Abstract
Zidovudine is an important component of first-line antiretroviral treatment regimens used to manage HIV and tuberculosis (TB) co-infection. Nail pigmentation is documented both in adult as well as pediatric HIV patients, but to the best of our knowledge, it has not been reported in 45-year-old women of HIV/TB co-infection. Such an adverse drugs reactions (ADR), although is harmless and reversible, psychological aspects of such ADR may be immense to the extent that it can negatively affect the compliance and result in therapeutic failure. Thus, it is worth reporting.
Keywords: Adverse drugs reactions, HIV and tuberculosis co-infection, nail hyper-pigmentation, Zidovudine
INTRODUCTION
More than half of the persons living with HIV infection in the United States will be ≥50 years of age by 2020, including postmenopausal women.[1] A “Cursed Duo,” i.e., TB/HIV co-infection is a major public health problem. Globally, one-third of the HIV patients are co-infected with tuberculosis (TB).[2] In India, NACO recommends a regimen containing zidovudine or stavudine along with lamivudine and efavirenz for such patients.[3]
Adverse drugs reactions (ADRs) are a great challenge to national anti-TB and HIV programme. Concomitant administration of highly active antiretroviral treatment (HAART) and anti-tubercular treatment possess significant challenge in the form of cumulative drug toxicities, drug-drug interactions at the pharmacokinetic and pharmacodynamic level leading to complexity of regimens and high pill burden. Advanced immunosuppression in HIV/TB co-infection is likely to increase the incidence of ADRs furthermore complicating the treatment outcomes and the natural history of TB/HIV co-infection. The ADRs can often negatively affect the compliance and can result in therapeutic failure.
Zidovudine is an important component of first-line antiretroviral treatment regimens used to manage HIV and TB co-infection. Nail pigmentation is documented both in adult as well as pediatric HIV patients.[4,5]
However, to the best of our knowledge, it has not been reported in 45-year-old women. Such an ADR, although is harmless and reversible, psychological aspects of such ADR may be immense to negatively affect the compliance. Furthermore, to better understand and create awareness regarding the health concerns of mature women living with HIV/TB co-infection as well as to formulate optimal treatment strategies for menopausal women living with HIV infection, the case is worth reporting.
CASE REPORT
A 45-year-old woman with 65 kg weight was a case of recently diagnosed TB/HIV co-infection. She was prescribed zidovudine (300 mg) + lamivudine (150 mg) + efavirenz (600 mg) and Category 2 DOTS Regimen: 2 (HRZES)3/1 (HRZE)/5 (HRE)3. She presented with nail pigmentation after 6 weeks of ingestion of above-mentioned therapy. It first appeared in greater toe nail, gradually increased in intensity over 2 weeks' time. Local examination revealed diffuse bluish-brown discoloration of thumbnails. It did not disappear on compression of nail plate. Nails were brittle and the pattern of nail pigmentation was diffuse in nature and there was no evidence of skin and mucus membranes being involved.
She had no previous history of any such ADR, hypertension, diabetes mellitus, any other chronic organic diseases, smoking or alcohol intake. The patient had even no history of any regular or long-term use of any drug. ELISA for HIV was positive. Polymerase chain reaction for RNA was positive. CD4+ count was 138 cells/mm3. Erythrocyte sedimentation rate — 47 mm (1 h), Mantoux test, and sputum samples for acid fast bacilli for three consecutive days were positive. A chest radiograph showed bilateral hilar opacities in both the lungs. A computed tomography scan of chest disclosed mediastinal lymphadenopathy.
Hemoglobin, 10.8 g%; total leukocyte count, 10,400/cumm; platelet count, 2.4 lac/cumm; blood urea, 19 mg/dl; serum creatinine, 0.8 mg/dl; blood sugar, 98 mg%; serum cholesterol, 180 mg%; triglycerides, 155 mg%; clotting and bleeding time was normal; serum uric acid, 6.6 mg/dl; bilirubin, 1 mg/dl; serum glutamic-oxaloacetic transaminase, 45 U/L; and serum glutamic pyruvic transaminase, 36 U/L. Thyroid profile was normal. Electrocardiogram and ultra-sonography were normal.
Temporal association between starting zidovudine HAART regimen and subsequent development of this ADR and appearance of such ADR could not be explained by any concurrent drug, disease, and chemical. Thus, a possible diagnosis of zidovudine-induced nail pigmentation was established. The de-challenge and re-challenge could not be done due to ethical and clinical constraint. No dose-related study was done in the current case because of similar reasons. The Naranjo's score was 7 and WHO causality assessment showed probable correlation with the current adverse event.[6,7] Severity of the reaction was assessed using Hartwig ADR severity assessment scale[8] which classified the said ADR into nonserious. Due to nonserious nature of the reaction, no change in the treatment was done in the current case except counseling.
DISCUSSION
Zidovudine-induced anemia, fatigue, malaise, myalgia, nausea, anorexia, headache, and insomnia are most common reported ADRs. In advanced HIV disease, bone marrow suppression, mainly anemia and granulocytopenia may also be encountered. However, chronic zidovudine administration has been relatively less documented to be associated with nail hyper-pigmentation [Figure 1].[9]
Figure 1.
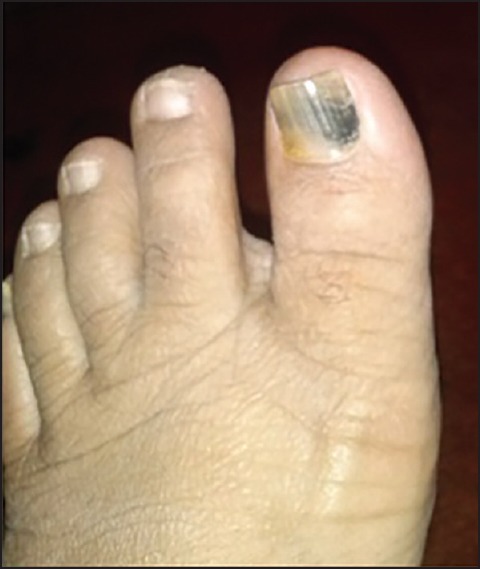
Showing nail hyper pigmentation in advancing age female patient due to zidovudine
0.95% and 1.95% of the incidence has been reported for hyper-pigmentation of skin and hyper-pigmentation of nail, respectively, among the patients receiving zidovudine.[10]
Drugs that are well-known to produce nail manifestations are cancer chemotherapeutic agents, psoralens, retinoids, tetracycline, antimalarials, and zidovudine.[11] Zidovudine is an important component of first-line antiretroviral treatment regimens used to manage HIV and TB co-infection. Nail pigmentation is documented both in adult as well as pediatric HIV.[4,5] Nail pigmentation occurs primarily in black patients. Unlike this, the current case was seen in Indian 45-year-old women. It appears to be reversible and relatively dose-dependent.[12] However, this was not studied in our case.
The underlying mechanism of nail pigmentation is not clear. Animal studies have shown that there are increased numbers of melanosomes within epidermal keratinocytes. Histopathologic findings of nail biopsy show deposits of brown pigmented granules containing melanin throughout the epidermis.[13]
Such an ADR although is harmless and reversible, psychological aspects of such ADR may be immense to hamper adherence to therapy and may lead to unnecessary investigations and treatment for misdiagnosis for such ADR as cyanosis or melanoma.
Thus, the current case report highlights and tries to draw attention and create awareness among prescribers regarding nonserious ADR of zidovudine-induced nail pigmentation in advancing age women of HIV/TB co-infection.
Financial support and sponsorship
PvPI, IPC- Ghaziabad, and NACO India.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Imai K, Sutton MY, Mdodo R, Del Rio C. HIV and menopause: A systematic review of the effects of HIV infection on age at menopause and the effects of menopause on response to antiretroviral therapy. Obstet Gynecol Int 2013. 2013 doi: 10.1155/2013/340309. 340309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Londhey VA. HIV and tuberculosis — A “cursed duo” in the HAART era. J Assoc Physicians India. 2009;57:681–2. [PubMed] [Google Scholar]
- 3.NACO. Antiretroviral Therapy Guidelines for HIV Infected Adults and Adolescents Including Post-exposure Prophylaxis — National AIDS Control Organization. 2013. [Last cited on 2014 Aug 23]. Available from: http://www.naco.gov.in .
- 4.Singh SK, Rai T. A case of zidovudine induced pigmentation on palms and soles. Indian Dermatol Online J. 2014;5:98–9. doi: 10.4103/2229-5178.126057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawre SM, Pore SM, Nandeshwar MB, Masood NM. Zidovudine-induced nail pigmentation in a 12-year-old boy. Indian J Pharmacol. 2012;44:801–2. doi: 10.4103/0253-7613.103306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 7.Edwards IR, Aronson JK. Adverse drug reactions: Definitions, diagnosis, and management. Lancet. 2000;356:1255–9. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 8.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229–32. [PubMed] [Google Scholar]
- 9.Gupta SB, Pujari SN, Joshi SR, Patel AK. AIDS Society of India Guidelines Development Committee. API consensus guidelines for use of antiretroviral therapy in adults (API-ART guidelines). Endorsed by the AIDS Society of India. J Assoc Physicians India. 2006;54:57–74. [PubMed] [Google Scholar]
- 10.Srikanth BA, Babu SC, Yadav HN, Jain SK. Incidence of adverse drug reactions in Human immunedeficiency virus-positive patients using highly active antiretroviral therapy. J Adv Pharm Technol Res. 2012;3:62–7. doi: 10.4103/2231-4040.93557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piraccini BM, Tosti A. Drug-induced nail disorders: Incidence, management and prognosis. Drug Saf. 1999;21:187–201. doi: 10.2165/00002018-199921030-00004. [DOI] [PubMed] [Google Scholar]
- 12.Obuch ML, Baker G, Roth RI, Yen TS, Levin J, Berger TG. Selective cutaneous hyperpigmentation in mice following zidovudine administration. Arch Dermatol. 1992;128:508–13. [PubMed] [Google Scholar]
- 13.Rahav G, Maayan S. Nail pigmentation associated with zidovudine: A review and report of a case. Scand J Infect Dis. 1992;24:557–61. doi: 10.3109/00365549209054640. [DOI] [PubMed] [Google Scholar]


