Abstract
Nimotuzumab (N) is a humanized anti‐epidermal growth factor receptor monoclonal antibody. This prospective, single‐armed, open label phase II study was conducted to evaluate the efficacy and safety of the combination of paclitaxel (T)/cisplatin (P) with nimotuzumab (N) as first‐line treatment in advanced esophageal squamous cell carcinoma (ESCC). Patients with pathologic confirmed unresectable locally advanced or metastatic ESCC were treated with the TPN regimen: nimotuzumab 200 mg weekly, paclitaxel 175 mg/m2 on day 1 and cisplatin 30 mg/m2 on days 1 and 2; repeat cycle every 3 weeks for six cycles. Radiotherapy was allowed to be admitted after four cycles of TPN treatment. The primary endpoint was the objective response rate (ORR). The secondary endpoint was the overall survival (OS), duration of disease control (DDC) and toxicities. From March 2011 to April 2013, a total of 59 patients were enrolled and 56 were eligible for the final analysis. Overall RR was 51.8% and disease control rate (DCR) (CR + PR + SD) was 92.9%. Local treatment (radiotherapy or surgery) followed by chemotherapy improved the duration of disease control for patients with metastatic disease and local‐regional advanced disease to 8.2 months and more than 23 months, respectively. The OS for patients with metastatic disease was 14.0 months (95% CI: 6.8–21.2 months). The most common G3/4 toxicities were neutropenia (46.4%), nausea (48.3%), alopecia (78.6%), anorexia (42.8%), vomiting (55.4%), arthralgia (62.5%) and anorexia (5%). Adding nimotuzumab to the standard TP regiment was safe, and well tolerated. The TPN regimen is an effective combination as the first‐line chemotherapy for the patients with advanced ESCC, and appears more active than current standard regimens.
Keywords: Advanced esophageal squamous cell cancer, chemotherapy, cisplatin, nimotuzumab, paclitaxel
Esophageal cancer is a leading cause of cancer death in China.1 Compared to that in European countries, the incidence of squamous cell carcinoma of the esophagus in China is far more dominant than that of adenocarcinoma.2 Even with the implementation of surveillance programs and increased education regarding prevention of the disease in high‐incidence areas, the majority of cases are diagnosed at advanced stages. The combination of fluorouracil and cisplatin has been the standard treatment for metastatic esophageal squamous cell carcinoma (ESCC). The efficacy is 25–35%, and the median overall survival is 8–9.5 months.3, 4, 5, 6, 7 Taxanes have gained considerable interest in recent years as well.8, 9, 10, 11 As the prognosis of advanced esophageal carcinoma is poor, with eventual resistance to all treatment options, there is a pressing need for the development of treatment options. The combination of paclitaxel and cisplatin has been demonstrated to be safe and effective in the treatment of advanced esophageal carcinoma.12, 13 Several studies have demonstrated that epidermal growth factor receptor (EGFR) overexpression is very common and associated with poor prognosis in ESCC. Therefore, anti‐EGFR targeting treatment should be included into combination treatment of ESCC.14, 15, 16 In several phase II studies, nimotuzumab, a humanized anti‐epidermal growth factor receptor monoclonal antibody h‐R3, has been shown to be effective and safe in the treatment of head and neck cancer, non‐small cell lung cancer (NSCLC) and esophageal cancer.17, 18, 19 Therefore, we conducted this prospective phase II trial to evaluate the efficacy and safety of the combination of nimotuzumab with paclitaxel and cisplatin (TPN) as the first line treatment in advanced ESCC.
Patients and Methods
All patients had pathologically confirmed unresectable local–regional advanced or recurrent and/or metastatic ESCC. Local‐regional advanced disease was defined as tumor lesions limited in one radiotherapeutic radiation field without distal organ metastases. Metastatic disease was defined as metastatic lesion(s) in other organ besides the esophagus or lymph nodes metastasis exceeding one radiotherapeutic radiation field. Patients were either chemotherapy‐naïve or had adjuvant chemotherapy more than 6 months before recurrence. No prior radiotherapy was allowed except radiotherapy at a non‐target lesion of the study more than 3 months before enrollment. Patients were required to have measurable disease according to the RECIST criteria (with the diameter of the lesion more than 10 mm by spiral CT or MRI, and the date of image within 15 days of enrollment). Further inclusion criteria included: age ranged between 18 and 75 years old; Karnofsky performance status ≥80; life expectancy ≥3 months; adequate renal function (serum creatinine ≤1.5× the upper limit of normal [ULN]), adequate hepatic function (total and direct bilirubin ≤2× the ULN), ALT and AST ≤5× the ULN, and alkaline phosphatase ≤2× the ULN or ≤5× the ULN in the setting of liver metastases; and adequate bone marrow function (absolute neutrophil count ≥1500/mm3, platelets ≥100 000/mm3, hemoglobin ≥9 g/dL). Female patients of childbearing potential had a negative serum pregnancy test before enrolment, and all fertile patients had to agree to use contraception during the study. The study was approved by the Ethics Committee at Peking University School of Oncology and each subject gave informed consent before inclusion. The study was registered at ClinicalTrials.gov with reference NCT01336049.
The exclusion criteria included the following: previous treatment of palliative chemotherapy or recurrence less than 6 months from time of last adjuvant chemotherapy/radiotherapy; known hypersensitivity to nimotuzumab, paclitaxel, cisplatin; brain or bone metastasis only; tumor with length ≥10 cm; liver metastasis occupies more than 50% of liver or lung metastasis covers more than 25% of lung; no measurable lesions only (e.g. pleural fluid and ascites); severe heart disease; other previous malignancy within past 5 years except non‐melanoma skin cancer; and with neurological or psychiatric abnormalities that affect cognitive.
Treatment plan
Nimotuzumab was administered intravenously over 60 min at 200 mg in 250 mL saline solution once a week. Paclitaxel 175 mg/m2 was given intravenously on day 1, and cisplatin 30 mg/m2 i.v. on day 1 and day 2 every 3 weeks. Paclitaxel infusions preceded the administration of cisplatin. Patients were premedicated with diphenhydramine hydrochloride 40 mg by i.v. 30 min before the first dose of nimotuzumab. Dexamethasone 10 mg, cimetidine 400 mg and diphenhydramine hydrochloride 50 mg were given 30–60 min before paclitaxel infusion. Over a course of 8 h, and the day after, 2–3 L 0.9% saline (with 20 mL KCl 15%) was given as hydration with cisplatin infusion.
Radical radiotherapy was allowed to be admitted after four cycles of TPN treatment on the condition that patients had only local‐regional advanced disease. Nimotuzumab was also recommended to be administered during radiotherapy. If a patient's disease was considered to be resectable, then surgery was recommended. Palliative radiotherapy could be chosen to control the symptoms for patients with metastatic disease after four to six cycles of treatment if there is no disease progression. Dose modification was preplanned in the protocol based on adverse events.
The primary objectives of this trial were to monitor the objective response rate (ORR). Spiral CT imaging or MRI were performed every two cycles of treatment for efficacy evaluation according to the Response Evaluation Criteria in Solid Tumors objective efficacy evaluation standards (version 1.0). The second objectives were to monitor overall survival (OS), duration of disease control (DDC) and toxicity. OS is defined to the length of time from first dose of treatment until the time of death; duration of disease control (DDC) was measured from the date of initiating chemotherapy to progression no matter what treatments patients accepted after chemotherapy. The follow‐up visit of DDC was performed every two cycles. Toxicities were evaluated according to the National Cancer Institute Common Toxicity Criteria version 2.0.
Statistical methods
This was a prospective phase II clinical trial. The ORR was expected to be improved by 20% in patients with advanced ESCC. Based on ORR of 35% in chemotherapy (paclitaxel and cisplatin) alone, the ORR was expected to be 55% with the addition of nimotuzumab. We assumed that for a one‐sided test α = 0.05; the power was 0.80 and at least 41 cases needed to be included. Considering that 20% of enrolled patients were missing during follow‐up, 50 cases were required to ensure 41 cases for analysis.
Results
Patient and tumor characteristics
From March 2011 to April 2013, 59 patients were enrolled and 56 were eligible for evaluation (Fig. 1). A total of three patients were ineligible, and were, hence, excluded from the analysis: one patient was found with concurrent thyroid disease, one patient had received radiotherapy 2 months before enrollment and one patient did not receive nimotuzumab treatment. Patient and tumor characteristics of 56 eligible patients are listed in Table 1. All the patients included were evaluated for safety and tumor response. The median age of the enrolled patients was 61.5 years (range, 42–75 years), with 46 males and 10 females, and most patients (96.4%) had a good performance status (ECOG 0‐1). Nine patients had received prior curative intended surgery and, of these, one patient had received adjuvant radiotherapy and one patient had received adjuvant chemotherapy. Lymph nodes (98.2%) were the main target site of metastases, followed by liver (14.3%) and lung (14.3%).
Figure 1.
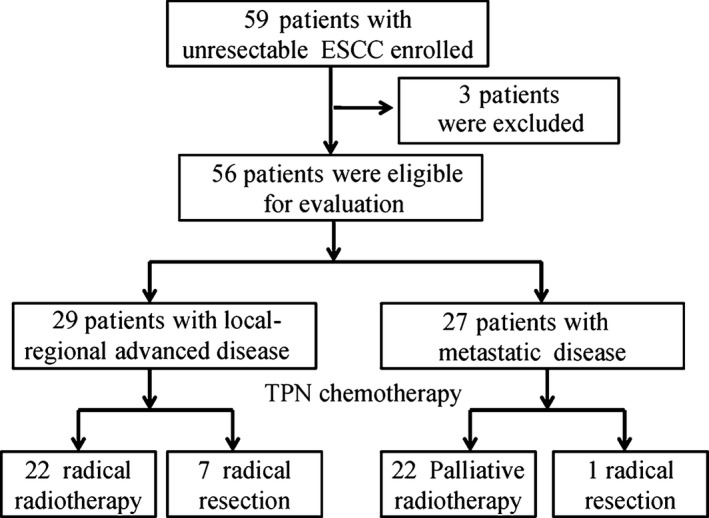
Study enrollment flow chart.
Table 1.
Characteristics of patients with ESCC (n = 56)
| Characteristics | Number of patients (%) |
|---|---|
| Age (years) | |
| Median | 61.5 |
| Range | 42–75 |
| Gender | |
| Male | 46 (82.1) |
| Female | 10 (17.9) |
| ECOG performance status | |
| 0 | 20 (35.7) |
| 1 | 34 (60.7) |
| 2 | 2 (3.6) |
| Metastatic sites | |
| Liver | 8 (14.3) |
| Lung | 8 (14.3) |
| Lymph nodes | 55 (98.2) |
| Previous treatment | |
| Esophagectomy | 9 (16.1) |
| Adjuvant chemotherapy | 1 (1.8) |
| Adjuvant radiotherapy | 1 (1.8) |
| Location of primary tumor | |
| Upper | 5 (8.9) |
| Middle | 32 (57.1) |
| Lower | 19 (33.9) |
| Tumor grade | |
| Well differentiated | 1 (1.8) |
| Moderate differentiated | 26 (46.4) |
| Poor differentiated | 29 (51.8) |
ESCC, esophageal squamous cell carcinoma.
Efficacy
A total of 191 treatment cycles with a median of four cycles (1.0–6.0) per patient were administered. All patients were evaluated for tumor response: 28 patients achieved partial response (PR), 23 patients had stable disease (SD) and four patients had progressive disease (PD), with an ORR of 51.8% (95% CI, 0.38–0.65) and a disease control rate (CR + PR + SD) of 92.9% (95% CI, 0.83–0.98). There were 29 patients with local‐regional advanced disease, among which 12 patients had PR (41.4%, [95% CI, 0.24–0.61]) and 15 patients had SD. Of 27 patients with metastatic lesion(s), 17 patients achieved PR (63.0% [95% CI, 0.42–0.81]) and 8 patients SD. (Table 2).
Table 2.
Clinical response of TPN treatment in ESCC (N = 56)
| Response | Number of patients | %/Month | 95% CI |
|---|---|---|---|
| All patients | 56 | ||
| PR | 29 | 51.8 | |
| SD | 23 | ||
| PD | 4 | ||
| ORR | 29/56 | 51.8 | 0.38–0.65 |
| DCR | 52/59 | 92.9 | 0.83–0.98 |
| DDC(m) | 10.8 | 5.9–15.7 | |
| OS(m) | 20.2 | 11.5–28.9 | |
| Local‐regional disease | 29 | ||
| PR | 12 | ||
| SD | 15 | ||
| PD | 2 | ||
| ORR | 12/29 | 41.4 | 0.24–0.61 |
| DDC(m) | 23.0 | 8.98–37.1 | |
| OS(m) | Not reached | ||
| Metastatic disease | 27 | ||
| PR | 17 | ||
| SD | 8 | ||
| PD | 2 | ||
| ORR | 17/27 | 63.0 | 0.42–0.81 |
| DDC(m) | 8.2 | 5.3–11.2 | |
| OS(m) | 14.0 | 6.8–21.2 |
ESCC, esophageal squamous cell carcinoma; TPN, nimotuzumab with paclitaxel and cisplatin. PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate; DDC, duration of disease control; OS, overall survival.
Survival was followed until January 2014 and the median follow‐up time was 24.1 (8.8–34.1) months. A total of 33 (58.9%) patients experienced disease progression and 29 (51.8%) patients died, including one death due to complications in surgery.The median DDC and OS in all the patients were 10.8 months (95% CI, 5.9–15.7 months) and 20.2 months (95% CI, 11.5–28.9 months), respectively. Among patients with local‐regional advanced disease, the median DDC time was 23.0 months (95% CI, 8.98–37.1 months), and OS was not reached by the time of the data analysis. Among 27 patients with metastatic disease, median DDC and OS time were 8.2 (95% CI, 5.3–11.2) months (Fig. 2) and 14.0 (95% CI, 6.8–21.2) months (Fig. 3).
Figure 2.
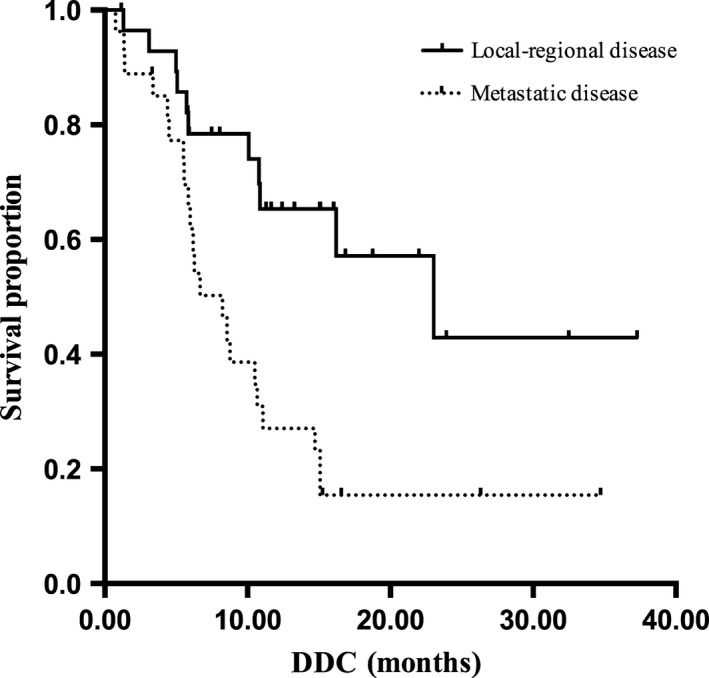
The median duration of disease control (DDC) of esophageal squamous cell carcinoma (ESCC) patients with local‐regional advanced disease and metastatic disease accepted nimotuzumab with paclitaxel and cisplatin (TPN) treatment.
Figure 3.
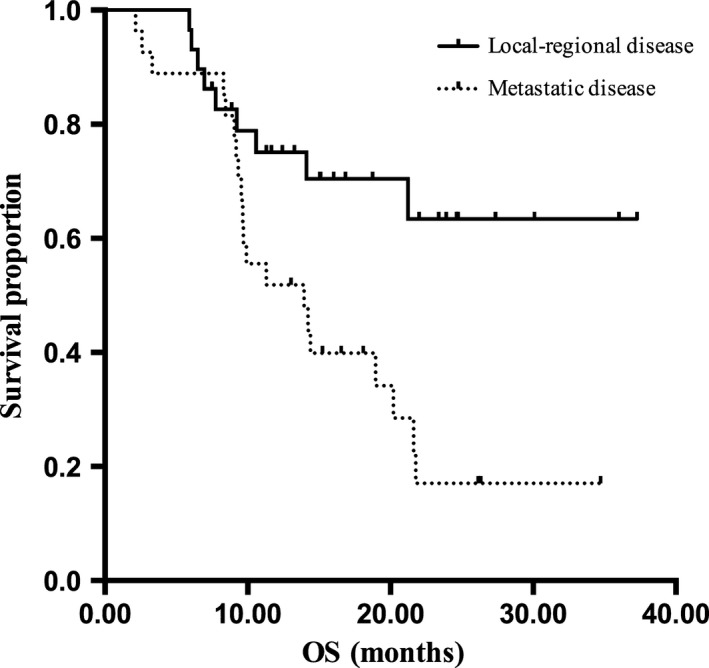
The overall survival of esophageal squamous cell carcinoma (ESCC) patients with local‐regional advanced disease and metastatic disease receiving nimotuzumab with paclitaxel and cisplatin (TPN) treatment.
Toxicity
The main hematological and non‐hematological toxicities of all patients are summarized in Table 3. The most common grade 3/4 hematological adverse events were neutropenia (46.4%) and leukopenia (25%). Nearly all the patients experienced at least one non‐hematological adverse event during the study, most of which were mild to moderate in severity. Nausea (48.3%), alopecia (78.6%), anorexia (42.8%), fatigue (69.6%), vomiting (55.4%) and arthralgia (62.5%) were the most common non‐hemotological adverse effects (all grades). There was no treatment‐related deaths during the study or within 28 days following the last study dose.
Table 3.
Adverse events of TPN as first line treatment for ESCC (N = 56)
| Adverse events | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Grade 4 (%) |
|---|---|---|---|---|
| Neutropenia | 9 (16.1) | 5 (8.9) | 19 (33.9) | 7 (12.5) |
| Leukopenia | 12 (21.4) | 15 (26.8) | 12 (21.4) | 2 (3.6) |
| Thrombopenia | 4 (7.1) | 2 (3.6) | 0 | 0 |
| Anaemia | 18 (32.1) | 2 (3.6) | 1 (1.8) | 0 |
| Febrile neutropenia | 1 (1.8) | 0 | 2 (3.6) | 0 |
| Fatigue | 31 (55.4) | 7 (12.5) | 1 (1.8) | 0 |
| Anorexia | 20 (35.7) | 4 (7.1) | 1 (1.8) | 0 |
| Stomatitis | 6 (10.7) | 2 (3.6) | 0 | 0 |
| Nausea | 24 (42.9) | 3 (5.4) | 0 | 0 |
| Vomiting | 20 (35.7) | 8 (14.3) | 3 (5.4) | 0 |
| Dry Skin | 9 (16.1) | 0 | 0 | 0 |
| Constipation | 6 (10.7) | 1 (1.8) | 0 | 0 |
| Alopecia | 17 (30.4) | 27 (48.2) | 0 | 0 |
| Skin pruritus | 15 (26.8) | 0 | 0 | 0 |
| Skin pigmentation | 3 (5.4) | 0 | 0 | 0 |
| Arthralgia | 24 (42.9) | 3 (5.4) | 1 (1.8) | 0 |
| Neuropathy | 15 (26.8) | 3 (5.4) | 2 (3.6) | 0 |
| Liver function change | 5 (8.9) | 2 (3.6) | 0 | 0 |
| Hematuria | 1 (1.8) | 0 | 0 | 0 |
| Proteinuria | 1 (1.8) | 0 | 0 | 0 |
ESCC, esophageal squamous cell carcinoma; TPN, nimotuzumab with paclitaxel and cisplatin.
Subsequent treatment
Among 29 patients with local‐regional advanced disease, 22 received radical radiotherapy and seven patients underwent radical resection following chemotherapy. Among 27 patients with metastatic disease, 22 cases received palliative radiotherapy, including 5 for metastatic lesions and 17 for primary lesions; one patient underwent radical resection.
Discussion
Esophageal squamous cell carcinoma is one of the most common forms of malignant tumor in China. Most patients' disease is in the advanced stages when diagnosed, and they are, hence, not eligible for surgery, and their prognosis is poor. The natural disease history is 6–10 months.13 Even after surgical resection, more than half of patients still suffer from recurrence and metastasis. Chemotherapy and radiotherapy are the main treatment methods for patients not eligible for surgery and for those with occurrence. Radiotherapy plays an irreplaceable role in the local treatment, while chemotherapy mainly aims to control the disease systemically. Taxane plus cisplatin has been widely used for ESCC, with better efficacy and safety profile than classical 5‐FU and cisplatin combination. However, there has been no major breakthrough in the overall survival rates for advanced ESCC.
The EGFR signal pathway plays an important role in the occurrence and development of epithelial‐derived tumors, including ESCC. It has been shown that the overexpression of EGFR is common in ESCC, with an incidence of 50–70%,14, 15, 20 and the overexpression is significantly related to prognosis.15, 16, 20 Nimotuzumab is a recombinant humanized monoclonal IgG1 antibody against human EGFR and blocks the binding of EGF and transforming growth factor‐a to EGFR. Nimotuzumab has shown clinical efficacy in head and neck cancer, NSCLC, or glioma as combination therapy with radiotherapy or chemo‐radiotherapy.17, 18, 21 Clinical trials of nimotuzumab have also been performed for ESCC. A phase II study that compared nimotuzumab plus concurrent chemoradiotherapy with fluorouracil and cisplatin in the treatment of stage III/IV ESCC resulted in a great improvement in efficacy (47.8 vs 15.4%, P = 0.014), the disease control rate (60.9 vs 26.9%, P = 0.017) and median survival (8.1 vs 2.97 months) in the nimotuzumab group.19 In another study that reported the results of 19 cases who received nimotuzumab in combination with fluorouracil and cisplatin, the efficacy was 42.1%, the disease control rate was 68.4%, and the main adverse effects were bone marrow suppression and gastrointestinal reactions.22
The combination of nimotuzumab, with TP regimen in this study demonstrated very encouraging results in the treatment of patients with advanced and metastatic ESCC. The overall response rate was 51.8% (95% CI: 0.38–0.65), with a disease control rate of 92.9% in the whole study group. In the patients with metastatic disease, the combination achieved an ORR of 63%, DDC of 8.2 months, and median OS of 14 months. The combination of nimotuzumab with TP attained the goal of the study design with statistical significance (the least efficacy >35%). The results for the combination of nimotuzumab with TP from this study suggest that it is more effective than the combination of nimotuzumab with 5‐FU and cisplatin in metastatic ESCC.
Of the 29 patients with local‐regional advanced cancer, 12 had PR (41.4%), and, among them, seven patients (24.1%) underwent radical surgery after chemotherapy; the remaining 22 patients received radical radiotherapy. The DDC in the subgroup was 23 months and the median OS had not been reached by the time of the data analysis. The study indicates that nimotuzumab in combination with paclitaxel and cisplatin is effective and may be a choice of pre‐operative or pre‐irradiative treatment for locally advanced ESCC.
Patients tolerated this triple regimen well and showed good compliance. Nimotuzumab in combination with TP regimen accompanied with 46.4% grade 3/4 granulocytopenia and 5.4% neutropenia fever. There were no therapy‐related deaths. Other common adverse events were fatigue and gastrointestinal reaction. Nimotuzumab‐related skin draught and pruritus, which were reported previously in other studies, were not noticed in the present study.23, 24 Interestingly, unlike for other EGFR inhibitors, an acne‐like rash was not observed. In general, the study regimen was well‐tolerated.
In conclusion, the combination of nimotuzumab with TP (TPN) is safe and effective with significantly improved ORR, DDC and OS in the treatment of patients with locally advanced initially unresectable and metastatic ESCC. A randomized phase III study is warranted for further confirming the efficacy of this combination.
Disclosure Statement
All authors declare that there is no conflict of interest.
Acknowledgments
The authors thank the participating patients and their families, as well as the network of research nurses, trail coordinators and BioNTech Company for assistance during the collection of data.
Cancer Sci 107 (2016) 486–490
Funding Information
No sources of funding were declared for this study.
References
- 1. Zhao P, Dai M, Chen WQ, Li N. Cancer trends in China. Jpn J Clin Oncol 2010; 40: 281–5. [DOI] [PubMed] [Google Scholar]
- 2. Liu W, Hao XS, Jin Y et al Analysis of clinicopathologic features of esophageal cancer patients after surgery – a report of 4329 cases. Zhongguo Zhong Liu Lin Chuang 2008; 35: 241–4. (In Chinese.) [Google Scholar]
- 3. Bleiberg H, Conroy T, Paillot B et al Randomised phase II study of cisplatin and 5‐fluorouracil (5‐FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer 1997; 33: 1216–20. [DOI] [PubMed] [Google Scholar]
- 4. Iizuka T, Kakegawa T, Ide H et al Phase II evaluation of cisplatin and 5‐fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japanese Esophageal Oncology Group Trial. Jpn J Clin Oncol 1992; 22: 172–6. [PubMed] [Google Scholar]
- 5. Ilson DH. Oesophageal cancer: new developments in systemic therapy. Cancer Treat Rev 2003; 29: 525–32. [DOI] [PubMed] [Google Scholar]
- 6. Polee MB, Kok TC, Siersema PD et al Phase II study of the combination cisplatin, etoposide, 5‐fluorouracil and folinic acid in patients with advanced squamous cell carcinoma of the esophagus. Anticancer Drugs 2001; 12: 513–7. [DOI] [PubMed] [Google Scholar]
- 7. Hayashi K, Ando N, Watanabe H et al Phase II evaluation of protracted infusion of cisplatin and 5‐fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407). Jpn J Clin Oncol 2001; 31: 419–23. [DOI] [PubMed] [Google Scholar]
- 8. Ilson DH, Ajani J, Bhalla K et al Phase II trial of paclitaxel, fluorouracil, and cisplatin in patients with advanced carcinoma of the esophagus. J Clin Oncol 1998; 16: 1826–34. [DOI] [PubMed] [Google Scholar]
- 9. Tanaka T, Fujita H, Sueyoshi S et al Second‐line combination chemotherapy with docetaxel for cisplatin‐pretreated refractory metastatic esophageal cancer: a preliminary report of initial experience. Chemotherapy 2007; 53: 449–53. [DOI] [PubMed] [Google Scholar]
- 10. Gong Y, Ren L, Zhou L et al Phase II evaluation of nedaplatin and paclitaxel in patients with metastatic esophageal carcinoma. Cancer Chemother Pharmacol 2009; 64: 327–33. [DOI] [PubMed] [Google Scholar]
- 11. Fujita Y, Hiramatsu M, Kawai M et al Evaluation of combined docetaxel and nedaplatin chemotherapy for recurrent esophageal cancer compared with conventional chemotherapyusing cisplatin and 5‐fluorouracil: a retrospective study. Dis Esophagus 2008; 21: 496–501. [DOI] [PubMed] [Google Scholar]
- 12. Zhang X, Shen L, Li J, Li Y, Li J, Jin M. A phase II trial of paclitaxel and cisplatin in patients with advanced squamous‐cell carcinoma of the esophagus. Am J Clin Oncol 2008; 31: 29–33. [DOI] [PubMed] [Google Scholar]
- 13. Levard H, Pouliquen X, Hay JM et al 5‐Fluorouracil and cisplatin as palliative treatment of advanced oesophageal squamous cell carcinoma. A multicentre randomised controlled trial. The French Associations for Surgical Research. Eur J Surg 1998; 164: 849–57. [DOI] [PubMed] [Google Scholar]
- 14. Hanawa M, Suzuki S, Dobashi Y et al EGFR protein overexpression and gene amplification in squamous cell carcinomas of the esophagus. Int J Cancer 2006; 118: 1173–80. [DOI] [PubMed] [Google Scholar]
- 15. Gibault L, Metges JP, Conan‐Charlet V et al Diffuse EGFR staining is associated with reduced overall survival in locally advanced oesophageal squamous cell cancer. Br J Cancer 2005; 93: 107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ozawa S, Ueda M, Ando N et al Prognostic significance of epidermal growth factor receptor in esophageal squamous cell carcinomas. Cancer 1989; 63: 2169–73. [DOI] [PubMed] [Google Scholar]
- 17. Crombet T, Torres L, Neninger E et al Pharmacological evaluation of humanized anti‐epidermal growth factor receptor, monoclonal antibody h‐R3, in patients with advanced epithelial‐derived cancer. J Immunother 2003; 26: 139–48. [DOI] [PubMed] [Google Scholar]
- 18. Crombet T, Osorio M, Cruz T et al Use of the humanized anti‐epidermal growth factor receptor monoclonal antibody h‐R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. J Clin Oncol 2004; 22: 1646–54. [DOI] [PubMed] [Google Scholar]
- 19. Ramos‐Suzarte M, Lorenzo‐Luaces P, Lazo NG et al Treatment of malignant, non‐resectable, epithelial origin esophageal tumours with the humanized anti‐epidermal growth factor antibody nimotuzumab combined with radiation therapy and chemotherapy. Cancer Biol Ther 2012; 13: 600–5. [DOI] [PubMed] [Google Scholar]
- 20. Wang KL, Wu TT, Choi IS et al Expression of epidermal growth factor receptor in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Cancer 2007; 109: 658–67. [DOI] [PubMed] [Google Scholar]
- 21. Ramos TC, Figueredo J, Catala M et al Treatment of high‐grade glioma patients with the humanized anti‐epidermal growth factor receptor (EGFR) antibody h‐R3: report from a phase I/II trial. Cancer Biol Ther 2006; 5: 375–9. [DOI] [PubMed] [Google Scholar]
- 22. Ling Y, Chen J, Tao M et al A pilot study of nimotuzumab combined with cisplatin and 5‐FU in patients with advanced esophageal squamous cell carcinoma. J Thorac Dis 2011; 4: 58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Babu KG, Prabhash K, Vaid AK et al Nimotuzumab plus chemotherapy versus chemotherapy alone in advanced non‐small‐cell lung cancer: a multicenter, randomized, open‐label Phase II study. OncoTargets Ther 2014; 7: 1051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma Ning‐Yi, Cai Xu‐Wei, Xiao‐Long Fu et al Safety and efficacy of nimotuzumab in combination with radiotherapy for patients with squamous cell carcinoma of the esophagus. Int J Clin Oncol 2014; 19: 297–302. [DOI] [PubMed] [Google Scholar]


