Abstract
Background:
Perturbations of antioxidant levels and lipid peroxidation, but not oxidative DNA damage as a biomarker of oxidative stress have been reported in uterine myoma patients.
Aims:
The study aimed at examining the patterns and influence of oxidative stress/damage biomarkers, 8-isoprostane (8-IP) and 8-hydroxy-2'-deoxyguanosine (8OHdG), on the ovulatory and luteal phases of normal and fibroid women.
Settings and Design:
Twenty women diagnosed of fibroids (1–5 years) and 20 nonfibroid women were age-matched. Selection was randomly done at the National Obstetrics and Gynaecology Department.
Subjects and Methods:
Three successive samples of urine were taken at 8:00 am on the 14th, 18th, and 21st days of the menstrual cycle. Mid-stream urine was collected from subjects, after they had cleaned genitals. The samples were kept in an ice chest, transported to the laboratory, and stored at − 70°C until the time of analysis. Samples were analyzed by ELISA technique using commercial kits for 8OHdG and 8-IP. Results were calculated using a computer program.
Statistical Analysis Used:
Statistical Package for Social Sciences, version 20.0, was used for data management and statistical analysis. The results were expressed as mean ± standard deviation. Differences in continuous data were compared using Student's t-test (two groups) and one-way ANOVA (three or more groups) followed by Bonferroni post hoc test. Relationship between variables was ascertained by Spearman's correlation coefficient. All results were considered significant at 5% level of probability.
Results:
Significant differences were observed between day 14 and day 21 in control and test groups' estrogen levels (P = 0.0047 and P = 0.004, respectively). Significant progesterone differences were observed between control and test groups on the same days (P = 0.002 and P = 0.001, respectively). A positive correlation was observed between day 21 estrogen and progesterone levels (P = 0.0003) of the control group. Test group had higher levels of 8-IP and 8OHdG than control groups on day 21, with 8OHdG at maximum in the test group but minimum in the control group. The influence of 8OHdG was seen by a negative correlation with estrogen and progesterone on day 21 (P = 0.0002) and a positive correlation between 8OHdG and 8-IP on the same day in the test group. Finally, there was a positive correlation between 8-IP and 8OHdG on day 14, but a negative correlation on day 21 (P = 0.0016).
Conclusions:
Oxidative damage was absent in the control group but was very much present in the test group on day 14 and day 21 with progesterone and estrogen acting in concert with oxidative damage biomarkers. An inverse pattern of biomarkers was observed between control and fibroid groups. Oxidative stress biomarkers influenced hormonal levels and pattern of the fibroid group.
Keywords: 8-hydroxy-2'-deoxyguanosine, 8-isoprostane, estrogen, fibroid, oxidative damage, oxidative stress, progesterone
INTRODUCTION
Uterine leiomyomas (fibroids) are the most common types of reproductive tract tumor of women. Uterine fibroids are noncancerous tumors consisting of fibers or fibrous tissue that arise in the uterus. Modern imaging techniques and careful pathological examination of specimens indicate that the incidence of uterine fibroids is as high as 70%, suggesting that these tumors are far more prevalent than previously estimated by clinical cases (20–50%).[1] Racial differences do exist. In a study involving 95,061 premenopausal nurses with no history of uterine leiomyoma, there was an approximately 2–3 times greater incidence rate among coloured women than non-coloured women when they were followed prospectively.[2] Examination of hysterectomy specimens by Kjerulff et al.[3] revealed 89% of the coloured women and 59% of the non-coloured women had leiomyomas. Finally, ultrasound examination by Baird et al.[4] showed that 73% of coloured women and 48% of non-coloured women had uterine fibroids. It is clear that uterine leiomyoma is higher in coloured women than non-coloured women.
In Nigeria, 60.6% of uterine fibroid was reported in a retrospective clinicopathological analysis of uterine leiomyomata over a 5-year period (1996–2000).[5] In Ghana data available at the Korle-Bu Teaching Hospital, Ghana's largest teaching hospital indicates that as of June 1998, 384 out of 726 major selective gynecological operations carried out at the hospital were fibroid related cases.[6] Fibroids rarely undergo malignant degeneration to become a sarcoma. The incidence of malignant degeneration is as low as 1.0% and has been estimated to be 0.2%.[7] Regardless of their generally benign neoplastic characteristics, uterine fibroids are responsible for significant morbidity in a large segment of the female population. The clinical effects of these tumors are related to their local mass effect, resulting in pressure on adjacent organs, fatigue, prolonged or heavy menstrual bleeding, pelvic pressure or pain, and in rare cases, reproductive dysfunction.[8] It is well established that human uterine leiomyomas possess receptors for estrogen and progesterone and grow under the influence of ovarian steroid hormones.[9,10,11]
Estrogen dominance has been implicated in the pathogenesis. It has been proposed that decreased apoptosis may contribute to the growth and maturation of leiomyomas.[12] One proto-oncogene product, Bcl-2, an integral membrane protein in the endoplasmic reticulum, nuclear envelope, and in the outer membranes of the mitochondria,[13] functions to inhibit apoptosis and it is abundantly expressed in leiomyoma.[12] Reduced apoptosis leads to reduced cell turnover in the myometrium and results in neoplastic accumulation of cells that eventually become fibrotic due to cellular senescence. Despite the afore-mentioned mechanisms, little is known concerning their etiology and molecular basis for the development and growth.
Oxidative stress has been linked to the pathophysiology of more than 100 human diseases, as well as the aging process.[14] However, its involvement in uterine leiomyomas has not been firmly established. Oxidative stress and prolidase activity have been measured in women with uterine fibroids. An increased anti-oxidative repair system in fibroid tissue compared to myometrium has been demonstrated.[15] Furthermore, perturbations of anti-oxidants such as superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase were found to be present in uterine leiomyoma, although lower than that observed in premalignant and malignant lesions of other uterine diseases.[16]
Further, oxidative damage is seen in proteins and DNA with the latter forming adducts such as 8-hydroxy-2'-deoxyguanosine (8OHdG). The presence of 8OHdG has been observed in the biopsies of leiomyoma with levels slightly higher than nonfibroid adjoining tissues of the endometrium.[17] In the Japanese quail model of fibroid, potential oxidative stress adducts such as 8-isoprostane (8-IP) (a lipid peroxidation biomarker) and 8OHdG were measured and found to be significantly reduced, accompanied by fibroid size reduction in the models that were fed selenium antioxidant.[18] Although the animal model suggests the possible role of oxidative stress and damage in fibroid development, the phenomenon is largely untested in humans. The aim of the study was to determine the urinary patterns of oxidative damage biomarkers in women clinically diagnosed of having fibroid during the ovulatory and luteal phases of the menstrual cycle.
SUBJECTS AND METHODS
The study was approved by the Ethics and Protocol Review Committee of the School of Allied Health Sciences. In addition, informed consent was obtained from all subjects. Twenty reproductive-aged women (21–45 years) diagnosed of fibroids (1–5 years) were selected at random from the out-patient Gynaecological Department of the National Teaching Hospital. Twenty other apparently healthy women served as aged-matched controls. Both groups were in the secretory phase of their menstrual cycles. Subjects completed a questionnaire which aided researchers in knowing the time of the cycle for sample collection.
Sample collection
Three successive samples of urine were taken at 8:00 am on the 14th, 18th, and 21st days of the menstrual cycle. Mid-stream urine was collected from subjects after they had cleaned genitals. The samples were kept in an ice chest, transported to the laboratory, and stored at −70°C until the time of analysis.
Biochemical assays
8-hydroxy-2'-deoxyguanosine determination
8OHdG test was performed using a competitive ELISA method with kits from Genox Inc (Baltimore, USA). The assay was performed according to the manufacturer's instructions. In brief, 50 µl of the anti-8-OHdG monoclonal antibody, samples as well as standards were added to a microtiter plate which had been precoated with 8OHdG and incubated at 37°C for 1 h. One hundred microliters of an enzyme-labeled secondary antibody was added to the plate and incubated at 37°C for another hour. Intermittent washing was carried out. One hundred microliters of freshly prepared chromatic substrate solution was added and incubated for 15 min in the dark which resulted in color development proportional to the amount of anti-8OHdG in the sample. The reaction was terminated by the addition of 1 M phosphoric acid and the absorbance was measured at 450 nm using a microplate reader.
8-isoprostane determination
The immunoassay reagent kit from Cayman Chemical Company (Michigan, USA) was used according to the manufacturer's instructions. The 8-IP assay was based on the principle of competitive binding between sample 8-IP, 8-IP acetyl cholinesterase (AChE) conjugate, and 8-IP tracer for a limited number of 8-IP-specific rabbit anti-serum binding sites. Fifty microliters of samples was added to each well and 50 µL of 8-IP AChE tracer was added to all wells except total activity and blank wells. Fifty microliters of 8-IP enzyme-immunoassay antiserum was added to all wells except total activity, nonspecific binding, and blank wells. The plates were covered and incubated at 4°C for 18 h and then washed 5 times with buffer. Two hundred microliters of Ellman's reagent was added to each well and 50 µL of tracer was added to total activity wells only. Plates were covered with plastic films for 90 min and absorbance was read at 420 nm. The amount of 8-IP tracer bound was inversely proportional to the concentration of 8-IP in the sample.
Estrogen/progesterone determination
The serum samples were analyzed for estrogen and progesterone using the ELISA methods. The respective immunoassay reagent kits were obtained from Cayman Chemical Company (Michigan, USA). The procedure was similar to that described previously for 8-IP except for the primary monoclonal antibody estrogen or progesterone that was bound to the solid phase.
Statistical analysis
Statistical Package for Social Sciences, version 20.0 (Armonk, NY: IBM Corp.), was used for data management and statistical analysis. The results were expressed as mean ± standard deviation. Differences in continuous data were compared using Student's t-test (two groups) and one-way ANOVA (three or more groups) followed by Bonferroni post hoc test. Relationship between variables was ascertained by Spearman's correlation coefficient. All results were considered significant at 5% level of probability.
RESULTS
The mean age was 33.6 ± 11.2 years. Participants were fairly healthy people with the exception of the presence of the fibroid in those belonging to the experimental group.
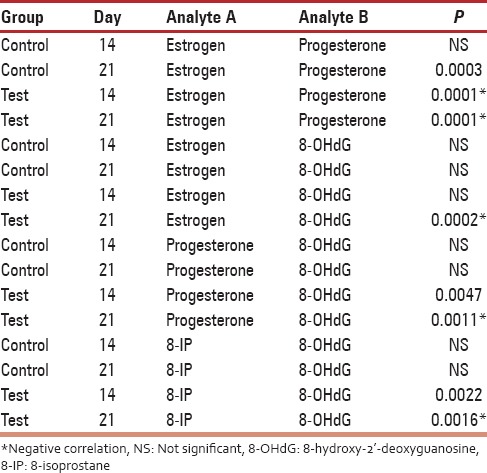
Urine estrogen levels were low and declined from day 14 to 21 in the control group with the highest level being 54.44 ± 42.16 ng/mL. On the contrary, there was a significant increase in estrogen levels of fibroid patients with the highest levels observed on day 21 (661.63 ± 444.71 ng/mL) [Figure 1]. Differences were statistically significant (P = 0.004) [Table 1]. Progesterone levels in controls increased from day 14 (38.50 ± 12.22 ng/mL) to day 21 (143.22 47.88 ng/mL). An inverse relationship was observed in the test group which started high (81.64 ± 48.26 ng/mL) on day 14, but declined progressively by day 21 (41.46 ± 29.96 ng/ml) [Figure 2]. On day 14, 8OHdG levels for both control and test groups were approximately 2.5 ng/mL. However, by day 18, the control group had declined to 2.16 ± 0.79 ng/mL while that of the test group had increased to 2.91 ± 0.96 ng/mL. By day 21, control and test groups were 2.50 ± 0.88 and 16.00 ± 2.96 ng/mL, respectively [Figure 3]. Urinary 8-IP was the only biomarker that had a similar pattern from day 14 to day 21. Levels in the test group were always uniformly higher than the control group. A progressive decline was observed; 98.61 ± 2.37 ng/mL to 81.12 ± 77.00 ng/mL in the control group and 116.65 ± 59.69 ng/mL to 105.13 ± 64.35 ng/mL in the test group. Differences were not statistically significant at any time-point [Figure 4]. A negative correlation was observed for the test group between estrogen and progesterone on days 14 and 21. In addition, a negative correlation was observed between progesterone and 8OHdG of the test group on day 21 [Table 2]. However, a positive correlation was seen between estrogen and progesterone only on day 21, with no corresponding correlation between progesterone and 8OHdG on that day for the control group. Furthermore, no correlation was found between estrogen and 8OHdG and 8-IP and 8OHdG for the control group. For the test group, a positive correlation was seen between 8OHdG and 8-IP on day 14. However, on day 21, this association was negative [Table 2].
Figure 1.
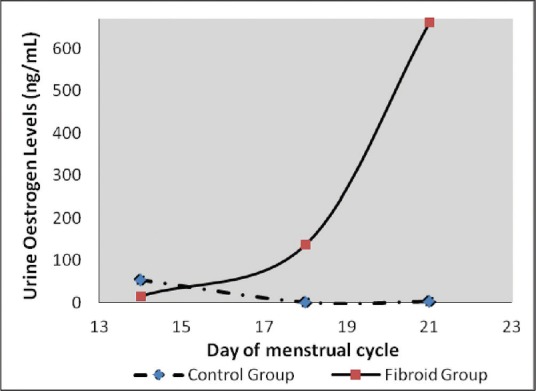
A graph showing urinary estrogen levels during the ovulatory and luteal phases of the menstrual cycle. Levels in control group were very low while that of the fibroid group was highly elevated during the luteal phase
Table 1.
A comparison between mean values of the Control group and the Test group for the various analytes on day 14 and 21 of the menstrual cycle
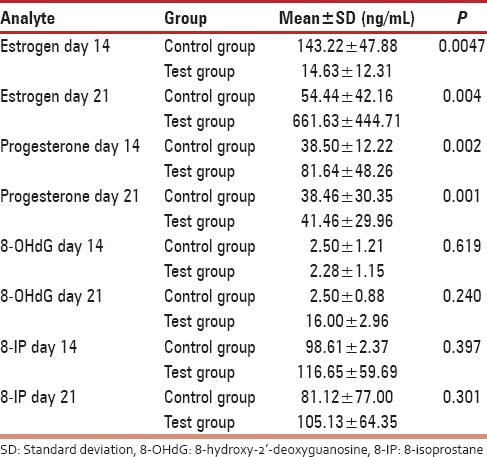
Figure 2.
A graph showing urinary progesterone levels during the ovulatory and luteal phases of the menstrual cycle. A gradual decline was observed in the fibroid group which was very low on day 21, while that of the control group steadily increased from basal levels during the luteal phase
Figure 3.
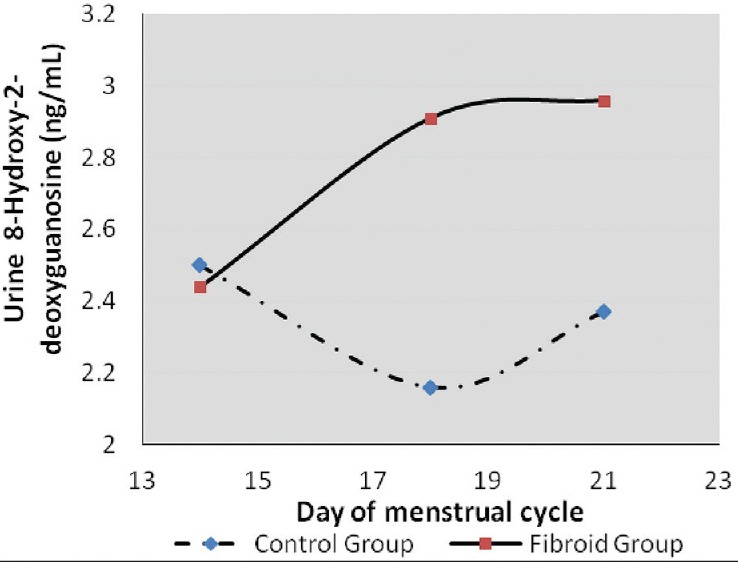
A graph showing urinary 8-hydroxy-2'-deoxyguanosine levels during the ovulatory and luteal phases of the menstrual cycle. During the luteal phase, oxidative DNA damage was low in the control group (day 18) while peak values were attained in the fibroid group (day 21)
Figure 4.
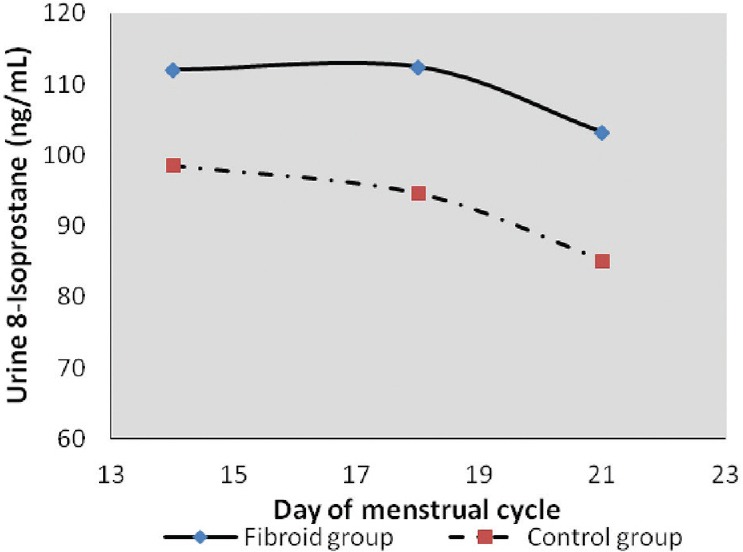
A graph showing urinary 8-isoprostane levels during the ovulatory and luteal phases of the menstrual cycle. Lipid peroxidation patterns were similar in both the control and test groups. Both groups showed a decline from the ovulatory to the luteal phase. However, levels were higher in the test group at all time-points compared to the control group
Table 2.
Correlation analysis between the urinary hormonal and oxidative damage biomarkers on day 14 and 21 of menstrual cycle of control and test groups
DISCUSSION
For the past decade, the association between uterine fibroid and oxidative stress has been an area of research interest. The most often used biomarker of oxidative DNA damage is 8OHdG. Work done on nonhuman species seems to suggest the involvement of oxidative stress in uterine leiomyoma. The Japanese study demonstrated a significant increased level of 8OHdG in quails that developed fibroid.[19] Our unpublished data using Eker rats also gave significant differences between rats that carried the genes for fibroid development and those that did not. The underlining mechanism has always been perturbations in hormonal levels. Such imbalances were observed in this study [Figures 1 and 2]. The test group had more than 10 times urine estrogen levels found in the control group on day 21. During the follicular phase, estrogen levels rise before the peak levels of LH. The estrogen level in this study on day 14 was higher in the control group compared to the test group. Differences were significant. The continual rise in estrogen as seen in [Figure 1] continued to promote the luteal phase. This may account for further preparation of the endometrial lining of the fibroid group than normal with subsequent heavy bleeding when the cycle is over, as seen in fibroid cases. Alternatively, the low progesterone level seen in the fibroid group may also account for poor endometrial lining during the luteal phase.
Lipid peroxidation in the test group was always higher at all time-points than the control group, suggestive of a higher degree of oxidative lipid damage in that group, hence a higher level of oxidative stress. However, an inverse pattern was obtained with 8OHdG levels. On day 14, both groups had similar levels. Furthermore, by day 18, levels of 8OHdG had surged in the fibroid group when compared to the control group. The high level of 8OHdG from day 18 coincided with the time progesterone increased. The presence of oxidative stress/damage may account for some suppression of progesterone at that time of the cycle. On the contrary, on day 18, the level of 8OHdG in the control group was rather low. Thus, during the process of fertilization and implantation, oxidative stress/damage is very low and does not interfere with the process. Although oxidative stress has been implicated in over 100 diseases, its involvement in the human process of fibroid development is still an immature hypothesis. Furthermore, although serum protein carbonyl groups and advanced oxidized protein products were higher in leiomyoma patients than in the control group followed by a decrease in antioxidants of these patients, oxidative stress was said to be significant in the patient group.[20] Mapping histological levels of 8OHdG in female reproductive organs, the percentage of DNA damage in fibroid tissue was 15 times higher compared with normal cervix.[17] It is indeed possible that oxidative stress plays an important role in the pathogenesis; however, urinary levels may not demonstrate significant differences as seen in this study.[21] Nevertheless, patterns that seem to interfere with the menstrual cycle's hormonal levels of fibroid patients have been demonstrated in this study. A similar inverse relationship was observed in the study of Vural et al.,[15] where catalase and prolidase were significantly higher in fibroid tissues compared to normal subjects but were lower in the serum compared to normal subjects. Other indirect associations have been shown where fibroid Japanese quails that were administered selenium antioxidant had significantly smaller tumors than those that did not receive supplementation.[18] Such perturbations of antioxidant levels as a biomarker of oxidative stress have also been reported in uterine myoma patients as well as lipid peroxidation, but not oxidative DNA damage.[22,16]
This study suggests that on the day of ovulation, both fibroid and nonfibroid females have the same level of 8OHdG. However, the luteal phase of the former is accompanied by a surge in 8OHdG and a depression of the same biomarker in the latter. There was a negative correlation between estrogen and progesterone on days 14 and 21 in the fibroid group [Table 2]. Thus, on day 21, when progesterone was suppose to peak, any increase in estrogen suppressed progesterone and this perhaps accounts for poor endometrial lining.[23] Similarly, on day 21, as estrogen diminished in the test group and the negative correlation observed implies that 8OHdG increased. Thus, oxidative damage was heightened during the luteal phase and may account for the poor endometrial lining. This was further seen by the negative correlation between progesterone and 8OHG on day 21. Therefore, oxidative damage in the fibroid group begins in the proliferative phase and continues to the luteal phase. This effect was not seen in the control group which suggests the absence of oxidative stress during the luteal phase. A further association was seen between 8-IP and 8OHdG in the test group. This positive association was seen on day 14. As lipid peroxidation increased, oxidative damage also increased. However, on day 21, a negative correlation was observed. Thus, the predominant oxidative stress biomarker at that level appeared to be 8OHdG. All put together, oxidative damage was absent in the control group, but was very much present in the test group on day 14 and day 21 with progesterone and estrogen acting in concert with oxidative damage biomarkers. The need for good levels of antioxidants in the reproductive years of the female is apparent to possibly avert the involvement of oxidative stress/damage in the development of fibroid.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Berry W, Doernte A, Conner M, Barnes M, Oates S. Spontaneously occurring fibroid tumors of the laying hen oviduct. Poult Sci. 2006;85:1969–74. doi: 10.1093/ps/85.11.1969. [DOI] [PubMed] [Google Scholar]
- 2.Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967–73. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 3.Kjerulff KH, Langenberg P, Seidman JD, Stolley PD, Guzinski GM. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J Reprod Med. 1996;41:483–90. [PubMed] [Google Scholar]
- 4.Baird DD, Schectman JM, Dixon D, Sandler DP, Hill MC. African Americans at higher risk than whites for uterine fibroids: Ultrasound evidence. Am J Epidemiol. 1998;147:S90. [Google Scholar]
- 5.Mohammed A, Shehu SM, Mayun AA, Tiffin IU, Alkali G, Abubakar AL. Uterine leiomyomata: A five year clinicopathological review in Zaria, Nigeria. Niger J Surg Res. 2005;7:206–8. [Google Scholar]
- 6.Fibroid Cases on the Increase. Ghana New Agency; October. 1999. [Last accessed on 2014 Jan 24]. Available from: http://www.modernghana.com/news2/7734/3/reggy-zippy.explodes.again.html .
- 7.Spanoudaki A, Oikonomou A, Dimitrova K, Prassopoulos P. Pedunculated uterine leiomyoma mimicking abdominal mass: A case report. Cases J. 2008;1:315. doi: 10.1186/1757-1626-1-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haney AF. Clinical decision making regarding leiomyomata: What we need in the next millenium. Environ Health Perspect. 2000;108(Suppl 5):835–9. doi: 10.1289/ehp.00108s5835. [DOI] [PubMed] [Google Scholar]
- 9.Jefferson WN, Doerge D, Padilla-Banks E, Woodling KA, Kissling GE, Newbold R. Oral exposure to genistin, the glycosylated form of genistin, during neonatal life adversely affects the female reproductive system. Environ Health Perspect. 2009;117:1883–9. doi: 10.1289/ehp.0900923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandon DD, Bethea CL, Strawn EY, Novy MJ, Burry KA, Harrington MS, et al. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am J Obstet Gynecol. 1993;169:78–85. doi: 10.1016/0002-9378(93)90135-6. [DOI] [PubMed] [Google Scholar]
- 11.Brandon DD, Erickson TE, Keenan EJ, Strawn EY, Novy MJ, Burry KA, et al. Estrogen receptor gene expression in human uterine leiomyomata. J Clin Endocrinol Metab. 1995;80:1876–81. doi: 10.1210/jcem.80.6.7775635. [DOI] [PubMed] [Google Scholar]
- 12.Maruo T, Ohara N, Wang J, Matsuo H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum Reprod Update. 2004;10:207–20. doi: 10.1093/humupd/dmh019. [DOI] [PubMed] [Google Scholar]
- 13.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 14.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–23. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 15.Vural M, Camuzcuoglu H, Toy H, Camuzcuoglu A, Aksoy N. Oxidative stress and prolidase activity in women with uterine fibroids. J Obstet Gynaecol. 2012;32:68–72. doi: 10.3109/01443615.2011.633718. [DOI] [PubMed] [Google Scholar]
- 16.Pejic S, Kasapovic J, Todorovic A, Stojiljkovic V, Pajovic SB. Lipid peroxidation and antioxidant status in blood of patients with uterine myoma, endometrial polypus, hyperplastic and malignant endometrium. Biol Res. 2006;39:619–29. [PubMed] [Google Scholar]
- 17.de Carvalho LF, Abrão MS, Biscotti C, Sharma R, Agarwal A, Falcone T. Mapping histological levels of 8-hydroxy-2'-deoxyguanosine in female reproductive organs. J Mol Histol. 2013;44:111–6. doi: 10.1007/s10735-012-9454-7. [DOI] [PubMed] [Google Scholar]
- 18.Tuzcu M, Sahin N, Ozercan I, Seren S, Sahin K, Kucuk O. The effects of selenium supplementation on the spontaneously occurring fibroid tumors of oviduct, 8-hydroxy-2'-deoxyguanosine levels, and heat shock protein 70 response in Japanese quail. Nutr Cancer. 2010;62:495–500. doi: 10.1080/01635580903441303. [DOI] [PubMed] [Google Scholar]
- 19.Foster DN, Nestor KE, Saif YM, Bacon WL, Moorhead PD. Influence of selection for increased body weight on the incidence of leiomyomas and leiomyosarcomas in Japanese quail. Poult Sci. 1989;68:1447–53. doi: 10.3382/ps.0681447. [DOI] [PubMed] [Google Scholar]
- 20.Santulli P, Borghese B, Lemaréchal H, Leconte M, Millischer AE, Batteux F, et al. Increased serum oxidative stress markers in women with uterine leiomyoma. PLoS One. 2013;8:e72069. doi: 10.1371/journal.pone.0072069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foksinski M, Gackowski D, Rozalski R, Olinski R. Cellular level of 8-oxo-2'-deoxyguanosine in DNA does not correlate with urinary excretion of the modified base/nucleoside. Acta Biochim Pol. 2003;50:549–53. [PubMed] [Google Scholar]
- 22.Chiou JF, Hu ML. Elevated lipid peroxidation and disturbed antioxidant enzyme activities in plasma and erythrocytes of patients with uterine cervicitis and myoma. Clin Biochem. 1999;32:189–92. doi: 10.1016/s0009-9120(98)00110-6. [DOI] [PubMed] [Google Scholar]
- 23.Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine messenger ribonucleic acid and protein are overexpressed in human regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]


