Abstract
Objectives
To determine the role of adherence and its significance in the relationship between self-efficacy and self-management of diabetic patients undergoing coronary artery bypass graft (CABG) in Taiwan.
Design
Descriptive and correlational survey design.
Setting
Three outpatient clinics in Taiwan.
Participants
Patients diagnosed with diabetes undergoing CABG at least 6 months before the study, 18 years of age or older, able to communicate verbally without any psychiatric problems, and with a life expectancy longer than 1 year.
Main outcome measures
Self-management assessment (self-efficacy for managing disease and adherence to guidelines and medication measured on a scale of 0–8), the higher aspects of self-management (keeping appointments, taking medication properly and keeping follow-up appointments) and the lower aspects of self-management (inability to share decisions with primary physician, inability to take correct actions when symptoms worsen and inability to adapt habits to improve health).
Results
The mean score obtained for self-management among the 166 participants was 6.48, with 57 (34.3%) of them showing non-adherent behaviour. Self-efficacy accounts for 38% (R2=0.380, F(1,103)=63.124, p < 0.001), and 54% of good self-management was explained by self-efficacy and adherence in managing disease (R2=0.540, F(2,102)=56.937, p<0.001). Adherence accounts for 16% of better self-management, age and education combined account for 4.9% (R2=0.589, F(6.98)=23.399, p<0.001), and lifestyle items account for 5.2% (R2=0.641, F(14,90)=11.457, p<0.001). Disease-related variables contribute 3.4% (R2=0.674, F(17,87)=10.599, p<0.001). Thus self-efficacy, adherence, age, education, primary care provider and systolic pressure are considered significant predictors of self-management. With the exception of adherence, none of the variables had a statistically significant mediating effect.
Conclusions
The results confirm strong relationships between self-efficacy, adherence and self-management, with adherence having a significant mediating effect in post-CABG patients with diabetes in Taiwan.
Keywords: ALLIED SPECIALITIES
Introduction
Cardiovascular diseases are the leading cause of death worldwide, accounting for 29% of all deaths according to WHO.1 Specifically, 17.3 million people die annually from cardiovascular diseases, with 7.2 million from coronary artery disease (CAD). In Taiwan, CAD accounts for more than 10 000 deaths per year. Coronary artery bypass graft (CABG) is an effective way to treat CAD; thus the number of CABGs has increased over the years.2 3
Diabetes mellitus is a significant predictor of CAD,4 and approximately one-third of CABG patients are diagnosed with diabetes.5 Patients with diabetes undergoing CABG have slower recovery than the general population because of more complications after surgery and cardiac adverse events, resulting in a higher mortality.4 6 7–11
Type 2 diabetes mellitus is a metabolic as well as a vascular disease. More frequent restenosis and increased atherosclerosis progression after revascularisation occur in diabetic patients undergoing CABG compared with non-diabetic patients. Thus, to maintain health, diabetic patients must adhere to the medication regimen and follow-up visits. Hyperglycaemia alone significantly increases cardiac events; therefore glucose control (maintaining optimal glycated haemoglobin (HbA1C)) is related to positive post-CABG outcomes.8 12 13
About 70% of diabetic patients do not believe they are at risk of cardiac events.14 Cramer et al reviewed studies of patient compliance/persistence with cardiovascular or anti-diabetes medication published since 2000.12 They found that patients not adhering to such medication is a significant problem; ∼30% of patients do not take their medication as specified and only 59% do take it at least 80% of the time. Although compliance is defined interchangeably with adherence in various studies,15 16 adherence is used more commonly.17 Adherence means that patients actively participate in their disease management and do what it is required of them.17 18
In Taiwan, diabetic patients do not manage their disease appropriately; only 14% of elderly diabetic patients have HbA1C values within the optimal range of <6.5.19 Therefore assessing post-CABG diabetic patients on medication and improving their adherence to treatment and medication is very important.
Adherence promotes good self-management, which is the key to health improvement, achieved by guiding patients to take an active role in their treatment and take responsibility for day-to-day disease management.20 21 Good self-management has the potential to cut healthcare costs through the prevention of long-term complications and reduction of the physical and psychological burden of the disease.15 22 23 Self-management can occur over the entire continuum of care, which spans from hospital to home care. Traditionally, patients received medical care from medical personnel; however, self-management has increased in popularity, as it reduces the burden of chronically ill patients as well as reducing the burden of caregivers and the healthcare system.24
Self-management can be influenced by the patient's beliefs about its ease or difficulty.23 This belief is termed self-efficacy, which focuses on individuals’ convictions that they have control over their behaviour through their motivation to achieve desired outcomes25 26 Self-efficacy was developed on the basis of four sources: past successful experiences, empathetic experiences from social models, social persuasion, and physical and mental status.25 27 Because self-efficacy can lead to behavioural changes, it plays an important role in the self-management of chronic disease.21 28 According to Bandura,27 self-efficacy provides confidence to achieve the behavioural change needed to respond to challenges. Self-efficacy is positively related to health behaviour and to change in health status.29 Thus assessing and enhancing self-efficacy are important for patients who need to engage in better self-management.
The important factors that affect self-management have been identified in a theory of diabetic self-care management developed by Sousa and Zauszniewski in 2005.31 Their model was derived from Orem's self-care (1995) and Bandura's self-efficacy (1997) theories,26 32 which reveal that influencing factors are part of constructs that include both internal (personal) and external (environmental) factors related to the self-care process. The important constructs are Bandura's personal and social systems and Orem's basic conditioning factors. Orem proposed that the basic conditioning factors are age, gender, developmental state, health status, sociocultural orientation, healthcare system, family system, pattern of living, the environment, and resource availability and adequacy.32 Based on the diabetic self-care management model,31 adherence and self-efficacy are the internal factors in this study.
In summary, self-management is critically important for promoting better outcomes for post-CABG patients with diabetes. Adherence and self-efficacy correlate with self-management. However, the contribution of adherence, self-efficacy, demographic factors, lifestyle and disease characteristics to self-management is not known. Therefore the primary objective of this research is to determine the predictors of self-management in post-CABG patients with diabetes. The aims are to (a) examine the contribution of adherence, self-efficacy, demographic variables, lifestyle and disease characteristics to self-management, (b) determine the reasons for patient non-adherence, and (c) examine the mediating effect of adherence and other influencing variables on the relationship between self-efficacy and self-management.
Methods
Design overview
The study used a cross-sectional, descriptive and correlational survey design. Ethics approval to conduct the research was obtained from the appropriate institutional review boards, and all participants gave their informed consent. Data were gathered by four self-administered questionnaires, described below, in three outpatient clinics while patients were waiting for a doctor's appointment or after they had been seen by the doctor before leaving the clinic. The research assistant, who was institutional review board certified and trained in assisting patients to fill out the questionnaires, was also responsible for collecting data from all the clinics.
Participants
The study sample consisted of 166 diabetic patients who underwent CABG in three outpatient clinics and were seen for follow-up between August 2010 and March 2011. The inclusion criteria for the study were (a) diagnosed with diabetes, (b) underwent CABG at least 6 months before the study, (c) 18 years of age or older, and (d) able to communicate verbally. Patients were excluded from the study on the basis of the following criteria: (a) psychiatric problems confirmed by a psychiatrist or (b) a comorbid condition such as malignancy or a major trauma that would limit life expectancy to less than 1 year.
For estimating the sample size, G*Power V.3.1 was used (Department of Criminology, University of Melbourne, Parkville, Victoria, Australia). The significance level was set at α=0.05, the statistical power at (1−β)=0.80, and effect size at 0.25. Effect size was determined as described in the study by Loring et al study,24 which examined health status and healthcare utilisation of a chronic disease self-management programme.
Measurements
Demographic questionnaire
The questionnaire collected demographic, lifestyle and disease information. Demographic data included age, gender, marital status, education, religion and socioeconomic status. Lifestyle information included alcohol use, tobacco use, primary care provider, employee status and exercise habits. Disease-related characteristics included time since diagnosis of diabetes, adverse effects of medication, coexisting diseases, laboratory results, and related clinical data.
Partners in Health Scale (PHS)
General self-management was assessed using the PHS, which addresses generic self-management for various chronic illnesses. The PHS consists of 11 items that address different aspects of disease self-care, including obtaining disease-related information, sharing in decisions, taking medication, understanding of and ability to monitor symptoms and respond to symptom changes, and making and keeping appointments. The scale uses a Likert-type scale with responses ranging from 0 (very good) to 8 (poor). Total scores can range from 0 (very good self-management) to 88 (poor self-management). The scale has been used in various studies across different countries.24 33–35 The reliability of the scale has been determined in several chronically ill populations, with a Cronbach's α of 0.92.23
Self-Efficacy for Managing Chronic Disease Scale (SES)
Confidence in one's ability to manage chronic illness was assessed using a modified version of the SES.23 The SES consists of 33 items divided into 10 subscales with confidence levels for doing a certain activity: (a) exercising regularly; (b) obtaining information about disease; (c) obtaining help from the community, family and friends; (d) communicating with physicians; (e) managing the disease in general; (f) doing chores; (g) engaging in social/recreational activities; (h) managing symptoms; (i) managing shortness of breath; and (j) controlling/managing depression.
The scale uses a Likert-type scale, and responses range from 1 (not at all confident) to 10 (totally confident). Responses were summed, and a mean score, ranging from 1 (low self-efficacy) to 10 (high self-efficacy), was calculated. The scale has been tested with several diverse chronically ill groups.23 35 36 The reliability of the scale (Cronbach's α=0.89) has been determined.24
Cardiovascular Diabetic Adherence Scale (CDAS)
Patient adherence was assessed using the CDAS, which was developed by an expert panel composed of one nursing faculty member, one cardiac surgeon and four registered nurses with over 10 years of experience. The CDAS consists of nine items that focus on diabetic and post-CABG adherence. The CDAS uses a Likert-type scale, and responses range from 1 (not at all compliant) to 5 (totally compliant). The total score was 45, and a score bellow 37 was considered non-adherent. Content validity was confirmed by an expert panel, who determined a 0.93 content validity index. The Cronbach's α was 0.627 for internal consistency. The confirmatory factor analysis showed goodness-of-fit index (GFI)=0.95, comparative fit index (CFI)=0.95, root mean square error of approximation (RMSEA)=0.06 and standardised root mean square residual (SRMR)=0.064, meaning an acceptable fit.
Statistical analysis
SPSS V.17.0 software was used for data analysis. Descriptive statistics were performed to analyse demographic data. Hierarchical regression analysis was used, with self-management chosen as the dependent variable, and self-efficacy, adherence level and other patient characteristics as independent variables. A multiple mediation script was used to present the mediating effect on self-management. Lastly, confirmatory factor analysis and coefficient α were used to ensure the psychometric properties of the instrument. The level of statistical significance for analyses was set at p<0.05.
Results
Psychometric properties of translating instruments
Two of the four questionnaires, the PHS for measuring self-management and SES for measuring self-efficacy, were originally in English. They were translated into Chinese by a health professional who is proficient in English but whose primary language is Chinese. In addition, a bilingual expert with a healthcare background was asked to examine whether any of the translations were inadequate and to correct them. The instrument was translated back into English by an independent translator, who is a native speaker without a medical background. This was carried out to ensure the validity of the Chinese translation.
Confirmatory factor analysis was used to assess whether the factor structure of the PHS (GFI=0.94; CFI=0.99; RMSER=0.068; standardised RMR=0.043) and SES (GFI=0.99; CFI=0.96; RMSER=0.13; standardised RMR=0.061) is the same in English as it is in Chinese. Lastly, Cronbach's α was 0.891 for the Chinese version of the PHS and 0.973 for the Chinese version of SES in the present study.
Demographics
As presented in table 1, the mean age of the participants was 67.5, and 80.1% were male. With regard to lifestyle, 25.9% of the participants never used tobacco, and 28% had never used alcohol. Fewer than half (40.4%) of the participants exercised regularly.
Table 1.
Demographics of study participants
| Variable | Number | Percentage |
|---|---|---|
| Gender | ||
| Male | 133 | 80.1 |
| Female | 33 | 19.9 |
| Age (mean±SD 68±9.5) | ||
| <50 years | 8 | 4.8 |
| 51–60 years | 38 | 22.9 |
| 61–70 years | 57 | 34.3 |
| >70 years | 66 | 37.9 |
| Education | ||
| Non-literate | 22 | |
| Elementary | 50 | 43.4 |
| High school | 58 | 34.9 |
| Associate degree or above | 36 | 21.7 |
| Marital status | ||
| Married | 146 | 87.9 |
| Unmarried | 4 | 2.5 |
| Separated/divorce | 16 | 9.6 |
| Employee status | ||
| Yes | 27 | 16.3 |
| No | 139 | 83.7 |
| Primary caregiver | ||
| Self | 85 | 51.2 |
| Family/others | 81 | 48.8 |
| Exercise | ||
| Never/seldom | 54 | 32.5 |
| Not regular | 45 | 27.1 |
| Regularly | 67 | 40.4 |
| Tobacco use | ||
| Never | 43 | 25.9 |
| Quit | 113 | 68.1 |
| Regular | 10 | 6 |
| Alcohol use | ||
| Never | 47 | 28.3 |
| Quit | 108 | 65.1 |
| Regular | 11 | 6.6 |
Disease characteristics
As seen in table 2, 73.5% of participants had hypertension, and 11.4% had renal disease. One hundred patients (60.2%) had postoperative discomfort and diabetic complications. Notably, the majority of the patients (88.5%) took four to 10 different kinds of pills.
Table 2.
Disease characteristics of the study participants
| Variable | Number | Percentage |
|---|---|---|
| Comorbidities | ||
| Hypertension | 122 | 73.5 |
| Renal disease | 19 | 11.4 |
| Cerebrovascular accident/stroke | 8 | 4.8 |
| Liver diseases | 7 | 4.2 |
| Hyperlipidaemia | 3 | 1.8 |
| Other | 32 | 16.6 |
| Number if coexisting disease | ||
| Up to there | 139 | 83.7 |
| More than three | 27 | 16.3 |
| Diabetic complication | ||
| Yes | 66 | 39.8 |
| No | 100 | 60.2 |
| Number of medications | ||
| <3 | 6 | 3.6 |
| 4–7 | 93 | 56 |
| 8–10 | 54 | 32.5 |
| 11–14 | 8 | 4.8 |
| >15 | 5 | 3 |
| Time since diabetes diagnosed | ||
| <1 year | 14 | 8.4 |
| 1–5 years | 38 | 22.9 |
| 6–10 years | 48 | 28.9 |
| >10 years | 66 | 39.8 |
| Time since surgery | ||
| <1 year | 46 | 27.7 |
| 1–5 years | 70 | 42.1 |
| 6–10 years | 30 | 18.1 |
| >10 years | 20 | 12.1 |
| Body weight | ||
| Below average | 16 | 9.6 |
| Normal | 106 | 63.9 |
| Overweight | 44 | 26.5 |
Self-management
The score of each item in this scale ranges from 0 to 8, the lowest indicating the best self-management. The better self-management aspects were: keeping appointments (1.07±1.76); taking prescribed medicine (1.20±1.98); setting follow-up appointments (1.42±2.01). The lower aspects of self-management were: patients being able to share decisions with their primary physician (3.36±2.44); taking correct actions when symptoms worsen (3.08±2.11); adapting habits to improve health (3.01±2.11).
Self-efficacy for managing disease
The mean score obtained was 6.48. The top three items for which the participants had the highest confidence were managing symptoms, engaging in social and recreational activities, and obtaining help from the community, family and friends. The top three items for which participants had the least confidence were exercising regularly, managing shortness of breath, and controlling/managing depression.
Adherence
The top three items that patients adhered to most closely were abstinence from tobacco and alcohol and keeping scheduled appointments. The top three items that patients adhered to least were regular exercise, checking their blood sugar, and diet. Of the 166 patients, 57 (34.3%) showed non-adherent behaviour.
Reasons for non-adherence
As seen in table 3, the most common reasons for non-adherence were forgetting to take medicine, difficulty in changing habits, lack of energy, and no help.
Table 3.
Reasons for non-adherence
| Variable | Number | Percentage | Ranking |
|---|---|---|---|
| Forgetting to take the medicine | 103 | 62 | 1 |
| Having difficulty changing habits | 98 | 59 | 2 |
| Lack of energy | 98 | 59 | 2 |
| Having no one to help | 64 | 38.6 | 3 |
| The symptoms were not worse | 43 | 25.9 | 4 |
| It's bothersome to take medicine for a long time | 30 | 18.1 | 5 |
| The symptoms did not improve | 24 | 14.5 | 6 |
| Having the disease for a long time | 24 | 14.5 | 6 |
| The diseases that I have cannot be treated to produce complete recovery | 23 | 13.9 | 7 |
| Too many medicines to take | 15 | 9 | 8 |
| Taking different medicines at different time points was too complicated | 14 | 8.4 | 9 |
| Too busy to take medicine | 12 | 7.2 | 10 |
| It is too far from my home to the hospital | 7 | 4.2 | 11 |
| Adverse effect of the medicines | 5 | 3 | 12 |
Predictors of self-management
Stepwise regression was first used to predict the demographics, lifestyle factors and disease characteristics that correlated with self-management. Next, hierarchical regression was performed, with self-management as the dependent variable to determine the relationship with other variables. As presented in table 4, five models were used. Joint mediation effect analysis was used to determine the effect of adherence and other variables on the relationship between self-efficacy and self-management.
Table 4.
Determinants of self-management
| Model | Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| variable | β | t | p Value | β | t | p Value | β | t | p Value | β | t | p Value | β | t | p Value |
| Self-efficacy | −0.616 | −7.945 | <0.001 | −0.518 | −7.502 | <0.001 | −0.496 | −7.385 | <0.001 | −0.413 | −5.356 | <0.001 | −0.410 | −5.387 | <0.001 |
| Adherence | −0.412 | −5.964 | <0.001 | −0.417 | −6.231 | <0.001 | −0.408 | −5.940 | <0.001 | −0.349 | −4.888 | <0.001 | |||
| Demographics | |||||||||||||||
| Age | −0.181 | −2.719 | 0.008 | −0.196 | −2.906 | 0.005 | −0.201 | −2.783 | 0.003 | ||||||
| Education | −0.258 | −2.304 | 0.023 | −0.239 | −2.111 | 0.038 | −0.273 | −2.447 | 0.015 | ||||||
| Lifestyle | |||||||||||||||
| Primary care | 0.184 | 2.468 | 0.015 | 0.147 | 2.166 | 0.045 | |||||||||
| Employee status | −0.113 | −1.514 | 0.134 | −0.054 | −1.291 | 0.467 | |||||||||
| Tobacco use | −0.023 | −0.295 | 0.769 | −0.017 | −0.120 | 0.826 | |||||||||
| Disease characteristics | |||||||||||||||
| Adverse | 0.064 | 0.988 | 0.356 | ||||||||||||
| Coexisting disease | 0.139 | 0.735 | 0.054 | ||||||||||||
| Systolic pressure | 0.139 | 2.092 | 0.045 | ||||||||||||
| R2 | 0.380 | 0.540 | 0.589 | 0.641 | 0.674 | ||||||||||
| F | 63.124 | 59.937 | 23.399 | 11.457 | 10.599 | ||||||||||
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||
| ΔR2 | 0.380 | 0.160 | 0.049 | 0.052 | 0.034 | ||||||||||
| ΔF | 63.124 | 35.567 | 2.899 | 1.616 | 3.13 | ||||||||||
| Mediation effects | |||||||||||||||
| Indirect value | 18.5 | 8.3 | 10.8 | 3.2 | |||||||||||
| 95%CI | −0.0472 to −0.0115 | −0.0255 to 0.0021 | −0.0297 to 0.0100 | −0.0546 to 0.0577 | |||||||||||
| Δp | <0.001 | <0.001 | 0.026 | 0.131 | 0.034 | ||||||||||
In model 1, self-efficacy accounts for 38% (R2=0.380, F(1,103)=63.124, p<0.001). In model 2, 54% of good self-management was explained by self-efficacy and adherence in managing diseases (R2=0.540, F(2,102)=56.937, p<0.001). In model 2, adherence accounts for 16% of better self-management. As demonstrated in models 3 and 4, age and education together account for only 4.9% (R2=0.589, F(6.98)=23.399, p<0.001), and lifestyle items account for only 5.2% (R2=0.641, F(14,90)=11.457, p<0.001). Model 5 revealed that disease-related variables contribute only 3.4% (R2=0.674, F(17,87)=10.599, p<0.001). Thus self-efficacy, adherence, age, education, primary care provider and systolic pressure are considered significant predictors of self-management.
As presented in table 4 and figure 1, only adherence was statistically significant and had an 18.5% mediating effect on the relationship between self-efficacy and self-management (95% CI=−0.0472 to −0.0115). Demographic items had an 8.3% mediating effect (95% CI=−0.0255 to 0.0021), lifestyle items had a 10.8% mediating effect (95% CI=−0.0297 to 0.0100), and disease characteristics had a 3.2% (95% CI=−0.0546 to 0.0577) mediating effect. However, with the exception of adherence, none of these variables had a statistically significant mediating effect.
Figure 1.
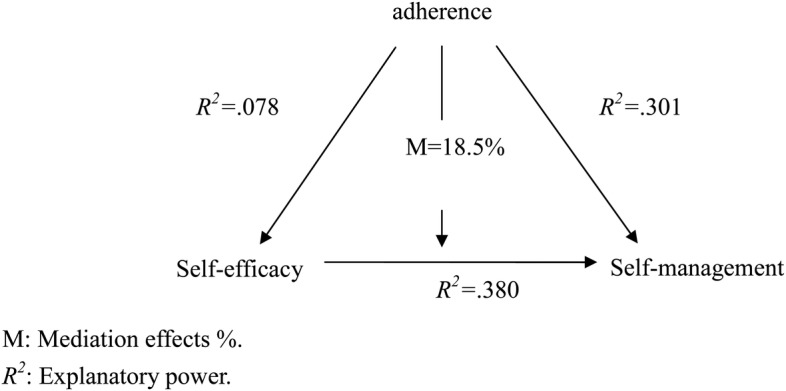
Mediating role of adherence in relationship between self-efficacy and self-management. M, mediation effects (%); R2, explanatory power.
Discussion
The mean score for self-efficacy in managing disease is 6.48, similar to previous research.23 24 37 Pang et al conducted a cross-sectional study to determine the factors that influence disease management self-efficacy in 44 individuals with a spinal cord injury in Taiwan,37 obtaining a mean score of 6.5 out of 10. Gallager et al25 and Lorig et al24 reported a similar level of self-efficacy in a chronic-disease population in the West.
The total score for self-management in our study was 25.08, similar to a score of 23.93 obtained by Gallagher et al. In addition, our study found self-efficacy to be a strong predictor of better self-management.25 Gallagher et al used a prospective and descriptive design to examine levels of self-management in 300 patients with chronic illness, showing that patients with low self-efficacy, a poor sense of coherence, and older age tend to have poor self-management.25 In addition, in a more recent study, Zhong et al found that strong self-efficacy was positively associated with good self-management.28 Thus promoting self-efficacy can have a profound influence on self-management.
With regard to adherence, 34.4% of our participants were non-adherent. This result is consistent with that of Cramer et al, who found that 41% of their participants neglected prescribed medication.12 Diet, control and exercise were non-adherent items, also seen in previous research.38–40 In our study, forgetting to take medicine was the most common reason for non-adherence. Topinkova et al conducted a cross-sectional comparative study in 3881 elderly people receiving home-care services in 11 European countries. Non-adherence to prescriptions was the most common issue among patients.41 Topinkova et al recommended simplifying the drug regimen as much as possible for seniors undergoing polypharmacotherapy.41 This is relevant to our study, as the majority of patients take between four and 10 kinds of pills.
Our study found that adherence had a 10.8% mediating effect on the relationship between self-efficacy and self-management. In relation to our findings, Zhong et al found that adherence to medication was influenced by attitude toward self-management.28 Furthermore, Costantini et al noted that, when practitioners include the patients’ beliefs and concerns in the treatment plan, treatment outcomes are enhanced.20 Involvement of patients and their families in making decisions for their care plan and assessment of patients’ self-management ability are crucial to the success of the treatment plan.
Participants were recruited from three hospitals, all located in Taipei, Taiwan. The characteristics of an urban sample might be different from those of a rural sample. Larger samples are recommended in future studies to better represent the population being investigated. The adherence instrument was used for the first time in this study, only with this sample's specific population. The confirmatory factor analysis showed a good fit of data to one concept. However, inputting patients’ opinions when the adherence instrument is developed and also after the study is completed is recommended to increase reliability. The self-efficacy and self-management instruments were translated from English, and a pilot test before use might be needed to confirm the psychometric properties. This paper focused only on diabetic patients who underwent CABG, and the difference in health needs and self-management between diabetic and non-diabetic patients is not known.
Conclusions
The results confirm strong relationships between self-efficacy, adherence and self-management in post-CABG patients with diabetes in Taiwan. This finding should be valuable to clinicians, and related strategies should be able to be developed on its basis.
In addition, areas of weakness in self-efficacy and reasons for non-adherence were identified. Primary care providers can use this information to help patients to maintain a healthier lifestyle and to enhance adherence. In this study, eight patients lived alone, requiring hospital-based home services and locating of community resources to help them meet healthcare needs. Further, forgetting to take medicine was the most common reason reported for non-adherence. The majority of the participants took between four and 10 different kinds of pills. Thus we recommend that the primary physician consults with the multidisciplinary team to review the list of medications. In addition, involvement of family members or volunteers or the use of a mobile phone alarm may help patients to take their medicines at the proper times. The results of this study should provide healthcare professionals with a better understanding of the importance of enhancing self-efficacy and adherence and the use of patient demographics and disease characteristics to promote better self-management.
What is already known on this topic.
Self-efficacy in managing disease is the main contributor to better self-management revealed by previous studies in both Asia and North America.
What this study adds.
In this study we found self-efficacy to be a strong predictor of better self-management, with adherence having a significant mediating effect on the relationship between self-efficacy and self-management.
Acknowledgments
We would like to express our sincere appreciation to Kuan-Chia Lin for statistical consultation. We thank Kuan-Chia Lin for help with data management.
Footnotes
Contributors: HHT, RYL: conception and design of study. HHT, RYL, HCH: data analysis. WJ, JYL: setting inclusionary criteria and recruiting participants. All authors were involved in interpretation of data. HHT, DLC: drafting of the manuscript. All authors approved the final version to be published.
Funding: The study was supported by grants from the National Science Council in Taiwan. The study sponsors were not involved in the study design, the collection, analysis and interpretation of data, the writing of the article or the decision to submit it for publication. The authors were independent of the study sponsors.
Ethics approval: The study was approved by appropriate institutional review boards.
Patient consent: Obtained.
Competing interests: None.
References
- 1.World Health Organization. WHO statistical information system (WHOSIS). 2009. http://www.who.int/whosis/en/index.html (accessed 1 Jul 2010).
- 2.Department of Health, Executive Yuan. 2010 National health insurance annual mortality report. Retrieved 16 July 2011. http://www.doh.gov.tw/CHT2006/DM/DM2_2_p02.aspx?class_no=440&now_fod_list_no=10642&level_no=3&doc_no=73104
- 3.Department of Health, Executive Yuan. 2010 National health insurance annual medical utilization report: Major surgeries of outpatients and inpatients Retrieved 16 July 2011. http://www.doh.gov.tw/CHT2006/DM/DM2_2.aspx?now_fod_list_no=10071&class_no=440&level_no=3=3
- 4.Hurst RT, Lee RW. Increased incidence of coronary atherosclerosis in type 2 diabetes mellitus: mechanisms and management. Ann Intern Med 2003;139:824–34. [DOI] [PubMed] [Google Scholar]
- 5.DAI Study Group. The prevalence of coronary heart disease in type 2 diabetic patients in Italy: the DAI study. Diabet Med 2004;21:738–45. [DOI] [PubMed] [Google Scholar]
- 6.Button E, Keaton P. Glycemic control after coronary bypass graft: using intravenous insulin regulated by a computerized system. Crit Care Nurs Clin North Am 2006;18:257–65. [DOI] [PubMed] [Google Scholar]
- 7.Brandt M, Harder K, Walluscheck KP, et al. Coronary artery bypass surgery in diabetic patients. J Cardiovasc Surg 2004;19:36–40. [DOI] [PubMed] [Google Scholar]
- 8.Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA 2009;23:1501–8. [DOI] [PubMed] [Google Scholar]
- 9.Pell JP, Pell CH, Jeffery RR, et al. Comparison of survival following coronary artery bypass grafting vs. percutanous coronary intervention in diabetic and non-diabetic patients: retrospective cohort study of 6320 procedures. Diabet Med 2004;21:790–2. [DOI] [PubMed] [Google Scholar]
- 10.Woods S, Eppley C, Engel A. The influence of diabetes mellitus in patients undergoing coronary artery bypass grafting surgery: a prospective cohort study. Am Surg 2008;74:839–44. [PubMed] [Google Scholar]
- 11.Woods S, Smith M, Sohail S, et al. The influence of diabetes mellitus in patients undergoing coronary artery bypass grafting surgery: an 8-year prospective cohort study. Chest 2004;126:1789–95. [DOI] [PubMed] [Google Scholar]
- 12.Cramer JA, Benedict A, Muszbek N, et al. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract 2008;62:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamagishi SL, Nalamura K, Matsui T, et al. Role of postprandial hyperglycemia in cardiovascular disease in diabetes. Int J Clin Pract 2006;61:83–7. [DOI] [PubMed] [Google Scholar]
- 14.Merz CN, Buse JB, Tuncer D, et al. Physician attitudes and practices and patient awareness of the cardiovascular complications of diabetes. JACC 2002;40:1877–81. [DOI] [PubMed] [Google Scholar]
- 15.Costantini L. Compliance, adherence, and self-management: is a paradigm shift possible for chronic kidney disease clients? CANNT J 2006;16:22–6. [PubMed] [Google Scholar]
- 16.Berg J, Evangelista LS, Carruthers D, Dunbar-Jacob JM, et al. , eds. Chronic illness: impact and interventions. Sudbury, MA: Jones and Bartlett Publishers, 2006:221–52. [Google Scholar]
- 17.Chen MM. The barriers to take medications as prescribed of elderly patients with multiple chronic disease. Tainan: National Cheng Kung University, 2008. [Google Scholar]
- 18.Patel RP, Taylor SD. Factors affecting medication adherence in hypertensive patients. Ann Pharmacother 2002;36:40–5. [DOI] [PubMed] [Google Scholar]
- 19.Tai TY, Chuang LM, Tsai ST, et al. The Diabetic Mellitus (Taiwan) Study Group. Treatment of type 2 diabetic mellitus in primary care setting in Taiwan: comparison with secondary and tertiary care. J Formos Med Assoc 2006;105:105–12. [DOI] [PubMed] [Google Scholar]
- 20.Creer T, Christian W. Chronic diseases and handicapped children. Champaign, IL: Research Press, 1976. [Google Scholar]
- 21.Lorig KR, Holman H. Self-management education: history, definition outcomes, and mechanisms. Ann Behav Med 2003;26:1–7. [DOI] [PubMed] [Google Scholar]
- 22.Brownson CA, Hoerger TJ, Fisher EB, et al. Cost effectiveness of diabetics’ self-management programs in community primary care settings. Diabetes Educ 2009;35:761–9. [DOI] [PubMed] [Google Scholar]
- 23.Lorig K, Ritter P, Stewart AL, et al. Chronic disease self-management program: 2-year health status and health care utilization. Med Care 2001;39:1217–23. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher R, Donoghue J, Chenoweth L, et al. Self-management in older patients with chronic illness. Int J Nurs Pract 2008;14:373–82. [DOI] [PubMed] [Google Scholar]
- 25.Wade V, Jackson D, Daly J. Coronary heart disease in aboriginal communities: towards a model for self-management. Contemp Nurse 2003;15:300–9. [DOI] [PubMed] [Google Scholar]
- 26.Bandura A. Self-efficacy: insights. Harvard mental health letter. Soc Behav Personal 1997;13:4–8. [Google Scholar]
- 27.Edwatds R, Rclfair J, Cecil H, et al. Reliability and validity of a self-efficacy instrument specific to sickle cell disease. Behav Res Ther 2000;38:951–63. [DOI] [PubMed] [Google Scholar]
- 28.Bandura A. Self-efficacy in changing societies. New York: Cambridge University Press, 1995. [Google Scholar]
- 29.Zhong X, Tanasugarn C, Fisher EB, et al. Awareness and practices of self-management and influence factors among individuals with type 2 diabetes in urban community settings in anhui province, china. Southeast Asian J Trop Med Public Health 2011;42:185–6, 184, 187–96. [PubMed] [Google Scholar]
- 30.Atak N, Gurkan T, Kose K. The effect of education on knowledge, self-management behaviors and self-efficacy of patients with type 2 diabetes. AJAN 2007;26:66–74. [Google Scholar]
- 31.Sousa VD, Zauszniewski JA . Toward a theory of diabetes self-care management. JTCT 2005;9:61–7. [Google Scholar]
- 32.Orem DE. Nursing: concepts of practice. 5th edn St Louis, MO: Mosby, 1995. [Google Scholar]
- 33.Battersby M, Ask A, Reece M, et al. The partners in health scale: development and psychometric properties of a generic assessment scale for chronic condition self-management. AJPH 2003;9:41–52. [Google Scholar]
- 34.Battersby M, Harvey P, Mills PD, et al. SA health plus: a controlled trial of a statewide application of a generic model of chronic illness care. Milbank Q 2007;85:37–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petkov J, Harvey P, Battersby M. The internal consistency and construct validity of the partners in health scale: validation of a patient rated chronic condition self-management measure. Qual Life Res 2010;19:1079–85. [DOI] [PubMed] [Google Scholar]
- 36.Lorig K, Holman H, Sobel D, et al. Living a healthy life with chronic conditions: self-management of heart disease, diabetes, asthma, bronchitis, emphysema and others. Boulder, CO: Bull publishing company, 2000. [Google Scholar]
- 37.Pang MY, Eng JJ, Lin KH, et al. Association of depression and pain interference with disease-management self-efficacy in community-dwelling individuals with spinal cord injury. J Rehabil Med 2009;41:1068–73. [DOI] [PubMed] [Google Scholar]
- 38.Jiang CI. The effect of the family support on the compliance of patients with chronic illness. Taipei Medical University, 2003. [Google Scholar]
- 39.Tsai CL. The impact of patients’ adherence outcome and utilization under the shared care disease management program for the diabetes—an example from a regional teaching hospital. Asia University, 2005. [Google Scholar]
- 40.Chang WC. Effect of compliant behaviors on blood glucose control and quality of life in diabetic patients. Chiayi: Meiho University, 2007. [Google Scholar]
- 41.Topinkova E, Fialova D, Carpenter GI, et al. [Cross-national comparison of drug compliance and non-compliance associated factors in the elderly with polypharmacotherapy]. Cas Lek Cesk 2006;145:726–32. [PubMed] [Google Scholar]


