, and a third vacutainer with fluoride as additive. Informed consent was taken from all subjects as per the ethical guidelines of the institute. Plasma and serum were separated. Serum was stored at −80°C till analysis. Plasma glucose was estimated by GOD-PAP (glucose oxidase/peroxidase-4-aminophenazonephenol; Randox) method, serum cholesterol by CHOD-PAP (cholesterol oxidase/p-aminophenazone; Randox) method, and serum triglycerides by GPO-PAP (glycerol phosphate oxidase-peroxidase aminophenazone; Randox) method. HDL was estimated by the precipitation method using phosphotungstate/magnesium-precipitation of apolipoprotein B containing lipoproteins, followed by estimation of cholesterol in the supernatant by enzymatic method. LDL was calculated using Friedwald formula. Serum apolipoprotein A1, B, E and lipoprotein (a) were estimated using immunoturbidimetric kits from RANDOX. HsCRP and insulin in serum were measured by ELISA.
LDL particle size was determined in EDTA plasma by electrophoresis.16 Briefly, a 3% slab gel was prepared by mixing 3.7 ml of solution 1, 3.7 ml of solution 2, 0.1 ml of tetramethyl ethylenediamine (TEMED) and 0.5 ml of ammonium per sulphate (APS). Samples prestained with Sudan Black were electrophoresed along run standards of known diameter, carboxylated polystyrene beads (diameter 40.0 nm), apoferritin (diameter 12.2 nm) and thyroglobulin (diameter 17.0 nm). Electrophoresis was performed in cold (4–8°C) using Tris borate EDTA (TBE) buffer (90 mM tris base, 80 mM boric acid, and 3 mM EDTA, pH 8.3). The gel was prerun for 10 min at 50 V. Samples were run initially at 70 V for 30 min, followed by 125 V for 1 h, and 200 V for 1.5 h. Gel was allowed to remain in darkness for 1 h. Densitometry was performed in a Helena electrophoresis data center (EDC) system (Helena Laboratories). Particle diameter corresponding to LDL peaks was calculated from a calibration curve prepared from standards of known diameters which were incorporated in every run.
Statistical analysis
Statisticial analysis was carried out using SPSS (V.16) (SPSS Inc, Chicago, Illinois, USA) software. Normality of the sampling distribution of each variable was tested with the Lilliefors test for normality (a modified Kolmogorov-Smirnov test). Baseline characteristics and biochemical estimates in men and women were compared using Student t test for continuous variables, and χ2 test for categorical variables. p Value less than 0.05 was considered significant. The distributions of all the biochemical variables were not normal, and values were log transformed for analysis. The Pearson correlation coefficients were computed to assess the association between LDL size and other variables. Standard regression analysis was carried out to study the independent association between LDL size and other variables.
Results
The characteristics of the employees of the industry under study are listed in table 1. Fifty-five percent of the subjects were men, and 45% were women. The mean waist circumference, blood pressure, triglycerides (TAG), cholesterol, hsCRP, apolipoprotein A, B and E and insulin were higher in men, whereas, mean HDL was higher in women. Women also had a significantly higher mean LDL particle diameter as compared with men. The distribution of LDL particle size was Gaussian with a coefficient of variation (CV) of 1.99%. Subjects who smoked tobacco had a significantly lower (p=0.029) mean LDL particle diameter (26.56±0.48 nm) as compared with non-smokers (26.72±0.53 nm). Metabolic syndrome, as classified by International Diabetes Federation criteria using cutoffs specified for Indians, was present in 16.3% of this young population. Mean LDL particle diameter (26.56±0.54 nm) was significantly lower in these subjects as compared with normal subjects (26.71±0.52 nm, p=0.035).
Table 1.
Characteristics of the study population
| Men | Women | p Value | |
|---|---|---|---|
| n=316 | n=260 | ||
| BMI (kg/m2) | 23.35 (3.00) | 23.13 (4.14) | 0.466 |
| WC (cm) | 84.10 (9.14) | 76.11 (12.63) | 0.000 |
| SBP (mm Hg) | 120.88 (10.69) | 110.79 (11.09) | 0.000 |
| DBP (mm Hg) | 74.96 (9.04) | 70.65 (9.45) | 0.000 |
| Glucose (mg/dl) | 96.74 (16.67) | 96.36 (23.57) | 0.425 |
| Cholesterol (mg/dl) | 178.14 (35.09) | 167.94 (35.47) | 0.000 |
| TAG (mg/dl) | 138.53 (80.68) | 104.44 (45.20) | 0.000 |
| HDL (mg/dl) | 41.91 (7.82) | 45.92 (9.79) | 0.000 |
| LDL (mg/dl) | 108.52 (29.54) | 101.14 (30.77) | 0.002 |
| LDL particle diameter | 26.64 (0.53) | 26.76 (0.50) | 0.033 |
| APO A1 (mg/dl) | 158.06 (41.01) | 142.10 (41.24) | 0.000 |
| APO B (mg/dl) | 98.77 (36.11) | 66.68 (28.97) | 0.000 |
| APO E (mg/dl) | 2.08 (1.57) | 1.26 (1.35) | 0.000 |
| Lipoprotein (a) (mg %) | 7.74 (8.52) | 6.70 (9.42) | 0.649 |
| hsCRP (mg/l) | 0.49 (1.59) | 0.40 (0.79) | 0.000 |
| Insulin (µIU/ml) | 7.37 (5.86) | 5.36 (3.76) | 0.000 |
Values were log transformed for analysis.
BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; TAG, triglycerides; HDL, high density lipoprotein; LDL, low density lipoprotein; APO, apolipoprotein; CRP; C-reactive protein.
A total of 34.6% of the subjects were nonvegetarians as assessed by fish, meat and chicken consumption. Subjects were asked if they ate chicken, fish and meat occasionally or regularly. Mean LDL particle diameter did not differ between subjects who consumed meat, chicken, fish and egg, and those who did not; 2.7% of the subjects ate meat regularly. Although mean particle diameter was lower in these subjects as compared with others (26.48±0.76 nm vs 26.69±0.52 nm), the difference was not statistically significant.
There was a strong positive correlation of LDL peak particle diameter with physical activity (p=0.015). Systolic blood pressure, diastolic blood pressure, and smoking showed a negative correlation with LDL particle diameter, which was significant (table 2).
Table 2.
Pearson correlation between low density lipoprotein (LDL) particle diameter and other clinical and anthropometric variables
| LDL- PD | ||
|---|---|---|
| Variable | Pearson correlation coefficient | p Value |
| Age | 0.078 | 0.144 |
| Smoking | −0.116 | 0.029 |
| Alcohol use | −0.002 | 0.965 |
| Physical activity | 0.130 | 0.015 |
| BMI | −0.071 | 0.181 |
| Waist circumference | −0.052 | 0.327 |
| SBP | −0.154 | 0.004 |
| DBP | −0.124 | 0.019 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Bold font shows the significant correlations and their p-value.
Among the biochemical risk factors for CVD studied, triglycerides and cholesterol levels showed a strong negative correlation with peak LDL particle diameter (table 3). Triglycerides/HDL which can be considered a surrogate marker for LDL particle diameter also showed significant positive correlation with particle diameter. Lipoprotein (a) correlated negatively with LDL particle diameter, and this correlation was significant. Multiple regression analysis was performed to explore the relationship of LDL particle diameter with parameters which showed significant associations in univariate analysis. Only physical activity, triglycerides and lipoprotein (a) significantly predicted LDL particle diameter (table 4).
Table 3.
Correlation of LDL particle diameter with lipids, apolipoproteins, hsCRP and insulin
| LDL- PD | ||
|---|---|---|
| Variable | Pearson correlation coefficient | p Value |
| Glucose (mg/dl) | −0.089 | 0.149 |
| Cholesterol (mg/dl) | −0.118 | 0.026 |
| Triglycerides (mg/dl) | −0.273 | 0.000 |
| HDL (mg/dl) | 0.069 | 0.193 |
| LDL (mg/dl) | −0.089 | 0.096 |
| TG/HDL | −0.270 | 0.000 |
| APOA1 (mg/dl) | 0.046 | 0.395 |
| APO B (mg/dl) | −0.009 | 0.870 |
| APO E (mg/dl) | 0.020 | 0.714 |
| Lipoprotein (a) (mg/dl) | −0.119 | 0.031 |
| hsCRP (mg/l) | −0.032 | 0.553 |
| Insulin (µIU/ml) | 0.008 | 0.885 |
HDL, High density lipoprotein; LDL, low density lipoprotein; APO, apolipoprotein; CRP; C-reactive protein.
Bold font shows the significant correlations and their p-value.
Table 4.
Stepwise multiple regression analysis of correlates of low density lipoprotein (LDL) particle size
| Independent parameters | R | r2 | |
|---|---|---|---|
| Step 1 | Triglycerides | 0.27 | 0.07 |
| Step 2 | Physical activity, TAG | 0.34 | 0.12 |
| Step 3 | Physical activity, TAG, Lp(a) | 0.38 | 0.15 |
The independent parameters excluded were cholesterol and smoking.
TAG, triglycerides.
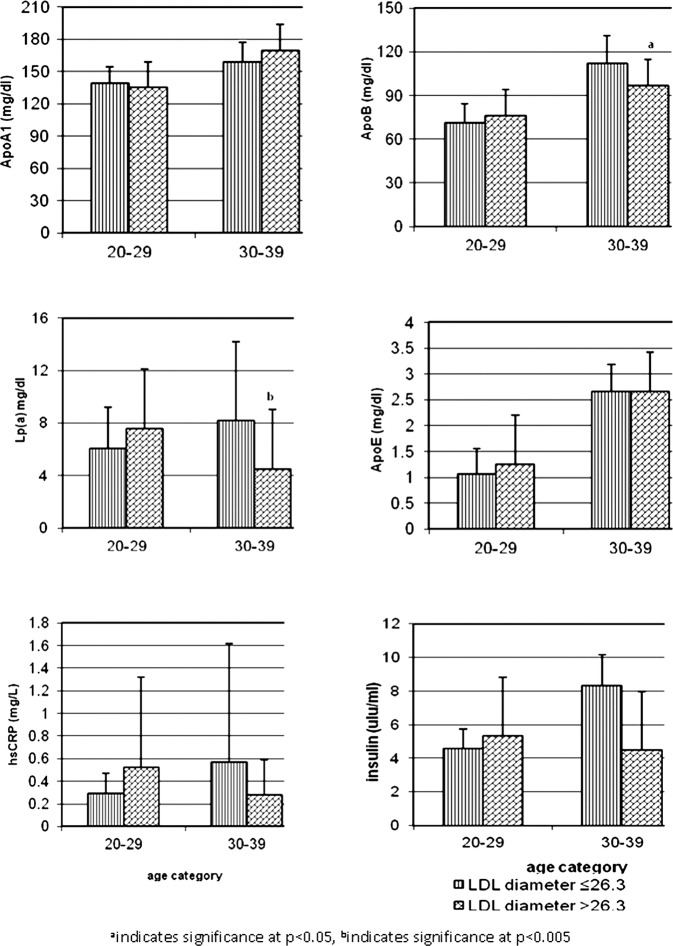
Based on LDL particle diameter, the population was divided into those with LDL particle diameter less than or equal to 26.3 nm (small/intermediate LDL), and those with particle diameter more than 26.3 nm (large LDL);17 27.4% of the men and 24.0% of women had small dense/intermediate phenotype. Figure 1 depicts the distribution of cardiovascular risk factors with different LDL phenotype in the two age categories. Atherogenic B phenotype was associated with higher mean Apo B and Lp(a) levels in subjects in the 30–39 years age category. The ApoB/ApoA1 ratio was also significantly higher in subjects with atherogenic phenotype (0.76±0.43 vs 0.60±0.38, p=0.022). Subjects with B LDL phenotype also had higher hsCRP and insulin levels; the difference, however, was not statistically significant.
Figure 1.
Cardiovascular risk factors within different LDL phenotypes in subjects in different age categories: aindicates significance at p<0.05, bindicates significance at p<0.005.
Discussion
Data on distribution of LDL in resident Indian population are limited. In this study of a young resident Indian industrial population, using polyacrylamide slab gel electrophoresis to characterise LDL particle size, we found a prevalence of small, dense, LDL phenotype (LDL size ≤26.3 nm) in 27.4% of men and 24.0% of women. Indians have higher triglycerides and lower HDL as compared with Caucasians. Small, dense, LDL is part of this atherogenic/metabolic profile. Differences in prevalence of small, dense, LDL have been reported in different ethnic populations.18 A prevalence of small LDL particle size of 20%–27% is reported in European populations,15 27%–33% in Americans,3 19 23%–27% in Japanese and Korean, and 7% in Mongolians. In a study carried out in a small number (n=39) of Asian Indians residing in UK, a prevalence of small, dense, LDL was reported in 44%, as compared with 21% in whites.20 In a recent study, prevalence of small, dense, LDL of 12.5%–14.2% was reported in migrant Indians, as compared with 15.7%–17.2% in rural Indians.21 In another study comparing normolipidemic migrant, Asian Indians and Caucasians, a 15% frequency of pattern B was reported in Indians as compared with 41% in Caucasians.22 Genetic makeup and environment factors are important determinants of sdLDL. A number of studies have looked at factors associated with small, dense, LDL. Gender has been reported to be a determinant of LDL size, and decreased activity of hepatic lipase is the possible reason for larger LDL diameter in women23 as compared with men. A similar association was observed in the present study. A smaller mean LDL particle diameter was found in the present study in subjects with metabolic syndrome. Small, dense, LDL has been reported in cross-sectional studies to be associated with metabolic syndrome,24 and in a recent follow-up study, small, dense, LDL was found to be independent predictors of cardiovascular and cerebrovascular events in subjects with metabolic syndrome.25 Metabolic syndrome is widely prevalent in Indians, and small, dense, LDL may be an important component of the syndrome in Indians. Among the components of metabolic syndrome, triglycerides were significantly correlated with small, dense, LDL, and so was triglyceride/HDL. A high prevalence of small, dense, LDL (21.4%) was seen even in subjects with low triglycerides (<133 mg/dl). In a study comparing Koreans, Mangolians and Japanese, an ethic difference in prevalence of small, dense, LDL was seen in subjects with low triglycerides (<133 mg/dl), and the difference in the diet consumed was attributed to it.26 Indians also consume a high carbohydrate diet, and this excess carbohydrate is converted to fat through de novo lipogenesis, thereby increasing very low density lipoprotein (VLDL) production and small, dense, LDL formation. Lipoprotein (a) was also significantly correlated with small, dense, LDL in the present study. Moon et al27 found a similar correlation between sdLDL and Lp(a). Increased oxidative stress is likely to increase both sdLDL and Lp(a), and may explain the correlation between the two. Physical activity was another important determinant of small, dense, LDL. Daily physical activity showed correlation with LDL particle diameter with higher particle diameter in subjects who were physically more active, and 39% of the subjects did moderate to heavy physical activity. Those who did very light to light physical activity had a mean particle diameter of 26.63±0.48 nm as compared with 26.78±0.58 nm in those who did moderate to heavy physical activity. Moderate intensity exercise leads to increase in lipase activity in muscle and liver, followed by increase in lipid uptake and oxidation by muscles, and improved insulin action, lower triglycerides, and large buoyant LDL.28 Among the apolipoproteins, only apo B showed some association with LDL particle diameter. Other factors, like insulin and hsCRP, did not show any correlation with LDL size.
The limitation of this study is that the population is mainly composed of industrial employees and their family members, and may not be representative of the general population.
In conclusion, triglycerides, physical activity and lipoprotein (a) emerged to be important determinants of small, dense, LDL in this young Indian population.
Acknowledgments
We thank all the participants in our study.
Footnotes
Contributors: RL designed and planned the study and wrote the draft manuscript; PD participated in the design of the study and reviewed the manuscript; MT and RG carried out the biochemical analysis; KSR was involved in the design of the study and revised the manuscript critically. All authors read and approved the final manuscript.
Funding: The funding for the study was provided by Indian Council of Medical Research (ICMR), New Delhi, India.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: Ethics committee, AIIMS, New Delhi.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, National Cholesterol Education Program. Second report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). Circulation 1994;89:1329–445. [DOI] [PubMed] [Google Scholar]
- 2.Krauss RM, Burke DJ. Identification of multiple classes of plasma low density lipoproteins in normal human subjects. J Lipid Res 1982;23:97–104. [PubMed] [Google Scholar]
- 3.Rizzo M, Berneis K. Low density lipoprotein size and cardiovascular risk assessment. QJM 2006;99:1–14. [DOI] [PubMed] [Google Scholar]
- 4.Lamarche B, St-Pierre AC, Ruel IL, et al. A prospective population based study of low density lipoprotein particle size as a risk factor for ischemic heart disease in men. Can J Cardiol 2001;17:859–65. [PubMed] [Google Scholar]
- 5.Toft-Petersen AP, Tilsted HH, Aarøe J, et al. Small dense LDL particles—a predictor of coronary artery disease evaluated by invasive and CT-based techniques: a case-control study. Lipids Health Dis 2011;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chait A, Brazg RL, Tribble DL, et al. Susceptibility of small dense, low density lipoproteins to oxidative modification in subjects with the atherogenic lipoprotein phenotype pattern B. Am J Med 1993;94:350–6. [DOI] [PubMed] [Google Scholar]
- 7.Campos H, Arnold KS, Balestra ME, et al. Differences in receptor binding of LDL subfractions. Arterioscler. Thromb Vasc Biol 1996;16:794–801. [DOI] [PubMed] [Google Scholar]
- 8.Anber V, Griffin BA, McConnell M, et al. Influence of plasma lipid and LDL subfraction profile on the interaction between low density lipoprotein with human arterial wall proteoglycans. Artherosclerosis 1997;124:261–71. [DOI] [PubMed] [Google Scholar]
- 9.Bjornheden T, Babyi A, Bondjers G, et al. Accumulation of lipoprotein fractions and subfractions in the arterial wall, determined in an in vitro perfusion system. Atherosclerosis 1996;123:43–56. [DOI] [PubMed] [Google Scholar]
- 10.Vakkilainen J, Makimattila S, Seppala-Lindroos A, et al. Endothelial dysfunction in men with small LDL particles. Circulation 2000;102:716–21. [DOI] [PubMed] [Google Scholar]
- 11.Miller BD, Alderman EL, Haskell WL, et al. Predominanace of dense low density lipoprotein particles predicts angiographic benefit of therapy in the Stanford Coronary Risk Intervention Project. Circulation 1996;94:2146–53. [DOI] [PubMed] [Google Scholar]
- 12.Campos H, Roederer GO, Lussier-Cancan S, et al. Predominance of large LDL and reduced HDL2 cholesterol in normolipidemic men with coronary artery disease. Arterioscler Thromb Vasc Biol 1995;15:1043–8. [DOI] [PubMed] [Google Scholar]
- 13.Gray RS, Robbins DC, Wang W, et al. Relation of LDL size to the insulin resistance syndrome and coronary heart disease in American Indians, the strong heart study. Arterioscler Thromb Vasc Biol 1997;17:2713–20. [DOI] [PubMed] [Google Scholar]
- 14.Ruotolo G, Tettamanti C, Garancini MP, et al. Smaller denser LDL particles are not a risk Factor for cardiovascular disease in healthy nonagenarian women of Cremona population study. Atherosclerosis 1998;140:65–70. [DOI] [PubMed] [Google Scholar]
- 15.Mykkanen L, Kuusisto J, Haffner SM, et al. LDL size and risk of coronary heart disease in elderly men and women. Arterioscler Thromb Vasc Biol 1999;19:2742–8. [DOI] [PubMed] [Google Scholar]
- 16.Singh Y, Lakshmy R, Gupta R, et al. A rapid 3% polyacrylamide slab gel electrophoresis method for high through put screening of LDL phenotype. Lipids Health Dis 2008;7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirany SV, Othman Y, Kutscher P, et al. Comparison of low-density lipoprotein size by polyacrylamide tube gel electrophoresis and polyacrylamide gradient gel electrophoresis. Am J Clin Pathol 2003;119:439–5. [DOI] [PubMed] [Google Scholar]
- 18.Goedecke JH, Utzschneider K, Faulenbach MV, et al. Ethnic differences in serum lipoproteins and their determinants in South African women. Metabolism 2010;59:1341–50. [DOI] [PubMed] [Google Scholar]
- 19.Lamarche B, Tchernof A, Moorjani S, et al. Small dense low density lipoprotein particles as predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation 1997;95:69–75. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni KR, MArkovitz JH, Nanda NC, et al. Increased prevalence of smaller and denser LDL particles in Asian Indians. ATVB 1999;19:2749–55. [DOI] [PubMed] [Google Scholar]
- 21.Patel JV, Caslake MJ, Vyas A, et al. Triglycerides and small dense low density lipoprotein in the discrimination of coronary heart disease risk in South Asian populations. Atherosclerosis 2010;209:579–84. [DOI] [PubMed] [Google Scholar]
- 22.Abate N, Garg A, Enos EA, et al. Physio-chemical properties of low density lipoproteins in normolipidemic Asian Indian men. Horm Metab Res 1995;27:326–31. [DOI] [PubMed] [Google Scholar]
- 23.Nikkila M, Pitkajarvi T, Koivula T, et al. Women have larger and less atherogenic low density lipoprotein particle size than men. Atherosclerosis 1996;119:181–90. [DOI] [PubMed] [Google Scholar]
- 24.Gentile M, Panico S, Jossa F, et al. Small dense LDL particles and metabolic syndrome in a sample of middle-aged women. Findings from Progetto Atena. Clin Chim Acta 2008;388:179–83. [DOI] [PubMed] [Google Scholar]
- 25.Rizzo M, Pernice V, Frasheri A, et al. Small, dense low-density lipoproteins (LDL) are predictors of cardio- and cerebro-vascular events in subjects with the metabolic syndrome. Clin Endocrinol (Oxf) 2009;70:870–5. [DOI] [PubMed] [Google Scholar]
- 26.Anuurad E, Shiwaku K, Enkhmaa B, et al. Ethnic differences in the formation of small LDL particles in Asians: a comparison of Koreans, Japanese and Mongolians. Eur J Clin Invest 2004;34:738–46. [DOI] [PubMed] [Google Scholar]
- 27.Moon JY, Kwon HM, Kwon SW, et al. Lipoprotein(a) and LDL particle size are related to the severity of coronary artery disease. Cardiology 2007;108:282–9. [DOI] [PubMed] [Google Scholar]
- 28.Couillard C, Despres J, Lamarche B, et al. Effects of endurance training on plasma HDL cholesterol levels depend on levels of triglycerides: evidence from men of the Health, Risk Factors, Exercise Training and Genetics (HERITAGE) Family Study. Arterioscler Thromb Vasc Biol 2001;21:1226–32. [DOI] [PubMed] [Google Scholar]