Abstract
Arynes participate in three‐component coupling reactions with N, S, P, and Se functionalities to yield 1,2‐heteroatom‐difunctionalized arenes. Using 2‐iodophenyl arylsulfonates as benzyne precursors, we could effectively add magnesiated S‐, Se‐, and N‐nucleophilic components to the strained triple bond. In the same pot, addition of electrophilic N, S, or P reagents and a copper(I) catalyst trapped the intermediate aryl Grignard to produce a variety of 1,2‐difunctionalized arenes.
Keywords: arynes, benzyne, Grignard reaction, heterocycles, multicomponent reactions
1,2‐Heteroatom‐functionalized arenes represent privileged structures in pharmaceuticals and catalysis (Scheme 1).1 Covering a vast area of chemical structure and function, their synthesis usually entails multistep sequences with attendant multiple purification steps. In the 2‐functionalized aniline series, for example, an ortho‐halo‐nitrobenzene is commonly used for initial SNAr C—X bond formation, followed by nitro reduction and a second C—N bond forming reaction at the amine functional group.2 More recently, sequential metal‐catalyzed C—N and C—S bond formations have been developed on suitably differentiated haloarene precursors.3 Our interest in benzyne chemistry4 led us to speculate whether this compound class could be made in a single operation through a three component coupling of two heteroatom moieties and an aryne.5
Scheme 1.
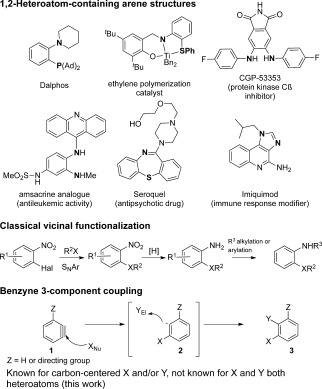
1,2‐Heteroatom‐functionalized arenes and proposed benzyne three‐component coupling approach.
Nucleophilic addition to benzyne to form a reactive aryl anion intermediate 2, followed by trapping with an electrophilic component, is a fundamental reaction mode in benzyne chemistry that has seen extensive application in synthesis.6, 7 Perhaps surprisingly, the three‐component coupling of benzyne and two heteroatom moieties has yet to be described as a general method.8, 9 The development of this reaction would yield valuable heteroatom‐functionalized arenes in a single step, with broad application in synthesis.
We chose to study tandem S‐ and N‐addition to benzyne in the first instance. Important precedent from Knochel and co‐workers had shown that magnesium thiolates undergo efficient addition to benzynes generated from 2‐iodophenyl arylsulfonates 4 with iPrMgCl, with the resulting adducts being trapped with simple carbon electrophiles in good yield.10 Using this chemistry for the initial nucleophilic addition, we then planned to try O‐benzoyl N,N‐dialkylhydroxylamines as the nitrogen source. These electrophilic aminating agents are simple to prepare as stable, crystalline solids, and have recently been shown to have excellent versatility in C—N bond formation.11
Treatment of 2‐iodophenyl‐sulfonate 4 with two equivalents of iPrMgCl in the presence of 4‐tert‐butyl‐benzenethiolate 5 at −78 °C in THF, followed by warming of the mixture to 0 °C, afforded the expected 2‐magnesiated benzothioether 6 (Scheme 2). Pleasingly, dropwise addition of this intermediate Grignard to a mixture containing one equivalent of O‐benzoyl N‐hydroxylpiperidine and catalytic CuCl2 (5 mol %) in dry THF, yielded the desired 2‐(2‐piperidine phenyl)benzenethioether 7 a in 35 % yield.
Scheme 2.
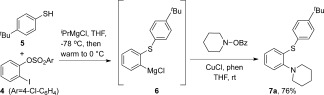
Tandem S,N‐functionalization of benzyne. phen=phenanthroline.
An optimization study (see the Supporting Information, SI) focusing on reaction stoichiometry and catalyst choice improved the yield to 76 %, using a CuCl catalyst (10 mol %) and phenanthroline ligand (10 mol %) system. Catalyst loading was a key parameter, as larger amounts of CuCl led to copious homocoupling of the intermediate Grignard 6. No product 7 a was detected in the absence of copper, as C‐arylation of the O‐benzoylhydroxylamine reagent is preferred to yield the ketone.12 We were pleased to find that the reaction was general for a range of aromatic, pyridine, and thiophene thiols, with electron‐donating (OMe, tBu) and electron‐withdrawing (F, Br) substituents being well accommodated (7 a–i, 51–76 % yield, Figure 1). Phenyl selenide was also productive in the coupling, affording the expected ortho‐seleno‐aminated product 7 j in 59 % yield.
Figure 1.
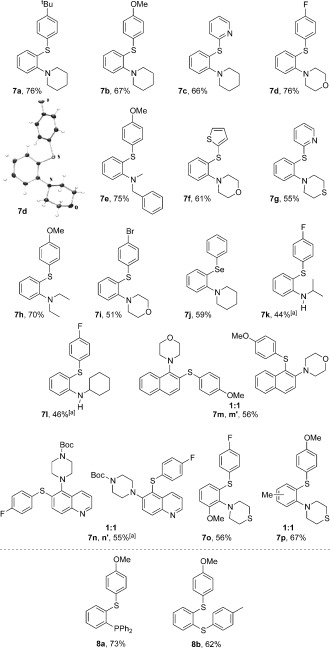
Scope of the (2‐aminophenyl)benzothioether substrate. Reaction conditions: thiol (1 mmol), iPrMgCl (2.2 mmol), and aryne precursor (1.2 mmol) were stirred at −78 °C for 45 min, then warmed to 0 °C. The resulting intermediate Grignard was quenched with 1.5 mol of R1R2N—OBz in the presence of CuCl (10 mol %) and phenanthroline (10 mol %; see SI). [a] An additional zincation step was performed by adding ZnCl2 (0.5 equiv) after the initial thiolate addition. The thermal ellipsoids of the X‐ray structure of 7 d were set at 50 %.19
In terms of the electrophilic amine moiety, the reaction worked well for introducing cyclic (morpholine, thiomorpholine, and piperidine) and acyclic secondary amines (N,N‐diethyl and N,N‐methyl benzyl amines). Primary O‐benzoyl hydroxylamines, however, worked poorly in the reaction. To address this shortcoming, we experimented with the transmetalation of intermediate 6 with zinc; pioneering work from Johnson has shown that arylzinc compounds can react with primary O‐benzoyl hydroxylamines under copper catalysis to give secondary anilines.13 We were pleased to find that the addition of ZnCl2 (0.5 equiv) to the reaction mixture was successful, enabling iPrNHOBz and CyNHOBz to be used as electrophiles in the reaction (7 k, 7 l) in 44 and 46 % yield, respectively.
Substituted arynes could be successfully employed, with 3‐methoxybenzyne reacting smoothly to give the expected 1,2,3‐O,N,S‐functionalized product 7 o as a single regioisomer.14 Using 1,2‐naphthyne as the starting material, by contrast, gave the thioaminated products 7 m and 7 m′ in 56 % yield, but as a separable mixture of regioisomers (1:1). Addition to 1,2‐naphthyne often favors the 2‐position (e.g., for neutral nitrogen nucleophiles),4d,e,h but selectivity can vary according to the nature of the nucleophile.15 Here, the strong thiolate nucleophile shows little discrimination (cf. magnesium amide addition). We were pleased to extend the reaction to 5,6‐quinolyne, a hetaryne that has scarcely been exploited in the literature.16 The three‐component coupling afforded the piperazinyl‐mercaptoquinolines (7 n, 7 n′) in 55 % yield (using ZnCl2 as an additive for the amination step), again giving a 1:1 mixture of regioisomers.17 Starting material 4‐methylbenzyne afforded a 66 % yield of 7 p in the expected 1:1 mixture of isomers, exhibiting the regiodivergence typical of additions to meta‐substituted benzynes. Finally, we demonstrated that electrophilic P and S sources were effective in the three‐component coupling, synthesizing the S,P adduct 8 a using CuCl (2 mol %) and ClPPh2, and the mixed S,S adduct 8 b through quenching with tolyldisulfide in the presence of CuCl (10 mol %).
Having established a working thio‐ and seleno‐amination system, we turned our attention to benzyne double amination. Access to this motif through stepwise metal‐catalyzed C—N bond forming chemistry is underdeveloped, making a prospective aryne three‐component coupling route particularly interesting.18 Using magnesiated secondary anilines as the initial nucleophiles, we were pleased to find that our established three‐component coupling conditions translated well to diamine synthesis (Scheme 3). Although yields were slightly lower than the thio system, we could successfully access a variety of 1,2‐diamino benzenes (10 a–l) in 30–73 % yield. It is likely that the initial aniline addition adduct presents additional steric hindrance to the second amination step, relative to the thio analogues, leading to some attenuation in yields. Zinc transmetalation was beneficial for the cyclic morpholine, piperidine, and piperazine products 10 g–i, plus the primary isopropylamine adduct 10 j. The effect was not general, however, with acyclic secondary amines 10 b,c,f reacting markedly worse under zincation conditions. The use of 1,2‐naphthyne in the diamination contrasted with the thioamination, giving a single regioisomeric product 10 k. The less reactive magnesium anilide is evidently able to discriminate between the differences in steric demand at either aryne position, adding to the more accessible aryne 2‐position. Starting with 3‐methoxy benzyne gave the O,N,N product 10 l as a single regioisomer, albeit in low yield. As with the thiolate addition system, we could also use phosphorus and sulfur electrophiles to access the N,P and N,S products 11 a and 11 b, respectively. The polarity reversal in synthesizing 11 b is complimentary to thiolate addition and trapping with R1R2NOBz reagents, as the O‐benzoyl hydroxylaniline reagents are less readily synthesized.20
Scheme 3.
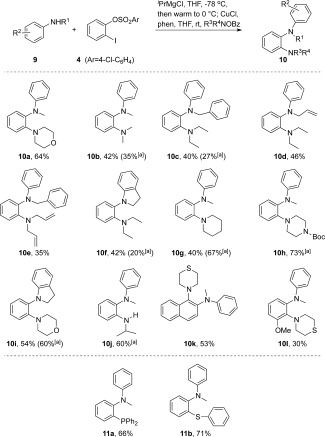
Synthesis of 2‐aminoaniline. Reaction conditions: amine (1 mmol), iPrMgCl (2.2 mmol), and aryne precursor (1.2 mmol) were stirred at −78 °C for 45 min, then warmed to 0 °C. The resulting intermediate Grignard was quenched with R1R2N—OBz (1.5 mol) in the presence of CuCl (10 mol %) and phenanthroline (10 mol %; see SI). [a] An additional zincation step was performed by adding ZnCl2 (0.5 equiv) after the initial anilide addition. Bz=benzoyl, Boc=tert‐butyloxycarbonyl.
We applied our three component coupling protocol to the synthesis of vortioxetine, 13, an antidepressant drug that has recently received FDA and EMA approval (sold as brintellix; Scheme 4). The molecule had previously been prepared through double nucleophilic substitution of 1,2‐dichlorobenzene, mediated by ferrocene, in 17 % overall yield.21 Following our optimized procedure, 2,4‐dimethylthiophenol reacted with benzyne precursor 4 to generate an intermediate Grignard 11. Transmetalation with ZnCl2 enabled efficient copper‐catalyzed amination with the N‐Boc protected O‐benzoyl hydroxylamine derived from piperazine, affording a 78 % yield of 12 in a single manipulation. Simple TFA treatment removed the Boc group, affording the desired pharmaceutical 13 in 73 % overall yield in two steps from 4, without recourse to noble‐metal catalysis.
Scheme 4.
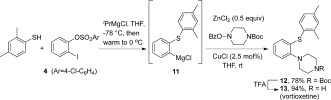
Vortioxetine synthesis. TFA=trifluoroacetic acid.
In conclusion, we have developed the aryne three‐component coupling reaction to encompass double heteroatom substitution, showcasing a new, one‐pot approach to S,N‐, N,N‐, Se,N‐, S,P‐, and N,P‐functionalized arenes that avoids the isolation of intermediates. The method encompasses a variety of thiols, arynes (including hetarynes), and amines, and uses inexpensive metal reagents and catalysts.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
miscellaneous_information
We thank TÜBİTAK‐BİDEB (scholarship to M.Ç.), Fundación Séneca (CARM, Spain) (Fellowship to J.‐A. G.‐L.) and the EPSRC (Leadership Fellowship to M.F.G.) for funding. Dr. James Raftery is thanked for X‐ray crystallography.
References
- 1.Synthesis of representative structures in Scheme 1:
- 1a. Lundgren R. J., Peters B. D., Alsabeh P. G., Stradiotto M., Angew. Chem. Int. Ed. 2010, 49, 4071–4074; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 4165–4168; [Google Scholar]
- 1b. Oakes D. C. H., Kimberley B. S., Gibson V. C., Jones D. J., White A. J. P., Williams D. J., Chem. Commun. 2004, 2174–2175; [DOI] [PubMed] [Google Scholar]
- 1c. Hennessy E. J., Buchwald S. L., J. Org. Chem. 2005, 70, 7371–7375; [DOI] [PubMed] [Google Scholar]
- 1d. Atwell G. J., Baguley B. C., Finlay G. J., Rewcastle G. W., Denny W. A., J. Med. Chem. 1986, 29, 1769–1776; [DOI] [PubMed] [Google Scholar]
- 1e. Warawa E. J., Migler B. M., U.S. Patent 4,879,288, 1988;
- 1f. Gertser J. F., et al., J. Med. Chem. 2005, 48, 3481–3491. [DOI] [PubMed] [Google Scholar]
- 2. Terrier F., Modern Nucleophilic Aromatic Substitution, Wiley‐VCH, Weinheim, 2013, ISBN 978–3527318612. [Google Scholar]
- 3.
- 3a. Tsvelikhovsky D., Buchwald S. L., J. Am. Chem. Soc. 2011, 133, 14228–14231; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3b. Dahl T., Tornøe C. W., Bang‐Andersen B., Nielsen P., Jørgensen M., Angew. Chem. Int. Ed. 2008, 47, 1726–1728; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 1750–1752. [Google Scholar]
- 4.
- 4a. Michel B., Greaney M. F., Org. Lett. 2014, 16, 2684–2684; [DOI] [PubMed] [Google Scholar]
- 4b. García‐López J.‐A., Greaney M. F., Org. Lett. 2014, 16, 2338–2341; [DOI] [PubMed] [Google Scholar]
- 4c. Hall C., Henderson J. L., Ernouf G., Greaney M. F., Chem. Commun. 2013, 49, 7602–7604; [DOI] [PubMed] [Google Scholar]
- 4d. Pirali T., Zhang F., Miller A. H., Head J. L., McAusland D., Greaney M. F., Angew. Chem. Int. Ed. 2012, 51, 1006–1009; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 1030–1033; [Google Scholar]
- 4e. McAusland D., Seo S., Pintori D. G., Finlayson J., Greaney M. F., Org. Lett. 2011, 13, 3667–3669; [DOI] [PubMed] [Google Scholar]
- 4f. Biswas K., Greaney M. F., Org. Lett. 2011, 13, 4946–4949; [DOI] [PubMed] [Google Scholar]
- 4g. Cant A. A., Roberts L., Greaney M. F., Chem. Commun. 2010, 46, 8671–8673; [DOI] [PubMed] [Google Scholar]
- 4h. Cant A. A., Bertrand G. H. V., Henderson J. L., Roberts L., Greaney M. F., Angew. Chem. Int. Ed. 2009, 48, 5199–5202; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 5301–5304. [Google Scholar]
- 5.Recent reviews on aryne chemistry:
- 5a. Tadross P. M., Stoltz B. M., Chem. Rev. 2012, 112, 3550–3577; [DOI] [PubMed] [Google Scholar]
- 5b. Gampe C. M., Carreira E. M., Angew. Chem. Int. Ed. 2012, 51, 3766–3778; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 3829–3842; [Google Scholar]
- 5c. Dubrovskiy A. V., Markina N. A., Larock R. C., Org. Biomol. Chem. 2013, 11, 191–218; [DOI] [PubMed] [Google Scholar]
- 5d. Hoffmann R. W., Suzuki K., Angew. Chem. Int. Ed. 2013, 52, 2655–2656; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 2717–2718; [Google Scholar]
- 5e. Holden (née Hall) C., Greaney M. F., Angew. Chem. Int. Ed. 2014, 53, 5746–5749; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 5854–5857; [Google Scholar]
- 5f. Bhunia A., Biju A. T., Synlett 2014, 25, 608–614; [Google Scholar]
- 5g. Goetz E., Shah T. K., Garg N. K., Chem. Commun. 2015, 51, 34–45. [DOI] [PubMed] [Google Scholar]
- 6.Seminal work: Huisgen R., Rist H., Justus Liebigs Ann. Chem. 1955, 594, 137. [Google Scholar]
- 7.Selected examples:
- 7a. Pansegrau P. D., Rieker W. F., Meyers A. I., J. Am. Chem. Soc. 1988, 110, 7178–7184; [Google Scholar]
- 7b. Tomori H., Fox J. M., Buchwald S. L., J. Org. Chem. 2000, 65, 5334–5341; [DOI] [PubMed] [Google Scholar]
- 7c. Yoshida H., Fukushima H., Ohshita J., Kunai A., Angew. Chem. Int. Ed. 2004, 43, 3935–3938; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2004, 116, 4025–4028; [Google Scholar]
- 7d. Jayanth T. T., Jeganmohan M., Cheng C.‐H., Org. Lett. 2005, 7, 2921–2924; [DOI] [PubMed] [Google Scholar]
- 7e. Larrosa I., Da Silva M. I., Gómez P. M., Hannen P., Ko E., Lenger S. R., Linke S. R., White A. J. P., Wilton D., Barrett A. G. M., J. Am. Chem. Soc. 2006, 128, 14042–14043; [DOI] [PubMed] [Google Scholar]
- 7f. Henderson J. L., Edwards A. S., Greaney M. F., J. Am. Chem. Soc. 2006, 128, 7426–7427; [DOI] [PubMed] [Google Scholar]
- 7g. Berti F., Crotti P., Cassano G., Pineschi M., Synlett 2012, 2463–2468; [Google Scholar]
- 7h. Truong T., Mesgar M., Le K. K. A., Daugulis O., J. Am. Chem. Soc. 2014, 136, 8568–8576; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7i. Nagaki A., Ichinari D., Yoshida J.‐i., J. Am. Chem. Soc. 2014, 136, 12245–12248. [DOI] [PubMed] [Google Scholar]
- 8.The two‐component coupling of benzyne and X—Y components through σ‐bond insertion is well described. Reviews:
- 8a. Peña D., Pèrez D., Guitián E., Angew. Chem. Int. Ed. 2006, 45, 3579–3581; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2006, 118, 3659–3661; [Google Scholar]
- 8b. Yoshida H., Takaki K., Synlett 2012, 1725–1732; recent examples: [Google Scholar]
- 8c. Alajarin M., Lopez‐Leonardo C., Raja R., Orenes R.‐A., Org. Lett. 2011, 13, 5668–5671; [DOI] [PubMed] [Google Scholar]
- 8d. Hendrick C. E., McDonald S. L., Wang Q., Org. Lett. 2013, 15, 3444–3447; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8e. Hwu J. R., Hsu Y. C., Chem. Eur. J. 2011, 17, 4727–4731; [DOI] [PubMed] [Google Scholar]
- 8f. Chen J., Murafuji T., Tsunashima R., Organometallics 2011, 30, 4532–4538; [Google Scholar]
- 8g. Shen C., Yang G., Zhang W., Org. Lett. 2013, 15, 5722–5725; [DOI] [PubMed] [Google Scholar]
- 8h. Rodríguez‐Lojo D., Cobas A., Peña D., Pérez D., Guitián E., Org. Lett. 2012, 14, 1363–1365; [DOI] [PubMed] [Google Scholar]
- 8i. Yoshida H., Kawashima S., Takemoto Y., Okada K., Ohshita J., Takaki K., Angew. Chem. Int. Ed. 2012, 51, 235–238; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 239–242; [Google Scholar]
- 8j. Yoshida H., Yoshida R., Takaki K., Angew. Chem. Int. Ed. 2013, 52, 8629–8632; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 8791–8794. [Google Scholar]
- 9.For heteroatom addition to benzyne followed by trapping with molecular iodine or oxygen, see:
- 9a. He Z., Jamison T. F., Angew. Chem. Int. Ed. 2014, 53, 3353–3357; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 3421–3425; [Google Scholar]
- 9b. Nagashima Y., Takita R., Yoshida K., Hirano K., Uchiyama M., J. Am. Chem. Soc. 2013, 135, 18730–18733. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Lin W., Sapountzis I., Knochel P., Angew. Chem. Int. Ed. 2005, 44, 4258–4261; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2005, 117, 4330–4333; [Google Scholar]
- 10b. Sapountzis I., Lin W., Fischer M., Knochel P., Angew. Chem. Int. Ed. 2004, 43, 4364–4366; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2004, 116, 4464–4466; [Google Scholar]
- 10c. Lin W., Ilgen F., Knochel P., Tetrahedron Lett. 2006, 47, 1941–1944; [Google Scholar]
- 10d. Lin W., Chen L., Knochel P., Tetrahedron 2007, 63, 2787–2797. [Google Scholar]
- 11.Reviews:
- 11a. Yan X., Yang X., Xi C., Catal. Sci. Technol. 2014, 4, 4169–4177; [Google Scholar]
- 11b. Louillat M.‐L., Patureau F. W., Chem. Soc. Rev. 2014, 43, 901–910. [DOI] [PubMed] [Google Scholar]
- 12. Campbell M. J., Johnson J. S., Org. Lett. 2007, 9, 1521–1524. [DOI] [PubMed] [Google Scholar]
- 13. Berman A. M., Johnson J. S., J. Am. Chem. Soc. 2004, 126, 5680–5681. [DOI] [PubMed] [Google Scholar]
- 14.For a recent discussion on aryne selectivity, see: Medina J. M., Mackey J. L., Garg N. K., Houk K. N., J. Am. Chem. Soc. 2014, 136, 15798–15805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.For examples of nonselective addition to 1,2‐naphthyne, see:
- 15a. Rao B., Zeng X., Org. Lett. 2014, 16, 314–317; [DOI] [PubMed] [Google Scholar]
- 15b. Jin T., Yamamoto Y., Angew. Chem. Int. Ed. 2007, 46, 3323–3325; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 3387–3389; [Google Scholar]
- 15c. Biju A. T., Glorius F., Angew. Chem. Int. Ed. 2010, 49, 9761–9764; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 9955–9958; [Google Scholar]
- 15d. Rémond E., Tessier A., Leroux F. R., Bayardon J., Juge S., Org. Lett. 2010, 12, 1568–1571; [DOI] [PubMed] [Google Scholar]
- 15e. Łaczkowski K. Z., García D., Peña D., Cobas A., Pérez D., Guitián E., Org. Lett. 2011, 13, 960–963; [DOI] [PubMed] [Google Scholar]
- 15f. Yoshioka E., Miyabe H., Tetrahedron 2012, 68, 179–189; [Google Scholar]
- 15g. Lakshmi B. V., Wefelscheid U. K., Kazmaier U., Synlett 2011, 345–348. [Google Scholar]
- 16.For quinolynes in synthesis, see:
- 16a. Kauffmann T., Boettcher F.‐P., Hansen J., Justus Liebigs Ann. Chem. 1962, 659, 102–109; [Google Scholar]
- 16b. Grig‐Alexa I.‐C., Garnier E., Finaru A.‐L., Ivan L., Jarry C., Léger J.‐M., Caubère P., Guillaumet G., Synlett 2004, 2000–2004; [Google Scholar]
- 16c. Collis G. E., Burrell A. K., Tetrahedron Lett. 2005, 46, 3653–3656; [Google Scholar]
- 16d. Saito N., Nakamura K., Shibano S., Ide S., Minami M., Sato Y., Org. Lett. 2013, 15, 386–389; [DOI] [PubMed] [Google Scholar]
- 16e. Fang Y., Larock R. C., Tetrahedron 2012, 68, 2819–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aryne distortion in 5,6‐quinolyne is known to be low, correlating with low regioselectivities in nucleophilic addition: Goetz A. E., Bronner S. M., Cisneros J. D., Melamed J. M., Paton R. S., Houk K. N., Garg N. K., Angew. Chem. Int. Ed. 2012, 51, 2758–2762; [Google Scholar]; Angew. Chem. 2012, 124, 2812–2816. [Google Scholar]
- 18.For an elegant rearrangement approach to the 1,2‐aminoaniline motif, see: Porzelle A., Woodrow M. D., Tomkinson N. C. O., Org. Lett. 2010, 12, 1492. [DOI] [PubMed] [Google Scholar]
- 19.CCDC 1028839 (7 d) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 20.The synthesis of O‐benzoyl N‐hydroxylanilines requires the preparation of N‐alkylated N‐hydroxyl anilines (either by N‐alkylation from N‐hydroxyl aniline or by treatment of a nitrosoalkane with PhMgBr), followed by an O‐benzoylation step. See for example:
- 20a. Porzelle A., Woodrow M. D., Tomkinson N. C. O., Eur. J. Org. Chem. 2008, 5135–5143; [Google Scholar]
- 20b. Endo Y., Hizatate S., Shudo K., Tetrahedron Lett. 1991, 32, 2803–2806. [Google Scholar]
- 21. Ruhland T., Smith G. P., Bang‐Andersen B., Pueschl A., Moltzen E. K., Andersen K., Int. Patent WO 2003029232A1, 2003.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
miscellaneous_information


