Abstract
The assessment of human epidermal growth factor receptor 2 (HER2) status is crucial for selecting patients with gastric cancer who may benefit from HER2‐targeted therapy. Accurate assessment using biopsy specimens is important for patients with advanced‐stage cancer. Intratumoral heterogeneity of HER2, however, is a major challenge in HER2 testing. Here, we aimed to examine whether assessment of HER2 status could be accurately carried out with currently used methods, namely, immunohistochemistry (IHC), FISH, and dual‐color in situ hybridization (DISH). Human epidermal growth factor receptor 2 status was evaluated in 108 biopsy tissues from patients with gastric adenocarcinoma and 70 matched surgical specimens by IHC, FISH, and DISH; HER2 amplification was detected in 11 (10.2%) out of 108 biopsy specimens. The IHC and FISH results were well correlated, and FISH and DISH results were consistent for all cases. The overall concordance rate of HER2 status between biopsy tissues and surgical specimens was 91.4%. All six discordant cases were false negative on biopsy; of these cases, five showed HER2 heterogeneity on surgical resection. Assessment of the HER2 status of biopsy tissues could predict the status of the whole tumor; however, a proportion of these cases may be discordant because of intratumoral heterogeneity.
Keywords: Biopsy specimens, dual‐color in situ hybridization, gastric cancer, human epidermal growth factor receptor 2, intratumoral heterogeneity
Short abstract
This study demonstrated that HER2 assessment in biopsy tissues may predict the HER2 status of the whole tumor by IHC, FISH, and DISH. However, some cases of discordance may occur because of intratumoral HER2 heterogeneity in gastric cancers. In cases of intratumoral heterogeneity, more accurate HER2 assessment can be achieved by analysis of whole sections and by using a combination of IHC and DISH, if possible.
The effect of trastuzumab in gastric cancer was first identified by the Trastuzumab for Gastric Cancer study (ToGA study); subsequently, this anticancer agent has been applied in the management of patients with advanced gastric cancer. A recent study showed improvement in the overall survival of patients with human epidermal growth factor receptor 2 (HER2)‐positive advanced gastroesophageal and gastric cancer who were treated with chemotherapy plus trastuzumab compared with overall survival in patients who received conventional chemotherapy alone.1 Therefore, accurate and detailed assessment of HER2 status using biopsy tissues and specimens from surgical resection in cases of advanced or metastatic gastric cancer plays an important role in patient care.
The evaluation of HER2 status has been carried out in a large number of studies using core needle biopsies in patients with breast cancer.2, 3, 4, 5 A recent study reported that the concordance rate of HER2 immunohistochemistry (IHC) and FISH between core needle biopsies and specimens from surgical resection is 96–98%.6 In contrast, the concordance rate of HER2 status between biopsy and surgical resection specimens in gastric cancers detected by in situ hybridization (ISH) ranges from 74.1% to 96.1%.7, 8, 9 Intratumoral heterogeneity is thought to be one of the causes of this relatively low concordance. While HER2 intratumoral heterogeneity may not always yield false‐negative results on biopsies, the effects of intratumoral heterogeneity and technical problems, such as complications with HER2 detection or lack of a sufficient number of biopsy specimens, on assessment of HER2 status have not yet been fully evaluated.
Three diagnostic techniques are currently approved for assessing HER2 status in clinical practice: IHC, FISH, or dual‐color ISH (DISH). Fluorescence in situ hybridization is the gold standard method for evaluating HER2 status. However, FISH requires dark‐field fluorescence microscopy, and it is therefore difficult to evaluate intratumoral heterogeneity in whole sections during FISH. In contrast, DISH is a relatively new method that can be used with a bright‐field microscope and preserves specimen integrity. Therefore, DISH is appropriate for morphological observations of large areas and simultaneous gene expression analysis.10, 11, 12 Because gastric cancer frequently shows intratumoral heterogeneity of HER2 and HER2 gene amplification is significantly correlated with histological subtype, DISH is a powerful method for analysis of HER2 status in gastric cancers.
Overexpression of HER2 protein in association with HER2 gene amplification has been found to promote tumorigenesis and is associated with poor prognosis in several human cancers.13, 14 A recent study reported that heterogeneous HER2 amplification in tumors is associated with poorer prognoses than homogeneous HER2 amplification in tumors for patients with breast cancer.15, 16 However, studies investigating the prognostic significance of HER2 in gastric cancer are still limited,17, 18, 19, 20, 21 and the characteristics and therapeutic effects of intratumoral heterogeneity have not yet been fully examined.
In the present study, we aimed to examine whether assessment of HER2 in biopsy specimens from patients with gastric cancer could be carried out accurately using IHC, FISH, and DISH and to determine whether intratumoral heterogeneity affected the results of HER2 assessment. Furthermore, we investigated the relationships between HER2 status and clinicopathological features in patients with gastric cancer and evaluated the prognostic significance of HER2 and intratumoral heterogeneity.
Materials and Methods
Case selection and tissue preparation
This study was approved by the Ethics Committee of Mie University Hospital (Tsu, Japan). A total of 108 consecutive patients with primary gastric adenocarcinoma who underwent biopsy at Mie University Hospital between 2009 and 2011 were enrolled. Of these patients, 70 (including 22 cases with metastatic lymph nodes) underwent surgical resection. All clinicopathological parameters, including patient age, gender, tumor location, tumor size, histological classification, pathological TNM stage, and lymphovascular invasion status, were reviewed. The lymphatic and vascular invasions were investigated using D2‐40 immunostaining and Victoria blue special staining, respectively. Histological classification was determined according to WHO and Lauren's classifications. In the biopsy series, we analyzed 249 tumor tissue pieces (a mean of 2.3 pieces for each case). In the surgical resection series, we analyzed whole sections of 70 tumor tissues and 202 regional lymph nodes from 22 cases with lymph node metastasis. All tissues were fixed with neutral‐buffered formalin for 24–48 h, routinely processed, and embedded in paraffin. The tumor tissue was cut from the most representative block of the lesion, avoiding areas with massive ulceration/necrosis. Tissue sections measuring 3‐μm thick were used for H&E staining, whereas sections measuring 4‐μm thick were used for IHC, and sections measuring 5‐μm thick were used for FISH and DISH.
Immunohistochemistry
Human epidermal growth factor receptor 2 IHC was carried out using an automated slide stainer (Bench‐Mark XT; Ventana Medical Systems, Tucson, AZ, USA). Tissue sections were deparaffinized and rehydrated, and antigens were retrieved for 36 min in EDTA (pH 9.0) at 95°C. The primary antibody was the PATHWAY HER2/neu (4B5) rabbit mAb (Ventana Medical Systems). 3,3′‐Diaminobenzidine‐tetrachloride was used as the chromogen, and the tissue sections were counterstained with hematoxylin II and Bluing Reagent. Human epidermal growth factor receptor 2 IHC was assessed according to the modified IHC scoring system used for the ToGA study.22 In surgical resections, an IHC score of 0 was given if there was no reactivity or membranous reactivity in less than 10% of tumor cells. An IHC score of 1+ was given if there was faint or barely detectable membranous reactivity in more than 10% of tumor cells, and stained tumor cells were reactive only in a part of the membrane. An IHC score of 2+ was given if weak to moderate complete or basolateral membranous reactivity was observed in more than 10% of tumor cells. An IHC score of 3+ was given if there was moderate to strong complete or basolateral membranous reactivity in more than 10% of tumor cells. In biopsy tissues, when either complete, basolateral, or lateral staining was observed between cell–cell contacts in a membrane, as described above, for at least one carcinoma cell cluster (more than five cells), the IHC score was given independent of the percentage of the reactive area. The definition of HER2‐positive was either an IHC score of 3+ or an IHC score of 2+ with positive results for FISH. Intratumoral heterogeneity of HER2 was defined as samples with 10–60% of tumor cells showing HER2 positivity. For our study, HER2 heterogeneity was defined as samples with 5–60% of tumor cells showing HER2 positivity. Biopsy tissues were considered to show HER2 heterogeneity when HER2‐positive cells were recognized only in some of the biopsy tissues.
Immunohistochemical staining for p53, MIB‐1, and D2‐40 was carried out using an automated slide stainer. For p53 IHC, we used a mouse mAb against p53 (clone DO‐7, diluted 1:100; Dako, Tokyo, Japan). The expression of p53 was assessed as follows: 3+ for more than 20% of the tumor cells showing nuclear immunostaining, 2+ for between 5% and 20% of tumor cells showing nuclear immunostaining, 1+ for less than 5% of tumor cells showing nuclear immunostaining, and 0 (none) for no staining. 0 and 1+ were regarded as low expression, whereas 2+ and 3+ were regarded as high expression.23 For the MIB‐1 index, we used mouse mAbs against ki‐67 (2ndGen, prediluted; Invitrogen, Tokyo, Japan). Cases with more than 20% of tumor cells showing positive staining were regarded as having a high proliferation index.15 For D2‐40 IHC, we used a mouse mAb against D2‐40 (clone D2‐40, diluted 1:100; Dako).
Fluorescence in situ hybridization
FISH was carried out using the HISTRA HER2 FISH Kit (JOKOH, Tokyo, Japan). Tissue sections were deparaffinized, rehydrated, and immersed in pretreatment solution for 40 min at 95°C. The tissue sections were then rinsed three times in deionized water for 3 min and incubated in protease solution for 10 min at 37°C. The enzymatic reaction was then stopped by placing the slides in deionized water three times for 3 min each. After dehydration with alcohol, a total of 10 μL HER2/CEP17 mixture probe (HER2 DNA probe labeled with Spectrum Orange and CEP17 DNA probe labeled with Spectrum Green) was applied to the tissue sections. The slides were co‐denatured for 5 min at 85°C and incubated at 37°C for 16–20 h using a ThermoBrite instrument (Abbott Molecular, Des Plaines, IL, USA). The slides were then washed in 2× SSC plus 0.2% NP‐40 at 72°C for 2 min, air dried, and counterstained with DAPI. The FISH signals were viewed with a confocal laser‐scanning microscope (LSM 710; Carl Zeiss, Oberkochen, Germany). The HER2/CEP17 ratio was determined by counting the HER2 signals and CEP17 signals in more than 40 nuclei for each tissue section. Amplification of the HER2 gene was defined as a HER2/CEP17 ratio of higher than 2.2. Negativity for HER2 amplification was defined as a HER2/CEP17 ratio of less than 1.8. The cut‐off values for CEP17 copy number were based on a report by Seol et al..15 Cases with signals in the range of 1.25–2.25 were defined as having disomy 17. Other cases were considered to have aneusomy 17, that is, either monosomy 17 (less than 1.25 signals per cell), low polysomy (more than 2.25 but less than 3.75 signals per cell), or high polysomy 17 (more than 3.75 signals per cell).
Dual‐color in situ hybridization
Dual‐color in situ hybridization was carried out using an automated slide stainer. Human epidermal growth factor receptor 2 signals were detected using an INFORM Dual ISH HER2 kit (Ventana Medical Systems). Pretreatment was carried out using heat treatment with citrate buffer (pH 6.0) for 12 min and enzyme treatment with ISH Protease 3 for 12 min. The HER2/CEP17 mixture probe was denatured for 20 min and hybridized for 6 h. Stringent washes were carried out at 72°C. The HER2 probe was reacted with SISH Chromogen for 8 min, and the CEP17 probe was reacted with Red Chromogen for 12 min. The tissue sections were counterstained with hematoxylin II for 8 min and Bluing Reagent for 4 min. The DISH signals were viewed with a light microscope (DP72; Olympus, Tokyo, Japan). Assessment of the HER2/CEP17 ratio was determined as described for FISH.
Analysis of HER2 gene amplified area in cases of HER2 intratumoral heterogeneity
We analyzed the tumor area of whole sections for HER2 heterogeneity using a DP25 microscope (Olympus), and the HER2‐amplified area was measured using Image J software.24
Results
Comparison of HER2 IHC and FISH results in biopsy specimens
The results for HER2 IHC and FISH in the 108 biopsies are shown in Table 1. From FISH analysis, HER2 amplification was observed in 11 cases (10.2%; seven with an IHC score of 3+, and four with an IHC score of 2+). Human epidermal growth factor receptor 2 amplification was not found in cases having an IHC score of 1+ or 0. Intratumoral HER2 heterogeneity was observed in 4 (3.7%) out of 108 biopsies (Table S1). The IHC score of each tissue spot coincided with results of FISH, and HER2 amplification was observed only in areas having an IHC score of 3+ or 2+ (Fig. 1).
Table 1.
Comparison of immunohistochemistry (IHC) and FISH results for assessment of human epidermal growth factor receptor 2 (HER2) status in biopsy specimens
| HER2 IHC score | Total | ||||
|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | ||
| FISH − (<1.8) | 74 | 18 | 5 | 0 | 97 |
| FISH + (>2.2) | 0 | 0 | 4 | 7 | 11 |
| Total | 74 | 18 | 9 | 7 | 108 |
Figure 1.
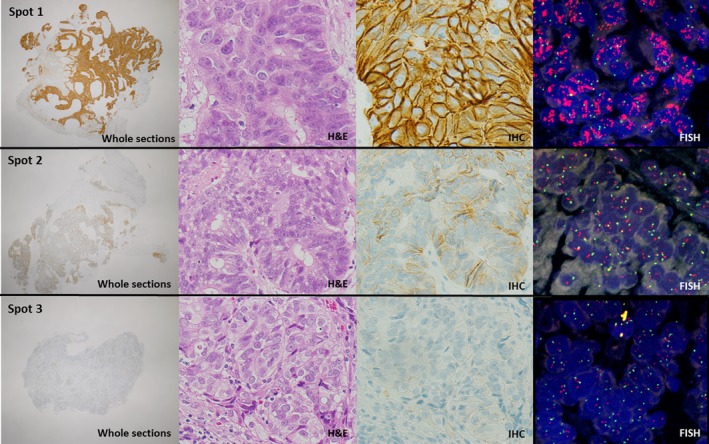
Intratumoral heterogeneity of human epidermal growth factor receptor 2 (HER2) (case no. 19). A representative case of gastric cancer showing heterogeneous HER2 expression in the biopsy specimen (3+, spot 1; 1+, spot 2; 0, spot 3) (whole sections, 2× objective; H&E, 40× objective; immunohistochemistry [IHC], 40× objective; FISH, 60× objective).
Comparison of FISH and DISH in biopsy specimens
The results for FISH and DISH in the 108 biopsies are shown in Table 2. The FISH and DISH results were consistent in all cases, and similarly, there was a significant correlation between IHC and DISH. In addition, there was a significant correlation between FISH signals and DISH signals (HER2, CEP17, and the HER2/CEP17 ratio; Pearson correlation coefficients of r = 0.939, P < 0.001; r = 0.303, P = 0.001; and r = 0.934, P < 0.001, respectively; Fig. S1).
Table 2.
Comparison of FISH and dual‐color in situ hybridization (DISH) results for assessment of human epidermal growth factor receptor 2 status in biopsy specimens
| IHC score | FISH | DISH | Total | ||
|---|---|---|---|---|---|
| Unamplified | Amplified | Unamplified | Amplified | ||
| 0 | 74 | 0 | 74 | 0 | 74 |
| 1+ | 18 | 0 | 18 | 0 | 18 |
| 2+ | 5 | 4 | 5 | 4 | 9 |
| 3+ | 0 | 7 | 0 | 7 | 7 |
| Total | 97 | 11 | 97 | 11 | 108 |
IHC, immunohistochemistry.
Comparison of HER2 status between biopsy and surgical resection specimens
The comparison of HER2 status between biopsy and matched surgical resection specimens is shown in Tables 3 and 4. The concordance rate of HER2 IHC was 80.0% (56/70). Out of the 70 cases, 10 cases showed HER2 IHC scores of 0 or 1+ in biopsies, but 2+ or 3+ in specimens from surgical resection. Conversely, two cases were scored as 2+ by HER2 IHC in biopsies, but 0 or 1+ in surgical resection specimens. The concordance rate of HER2 status between biopsy and surgical resection specimens by DISH was 91.4% (64/70). Six cases showed no amplification in biopsy specimens but amplification in specimens obtained by surgical resection. When specimens obtained from surgical resection were regarded as the gold standard, the predictive value of biopsy specimens for HER2 status was as follows: sensitivity of 50.0%, specificity of 100%, positive predictive value (PPV) of 100%, and negative predictive value (NPV) of 90.6%.
Table 3.
Concordance of human epidermal growth factor receptor 2 (HER2) immunohistochemistry (IHC) between biopsy and surgical resection specimens
| Score | HER2 IHC in surgical resection | Total | |||
|---|---|---|---|---|---|
| 0 or 1+ | 2+ | 3+ | |||
| HER2 IHC in biopsy | 0 or 1+ | 50 | 9 | 1 | 60 |
| 2+ | 2 | 4 | 1 | 7 | |
| 3+ | 0 | 1 | 2 | 3 | |
| Total | 52 | 14 | 4 | 70 | |
Table 4.
Concordance of human epidermal growth factor receptor 2 (HER2) status between biopsy and surgical resection specimens
| HER2 status in surgical resection | Total | |||
|---|---|---|---|---|
| Unamplified | Amplified | |||
| HER2 status in biopsy | Unamplified | 58 | 6 | 64 |
| Amplified | 0 | 6 | 6 | |
| Total | 58 | 12 | 70 | |
The staining characteristics of concordant positive cases and discordant positive cases are shown in Table 5. In discordant positive cases, intratumoral heterogeneity of HER2 status was found in 5 (83.3%) of six cases (Fig. 2). In contrast, concordant positive cases showed a homogeneous pattern in both biopsy and surgical resection specimens in 4 (66.7%) of six cases.
Table 5.
Discordant and concordant cases of human epidermal growth factor receptor 2 (HER2) status between biopsy and surgical resection specimens
| Case no. | Biopsy | Surgical resection | Amplified area, % | ||||
|---|---|---|---|---|---|---|---|
| HER2 IHC | HER2 status (HER2/CEP17 ratio) | WHO classification | HER2 IHC | HER2 status (HER2/CEP17 ratio) | WHO classification | ||
| Discordant cases | |||||||
| 10 | 0 | Unamplified (1.13) | Tubular | 2+ Heterogeneous | Heterogeneous (2.96) | Tubular | 17.4 |
| 15 | 0 | Unamplified (1.17) | Tubular | 2+ Heterogeneous | Heterogeneous (3.36) | Tubular | 5.4 |
| 38 | 0 | Unamplified (1.13) | Poorly cohesive | 2+ Heterogeneous | Heterogeneous (4.02) | Poorly cohesive (tubular component) | 9.3 |
| 71 | 2+ Heterogeneous | Unamplified (1.11) | Tubular | 2+ | Homogeneous (3.61) | Tubular | 89.9 |
| 79 | 1+ | Unamplified (1.10) | Tubular | 2+ Heterogeneous | Heterogeneous (2.68) | Papillary | 12.2 |
| 87 | 0 | Unamplified (1.36) | Tubular | 3+ Heterogeneous | Heterogeneous (7.69) | Tubular | 5.3 |
| Concordant cases | |||||||
| 6 | 3+ | Amplified (7.62) | Poorly cohesive | 3+ | Homogeneous (8.69) | Tubular (poorly component) | 100.0 |
| 41 | 3+ Heterogeneous | Amplified heterogeneous (8.92) | Tubular | 3+ Heterogeneous | Heterogeneous (8.86) | Tubular | 12.4 |
| 58 | 2+ Heterogeneous | Amplified (3.25) | Tubular | 2+ Heterogeneous | Homogeneous (3.42) | Tubular | 100.0 |
| 64 | 2+ Heterogeneous | Amplified (2.60) | Tubular | 2+ Heterogeneous | Homogeneous (2.20) | Tubular | 100.0 |
| 72 | 2+ | Amplified (4.29) | Tubular | 3+ | Homogeneous (4.60) | Tubular | 100.0 |
| 85 | 3+ Heterogeneous | Amplified heterogeneous (4.40) | Tubular | 2+ Heterogeneous | Heterogeneous (2.75) | Tubular | 47.9 |
Figure 2.
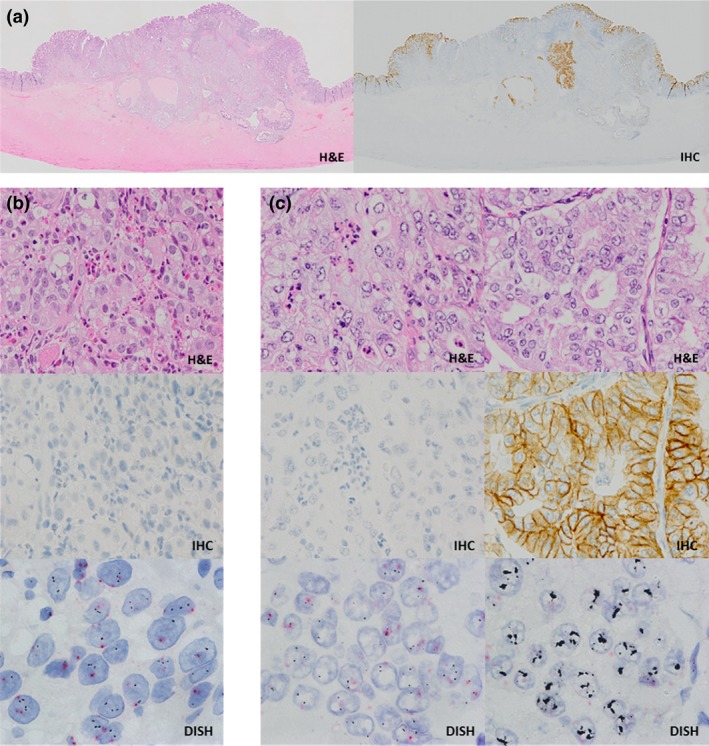
Discordant cases between biopsy and surgical resection specimens (case no. 87). (a) Intratumoral heterogeneity of human epidermal growth factor receptor 2 (HER2) in the surgical resection specimen (H&E, whole slide images (left); immunohistochemistry [IHC], whole slide images (right)). (b) The HER2 gene was not amplified in the biopsy specimen (H&E, 40× objective; IHC, 40× objective; dual‐color in situ hybridization [DISH], 100× objective). (c) The HER2 IHC positive area of the surgical resection specimen showed HER2 gene amplification (H&E, 40× objective; IHC, 40× objective; DISH, 100× objective).
HER2 status and clinicopathological factors
HER2 amplification was significantly associated with Lauren's intestinal type (P = 0.0038). Additionally, HER2 amplification tended to correlate with lymphatic invasion (P = 0.0817), but was not associated with age, gender, tumor location, tumor size, T or N factor, TNM stage, or vascular invasion (P > 0.05; Table S2).
High expression of p53 was detected in 11 (64.7%) of 17 cases of HER2 amplification and in 42 (46.2%) of 91 cases without HER2 amplification. However, no significant correlation was found between high expression of p53 and HER2 amplification (P = 0.1269). High MIB‐1 proliferation indices were detected in 15 (88.2%) of 17 cases of HER2 amplification and in 64 (70.3%) of 91 cases without HER2 amplification. No significant correlations were observed between the MIB‐1 index and HER2 amplification (P = 0.1050; Table S3).
HER2 status of primary tumor and lymph node metastasis
Of 22 cases with positive regional lymph node metastasis, overexpression of HER2 protein (score of 2+ or 3+ by IHC) was observed in seven cases (31.8%) in the primary tumor and eight cases (36.4%) in lymph node lesions (Table S4). HER2 gene amplification was found in five cases (22.7%) in the primary tumor and corresponding lymph nodes (Table S5).
Discussion
Currently, there are three methods for evaluating HER2 status: IHC, FISH, and DISH. In the ToGA trial, the concordance rate between IHC and FISH was 87%. Additionally, Cho et al.25 reported that the concordance between IHC and silver ISH is 95%. In our study, there was no FISH positivity in cases having IHC scores of 0 or 1+ (Table 1), and HER2 protein expression and gene amplification were significantly correlated. Moreover, the concordance rate between FISH and DISH was 100% (Table 2), and there was a significant correlation between the number of FISH signals and the number of DISH signals (Fig. S1). These findings suggested that the use of different techniques did not affect the detection of HER2 status.
Previous studies have reported that the concordance rate of HER2 status between biopsy and surgical resection specimens from patients with gastric cancer, as detected by ISH, ranges from 74.1% to 96.1%.7, 8, 9 Additionally, Pirrelli et al.9 reported that HER2 assessment in biopsy specimens has a sensitivity of 62.5%, specificity of 96.2%, a PPV of 71.4%, and an NPV of 94.4%. In comparison with these other studies, our study showed a high concordance rate of 91.4% between biopsy and surgical resection specimens; however, the sensitivity was lower (50%) in biopsy specimens. This variation may be related to the criteria used for evaluating the tissue sections. Intratumoral heterogeneity of HER2 is defined as 10–60% of cells showing positivity, based on current guidelines. However, we used the range of 5–60% in order to examine intratumoral heterogeneity in more detail. In our study, the predictive value for the range of 10–60% was as follows: sensitivity of 66.7%, specificity of 100%, PPV of 100%, and NPV of 95.3%. The concordance rate was 95.7%. These results are consistent with previous studies, and these findings suggested that HER2 assessment using biopsy tissues could be applied for prediction of the HER2 status of the whole tumor.
In this study, HER2 heterogeneity was found in 9 (52.9%) of 17 HER2‐positive cases. Five (83.3%) of the six discordant cases between biopsy and surgical resection specimens showed intratumoral HER2 heterogeneity (Table 5). Of these, 3 (50.0%) cases showed a HER2‐positive area of 5–10%. In contrast, HER2 heterogeneity with 5–10% positivity was not observed in concordant cases. These results suggested that intratumoral heterogeneity plays an important role in the discrepancy between biopsy tissues and specimens obtained from surgical resection. Moreover, these results suggest that cases with 5–10% intratumoral heterogeneity do not contribute to the false‐positive rate observed during evaluation of biopsy tissues. In concordant and discrepant cases, Wang et al.(8 ) reported that there was no significant relationship between the number of biopsies and consistency. In contrast, Rüschoff et al.26 stated that at least six to eight tumor tissue pieces were needed for adequate assessment of biopsy. In the present study, the mean numbers of tumor biopsy specimens in concordant and discordant cases were 2.75 and 1.83 (when a 5% cut‐off value was used) or 2.75 and 1.33 (when a 10% cut‐off value was used), respectively, and there was no significant relationship between the number of biopsy tissues and concordance (Fig. S2). However, the tumor area included in biopsy tissues tended to be larger in concordant cases than in discordant cases (5.9 ± 4.0 mm2 vs 2.2 ± 0.8 mm2 when a 5% cut‐off value was used; 5.9 ± 4.0 mm2 vs 2.9 ± 0.4 mm2 when a 10% cut‐off value was used, respectively; Fig. S3). These data suggest that the sampled tumor area may be more important than the number of biopsies during the evaluation of HER2 status. Furthermore, sensitivity, specificity, and κ values were 100% in cases using more than three (when a 10% cut‐off value was used) or more than four tumor tissue pieces (when a 5% cut‐off value was used; Fig. S4). Thus, we recommend that assessment of HER2 status using biopsy tissues should be carried out with more than three tissue pieces, including a sufficient tumor volume. When it is necessary to assess fewer than three tissue pieces, cases found to be negative for HER2 may need to undergo an additional biopsy based on the possibility of intratumoral heterogeneity. Further analyses are needed to determine the appropriate cut‐off values in cases of HER2 heterogeneity.
Some studies have reported that HER2 is a poor prognostic factor in gastric cancer.18, 19, 20 However, Barros‐Silva et al.17 and Kim et al.21 reported that HER2 expression is not associated with poor prognosis. Additionally, Cho et al.25 reported that HER2 is not a significant prognostic factor in patients with advanced gastric cancer. In our study, HER2 gene amplification was not related to various clinicopathological factors (Table S2). However, we found a significant relationship between HER2 gene amplification and clinicopathological factors in intestinal‐type cancer (Table S6). These data suggested that HER2 gene amplification may be an important prognostic factor in specific subtypes of gastric cancer, such as intestinal‐type cancer. Moreover, we found evidence of HER2 positivity in non‐invasive cancers, and all cases showing this feature also had intramucosal HER2 heterogeneity (Fig. S5). By careful observation of intramucosal lesions of invasive cancer with HER2 amplification, we found invasive cancers with intratumoral heterogeneity already possessed heterogeneity in the mucosa (Table S7). These data suggested that HER2 gene amplification could be a private mutation that may occur in relatively early stage of gastric cancer development.27 In breast cancer, HER2 overexpression in ductal carcinoma in situ has been reported to be associated with the risk of tumor invasion and several poor prognostic features.28, 29 However, the biological and clinical significance of HER2 in early gastric cancer is poorly understood. Further studies are needed to elucidate its biological and clinical significance.
A significant discordance in HER2 status between primary lesions and lymph node metastases has been reported in cancers of the breast, urinary bladder, and prostate.30, 31, 32 This discordance could be explained by genetic drift during tumor progression or the presence of intratumoral heterogeneity of HER2.29 In our study, the concordance rate of HER2 expression by IHC between the primary tumor and lymph node metastasis was 86.4% (Table S4), and discordance of HER2 gene amplification results was not observed (Table S5). In cases of HER2 heterogeneity, HER2 gene status in lymph node metastases showed intralesional heterogeneity similar to that of the primary tumor (Fig. S6). These findings suggested that HER2 testing of lymph node metastases could also be used to assess the HER2 status of the primary lesion.
In breast cancer, HER2 heterogeneity shows a cluster‐type or scatter‐type (mosaic pattern) distribution.33 From a therapeutic standpoint, genetically heterogeneous tumors have been shown to be sensitive to HER2‐targeted therapy.34, 35 Currently, the NSABP B‐47 trial of adjuvant trastuzumab therapy is being undertaken in patients with breast cancer showing low expression of HER2. In our study, HER2 heterogeneity was found to show a cluster‐type or scatter‐type distribution (Fig. S7). The mosaic pattern manifests as a mixed subpopulation of cells showing HER2 amplification within a population of cells lacking HER2 amplification (Fig. S8). Moreover, no significant differences in tumor growth were found between HER2‐amplified and ‐unamplified regions (Fig. S9). These findings suggest that there may not be competition for survival between subpopulations of cells with or without HER2 amplification. The therapeutic effects of HER2‐targeted therapy may not be as obvious in tumors showing HER2 heterogeneity; thus, the effects of HER2 heterogeneity on the therapeutic efficacy of HER2‐targeted treatments should be examined in clinical trials.
In summary, our study indicated that HER2 assessment in biopsy tissues may predict the HER2 status of the whole tumor by IHC, FISH, and DISH. However, some cases of discordance may occur because of intratumoral HER2 heterogeneity in gastric cancers. In cases of intratumoral heterogeneity, more accurate HER2 assessment can be achieved by analysis of whole sections and by using a combination of IHC and DISH, if possible.
Disclosure Statement
The authors have no conflict interest.
Supporting information
Fig. S1. Comparison of FISH and dual‐color in situ hybridization (DISH) in biopsy specimens.
Fig. S2. Number of biopsies in cases of human epidermal growth factor receptor 2 (HER2) heterogeneity (n = 10).
Fig. S3. Tumor area of human epidermal growth factor receptor 2 (HER2) heterogeneity in biopsies (n = 10).
Fig. S4. Sensitivity, specificity, and κ coefficient of human epidermal growth factor receptor 2 (HER2) status in correlation with the number of biopsy specimens.
Fig. S5. Intratumoral heterogeneity of human epidermal growth factor receptor 2 (HER2) in non‐invasive cancer (case no. 10).
Fig. S6. Percentage of human epidermal growth factor receptor 2 (HER2)‐amplified and ‐unamplified areas in a case of heterogeneity (case no. 41).
Fig. S7. Distribution of cells showing HER2 gene amplification in primary tumors and lymph node metastases.
Fig. S8. Heterogeneous mosaic pattern in human epidermal growth factor receptor 2 (HER2) immunohistochemistry (IHC) and dual‐color in situ hybridization (DISH).
Fig. S9. Regional differences in the MIB‐1 index in cases of human epidermal growth factor receptor 2 (HER2) heterogeneity (n = 7).
Table S1. Cases with intratumoral heterogeneity of HER2 gene amplification in biopsy specimens (n = 4).
Table S2. Comparison of human epidermal growth factor receptor 2 (HER2) status and clinicopathological factors.
Table S3. Comparison of human epidermal growth factor receptor 2 (HER2) status and p53 expression, MIB‐1 index.
Table S4. Comparison of human epidermal growth factor receptor 2 (HER2) immunohistochemistry between primary tumors and lymph node metastases.
Table S5. Comparison of human epidermal growth factor receptor 2 (HER2) status between primary tumors and lymph node metastases.
Table S6. Comparison of human epidermal growth factor receptor 2 (HER2) status and clinicopathological factors in intestinal‐type gastric cancers.
Table S7. Comparison of human epidermal growth factor receptor 2 (HER2) status between mucosal sites and invasive sites in invasive gastric carcinoma (n = 9).
Doc. S1. Supplementary materials and methods.
Cancer Sci 107 (2016) 536–542
Funding Information No sources of funding were declared for this study.
References
- 1. Sawaki A, Ohashi Y, Omuro Y et al Efficacy of trastuzumab in Japanese patients with HER2‐positive advanced gastric or gastroesophageal junction cancer: a subgroup analysis of the Trastuzumab for Gastric Cancer (ToGA) study. Gastric Cancer 2012; 15: 313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnedos M, Nerurkar A, Osin P, A'Hern R, Smith IE, Dowsett M. Discordance between core needle biopsy (CNB) and excisional biopsy (EB) for estrogen receptor (ER), progesterone receptor (PgR) and HER2 status in early breast cancer (EBC). Ann Oncol 2009; 20: 1948–52. [DOI] [PubMed] [Google Scholar]
- 3. Sutela A, Vanninen R, Sudah M et al Surgical specimen can be replaced by core samples in assessment of ER, PR and HER‐2 for invasive breast cancer. Acta Oncol 2008; 47: 38–46. [DOI] [PubMed] [Google Scholar]
- 4. Tamaki K, Sasano H, Ishida T et al Comparison of core needle biopsy (CNB) and surgical specimens for accurate preoperative evaluation of ER, PgR and HER2 status of breast cancer patients. Cancer Sci 2010; 101: 2074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chivukula M, Bhargava R, Brufsky A, Surti U, Dabbs DJ. Clinical importance of HER2 immunohistologic heterogeneous expression in core‐needle biopsies vs resection specimens for equivocal (immunohistochemical score 2+) cases. Mod Pathol 2008; 21: 363–8. [DOI] [PubMed] [Google Scholar]
- 6. Arnould L, Roger P, Macgrogan G et al Accuracy of HER2 status determination on breast core‐needle biopsies (immunohistochemistry, FISH, CISH and SISH vs FISH). Mod Pathol 2012; 25: 675–82. [DOI] [PubMed] [Google Scholar]
- 7. Lee S, de Boer WB, Fermoyle S, Platten M, Kumarasinghe MP. Human epidermal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resections. Histopathology 2011; 59: 832–40. [DOI] [PubMed] [Google Scholar]
- 8. Wang T, Hsieh ET, Henry P, Hanna W, Streutker CJ, Grin A. Matched biopsy and resection specimens of gastric and gastroesophageal adenocarcinoma show high concordance in HER2 status. Hum Pathol 2014; 45: 970–5. [DOI] [PubMed] [Google Scholar]
- 9. Pirrelli M, Caruso ML, Di Maggio M, Armentano R, Valentini AM. Are biopsy specimens predictive of HER2 status in gastric cancer patients? Dig Dis Sci 2013; 58: 397–404. [DOI] [PubMed] [Google Scholar]
- 10. Yan B, Yau EX, Choo SN et al Dual‐colour HER2/chromosome 17 chromogenic in situ hybridisation assay enables accurate assessment of HER2 genomic status in gastric cancer and has potential utility in HER2 testing of biopsy samples. J Clin Pathol 2011; 64: 880–3. [DOI] [PubMed] [Google Scholar]
- 11. Kiyose S, Igarashi H, Nagura K et al Chromogenic in situ hybridization (CISH) to detect HER2 gene amplification in breast and gastric cancer: comparison with immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH). Pathol Int 2012; 62: 728–34. [DOI] [PubMed] [Google Scholar]
- 12. Garcia‐Garcia E, Gomez‐Martin C, Angulo B et al Hybridization for human epidermal growth factor receptor 2 testing in gastric carcinoma: a comparison of fluorescence in‐situ hybridization with a novel fully automated dual‐colour silver in‐situ hybridization method. Histopathology 2011; 59: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu D, Hung MC. Overexpression of ErbB2 in cancer and ErbB2‐targeting strategies. Oncogene 2000; 19: 6115–21. [DOI] [PubMed] [Google Scholar]
- 14. Hogdall EV, Christensen L, Kjaer SK et al Distribution of HER‐2 overexpression in ovarian carcinoma tissue and its prognostic value in patients with ovarian carcinoma: from the Danish MALOVA Ovarian Cancer Study. Cancer 2003; 98: 66–73. [DOI] [PubMed] [Google Scholar]
- 15. Seol H, Lee HJ, Choi Y et al Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod Pathol 2012; 25: 938–48. [DOI] [PubMed] [Google Scholar]
- 16. Bartlett AI, Starcyznski J, Robson T et al Heterogeneous HER2 gene amplification: impact on patient outcome and a clinically relevant definition. Am J Clin Pathol 2011; 136: 266–74. [DOI] [PubMed] [Google Scholar]
- 17. Barros‐Silva JD, Leitao D, Afonso L et al Association of ERBB2 gene status with histopathological parameters and disease‐specific survival in gastric carcinoma patients. Br J Cancer 2009; 100: 487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song Y, Huang J, Wang JW. Relationship between HER2/neu gene amplification and protein expression and prognosis in patients with advanced gastric carcinoma. Chin J Cancer 2010; 29: 76–81. [DOI] [PubMed] [Google Scholar]
- 19. Yonemura Y, Ninomiya I, Yamaguchi A et al Evaluation of immunoreactivity for erbB‐2 protein as a marker of poor short term prognosis in gastric cancer. Cancer Res 1991; 51: 1034–8. [PubMed] [Google Scholar]
- 20. Park DI, Yun JW, Park JH et al HER‐2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci 2006; 51: 1371–9. [DOI] [PubMed] [Google Scholar]
- 21. Kim MA, Jung EJ, Lee HS et al Evaluation of HER‐2 gene status in gastric carcinoma using immunohistochemistry, fluorescence in situ hybridization, and real‐time quantitative polymerase chain reaction. Hum Pathol 2007; 38: 1386–93. [DOI] [PubMed] [Google Scholar]
- 22. Hofmann M, Stoss O, Shi D et al Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 2008; 52: 797–805. [DOI] [PubMed] [Google Scholar]
- 23. Hewedi IH, Radwan NA, Shash LS. Diagnostic value of progesterone receptor and p53 expression in uterine smooth muscle tumors. Diagn Pathol 2012; 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rasband WS, Image J, Bethesda, MD: U.S. National Institutes of Health. http://imagej.nih.gov/ij/,1997-2012. [Google Scholar]
- 25. Cho EY, Park K, Do I et al Heterogeneity of ERBB2 in gastric carcinomas: a study of tissue microarray and matched primary and metastatic carcinomas. Mod Pathol 2013; 26: 677–84. [DOI] [PubMed] [Google Scholar]
- 26. Rüschoff J, Hanna W, Bilous M et al HER2 testing in gastric cancer: a practical approach. Mod Pathol 2012; 25: 637–50. [DOI] [PubMed] [Google Scholar]
- 27. Sottoriva A, Kang H, Ma Z et al A Big Bang model of human colorectal tumor growth. Nat Genet 2015; 47: 209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roses RE, Paulson EC, Sharma A et al HER‐2/neu overexpression as a predictor for the transition from in situ to invasive breast cancer. Cancer Epidemiol Biomarkers Prev 2009; 18: 1386–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bartkova J, Barnes DM, Millis RR, Gullick WJ. Immunohistochemical demonstration of c‐erbB‐2 protein in mammary ductal carcinoma in situ. Hum Pathol 1990; 21: 1164–7. [DOI] [PubMed] [Google Scholar]
- 30. Santinelli A, Pisa E, Stramazzotti D, Fabris G. HER‐2 status discrepancy between primary breast cancer and metastatic sites. Impact on target therapy. Int J Cancer 2008; 122: 999–1004. [DOI] [PubMed] [Google Scholar]
- 31. Morris MJ, Reuter VE, Kelly WK et al HER‐2 profiling and targeting in prostate carcinoma. Cancer 2002; 94: 980–6. [PubMed] [Google Scholar]
- 32. Jimenez RE, Hussain M, Bianco FJ Jr et al Her‐2/neu overexpression in muscle‐invasive urothelial carcinoma of the bladder: prognostic significance and comparative analysis in primary and metastatic tumors. Clin Cancer Res 2001; 7: 2440–7. [PubMed] [Google Scholar]
- 33. Hanna WM, Ruschoff J, Bilous M et al HER2 in situ hybridization in breast cancer: clinical implications of polysomy 17 and genetic heterogeneity. Mod Pathol 2014; 27: 4–18. [DOI] [PubMed] [Google Scholar]
- 34. Ohlschlegel C, Zahel K, Kradolfer D, Hell M, Jochum W. HER2 genetic heterogeneity in breast carcinoma. J Clin Pathol 2011; 64: 1112–6. [DOI] [PubMed] [Google Scholar]
- 35. Allison KH, Dintzis SM, Schmidt RA. Frequency of HER2 heterogeneity by fluorescence in situ hybridization according to CAP expert panel recommendations: time for a new look at how to report heterogeneity. Am J Clin Pathol 2011; 136: 864–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Comparison of FISH and dual‐color in situ hybridization (DISH) in biopsy specimens.
Fig. S2. Number of biopsies in cases of human epidermal growth factor receptor 2 (HER2) heterogeneity (n = 10).
Fig. S3. Tumor area of human epidermal growth factor receptor 2 (HER2) heterogeneity in biopsies (n = 10).
Fig. S4. Sensitivity, specificity, and κ coefficient of human epidermal growth factor receptor 2 (HER2) status in correlation with the number of biopsy specimens.
Fig. S5. Intratumoral heterogeneity of human epidermal growth factor receptor 2 (HER2) in non‐invasive cancer (case no. 10).
Fig. S6. Percentage of human epidermal growth factor receptor 2 (HER2)‐amplified and ‐unamplified areas in a case of heterogeneity (case no. 41).
Fig. S7. Distribution of cells showing HER2 gene amplification in primary tumors and lymph node metastases.
Fig. S8. Heterogeneous mosaic pattern in human epidermal growth factor receptor 2 (HER2) immunohistochemistry (IHC) and dual‐color in situ hybridization (DISH).
Fig. S9. Regional differences in the MIB‐1 index in cases of human epidermal growth factor receptor 2 (HER2) heterogeneity (n = 7).
Table S1. Cases with intratumoral heterogeneity of HER2 gene amplification in biopsy specimens (n = 4).
Table S2. Comparison of human epidermal growth factor receptor 2 (HER2) status and clinicopathological factors.
Table S3. Comparison of human epidermal growth factor receptor 2 (HER2) status and p53 expression, MIB‐1 index.
Table S4. Comparison of human epidermal growth factor receptor 2 (HER2) immunohistochemistry between primary tumors and lymph node metastases.
Table S5. Comparison of human epidermal growth factor receptor 2 (HER2) status between primary tumors and lymph node metastases.
Table S6. Comparison of human epidermal growth factor receptor 2 (HER2) status and clinicopathological factors in intestinal‐type gastric cancers.
Table S7. Comparison of human epidermal growth factor receptor 2 (HER2) status between mucosal sites and invasive sites in invasive gastric carcinoma (n = 9).
Doc. S1. Supplementary materials and methods.


