Abstract
Background:
Cachexia can occur as part of many end-stage or chronic diseases, and chronic obstructive pulmonary disease. This study was aimed to evaluate the effect of Pentoxifylline in patients with cancer cachexia.
Materials and Methods:
The present study was conducted as a double-blind randomized controlled trial on 70 patients with advanced malignancy who loss of >5% of ideal or preillness body weight in the previous 2 months. Patients were assessed in two groups: case group, under treatment, using Pentoxifylline (400 mg) three times a day, for 2 months, and in the control group, patients received placebo. Age, sex, weight change, change in arm circumference and quality of life were assessed at baseline, week-4 and week-8.
Results:
The mean age of the patients was 56 ± 17.3 years and 47% were female. Weight and arm circumference decreased during follow-up in both groups, but these differences between case and controls were not statistically significant. Quality of life (QOL) score in the case group improved after 4 weeks then decreased at the end of treatment but in the control group QOL score decreased during 2 month treatment. In week-4 patients in the case group significantly reported higher score of QOL compare to patients in the control group (P = 0.029).
Conclusion:
Results of this study demonstrated that Pentoxifylline in the treatment of cancer cachexia did not have any effect in weight gain and arm circumference in cachectic patients. But in short-term (1 month) treatment, QOL was improved in these patients. And after 2 month treatment this was not effective compared to placebo.
Keywords: Cancer cachexia, malignancy, Pentoxifylline, quality of life
INTRODUCTION
Cachexia is a devastating syndrome of body wasting; the word of cachexia is derived from the Greek words kakos and hexia “bad condition”.[1,2] It is a caused by several factors of which comprises adipose tissue loss and skeletal muscle which may be compounded by anorexia leading to reduced food intake, a dysregulated metabolic state with increased basal energy expenditure and is resistant to conventional nutritional support.[3,4,5,6,7] Cachexia accompanies the end stage of several chronic diseases including chronic kidney disease and chronic heart failure, in particular, cancer[8,9] and has long been known as a key sign in many cancers.[2] Weight loss occurs in 30-80% of patients with cancer, and is severe, with the initial body weight loss of 10% in 15% of the patients.[10] The prevalence of cancer-related anorexia/cachexia syndrome increases from 50% to more than 80% before death, and in more than 20% of cancer patients it is the cause of death.[11,12] Also, in cancer patients, cachexia is estimated to be the cause of 20% to 40% of immediate death.[13,14,15,16] Previous studies showed that cancer cachexia is cause of reduces performance status, decreased response to chemotherapy impaired in quality of life (QOL), more frequent and severe toxicity of anticancer therapy and a high mortality rate in cancer patients.[17,18,19,20] Managing of cancer-related anorexia/cachexia is a complex challenge and unfortunately, in adults with advanced solid tumors, this remains an infrequent achievement. To date, the practice guidelines for the treatment and prevention of cancer-related anorexia/cachexia and its muscle wasting/loss, apart from growth hormone and some appetite stimulants, are lacking despite several years of coordinated efforts in basic and clinical research.[21,22]
The specific etiology and mechanisms of cancer cachexia despite its profound clinical impact are incompletely understood. Meanwhile cachexia can obvious in individuals with metastatic cancer as well as in individuals with localized disease. Also, cachexia does not appear to be the result of tumor size, type, or extent.[23] However, it is showed that the proinflammatory cytokines interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α play central roles in the pathophysiology of cancer-related anorexia/cachexia syndrome.[24,25] The role of TNF-α cachexia is still subject to debate, while some studies show TNF-α to be detectable in the serum of 36.5 patients with pancreatic cancer with the level of TNF-α in serum being adversely correlated with the body weight and body mass index, and serum protein and albumin levels.[26] Appetite is affected by many cytokines, including interleukin IL-1, and IL-6 as well as the TNF-α.[27] The cytokines cross the brain barrier, interact with the luminal surface of the brain endothelial cells to release substances that blunt the appetite.[28] TNF-α and IL-1 receptors are found in the hypothalamic areas of the brain, which is responsible for the regulation of food intake. Inhibitors of cyclo-oxygenase can block the anorexia caused by both TNF-α and IL-6, suggesting that a prostaglandin, such as PGE3, plays the role of a direct mediator of appetite suppression.[29] Pentoxifylline can inhibit the action of TNF. The production of TNF-α has been shown to be downregulated by Pentoxifylline which would be effective in the attenuation or reversal of the weight loss process seen in patients with cancer cachexia.[30,31] Therefore, the present study was designed to evaluate the effects of oral Pentoxifylline compared with placebo in patients with cancer cachexia.
MATERIALS AND METHODS
In this double-blind randomized controlled trial, between October 2011 and June 2012, 70 patients with advanced malignancy who loss of >5% of ideal or preillness body weight in the previous 2 months were studied. Patients of any gender, older than 18 years old with histologic or cytologic proof evidencing cancers other than brain cancer were eligible if they had incurable malignancy with a life expectancy ≥4 months. Other inclusion criteria also included: Weight loss perceived by the patient as a problem, patient should be alert and mentally competent. Patients who ongoing tube feeding or non-intravenous nutrition, patients affliction with edema or ascites, adrenal corticosteroids treatment (except for dexamethasone during chemotherapy) androgens, progestational agents, or other appetite stimulants within the previous month, brain metastases, pregnancy or lactation, mechanical obstruction of the alimentary tract, malabsorption, or intractable vomiting, anticipated alcohol or barbiturate use during the study period, poorly controlled hypertension or congestive heart failure, were exclude from the study. Patients were allowed to begin or continue chemo- or radio-therapy. Written informed consent, after the participating patients were explained about and informed of the purposes of the study, was obtained from them all. This study was investigated and approved in Isfahan University of Medical Sciences.
Patients meeting the inclusion criteria were random divided into two 35-member groups of case and control, using random-maker software “Random Allocation”. In the case group, 35 patients, under treatment, using Pentoxifylline (400 mg) three times a day, and in the control group, patients received placebo (formally, manufactured as the same as Pentoxifylline tablets, in Isfahan University, faculty of pharmacy,) three times a day. Duration of treatment was 2 months; also, patients were unaware of the treatment allocation and were followed up monthly by a doctor who was blinded to the study groups.
Age, sex, weight change, change in arm circumference and QOL were variables to be observed in this study. Variables were assessed at baseline, week-4 and week-8. Patients were weighed without shoes and in light clothes. Also, changes in QOL were determined using the SF-36 standard questionnaire that includes a global health status and QOL scale which was described before. Variables of the subscale were scored on the basis of a 0-100 (not at all to very much).
Statistical analyses were done by “SPSS-20”. Data are reported as Mean±SD and number (percent). Age, weight change, change in arm circumference and QOL score were compared between groups at baseline, week-4 and week-8 using Independent sample T-test. Sex in study groups was assessed applying the chi-square test and change in weight, arm circumference and QOL score during the evaluation time periods was compared between groups using repeated measurements of ANOVA. The level of significance is considered to be less than 0.05.
RESULTS
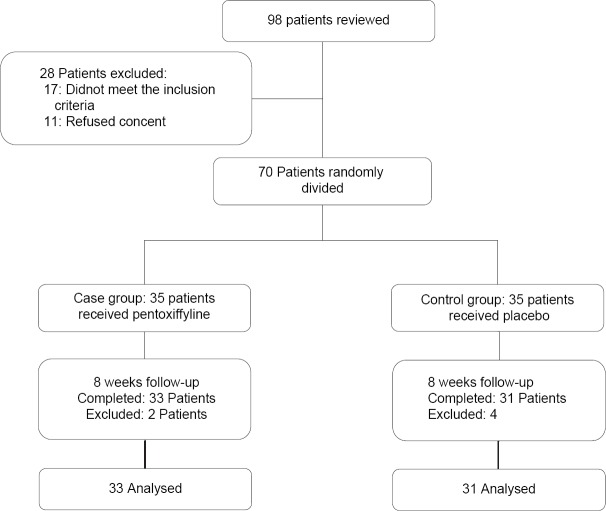
Of 98 reviewed patients 28 were not eligible and did not enter into the study. Also, during follow-up 4 patients in the case group (1 patient due to nausea and vomiting, 2 patients due to the resultant tachycardia and 1 patient did not desire to continue) and 2 patients in the control group (no desire to continue) were excluded, finally, 64 patients (31 cases and 33 controls) completed the study and analyzed [Figure 1].
Figure 1.
Patients who entered the study, divided into the study groups and analyzed
The mean age of the studied patients was 56 ± 17.3 years. Thirty-four patients (53%) were males and 30 patients (47%) were females. Comparison of characteristics and changes in weight, arm circumference and QOL score in studied groups are shown in Table 1. Mean of age and sex combination were not significant between studied groups. Weight and arm circumference decreased during follow-up in both groups, but these differences between case and controls were not statistically significant. QOL score in case group improved after 4 weeks then decreased at the end of treatment compared to baseline and week-4, in contrast in the control group QOL score decreased during 2 month treatment. The result of the test invested that differences in QOL score changes during the evaluation time periods was statistically significant between groups of case and control. Moreover, during study time periods, there was no significant differences in the mean score of QOL between the study groups at baseline and week-8. But in week-4 patients in the case group significantly reported higher score of QOL compared to patients in the control group [Figure 2].
Table 1.
Baseline characteristics and changes in weight, arm circumference and QOL score in patients with cancer cachexia by group
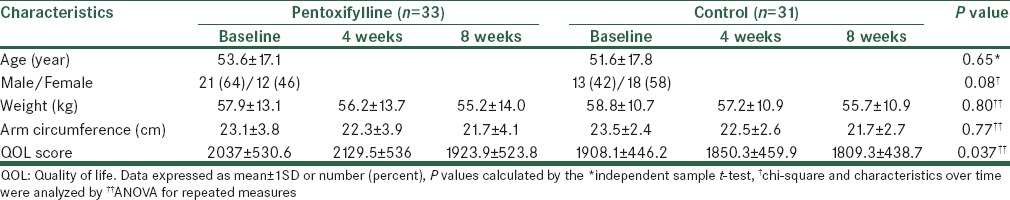
Figure 2.
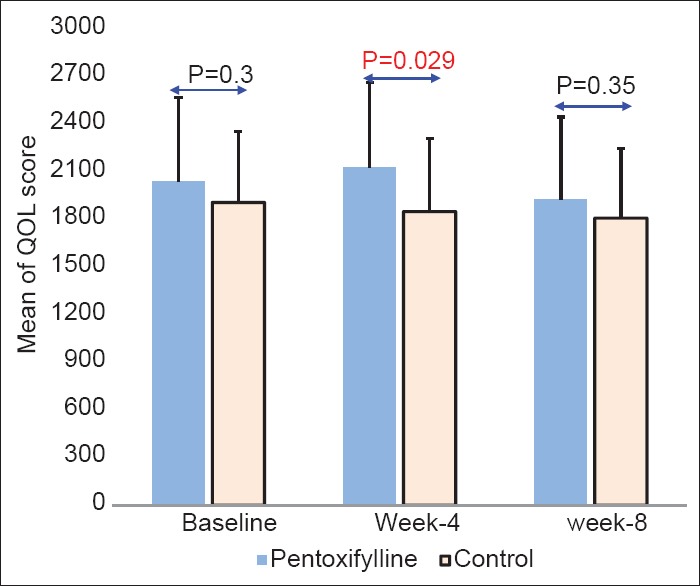
Comparison of mean on quality of life (QOL) between study groups. Error bars are expressed as SD. P-values calculated by the independent samples test. Statistical significant differences were found between groups in week-4
During the study period, three patients in case group reported side effects (nausea and vomiting and tachycardia) whereas in the control group side effect was not observed. However, there was difference in the incidence of side effects between both studied groups (P value = 0.21).
DISCUSSION
Cachexia can occur as part of many end-stage or chronic diseases, and chronic obstructive pulmonary disease. Results of the present study, which aimed to evaluate the effects of oral Pentoxifylline compare with placebo in patients with cancer cachexia, showed that, during follow-up, similar decreasing was occur in cases compare to controls for weight and arm circumference. After 4 weeks, QOL score in patients who received Pentoxifylline improved compare to baseline and decreased after 8 weeks compared to baseline and week-4. In the control group QOL score decreased during follow-up. QOL score in week-4 in the case group was significantly was higher than the control group but 2 months after treatment QOL score in both groups was similar. Our results showed the effect of that treatment with Pentoxifylline on patients QOL in short term (4 weeks).
However, treatment of the cancer as this will completely reverse the cachexia syndrome is the best management of cancer cachexia. Inappropriately, in adults with progressive solid tumors, this remains a rare achievement. Counteract weight loss by increasing nutritional intake could be another choice, but nutrition as a unimodal therapy was not totally able to reverse the wasting related to cachexia. Therefore, based on these results, with the purpose of providing symptomatic improvement, trials to manipulate the procedure of cachexia with a variety of pharmacological agents were managed.[32]
Currently, progestogens (medroxyprogesterone and megestrol) are considered the best available treatment option for cancer anorexia-cachexia syndrome. And in Europe, are approved for treatment of cancer- and AIDS-related cachexia. However, progestogens in their ability to treat cancer cachexia are limited. In patients treated with megestrol acetate in short term, lower than 30 percent appetite stimulation,[33] and even though weight and desire for food advance, there is no revealed development in QOL.[34,35] Also, other study demonstrates that weight and appetite increased significantly, and the QOL improved significantly, and proinflammatory cytokines levels decreased significantly, in male patients with head and neck cancer in advanced stage.[36]
In experimental models of cachexia with some positive results, drugs able to inhibit the synthesis and/or release of cytokines (eicosapentaenoic acid, and melatonin), anti-cytokine antibodies, anti-inflammatory cytokines interleukin (IL-12, and IL-15), and bortezomib have been tested. Inappropriately, limited or disappointing results have provided in other clinical studies in humans. In a meta-analysis in 2007[37] authors concluded that there were inadequate data to find whether oral eicosapentaenoic acid was better than placebo. Besides, it is showed that, no evidence was found in improving symptoms associated with the cachexia syndrome in patients with advanced cancer who received eicosapentaenoic acid combined with a protein energy supplementation versus in patients who received a protein energy supplementation in the presence of megestrol acetate.
It is reviewed that Pentoxifylline, melatonin, cyclo-oxygenase-2 inhibitors and thalidomide have been tested in experimental models of cachexia, with some positive results and in humans have provided limited and disappointing results.[13] It is showed that only thalidomide has been effective to reduce TNF-α level both in vitro and in vivo and to enhance weight gain in cancer patients with cachexia.[5,38]
Clinical management of cachexia on treatment by Pentoxifylline is limited. Even though some studies using animal models advise that Pentoxifylline is able to reduce the cytokine-induced toxicity of antineoplastic agents.[39] One clinical study, on 70 patients with an Eastern Cooperative Oncology Group performance status with cancer anorexia and/or cachexia (defined by a weight loss of > or =5 lb in the preceding 2 months or a caloric intake <20 kcal/kg/d) has shown that the drug was unsuccessful to improve appetite or increase the weight of cachectic patients, either, patients frequently reported some side effects.[31] Similar to Goldberg et al. study,[31] results of the present study investigated that weight gain in cancer patients with cachexia who treated with Pentoxifylline was same to the placebo group. But in short term QOL was improved in the Pentoxifylline group, although QOL was not assessed in Goldberg et al. study.[31] Side effects occurred in the small number of the Pentoxifylline group in the present study but patients in Goldberg et al. study[31] frequently reported gastrointestinal side effects.
Some limitations of study that should be considered are as follows, first, the time period for assessing weight loss varied over time for patients with weight loss ≥5 percent. This study focused on the presence of weight loss during previous 2 months whereas the clinical course of a patient losing the weight over 12 or more is very different than a patient losing ≥5 percent of weight over 1-2 months. Maybe another limitation of this study is its sample size that can affect the results of the study and we were unable to detect small differences between studied groups for weight gain. However, the reported clinical trials using animal models and in human have been relatively small and despite the unsuccessful clinical findings reported herein, it is still plausible that this cytokine is a mediator of this syndrome, because of the role of TNF-α in mediating weight loss and appetite in the setting of cancer. Therefore, larger randomized studies are necessary to assess the efficacy of Pentoxifylline in the treatment of cancer cachexia. Also, it seems that treatment regimens involving different combinations may be a better approach in treating cachexia than single therapy and will be more successful.
In conclusion, results of this study demonstrated that, Pentoxifylline in the treatment of cancer cachexia did not have any effect in weight gain and arm circumference in cachectic patients. Also, in short-term (1 month) treatment, QOL was improved in these patients but this was not effective in long-term (2 months) treatment compared to the placebo group. It is suggested that the role of Pentoxifylline in the treatment of cancer cachexia should be further defined and confirmed in clinical trials with important issues such as dosage, duration, best time to start treatment, weight gain, arm circumference and as the overall quality of life.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bosaeus I, Daneryd P, Svanberg E, Lundholm K. Dietary intake and resting energy expenditure in relation to weight loss in unselected cancer patients. Int J Cancer. 2001;93:380–3. doi: 10.1002/ijc.1332. [DOI] [PubMed] [Google Scholar]
- 2.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 3.Donohoe CL, Ryan AM, Reynolds JV. Cancer cachexia: Mechanisms and clinical implications. Gastroenterol Res Pract 2011. 2011 doi: 10.1155/2011/601434. 601434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantovani G, Macciò A, Madeddu C, Serpe R, Massa E, Dessì M, et al. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist. 2010;15:200–11. doi: 10.1634/theoncologist.2009-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan ZH, Simpson EJ, Cole AT, Holt M, MacDonald I, Pye D, et al. Oesophageal cancer and cachexia: The effect of short-term treatment with thalidomide on weight loss and lean body mass. Aliment Pharmacol Ther. 2003;17:677–82. doi: 10.1046/j.1365-2036.2003.01457.x. [DOI] [PubMed] [Google Scholar]
- 6.Argilés JM, Busquets S, García-Martínez C, López-Soriano FJ. Mediators involved in the cancer anorexia-cachexia syndrome: Past, present, and future. Nutrition. 2005;21:977–85. doi: 10.1016/j.nut.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Fearon KC, Voss AC, Hustead DS Cancer Cachexia Study Group. Definition of cancer cachexia: Effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83:1345–50. doi: 10.1093/ajcn/83.6.1345. [DOI] [PubMed] [Google Scholar]
- 8.Richey LM, George JR, Couch ME, Kanapkey BK, Yin X, Cannon T, et al. Defining cancer cachexia in head and neck squamous cell carcinoma. Clin Cancer Res. 2007;13:6561–7. doi: 10.1158/1078-0432.CCR-07-0116. [DOI] [PubMed] [Google Scholar]
- 9.Bruera E. Clinical management of anorexia and cachexia in patients with advanced cancer. Oncology. 1992;49(Suppl 2):35–42. doi: 10.1159/000227126. [DOI] [PubMed] [Google Scholar]
- 10.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–7. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 11.Inui A. Cancer anorexia-cachexia syndrome: Current issues in research and management. CA Cancer J Clin. 2002;52:72–91. doi: 10.3322/canjclin.52.2.72. [DOI] [PubMed] [Google Scholar]
- 12.Bennani-Baiti N, Walsh D. What is cancer anorexia-cachexia syndrome? A historical perspective. J R Coll Physicians Edinb. 2009;39:257–62. [PubMed] [Google Scholar]
- 13.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–71. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 14.Strasser F, Bruera ED. Update on anorexia and cachexia. Hematol Oncol Clin North Am. 2002;16:589–617. doi: 10.1016/s0889-8588(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 15.Argilés JM, Busquets S, Felipe A, López-Soriano FJ. Muscle wasting in cancer and ageing: Cachexia versus sarcopenia. Adv Gerontol. 2006;18:39–54. [PubMed] [Google Scholar]
- 16.Giordano KF, Jatoi A. The cancer anorexia/weight loss syndrome: Therapeutic challenges. Curr Oncol Rep. 2005;7:271–6. doi: 10.1007/s11912-005-0050-9. [DOI] [PubMed] [Google Scholar]
- 17.Uomo G, Gallucci F, Rabitti PG. Anorexia-cachexia syndrome in pancreatic cancer: Recent development in research and management. JOP. 2006;7:157–62. [PubMed] [Google Scholar]
- 18.Bennani-Baiti N, Davis MP. Cytokines and cancer anorexia cachexia syndrome. Am J Hosp Palliat Care. 2008;25:407–11. doi: 10.1177/1049909108315518. [DOI] [PubMed] [Google Scholar]
- 19.Persson C, Glimelius B. The relevance of weight loss for survival and quality of life in patients with advanced gastrointestinal cancer treated with palliative chemotherapy. Anticancer Res. 2002;22:3661–8. [PubMed] [Google Scholar]
- 20.Scott HR, McMillan DC, Brown DJ, Forrest LM, McArdle CS, Milroy R. A prospective study of the impact of weight loss and the systemic inflammatory response on quality of life in patients with inoperable non-small cell lung cancer. Lung Cancer. 2003;40:295–9. doi: 10.1016/s0169-5002(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 21.Lainscak M, Filippatos GS, Gheorghiade M, Fonarow GC, Anker SD. Cachexia: Common, deadly, with an urgent need for precise definition and new therapies. Am J Cardiol. 2008;101:8–10E. doi: 10.1016/j.amjcard.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 22.Boddaert MS, Gerritsen WR, Pinedo HM. On our way to targeted therapy for cachexia in cancer? Curr Opin Oncol. 2006;18:335–40. doi: 10.1097/01.cco.0000228738.85626.ac. [DOI] [PubMed] [Google Scholar]
- 23.Fox KM, Brooks JM, Gandra SR, Markus R, Chiou CF. Estimation of cachexia among cancer patients based on four definitions. J Oncol 2009. 2009 doi: 10.1155/2009/693458. 693458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLaughlin CL, Rogan GJ, Tou J, Baile CA, Joy WD. Food intake and body temperature responses of rats to recombinant human inter leukin-1 beta and a tripeptide inter leukin-1 beta antagonist. Physiol Behav. 1992;52:1155–60. doi: 10.1016/0031-9384(92)90475-h. [DOI] [PubMed] [Google Scholar]
- 25.Perboni S, Inui A. Anorexia in cancer: Role of feeding-regulatory peptides. Philos Trans R Soc Lond B Biol Sci. 2006;361:1281–9. doi: 10.1098/rstb.2006.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karayiannakis AJ, Syrigos KN, Polychronidis A, Pitiakoudis M, Bounovas A, Simopoulos K. Serum levels of tumor necrosis factor-alpha and nutritional status in pancreatic cancer patients. Anticancer Res. 2001;21:1355–8. [PubMed] [Google Scholar]
- 27.Plata-Salamán CR, Oomura Y, Kai Y. Tumor necros is factor and inter leukin-1 beta: Suppression of food in take by directaction in the central nervous system. Brain Res. 1988;448:106–14. doi: 10.1016/0006-8993(88)91106-7. [DOI] [PubMed] [Google Scholar]
- 28.Banks WA. Anorectic effects of calculating cytokines: Role of the vascular blood-brainbarrier. Nutrition. 2001;17:434–7. doi: 10.1016/s0899-9007(01)00507-x. [DOI] [PubMed] [Google Scholar]
- 29.Hellerstein MK, Meydani S, Meydani M, Wu K, Dinarello CA. Interleukin-1-inducedanorexiaintherat. Influence of prostaglandins. J Clin Invest. 1989;84:228–35. doi: 10.1172/JCI114145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strassmann G, Kambayashi T. Inhibition of experimental cancer cachexia by anti-cytokine and anti-cytokine-receptor therapy. Cytokines Mol Ther. 1995;1:107–13. [PubMed] [Google Scholar]
- 31.Goldberg RM, Loprinzi CL, Mailliard JA, O'Fallon JR, Krook JE, Ghosh C, et al. Pentoxifylline for treatment of cancer anorexia and cachexia? A randomized, double-blind, placebo-controlled trial. J Clin Oncol. 1995;13:2856–9. doi: 10.1200/JCO.1995.13.11.2856. [DOI] [PubMed] [Google Scholar]
- 32.Mantovani G, Madeddu C. Cancer cachexia: Medical management. Support Care Cancer. 2010;18:1–9. doi: 10.1007/s00520-009-0722-3. [DOI] [PubMed] [Google Scholar]
- 33.Loprinzi CL, Ellison NM, Schaid DJ, Krook JE, Athmann LM, Dose AM, et al. Controlled trial of megestrol acetate for the treatment of cancer anorexia and cachexia. J Natl Cancer Inst. 1990;82:1127–32. doi: 10.1093/jnci/82.13.1127. [DOI] [PubMed] [Google Scholar]
- 34.Loprinzi CL, Kugler JW, Sloan JA, Mailliard JA, Krook JE, Wilwerding MB, et al. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J Clin Oncol. 1999;17:3299–306. doi: 10.1200/JCO.1999.17.10.3299. [DOI] [PubMed] [Google Scholar]
- 35.Jatoi A, Windschitl HE, Loprinzi CL, Sloan JA, Dakhil SR, Mailliard JA, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: A North Central Cancer Treatment Group study. J Clin Oncol. 2002;20:567–73. doi: 10.1200/JCO.2002.20.2.567. [DOI] [PubMed] [Google Scholar]
- 36.Mantovani G, Macciò A, Bianchi A, Curreli L, Ghiani M, Santona MC, et al. Megestrol acetate in neoplastic anorexia/cachexia: Clinical evaluation and comparison with cytokine levels in patients with head and neck carcinoma treated with neoadjuvant chemotherapy. Int J Clin Lab Res. 1995;25:135–41. doi: 10.1007/BF02592554. [DOI] [PubMed] [Google Scholar]
- 37.Dewey A, Baughan C, Dean T, Higgins B, Johnson I. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD004597.pub2. CD004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon JN, Trebble TM, Ellis RD, Duncan HD, Johns T, Goggin PM. Thalidomide in the treatment of cancer cachexia: A randomised placebo controlled trial. Gut. 2005;54:540–5. doi: 10.1136/gut.2004.047563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balázs C, Kiss E. Immunological aspects of the effect of pentoxifylline (trental) (a brief review) Acta Microbiol Immunol Hung. 1994;41:121–6. [PubMed] [Google Scholar]