Abstract
Background
This study sought to investigate the prognostic value of the medial E (early transmitral flow velocity) to e′ (early diastolic mitral annulus velocity) ratio (E/e′) using the standard cutoff value of 15 among octogenarians stratified according to left ventricular ejection fraction (LVEF), atrial fibrillation (AF) and diabetes.
Methods
We examined a consecutive, single-centre cohort of 1197 subjects (male = 39.3%, female = 60.6%) between 80 and 89 years old (mean ± SD = 82.9 ± 2.81) who underwent transthoracic echocardiography from January 2009 to January 2011. E/e′ and LVEF were measured. These subjects were prospectively followed up for 29 months (mean ± SD = 12.8 ± 7.9). Primary endpoint was all-cause mortality.
Results
In univariate analysis, patients with underlying AF (AF vs no AF, p<0.001), diabetes (diabetes vs no diabetes, p<0.001), cancer (cancer vs no cancer, p<0.001), LVEF <45% (≥45% vs <45%, p<0.001) or an E/e′ ≥15 (≥15 vs <15, p<0.001) had a poorer prognosis. Gender had no significant effect on prognosis (p<0.08). In multivariate analysis, age, AF, diabetes, cancer, a LVEF <45% and E/e′ ≥15 were significant, independent predictors of a poor prognosis.
Conclusions
E/e′ is a predictor of mortality among octogenarians independently of LVEF, AF and diabetes.
Keywords: Imaging and Diagnostics
Introduction
Cardiovascular (CV) disease is the most common cause of morbidity and mortality among the elderly. The WHO projects that CV-related diseases will be responsible for 25 million deaths in 2030. CV disease accounts for 47% of all deaths annually in Europe.1 In Singapore, CV disease was responsible for 30.4% of all deaths in 2011.2 The looming issue of an increasingly ageing population furthers the threat of CV disease as a major health problem. There is vast evidence in the literature in the prognostic utility of echo-Doppler evaluation of diastolic function in a wide variety of patients.3 A normal filling pattern indicates an excellent prognosis, while an increased ratio of early mitral flow/early diastolic annulus velocity ratio (E/e′) indicates a poor prognosis in relation to increased mortality, increased risk of hospitalisation and congestive heart failure.4–8
Ageing is a common shared risk factor for atrial fibrillation (AF), diastolic dysfunction, hypertension, diabetes, and thus, have broad implications for CV health in the elderly with these underlying comorbidities.9–11 Moreover, patients with the diagnosis of diastolic dysfunction were more likely to have AF at the time of diagnosis12 and the prevalence of this arrhythmia increases significantly with age, reaching close to 1 in 10 in those over 80 years.13 In a multiethnic Asian study, AF has been shown to be a precipitant of acute heart failure admissions by 5.4% compared with hypertension (2.5%) and acute coronary syndromes (2.1%).14 Despite sharing ageing as a risk factor, there is a dearth of studies associating AF and diastolic dysfunction and the extent to which the available evidence could be generalised to an older Asian population is unknown. Thus, we explored the prognostic value of E/e′ using the standard cutoff of 15 in Singaporeans stratified according to left ventricular ejection fraction (LVEF), AF and diabetes.
Methods
Study population and data collection
We examined a consecutive, single-centre cohort of 1197 unselected octogenarians aged 80–89 (mean ± SD=82.9 ± 2.81 years) from Tan Tock Seng Hospital Singapore (men = 39.3%, female = 60.6%). Baseline characteristics of the study population are described in table 1. The recruitment period lasted for 2 years from January 2009 to January 2011. The study group included individuals who underwent clinically indicated transthoracic echocardiography as either inpatients or outpatients. Indications were computed based on the diagnosis on the echocardiographic request form. Patients with severe valvular regurgitation and stenosis, prosthetic valves, congenital heart disease, pacemaker or implantable cardioverter-defibrillator were excluded. AF was diagnosed based on past or present documentation on electrocardiography. Coronary artery disease (CAD) was defined as patient with history of CAD, myocardial infarction, positive cardiac stress imaging test or coronary revascularisation. Whereas diabetes was defined as fasting blood sugar ≥7.0 mmol/L, HbA1c≥6.5%, on oral hypoglycaemic medications, insulin or diet control, the hypertension category was based on drug history of antihypertensive or systolic blood pressure >140 mm Hg and diastolic blood pressure >90 mm Hg. Malignancy was diagnosed based on histological diagnosis, radiographic evidence of metastasis if histological evidence was unavailable or history of chemotherapy/radiotherapy. A complete echocardiography study was performed using standard views and techniques with a Vivid three scanner in second harmonic mode (GE Vingmed, Horten, Norway). Medial E/e′ and LVEF were measured based on the American Society of Echocardiography guidelines. Peak E was obtained in the apical four-chamber view, using pulsed-wave (PW) Doppler, with the sample volume placed at the mitral tips. For peak e′, PW tissue Doppler imaging was performed in the apical views and sample volume positioned at or 1 cm within the septal insertion site of mitral leaflets to cover the longitudinal excursion of the mitral annulus in both systole and diastole. A sweep speed of 100 mm/s at end-expiration was used in the assessment of peak E and e′. LVEF by echocardiography was determined using the Simpson's biplane method. Non-foreshortened apical two and four chamber views were used and manual tracing of LV end-systolic and end-diastolic frames were performed.15 The 1197 subjects were prospectively followed for 29 months (mean±SD=12.8±7.9). The primary endpoint was all-cause mortality. Longitudinal follow-up data were obtained by reviewing electronic medical records. Data were censored at the time of loss to follow-up or on the closing date of the study. Patients were divided into four groups (group 1: E/e′ <15 and LVEF ≥45%, group 2: E/e′ <15 and LVEF <45%, group 3: E/e′≥15 and LVEF ≥45% and group 4: E/e′≥15 and LVEF <45%). Survival estimates were stratified according to the LVEF groups, AF and diabetes. Subanalysis was performed for different quartiles of E/e′; this analysis was independent of the other covariates. Approval was obtained from the ethics committee under the National Health Group Domain Specific Review Board (NHG DSRB) in Singapore and complies with the Declaration of Helsinki.
Table 1.
Baseline characteristics of the study population (N=1197)
| Characteristics | Number | Mean ± SD or percentage |
|---|---|---|
| Number of patients | 1197 | – |
| Mean age (year) | 1197 | 82 ± 2.8 |
| Mean number of months of follow-up (months) | 1197 | 128 ± 7.9 |
| Gender (%) | ||
| Male | 471 | 39.4% |
| Female | 726 | 60.7% |
| Diagnoses (%) | ||
| Atrial fibrillation | 197 | 16.5% |
| Diabetes | 464 | 38.8% |
| Malignancy | 186 | 15.5% |
| Coronary artery disease | 389 | 32.5% |
| Hypertension | 583 | 48.7% |
| Indication of echocardiography (%) | ||
| Coronary artery disease | 200 | 16.7% |
| Post-myocardial infarction/coronary | 332 | 27.7% |
| Revascularization | ||
| Preoperative | 130 | 10.8% |
| Heart failure | 297 | 24.8% |
| Others | 238 | 19.8% |
| LVEF | ||
| <45% | 246 | 20.45% |
| >45% | 951 | 79.55% |
| E/e′ by LVEF groups | ||
| Group 1: E/e′ <15 and LVEF ≥45% | 571 | 47.7% |
| Group 2: E/e′ <15 and LVEF <45% | 205 | 17.1% |
| Group 3: E/e′ ≥15 and LVEF ≥45% | 380 | 31.8% |
| Group 4: E/e′ ≥15 and LVEF <45% | 41 | 3.4% |
| E/e′ by quartiles | ||
| 1st quartile: ≤13.2 | 303 | 25.3% |
| 2nd quartile: 13.2–17.2 | 296 | 24.7% |
| 3rd quartile: 17.2–23.7 | 300 | 25.1% |
| 4th quartile: ≥23.7 | 298 | 24.9% |
| Loss to follow-up | 35 | 2.9% |
| Events | 314 | 26.2% |
E/e′, early transmitral flow velocity (E) to early diastolic mitral annulus velocity (e′); LVEF, left ventricular ejection fraction.
Statistical analysis
Continuous data are expressed as the mean value±SD value and were compared using the independent Student's t test. Categorical data are presented as absolute values and percentages, and were compared using the χ2 test. Survival curves were plotted according to the Kaplan–Meier method, and survival times were compared using the log-rank test. Potential independent predictors of outcome were identified by univariate analyses using a Cox proportional hazards regression model. A multivariate Cox proportional hazards regression model was constructed using age, gender, AF, diabetes, malignancy and the groups as covariates. A separate multivariate model was built by adjusting for age, gender, AF, malignancy and LVEF. Stata V.10.1 (StataCorp LP, College Station, Texas, USA) and IBM SPSS V.19.0 (IBM Corp., Armonk, New York, USA) were used for all analyses.
Results
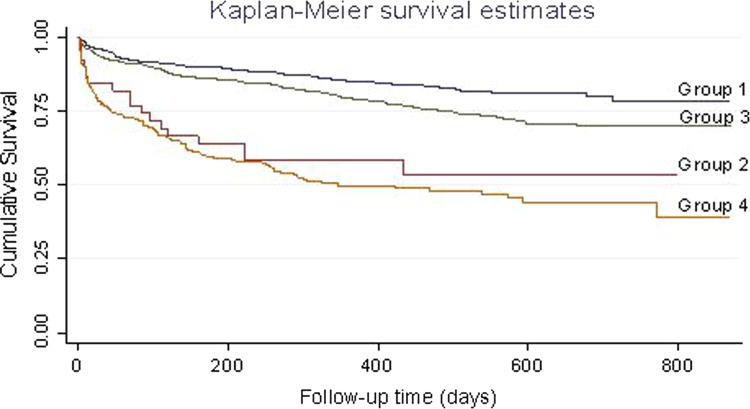
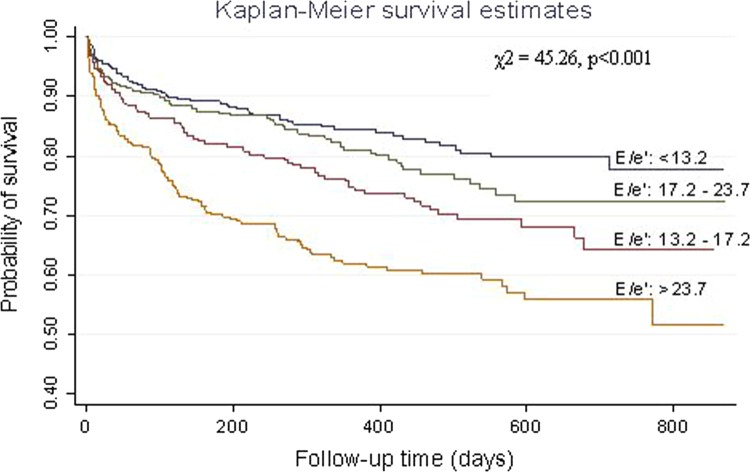
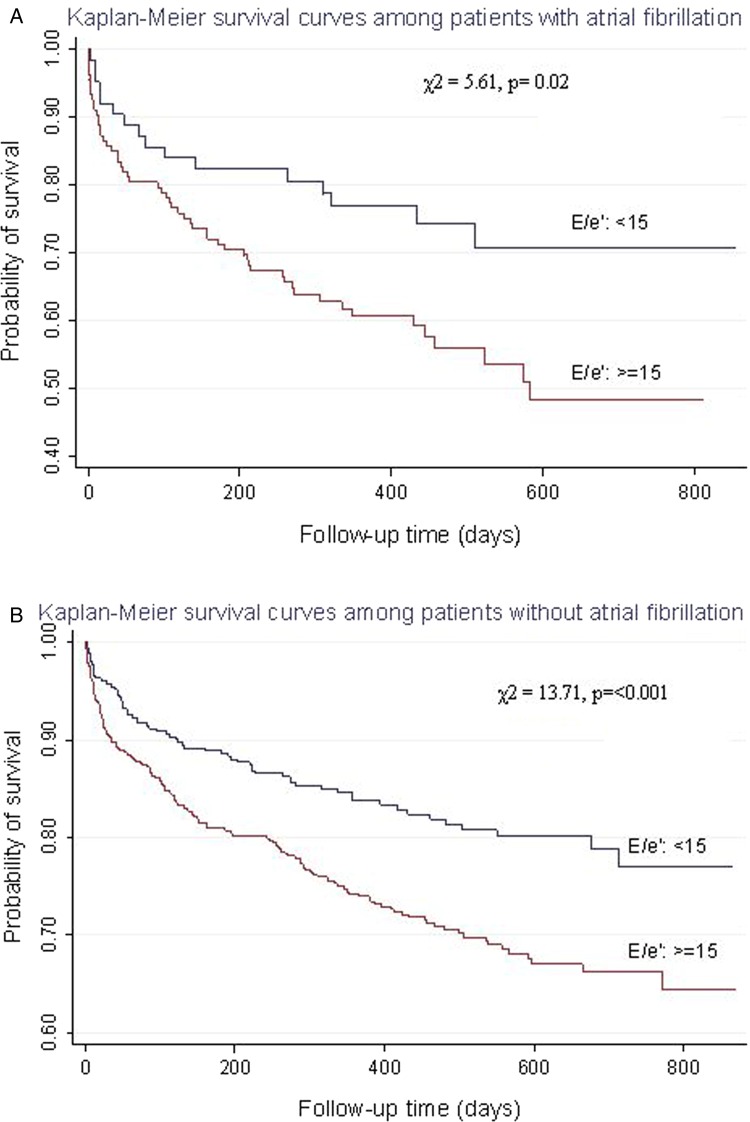
A total of 314/1197 (26.2%) subjects met the primary endpoint (all-cause mortality) and 35 (2.9%) were lost to follow-up. In the univariate analysis, a poorer prognosis was observed among patients with underlying AF (AF vs no AF, p<0.001), diabetes (diabetes vs no diabetes, p<0.001), cancer (cancer vs no cancer, p<0.001), a LVEF <45% (≥45% vs <45%, p<0.001) or an E/e′ ≥15 (≥15 vs <15, p<0.001). Gender had no significant effect on prognosis (p<0.08). When LVEF and E/e′ were analysed as a composite predictor, groups 2, 3 and 4 had a poorer prognosis than group 1 (E/e′ <15 and LVEF ≥45%), with group 4 (E/e′≥15 and LVEF <45%) showing the worst prognosis (HR=4.01; 95% CI 2.92 to 5.51; p<0.001) (figure 1). Group 3 (E/e′≥15 and LVEF ≥45%) had a HR of 1.46 (95% CI 1.07 to 1.98; p = 0.02), followed by Group 2 (E/e′ <15 and LVEF <45%) with a HR=3.16 (95% CI 1.84 to 5.42; p < 0.001). In multivariate analysis, age, AF, diabetes, cancer, LVEF <45% and an E/e′ ≥15 were significant independent predictors of poor prognosis. Analysis of E/e′ based on quartile values clearly established differences in mortality among subjects with an E/e′ <13.7 and >23.7 (p < 0.001; figure 2). Kaplan–Meier curves revealed that subjects with E/e′ ≥15 had a poorer prognosis compared with subjects with E/e′ values <15, even when stratified by either the presence or absence of diabetes mellitus (DM) (presence, p = 0.03; absence, p < 0.001) and the presence or absence of AF (presence, p = 0.02; absence, p < 0.001) (figure 3). Several reports have indicated that impairment of LV diastolic function can be present in subjects with diabetes even in the absence of alterations to LV systolic function.16
Figure 1.
Kaplan–Meier survival plots stratified according to E/e′ and left ventricular ejection fraction groups.
Figure 2.
Kaplan–Meier survival plots stratified according to different quartiles of E/e′ independent of left ventricular ejection fraction.
Figure 3.
Kaplan–Meier survival plots in patients with E/e′ <15 or ≥15 stratified according to atrial fibrillation (AF): (A) patients with AF, (B) patients without AF.
Discussion
This study indicates that E/e′ is a predictor of mortality among Singapore octogenarians independently of LVEF or the presence of AF or diabetes. We evaluated a population of individuals aged 80 and over, who presented either at the outpatient or inpatient clinics and underwent an indicated echocardiographic assessment at the discretion of the consulting physicians. Although E/e′ provides an instantaneous measure of LV filling pressures, it was an independent predictor of mortality after adjusting for age, gender, diabetes, malignancy and LVEF. In a previous study, subjects with left ventricular systolic dysfunction had similar CV and all-cause mortality rates compared with subjects with severe left ventricular diastolic dysfunction and the latter was an independent and incremental prognostic indicator of all-cause mortality.17 The strength of the study is twofold. First, it includes a very old Asian cohort that we come across in our day-to-day clinical practice. Moreover, this cohort is usually excluded in clinical trials in view of their multiple comorbidities. Second, Kaplan–Meier survival curves clearly demonstrate a survival difference among octogenarians with or without AF, despite the scarcity of data in the evaluation of diastolic dysfunction and AF. This favours the use of medial E/e′ as a parameter to further risk stratify patients with AF (HR = 0.58; 95% CI 0.44 to 0.76; p<0.001). In the presences of AF, a higher mitral E/e′ was predictive of the development of CV events independently of other CV comorbidities, thus mitral E/e′ appears to be clinically useful for risk stratification18 and this similarly concurred with our study in relation to mortality outcomes. A study conducted in Japan among 230 patients with a mean age of 72 (± 11 years) demonstrated E/e′ as a useful predictor of prognosis among patients with non-valvular AF.19 The association of diastolic dysfunction and AF is related to the increase in atrial pressure and volume overload resulting in atrial structural remodelling among patients with abnormal diastolic parameters.20 21 One of the largest study in this context is the Cardiovascular Health Study, which examined echo-Doppler derived diastolic parameters in 4480 older adults and reported that peak E-wave velocity and left atrial diameter were positively and nonlinearly associated, but E/e′ was not evaluated here.21 However, E-wave velocity alone in the setting of AF has its shortcomings as mitral inflow patterns are determined by loading conditions.20 Especially in the elderly, DM is often associated with arterial hypertension, which in turn is associated with diastolic dysfunction and unfavourable CV outcome even in the absence of alterations of LV systolic function.22–28 DM patients have been shown to have higher E/e′ compared with non-DM patients.29 In our study, among diabetic octogenarians, E/e′ >15 had worse outcomes compared with those without DM. Although our study population did not include a surgical cohort, the use of E/e′ among the elderly with regards to prognostication in patients with unoperated severe aortic stenosis showed survivors compared with non-survivors had a lower E/e′ ratio (12.19 ± 5.7 vs 16.87 ± 7.43, p<0.001) and a lower prevalence of E/e′ >15 (20% vs 55%, p<0.001). Among patients with LVEF ≥50%, the subgroup with E/e′ ≤15 and E/e′ >15 had 1 year survival rates of 73.8% and 47.8%, respectively (p = 0.027). Whereas in the patients with an LVEF <50%, those with E/e′ ≤15 and with E/e′ >15 demonstrated 70.6% and 22.3% 1 year survival, respectively (p=0.003).30
This study has a number of limitations. First, we did not distinguish between patients with symptomatic or asymptomatic heart failure. Second, the Doppler-derived parameters were not standardised between sonographers, and hence, may have provided a source of inconsistency during data collection. Third, medial E/e′ was computed as a single diastolic parameter, and was not correlated with other known diastolic indices such as, mitral valve deceleration time, pulmonary venous flow velocities and left atrial dimensions; this analysis could provide additional value in the prediction of mortality in this subpopulation of patients and warrants further study. Fourth, the primary endpoint failed to examine other clinical endpoints such as the time to first heart failure-related hospitalisation, readmission rates for heart failure and non-fatal myocardial infarction. Assessment of these clinical endpoints would be relevant for clinical practice. Fifth, correlation with other established cardiac biomarkers, such as brain natriuretic peptide may have been worthwhile. Finally, the presence of regional wall motion abnormalities was not taken into consideration, which may result in inaccuracy during computation of the E/e′ value, especially among patients with a LVEF <45%. In this study, Kaplan–Meier analysis for different quartile values of E/e′ revealed a significant difference in the mortality outcome between the highest and lowest ranges; however, our research did not venture into obtaining an explanation of why E/e′ of 13.2–17.2 had a worse outcome than E/e′ of 17.2–23.7. This observation could be scrutinised as part of another research study.
The current study revealed that E/e′ is a powerful, significant predictor of mortality among Singapore octogenarians, independently of LVEF, AF and diabetes. Despite the aforementioned limitations, this study has shed light on the prognostic utility of assessing E/e′ among octogenarians in daily clinical practice.
Acknowledgments
The authors wish to thank Mr Wilson Low, Clinical Research Unit for the statistical analysis and interpretation and Ms Kavitha Gunashekar, Clinical Research Unit, Tan Tock Seng Hospital for proof-reading the manuscript.
Footnotes
Competing interests: None.
Ethics approval: National Health Group Domain Specific Review Board, Singapore.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.European cardiovascular disease Statistics 2012. Brussels: European Heart Network, 2012. http://www.Ehnheart.Org/Cvd-Statistics.Html (19 Feb 2013). [Google Scholar]
- 2.Deaths From Cardiovascular Disease. Singapore: Singapore Heart Foundation, 2011. http://www.myheart.org.sg/article/about-the-heart-and-heart-disease/statistics/Singapore/75 (19th Feb 2013). [Google Scholar]
- 3.Little WC, Ohara T. Left atrial emptying reserve a mirror of LV diastolic function that predicts prognosis. JACC Cardiovasc Imaging 2011;4:389–91. [DOI] [PubMed] [Google Scholar]
- 4.Cerisano G, Bolognese L, Carrabba N, et al. Doppler-derived mitral deceleration time: an early strong predictor of left ventricular remodeling after reperfused anterior acute myocardial infarction. Circulation 1999;99:230–6. [DOI] [PubMed] [Google Scholar]
- 5.Moller JE, Sondergaard E, Poulsen SH, et al. Pseudonormal and restrictive filling patterns predict left ventricular dilation and cardiac death after a first myocardial infarction: a serial color M-mode Doppler echocardiographic study. J Am Coll Cardiol 2000;36:1841–6. [DOI] [PubMed] [Google Scholar]
- 6.Moller JE, Sondergaard E, Seward JB, et al. Ratio of left ventricular peak E-wave velocity to flow propagation velocity assessed by color M-mode Doppler echocardiography in first myocardial infarction: prognostic and clinical implications. J Am Coll Cardiol 2000;35:363–70. [DOI] [PubMed] [Google Scholar]
- 7.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165–93. [DOI] [PubMed] [Google Scholar]
- 8.Nijland F, Kamp O, Karreman AJ, et al. Prognostic implications of restrictive left ventricular filling in acute myocardial infarction: a serial Doppler echocardiographic study. J Am Coll Cardiol 1997;30:1618–24. [DOI] [PubMed] [Google Scholar]
- 9.Redfield MM, Jacobsen SJ, Burnett JC, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of heart failure epidemic. JAMA 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 10.Cahill JM, Horan M, Quigley P, et al. Doppler-echocardiographic indices of diastolic dysfunction in heart failure admissions with preserved left ventricular systolic function. Eur J Heart Fail 2002;4:473–8. [DOI] [PubMed] [Google Scholar]
- 11.Stevens SM, Farzaneh-Far R, Na B, et al. Development of an echocardiographic risk-stratification index to predict heart failure in patients with stable coronary artery disease: the Heart and Soul study. JACC Cardiovasc Imaging 2009;2:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michael AR, John SG, Susan RH, et al. Echocardiographic diastolic parameters and risk of atrial fibrillation: the Cardiovascular Health Study. Eur J Heart Fail 2012;33:904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christopher JM, Bernard JG. A practical approach to the management of patients with atrial fibrillation. Heart Asia 2010;2:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kui Toh G, Cao Yan, Ping Ping G. Precipitant in acute heart failure in a multiethnic Asian urban cohort study. Heart Asia 2011;3:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherif FN, Christopher PA, Thierry CG, et al. ASE recommendations of the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165–93. [DOI] [PubMed] [Google Scholar]
- 16.Russo C, Jin Z, Homma S, et al. Effect of diabetes and hypertension on left ventricular diastolic function in a high-risk population without evidence of heart disease. Eur J Heart Fail 2010;12:454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Safar ME, Iaria P, et al. Prevalence and prognosis of left ventricular diastolic dysfunction in the elderly: the PROTEGER study. Am Heart J 2010;160:471–8. [DOI] [PubMed] [Google Scholar]
- 18.Tsujimoto S, Miyasaka Y, Suwa Y, et al. Mitral E/e’ ratio as an independent predictor for cardiovascular events in patients with atrial fibrillation. J Am Coll Cardiol 2012;59(13s1):E1123. [Google Scholar]
- 19.Okura H, Takada Y, Kubo T, et al. Tissue Dopper-derived index of left ventricular filling pressure, E/e’, predicts survival of patients with non-valvular atrial fibrillation. Heart 2006;92:1248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michiel R, David DM, Emelia JB. Novel risk factors for atrial fibrillation. Circulation 2012;125:e941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg MA, Gottdiener JS, Heckbert SR, et al. Echocardiographic diastolic parameters and risk of atrial fibrillation: the Cardiovascular Health Study. Eur Heart J 2012;33:904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Celentano A, Vaccaro O, Tammaro P, et al. Early abnormalities of cardiac function in non-insulin-dependent diabetes mellitus and impaired glucose tolerance. Am J Cardiol 1995;76:1173–6. [DOI] [PubMed] [Google Scholar]
- 23.Zabalgoita M, Ismaeil MF, Anderson L, et al. Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled diabetes mellitus. Am J Cardiol 2001;87:320–3. [DOI] [PubMed] [Google Scholar]
- 24.Boyer JK, Thanigaraj S, Schechtman KB, et al. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol 2004;93:870–5. [DOI] [PubMed] [Google Scholar]
- 25.Fang ZY, Schull-Meade R, Leano R, et al. Screening for heart disease in diabetic subjects. Am Heart J 2005;149:349–54. [DOI] [PubMed] [Google Scholar]
- 26.Levy D, Larson MG, Vasan RS, et al. The progression from hypertension to congestive heart failure. J Am Med Assoc 1996;275:1557–62. [PubMed] [Google Scholar]
- 27.Fouad FM, Slominski JM, Tarazi RC. Left ventricular diastolic function in hypertension: relation to left ventricular mass and systolic function. J Am Coll Cardiol 1984;3:1500–6. [DOI] [PubMed] [Google Scholar]
- 28.Inouye I, Massie B, Loge D, et al. Abnormal left ventricular filling: an early finding in mild to moderate systemic hypertension. Am J Cardiol 1984;53:120–6. [DOI] [PubMed] [Google Scholar]
- 29.Cesare R, Zhezhen J, Shunichi H, et al. Effect of diabetes and hypertension on left ventricular diastolic function in a high-risk population without evidence of heart disease. European J Heart Fail 2010;12:454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biner S, Rafique AM, Goykhman P, et al. Prognostic value of E/E’ ratio in patients with unoperated severe aortic stenosis. JACC Cardiovasc Imaging 2010;3:899–907. [DOI] [PubMed] [Google Scholar]