Abstract
The interaction of low‐energy electrons with biomolecules plays an important role in the radiation‐induced alteration of biological tissue at the molecular level. At electron energies below 15 eV, dissociative electron attachment is one of the most important processes in terms of the chemical transformation of molecules. So far, a common approach to study processes at the molecular level has been to carry out investigations with single biomolecular building blocks like pyrimidine as model molecules. Electron attachment to single pyrimidine, as well as to pure clusters and hydrated clusters, was investigated in this study. In striking contrast to the situation with isolated molecules and hydrated clusters, where no anionic monomer is detectable, we were able to observe the molecular anion for the pure clusters. Furthermore, there is evidence that solvation effectively prevents the ring fragmentation of pyrimidine after electron capture.
Keywords: biomolecular clusters, metastable compounds, negative‐ion formation, reaction mechanisms, solvent effects
Whenever highly energetic radiation interacts with biological tissue, many secondary species, mainly low‐energy electrons (LEEs) with kinetic energies of less than 15 eV, are produced. Sanche and co‐workers1, 2 have shown that LEEs interacting with plasmid DNA induce single‐strand breaks (SSBs) and double‐strand breaks (DSBs), thereby leading to cell damage with serious biological consequences, such as improper transcription and replication of genetic information, which can lead to mutations, cancer, or apoptosis.3 The yield of SSBs and DSBs showed resonance character and was assigned to initial temporary negative ion (TNI) formation by free‐electron attachment with subsequent bond dissociation processes, that is, dissociative electron attachment (DEA).1, 2 Within recent years, numerous DEA studies with biologically relevant molecules have been carried out in order to understand how LEEs can effectively fragment biologically related species.4–6 DNA and RNA bases have played an important role in improving our understanding of electron–DNA interactions.
Moreover, gas‐phase studies with nucleobases interacting with ions,7 photons,8 and potassium atoms9 have also drawn special attention. Such gas‐phase studies constitute a powerful method for describing fundamental processes at the molecular level. However, for an isolated system, the molecular dynamics initiated by a collision cannot be affected by the surrounding medium, which cannot be excluded in the condensed phase. Related to this, the reaction energetics may be modified; for example, vertical ionization energies in aqueous solution are altered from the gas‐phase values.10 Hence, there is an urgent need to link the gas phase to the condensed phase. Experimental studies on electron ionization,11 ion impact,12 and photoionization13 of biomolecular clusters have been reported, but to date, the fragmentation dynamics of a negatively charged biomolecular cluster formed through the capture of LEEs has not been investigated.
Herein, we describe the first detailed experimental study of the solvation effect in the attachment of low‐energy electrons to pure and hydrated clusters of a biomolecule, specifically, the nucleobase prototype molecule pyrimidine (Pyr, C4N2H4; Figure 1). We compare anion formation in isolated molecules with that in clusters, which enables the investigation of the effects of the surroundings on the processes induced in biological matter by LEEs.
Figure 1.
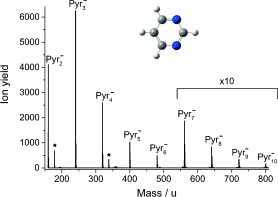
Negative‐ion mass spectrum for pure Pyr clusters obtained at an electron energy of approximately 0 eV. The whole range between Pyr2 − and Pyr3 −, as well as Pyr4 − and Pyr5 −, was measured, while other sections between the cluster anions were skipped. Pyr cluster anions with one water molecule attached are indicated with a star (see text).
Before presenting the results for clusters, we briefly summarize electron‐induced chemistry in a single Pyr molecule, which we also investigated owing to the lack of reported studies dealing with this topic. Like all nucleobases, Pyr does not form a parent anion that is detectable by mass spectrometry upon electron attachment because of rapid spontaneous electron emission or dissociation into stable fragments. Indeed, the electron affinity of Pyr was predicted to be slightly negative14 in agreement with the present results. The main fragment anions correspond to cleavage of the ring; with strong formation of CN−, as well as C3H2N− (the isobaric species C2N2 − may also form). The dehydrogenated parent anion (Pyr−H)− is also weakly detected. All three fragments are formed only at higher electron energies in two broad resonances at approximately 5.5 and 9 eV. By contrast, Modelli et al.15 observed three low‐lying π* resonances (at 0.39, 0.82, and 4.26 eV in electron transmission experiments; similar values are reported in Ref. 14). Obviously, these three resonances are short‐lived and cannot be detected by mass spectrometry. However, as proposed in Ref. 16, the embedding of a molecule in a cluster may stabilize its anion against spontaneous electron emission. When studying Rydberg electron transfer from laser‐excited xenon atoms, they observed that even one molecule of Pyr or water attached to the Pyr anion is sufficient for stabilization against spontaneous electron emission. Similarly Kelly et al.17 observed positive electron affinities for the hydrated Pyr anions (formed through the insertion of electrons into the expanding neutral gas jet of a supersonic expansion source) by means of photoelectron spectroscopy, and this result was also supported by DFT calculations. A negative‐ion mass spectrum for the attachment of free electrons with energies of about 0 eV to neutral pure Pyr clusters is shown in Figure 1. With our clustering conditions, it is possible to detect bare Pyr clusters of up to ten molecules. It should be noted that the regions between the stable cluster ions lack peaks corresponding to solvated fragment anions (even at higher electron energies). The only species weakly observed can be assigned to Pyr cluster anions with one water molecule attached, which is formed owing to a small amount of residual moisture. No signal for the Pyr anion (mass 80 u) resulting from the capture of electrons with low energy (close to zero eV) is observed, which is in agreement with previous studies.16, 17
Figure 2 a shows the ion yield of the dimer anion as a function of the incident electron energy. Reflecting the mass spectrum of Figure 1, an abundant zero eV peak is visible, which is located at this energy owing to a stabilizing interaction between the TNI and surrounding molecules in the cluster.18 In addition to the low‐energy feature, a signal arises at higher electron energies from about 4 up to 15 eV. The first peak at about 5.4 eV is above the three mentioned π* resonances and must thus be ascribed to a core‐excited resonance.
Figure 2.
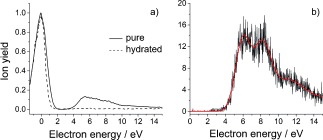
a) Negative‐ion yields of the Pyr dimer (m=160 u) for pure (full line) and hydrated (dashed line) clusters. b) Ion yield of the Pyr monomer anion only observable for pure clusters.
The π→π* transition in neutral Pyr occurs at about 5.2 eV.19 For the anionic system, a core‐excited resonance at about 5.5 eV has been reported,15 which is in very good agreement with the present results. In addition, several other excited states up to 10.5 eV have been predicted,20 which contribute to the present ion yield at higher energies. Remarkably, in this energy range, we are also able to observe the monomer anion of Pyr with main resonance peaks at 6.1 and 8.2 eV (Figure 2 b), that is, electronic excitation is required to observe this anion. By varying the expansion pressure and following the change of the ion yields, we determined that at least a neutral tetramer cluster is needed to stabilize this anion. We propose that the anion is in a metastable state above the neutral ground state, where spontaneous electron emission via a Franck–Condon transition is prevented owing to a larger equilibrium distance of the anion.21 Substantial energy relaxation within the cluster and evaporation of neutral monomers is required to reach this metastable state.
In order to investigate hydration effects, hydration of Pyr clusters was induced. The resulting mass spectrum at approximately 0 eV is shown in the mass range between 160 and 400 u in Figure 3. Strongly hydrated Pyr monomers and dimers are clearly recognizable, which in the case of the monomer indicates solvation with at least 17 water molecules. As in the case of pure Pyr clusters, no fragmentation products are observable above the Pyr monomer. However, we observed a change in the ion yield of intact cluster anions, as shown in Figure 2 a for Pyr2 −.
Figure 3.
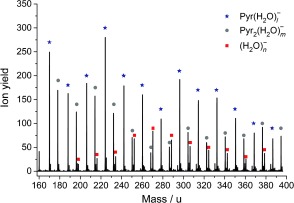
Negative‐ion mass spectrum of hydrated Pyr (monomer and dimer) at an electron energy of approximately 0 eV. (H2O)n − clusters formed upon DEA to the hydrated Pyr clusters are observable for n≥11.22
When the Pyr cluster is hydrated, it seems that the mentioned core‐excited resonance is suppressed. We also note that the monomer anion of Pyr observed for pure clusters vanishes upon hydration. We may ascribe this effect to steric shielding of Pyr by surrounding water molecules, that is, the required electronically excited states are no longer accessible. However, more likely is the decay of this resonance by emission of the captured electron to leave an electronically excited state of the water–Pyr complex. Recently it was proposed for hydrated DNA that such states are dissociative and lead to the formation of OH radicals.23 These radicals may induce a SSB in DNA, which, together with the strand‐breaking attachment of the released electron on the opposite strand, combines to a DSB that can lead to the development of cancerous cells.23
Finally, we considered the effect of solvation on the formation of fragment anions below the monomer anion discussed above. It turns out that the main fragment anions associated with ring cleavage, namely CN− and C3H2N−, are strongly suppressed in the solvated stage and only (Pyr−H)− remains. We note that this effect is enhanced by weakening of the intramolecular C—H bond as a result of the intermolecular hydrogen bonds.12 The resulting ion yield of (Pyr−H)− is shown in Figure 4, and the comparison shows that the ion yield maximum is shifted towards higher energies for clusters. This effect results from the energy‐dissipating environment of the cluster, which leads to stabilization of the anion. For the hydrated case, we also note that the most stable structures found show water molecules surrounding the Pyr N sites.17
Figure 4.
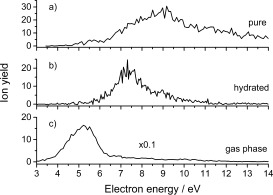
Ion yield of (Pyr−H)− resulting from DEA to pure Pyr clusters (a), hydrated Pyr clusters (b), and isolated Pyr (c).
In summary, we studied electron attachment to pure and hydrated Pyr clusters in order to be able to drawn conclusions about the fragmentation of a molecule in a biological environment. Comparison to the gas phase shows an extraordinary solvation effect; assuming similar behaviour with DNA and RNA bases, these biomolecular building blocks should be protected from LEE in irradiated cells by the surrounding water. However, we also observed that the stabilization of electronically excited anions of Pyr is strongly suppressed in case of hydration. This result supports the recently proposed route of DSB formation in hydrated DNA by initial electron attachment above energies of 5 eV.
Experimental Section
A double‐focusing mass spectrometer24 was used for the present measurements. The electron energy resolution was about 1 eV with beam currents of 10 μA. The electron energy scale was calibrated through measurements of anions of SF6. Clusters were created by means of supersonic expansion through a 50 μm nozzle. For better clustering conditions, the liquid Pyr sample (Sigma Aldrich, Vienna; stated purity ≥98 %) was heated up to about 30 °C and argon was used as the seeding gas (0.5 bar). The cluster chamber and ionization chamber were separated by a 1 mm skimmer. For the gas‐phase measurements, the sample was introduced directly to the ionization chamber.
This work was partially supported by the FWF, Wien (I1015, P24443, M1445) and DFG (FOR1789). F.F.S. acknowledges the Portuguese FCT‐MEC for post‐doctoral scholarship SFRH/BPD/68979/2010 and the grants UID/FIS/00068/2012 and PTDC/FIS‐ATO/1832/2012.
Contributor Information
Dr. Filipe Ferreira da Silva, Email: f.ferreiradasilva@fct.unl.pt
Prof. Dr. Stephan Denifl, Email: Stephan.Denifl@uibk.ac.at
References
- 1. Boudaïffa B., Cloutier P., Hunting D., Huels M. A., Sanche L., Science 2000, 287, 1658–1660. [DOI] [PubMed] [Google Scholar]
- 2. Alizadeh E., Sanche L., Chem. Rev. 2012, 112, 5578–5602. [DOI] [PubMed] [Google Scholar]
- 3. Pogozelski W. K., Tullius T. D., Chem. Rev. 1998, 98, 1089–1108. [DOI] [PubMed] [Google Scholar]
- 4. Baccarelli I., Bald I., Illenberger E., Gianturco F. A., Kopyra J., Phys. Rep. 2011, 508, 1–44. [Google Scholar]
- 5. Ptasińska S., Denifl S., Grill V., Märk T. D., Scheier P., Gohlke S., Huels M., Illenberger E., Angew. Chem. Int. Ed. 2005, 44, 1647–1650; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2005, 117, 1673–1676. [Google Scholar]
- 6. Tanzer K., Feketeová L., Puschnigg B., Scheier P., Illenberger E., Denifl S., Angew. Chem. Int. Ed. 2014, 53, 12240–12243; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 12437–12440. [Google Scholar]
- 7. De Vries J., Hoekstra R., Morgenstern R., Schlathölter T., Phys. Rev. Lett. 2003, 91, 053401. [DOI] [PubMed] [Google Scholar]
- 8. Jochims H. W., Schwell M., Baumgärtel H., Leach S., Chem. Phys. 2005, 314, 263–282. [Google Scholar]
- 9. Almeida D., da Silva F. F., Garcia G., Limao‐Vieira P., Phys. Rev. Lett. 2013, 110, 023201. [DOI] [PubMed] [Google Scholar]
- 10. Fernando H., Papadantonakis G. A., Kim N. S., LeBreton P. R., Proc. Natl. Acad. Sci. USA 1998, 95, 5550–5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim S. K., Lee W., Herschbach D. R., J. Phys. Chem. 1996, 100, 7933–7937. [Google Scholar]
- 12. Schlathölter T., Alvarado F., Bari S., Lecointre A., Hoekstra R., Bernigaud V., Manil B., Rangama J., Huber B., ChemPhysChem 2006, 7, 2339–2345. [DOI] [PubMed] [Google Scholar]
- 13. Golan A., Bravaya K. B., Kudirka R., Kostko O., Leone S. R., Krylov A. I., Ahmed M., Nat. Chem. 2012, 4, 323–329. [DOI] [PubMed] [Google Scholar]
- 14. Nenner I., Schulz G., J. Chem. Phys. 1975, 62, 1747. [Google Scholar]
- 15. Modelli A., Bolognesi P., Avaldi L., J. Phys. Chem. A 2011, 115, 10775–10782. [DOI] [PubMed] [Google Scholar]
- 16. Periquet V., Moreau A., Carles S., Schermann J. P., Desfrancois C., J. Electron Spectrosc. Relat. Phenom. 2000, 106, 141–151. [Google Scholar]
- 17. Kelly J. T., Xu S., Graham J., Nilles J. M., Radisic D., Buonaugurio A. M., Bowen K. H., Hammer N. I., Tschumper G. S., J. Phys. Chem. A 2014, 118, 11901–11907. [DOI] [PubMed] [Google Scholar]
- 18. Smyth M., Kohanoff J., Fabrikant I. I., J. Chem. Phys. 2014, 140, 184313. [DOI] [PubMed] [Google Scholar]
- 19. da Silva F. F., Almeida D., Martins G., Milosavljević A. R., Marinković B. P., Hoffmann S. V., Mason N. J., Nunes Y., Garcia G., Limão‐Vieira P., Phys. Chem. Chem. Phys. 2010, 12, 6717–6731. [DOI] [PubMed] [Google Scholar]
- 20. Mašín Z., Gorfinkiel J. D., J. Chem. Phys. 2012, 137, 204312. [DOI] [PubMed] [Google Scholar]
- 21.E. Illenberger, J. Momigny, Gaseous Molecular Ions: An Introduction to Elementary Processes Induced by Ionization, Steinkopff Verlag, Darmstadt/Springer, New York, 1992.
- 22. Knapp M., Echt O., Kreisle D., Recknagel E., J. Phys. Chem. 1987, 91, 2601–2607. [Google Scholar]
- 23. Alizadeh E., Orlando T. M., Sanche L., Annu. Rev. Phys. Chem. 2015, 66, 379–398. [DOI] [PubMed] [Google Scholar]
- 24. Almeida D., Kinzel D., da Silva F. F., Puschnigg B., Gschliesser D., Scheier P., Denifl S., García G., González L., Limão‐Vieira P., Phys. Chem. Chem. Phys. 2013, 15, 11431–11440. [DOI] [PubMed] [Google Scholar]


