Abstract
Objective:
Hyperlipidemia, extensively contributes in the progression of coronary heart diseases and atherosclerosis, but may be managed through alterations in the nutritional pattern. Several studies show that diet rich in polyphenols and antioxidants have antiatherogenic effects. Citrus sinensis and Citrus paradisi are widely known for health benefits and have found to produce antioxidant, anti-inflammatory, and hypolipidemic effects, hence current research was planned to determine the hypolipidemic effects of C. sinensis and C. paradisi in rats receiving diet rich in cholesterol.
Materials and Methods:
All rats were divided into 11 groups each comprising 10 animals: Normal control group and hyperlipidemic control. C. sinensis treated three groups, C. paradisi treated three groups, C. sinensis and C. paradisi combination treated two groups, and group treated atorvastatin. All rats in the respective groups were treated orally with sterile water, juices, and standard drug for 8 weeks and lipid profile was estimated at the end of dosing.
Results:
Cholesterol, triglycerides (TGs), and low-density lipoprotein (LDL) were decreased at all the three doses of C. sinensis and C. paradisi but rise in high-density lipoprotein (HDL) was only significant at 8 ml/kg, and 0.3 ml/kg, respectively. Animals received the combination doses of C. sinensis and C. paradisi also showed a highly significant fall in cholesterol, LDL, and TGs, however HDL level was significantly elevated by SPJ-2 combination.
Conclusion:
Results suggest that C. sinensis and C. paradisi possess antihyperlipidemic activity due to phytochemicals and other essential nutrients, hence may serve as cardioprotective by preventing thrombosis.
KEY WORDS: Grapefruit, hyperlipidemia, hypocholesterolemic, orange
Hyperlipidemia is a diverse group of disorder categorized by the increased levels of lipids and lipoproteins in the bloodstream, e.g., cholesterol, low-density lipoprotein (LDL), and very LDL (VLDL), which eventually leads to atherosclerosis.
Atherosclerosis and coronary heart diseases (CHD) are the most persistent reasons of illness and death all over the world, though number of causes such as diet rich in saturated fatty acid, high blood pressure, family history, age, and life pattern has an important role in causing ischemic heart disease. However, the increased levels of cholesterol, primarily total cholesterol (TC) and LDL are responsible for the onset of CHDs. A 20% decrease in the cholesterol level can decrease about 31% incidence of CHD and 33% of death rate[1] and cardiovascular disorders are the primary cause of death in the western countries.[2] Hypercholesterolemia is the main cause of cardiovascular disease (CVD)[3] and cellular cholesterol homeostasis is of vital importance in preventing CVD. The plasma cholesterol level can be controlled in numerous ways, e.g., decrease the absorption of dietary lipids, increase the elimination of cholesterol through fecal excretion, decrease cholesterol biosynthesis, and the removal of cholesterol from circulation. There are many studies, which show the valuable effects of HMG-CoA reductase inhibitors and acyl-CoA: Cholesterol acyl transferase (ACAT) inhibitors on hypercholesterolemia and atherosclerosis.[4,5] However, existing lipid-lowering drugs have several adverse effects.[1]
In the earlier days, extensive attention has been given to develop awareness about the bioactive constituents of plants and their benefits to health. Human diet from the herbal source comprises several compounds called as nutraceuticals, which though not regarded as nutrients but have an essential part in preserving health; some remarkable nutraceuticals are polyphenols, phytoestrogens, phytates, and polyunsaturated fatty acids.
The beneficial role of dietary polyphenol such as flavonoids in citrus fruit is the entity of rising scientific interest in human health. Naringin, a naturally occurring bioflavonoid in citrus fruits, has been reported to possess antimicrobial,[6] antimutagenic,[7] anticarcinogenic,[8] and anti-inflammatory[9] activities. The effective character of naringin has occupied significant consideration for its use as lipid-lowering and antiatherogenic agent.
Recent researchers have already explored the lipid-lowering property of naringin, intervened by inhibiting HMG-CoA reductase in rats[10,11] but selection of the animal model is significant to test the efficacy of nutraceutical in avoidance of specific disease. Hence, the current research investigates the probability of this functional compound in suppressing diet-induced hypercholesterolemia as efficiently as atorvastatin in rats that prone to develop hypercholesterolemia and atherosclerosis. Antihypercholesterolemic property of bioflavonoids had shown to alter plasma and tissue lipids, cholesterol-regulating enzymes, and tissue morphology in rabbits.[12]
The grapefruit has become famous for health benefits since late 19th century but formerly it was only cultivated as an ornamental plant. Grapefruit (Citrus paradisi) and sweet orange (Citrus sinensis) are the members of the Rutaceae family, their juice and pulp parts have shown hypolipidemic effects in the diet-induced hypercholesterolemia in rats. Grapefruit is a good source of Vitamin C, along with dietary fiber, Vitamin A, potassium, folate, and Vitamin B5. It also contains phytochemicals, including limonoids and lycopene.[13] People developed an interest in grapefruit because of its lipid-lowering abilities, which may be due to the high pectin contents of grapefruit, a soluble fiber that lowers blood cholesterol.[14]
Fujioka et al.,[15] in a pilot study showed that on average, participants eating half a grapefruit with each meal reduce about 3.6 pounds body weight and those drinking a serving of grapefruit juice 3 times a day lost 3.3 pounds. Moreover, several patients in the study lost more than 10 pounds. Grape fruit is not only a good source of Vitamin C and minerals but also contains protective chemicals, e.g., phenolic acid, limonoids, terpenes, monoterpenes, and bioflavonoids, effective against cancer and heart disease.
The chief bioflavonoid in grapefruit is Naringin[16,17] which gives grapefruit juice bitter taste. Naringin has diverse pharmacological properties such as antioxidant, lipid lowering,[18] anticarcinogenic,[17] and enzyme inhibition, e.g. CYP3A4 and CYP1A2, responsible for several drug interactions in vitro.[19] Pink and red grapefruits are rich in lycopene,[17,19] an antioxidant that seems to lessen the threat of prostate cancer.[20] Grapefruit has also shown relief in some people with rheumatoid arthritis[21] and other inflammatory disorders, which might be due to the presence of chemicals that block prostaglandins.
Orange being a rich source of nutrients has been known for a number of health and nutritional benefits; it contains nearly two hundred phytonutrients and flavonoids, which have shown activity against different types of cancers. They have also been reported to show strong anti-inflammatory and anti-oxidant properties and prevent bone loss.[19,22,23,24] The essential oils present in orange juice (C. sinensis) are composed of many constituents, including monoterpenes and sesquiterpenes with d-limonene as a major constituent.[25] Orange juice or its flavonoid has been reported to produce cholesterol-lowering effect in rabbits[26] and humans.[27] Its anti-inflammatory role in different disease conditions is also well documented.[28,29] Narirutin or Naringenin 7-O-rutinoside is another important flavonoid present abundantly in orange juice.[30] It is absorbed well and shows good bioavailability.[31] It has shown anti-inflammatory,[32,33] anti-allergic, and anti-asthmatic properties.[33,34] Orange and grape fruit, both are rich in flavonoids and vitamins, with strong anti-inflammatory and antioxidant properties.[19,22,23,24]
Hence, considering the reported biological activities, a recent study was designed to explore the antihyperlipidemic effect of C. paradisi, C. sinensis, and their combinations in animals kept on high cholesterol diet.
Materials and Methods
Animals
Adult male Wister rats with mean body weight of 220 ± 10 g were selected for the study and kept under controlled temperature at 23°C ± 2°C and humidity of 50–60%. Five rats were housed in each plastic cage having a size of 81 cm × 46 cm × 41 cm. Rats were maintained on a 12/12 h light and dark cycle during the experiment with nonstop access to rat chow and tap water. The use of animals in the study was in accordance to the guidelines of National Research Council for the care and use of Laboratory Animals[35] and permitted by the Board of Advance Studies and Research University of Karachi.
Dosing
Citrus sinensis juice
The commonly available variety of orange (C. sinensis) family Rutaceae was purchased from the local vendors. The fruit was recognized by Prof. Anjum Parveen Director, Plant Conservation Center, University of Karachi and specimen sample no C. S 10-10 was submitted to the Department of Pharmacognosy, University of Karachi. Fresh juice was squeezed after peeling the fruit and was used promptly after filtration. C. sinensis juice was administered orally in different doses, that is, 2, 5, and 8 ml/kg as per the body weight.
Citrus paradisi juice
The commonly available variety of grapefruit (C. paradisi) family Rutaceae was also purchased from the local vendors. The fruit was recognized by Prof. Anjum Parveen Director, Plant Conservation Center; University of Karachi and specimen sample no C.P 09-10 was submitted to the Department of Pharmacognosy, University of Karachi. Fresh juice was squeezed after peeling the fruit, which was used promptly after filtration. C. paradisi juice was given orally in three doses, that is, 0.1, 0.3, and 0.5 ml/kg according to the body weight.
Combinations of Citrus sinensis and Citrus paradisi
C. sinensis and C. paradisi juices were given in combination by mouth in two doses, that is, 2 ml/kg +0.1 ml/kg and 5 ml/kg +0.3 ml/kg, respectively, and were abbreviated as SPJ-1 (2 + 0.1 ml/kg) and SPJ-2 (5 + 0.3 ml/kg), respectively.
Induction of hyperlipidemia
Hyperlipidemia was induced as described by Sumbul and Ahmed,[36] by the addition of egg yolk (24%) of the whole feed of all rats, that is, hyperlipidemic control, test animals, and animals treated with standard drug for a period of 8 weeks.
Design of experiment
All rats were divided into 11 groups each comprising 10 animals; normal control group was given sterile water with regular diet, hyperlipidemic control group was given sterile water with high cholesterol diet, animals of test group were divided into eight groups; three groups received high cholesterol diet and different doses of C. sinensis, other three groups were given high cholesterol diet and C. paradisi in different doses, while remaining two groups received high cholesterol diet and combination of C. sinensis and C. paradisi juices. The animals of the standard group received high cholesterol diet and atorvastatin in the dose of 10 ml/kg.[37] All drugs were administered orally on once a daily basis for 8 weeks. At the end of the investigational period, animals were deprived of food overnight and sacrificed by decapitation and blood samples were collected in gel tubes.
Lipid profile
Serum of blood collected in gel tubes was separated by HuMax 14K centrifuge at 2000 rpm for 10 min and lipid profile was estimated within 3 h on Humalyzer 3000, semi-automatic chemistry analyzer, model number 16700 (Human Germany), with the help of standard kits from Human, Germany.
Cholesterol
Cholesterol in the serum was determined by CHOD-PAP method, enzymatic colorimetric test for cholesterol with Lipid Clearing Factor. 10 µl sample into cuvette was mixed with 1000 µl enzyme reagent and incubated for 5 min, and then the absorbance was measured within 60 min.[38]
Triglycerides
Triglycerides (TGs) were determined by GPO-PAP method, an enzymatic colorimetric test. 10 µl sample was mixed with 1000 µl mono-reagent in a cuvette and incubated for 10 min, then the absorbance was measured within 60 min.[38]
High-density lipoprotein-cholesterol
The majority of laboratories use several methods for careful precipitation and removal of VLDL and LDL, followed by the enzymatic measurement of high-density lipoprotein (HDL) in the supernatant fraction.[39] In this test, HDL-cholesterol, precipitant, and standard was used with human cholesterol Test Kit. 200 µl sample was mixed with 100 µl distilled water and 400 µl PREC HDL reagent, then incubated for 10 min and centrifuged at 3000 rpm for 10 min. 100 µl clear supernatant was taken in a separate tube having 1000 µl cholesterol reagent and the absorbance was measured within 60 min.[38]
Low-density lipoprotein-cholesterol
The LDL-cholesterol was measured by utilizing test kit of Human, Germany. All reagents were warmed at 37°C then pipette 10 µl sample + 750 µl enzyme into the cuvette. Mixed gently and incubated for 5 min, 250 µl substrate was added and incubated at 37°C, and then the absorbance was measured after 5 min.[40,41]
Histopathological examination
Specimens of the liver after removal from the body were preserved in 10% buffered formalin for at least 24 h before further processing. The blocks of organs in cassettes were processed in an automatic tissue processor (Gilford 101 system). The tissue sections were mounted on slides and dried gently in the oven at 60°C for 1 h. This was followed by standard staining procedure for histopathological examination. The slides were then finally examined under a light microscope for morphological changes and photographed by the camera mounted over the microscope for a permanent record.
Statistical analysis
Data entry and analysis were performed using Superior Performance Statistical Software (SPSS) version 17 IBM 2009. Data were presented as mean ± standard error of the mean with 95% confidence interval. ANOVA followed by post-hoc was performed for the comparisons of values with control. Values of P ≤ 0.05 were considered as significant and P ≤ 0.005 as highly significant.
Results
Table 1 displays the result of C. sinensis and atorvastatin on cholesterol, LDL, HDL, and TG in hyperlipidemia-induced rats and normal control animals. There was a highly significant decline in cholesterol, LDL, and TG levels by C. sinensis at all three doses, that is, 2, 5, and 8 ml/kg in comparison to hyperlipidemic controls, which were similar to the standard drug atorvastatin. However, HDL levels were only significantly increased at 8 ml/kg dose of C. sinensis, almost comparable to atorvastatin.
Table 1.
Effect of C. sinensis on lipid profile in rats
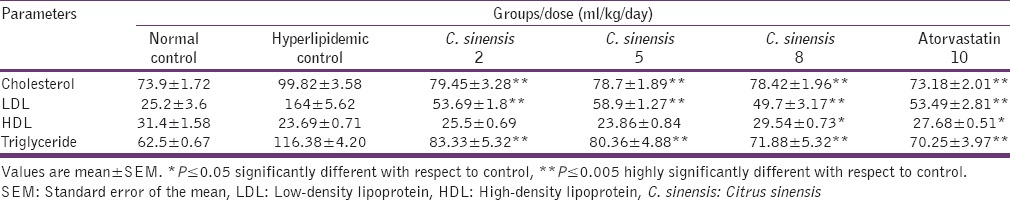
Table 2 shows the results of C. paradisi on cholesterol, LDL, HDL, and TG in hyperlipidemia-induced rats and normal control rats. There was a highly significant decrease in cholesterol, LDL, and TG level at 0.1, 0.3, and 0.5 ml/kg dose of C. paradisi in comparison to hyperlipidemic controls, which were similar to the standard drug atorvastatin. However, a significant increase in HDL level was only observed at 0.3 ml/kg dose of C. paradisi, almost similar to the effect of atorvastatin.
Table 2.
Effect of C. paradisi on lipid profile in rats
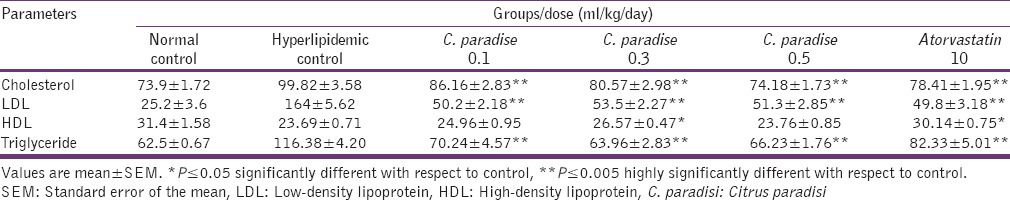
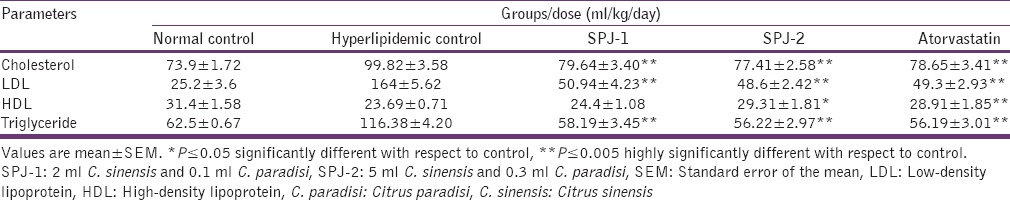
Table 3 reveals the results of two combination doses of C. sinensis and C. paradisi on cholesterol, LDL, HDL, and TG in hyperlipidemia-induced and normal control rats. There was a highly significant decrease in cholesterol, LDL, and TG level at SPJ-1 and SPJ-2 in comparison to hyperlipidemic control. This effect was almost similar to the standard drug atorvastatin; however HDL was only significantly increased at SPJ-2.
Table 3.
Impact of the two doses of combined C. sinensis and C. paradisi on lipid profile in rats
Histopathological examination
Histopathological examination of hepatic tissue in hyperlipidemia-induced control rats shows hepatocytes surrounded by lipid content with no cytoplasm [Figure 1]. The histopathological examination of hepatic tissue of animals received 0.1 ml/kg of C. paradisi shows macrovascular and microvascular steatosis with slight lipid deposition [Figure 2]. However, hepatic tissue of animals received 0.3 ml/kg and 0.5 ml/kg of C. paradisi, three doses of C. sinensis, and two combination doses of C. sinensis and C. paradisi did not reveal any microscopic changes in hepatic tissue [Figures 3 and 4].
Figure 1.

Hepatic tissue in hyperlipidemia-induced control rats showing hepatocytes surrounded by lipid content
Figure 2.
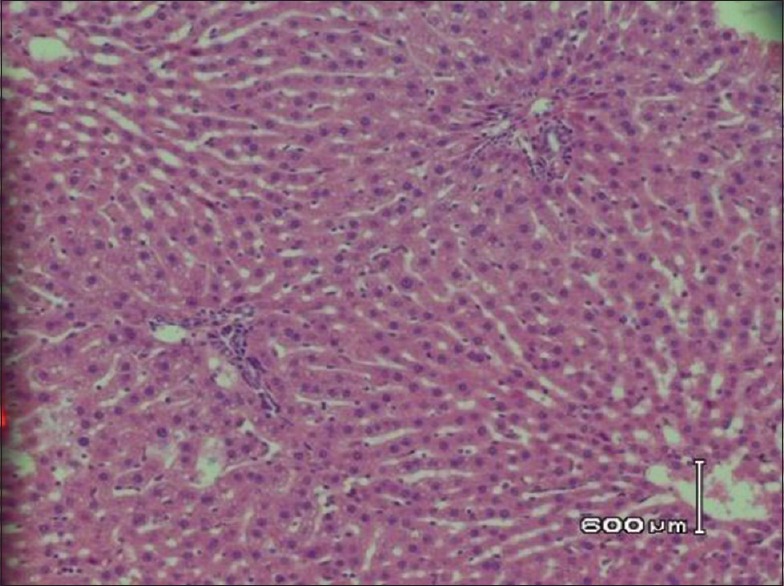
Hepatic tissue in hyperlipidemia-induced rats treated with 0.1 ml/kg of C. paradisi showing minor lipid contents
Figure 3.
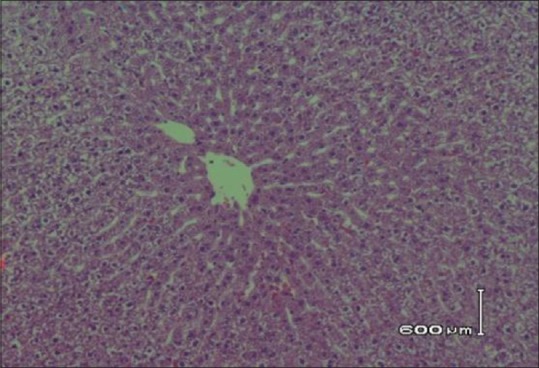
Hepatic tissues showing no lipid contents in animals that received 0.3 and 0.5 ml/kg of C. paradisi
Figure 4.
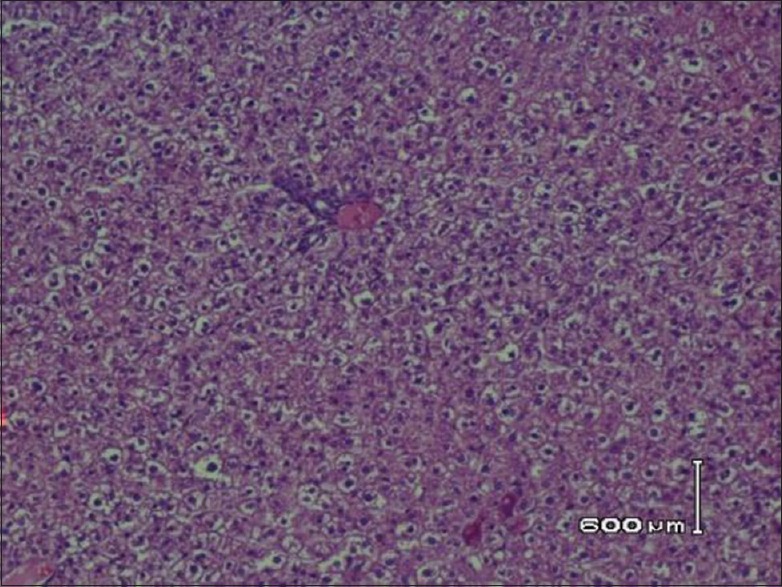
Hepatic tissues showing no lipid contents in animals that received combination doses of C. paradisi and C. sinensis
Discussion
Citrus flavonoids are a group of naturally occurring polyphenols in fruits and vegetables and have been reported to show a variety of biological effects[42] along with anti-hypercholesterolemic action,[43,44] several in vivo studies have already verified the hypocholesterolemic action of naringin in rats.
Hyperlipidemia is an established risk factor for CVD, particularly atherosclerosis. Coronary artery disease (CAD) is one of the chief reasons for premature death worldwide and is likely to be the most significant cause of death.[45] It is well documented that imbalance diet is an important cause of hyperlipidemias and atherosclerosis. Many animal and human studies have established the hypercholesterolemic properties of saturated fatty acids raising TC and changing lipoprotein pattern, however, mechanisms remain under study.
Cholesterol feeding has been often used to raise serum or tissue cholesterol levels to evaluate the hypercholesterolemia associated metabolic disturbances in different animal models.[46] Results of the present study show that keeping the animal on high cholesterol diet significantly increased the TC, TG, and LDL levels in serum as related to rats on a normal diet. However, animals that received high cholesterol diet with C. sinensis and C. paradisi juices showed considerable decline in the levels of TC, TG, and LDL, however, there was a significant promotion in plasma HDL levels, thus indicating the efficacy of juices in preventing atherosclerotic CAD.
Sufficient evidence is present with respect to the fact that HDL-cholesterol is inversely related to total body cholesterol and a decrease of plasma HDL-cholesterol level may accelerate the growth of atherosclerosis leading to ischemic heart diseases.[47] Flavonoids are described to increase HDL-C concentration and reduce LDL and VLDL levels in the hypercholesterolemic rats.[48] Hence, flavonoids and polyphenols found in C. sinensis and C. paradisi may be considered as promising in elevating HDL and reducing LDL and VLDL in the treated rats.
Flavonoids and Vitamin C are well documented to cause decrease in LDL and cholesterol level;[49,50,51,52] results of the present study showed highly significant reduction in plasma LDL, cholesterol, and TGs level in all the animal groups that received C. sinensis, C. paradisi, and their combination, this might be due to the high flavonoid content in these fruits. The hypocholesterolemic effect was almost similar to HMG-CoA reductase inhibitor atorvastatin, which was used as a positive control in this study.
Current research also showed a significant increase in HDL at 8 ml/kg of C. sinensis, 0.3 ml/kg of C. paradisi, and SPJ-2 combination, which was almost comparable to atorvastatin. This could be justified on the basis of the combined effects of Vitamin C, folate, and flavonoids since polyphenolic antioxidants present in the fruits exhibit a broad range of biological effects,[42] including hypocholesterolemic effect.[43,44] Naringin is one of the bioflavonoids in citrus and grapefruits that have been reported to have cholesterol lowering and antiatherogenic effect, may be initiated by preventing HMG-CoA reductase activity.[10,11]
ACAT is one of the cholesterol-regulating enzymes that catalyze the esterification of cholesterol with long chain fatty acids and is thought to play a vital role in the progress of atherosclerotic lesions.[53] Since the increased hepatic microsomal activity of ACAT is normally associated with high cholesterol diet,[25,54] it has been documented that naringin lowers the hepatic microsomal ACAT activity[10,50] and it has also been reported that naringin through decreasing the activity of ACAT1 and ACAT2 results in decreased accessibility of lipids for congregation of apoB-consisting lipoproteins and increased plasma HDL-concentration.[55] Liver plays an important part in the regulation of plasma LDL and cholesterol metabolism through biliary secretion of bile acids and cholesterol. Naringin may result in significantly higher levels of cholesterol metabolites, levels of cholesterol, and bile acids, which are not absorbed in the small intestine.[56] In general, endothelial dysfunction stage in coronary disease is related with total serum cholesterol levels,[57] moreover, HDL efficacy to reverse cholesterol transport might be significant for the function of endothelium as indicated in hypercholesterolemic patient that HDL plays a defensive role in endothelial function.[58]
A possible mechanism for lipid lowering effect of citrus flavonoids might be due to its antioxidant effects and due to its inhibition of uptake of oxidized LDL by macrophages, reduced LDL aggregation, and reduced oxidation of LDL cholesterol,[59] since macrophages play an important role in the early diagnosis.
Conclusion
Result of the recent study shows that juices of C. sinensis and C. paradisi produced a favorable effect of serum lipid profile in rats, since reduced serum TC, TG, LDL, and increased HDL, thus reducing the chances of atherogenesis. Hence, finding of this study provides some biochemical basis for the use of C. sinensis and C. paradisi juices as antihyperlipidemic agent with preventive and curative effects against hyperlipidemia; however more studies are required to gain insight into the possible mechanism of action.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kanakavalli K, Thillaivanan S, Parthiban P. Hypolipidemic activity of Nathaichoori Chooranam (NC) (Siddha drug) on high fat diet induced hyperlipidemic rats. Int J Pharm Res Sci. 2014;02:104–9. [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Mortality from coronary heart disease and acute myocardial infarction – United States, 1998. MMWR Morb Mortal Wkly Rep. 2001;50:90–3. [PubMed] [Google Scholar]
- 3.Wald NJ, Law MR. Serum cholesterol and ischaemic heart disease. Atherosclerosis. 1995;118(Suppl):S1–5. [PubMed] [Google Scholar]
- 4.Byington RP, Jukema JW, Salonen JT, Pitt B, Bruschke AV, Hoen H, et al. Reduction in cardiovascular events during pravastatin therapy. Pooled analysis of clinical events of the pravastatin atherosclerosis intervention program. Circulation. 1995;92:2419–25. doi: 10.1161/01.cir.92.9.2419. [DOI] [PubMed] [Google Scholar]
- 5.Delsing DJ, Offerman EH, van Duyvenvoorde W, van Der Boom H, de Wit EC, Gijbels MJ, et al. Acyl-CoA: Cholesterol acyltransferase inhibitor avasimibe reduces atherosclerosis in addition to its cholesterol-lowering effect in ApoE*3-Leiden mice. Circulation. 2001;103:1778–86. doi: 10.1161/01.cir.103.13.1778. [DOI] [PubMed] [Google Scholar]
- 6.Kim DH, Jung EA, Sohng IS, Han JA, Kim TH, Han MJ. Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch Pharm Res. 1998;21:17–23. doi: 10.1007/BF03216747. [DOI] [PubMed] [Google Scholar]
- 7.Higashimoto M, Yamato H, Kinouchi T, Ohnishi Y. Inhibitory effects of citrus fruits on the mutagenicity of 1-methyl-1,2,3,4-tetrahydro-beta-carboline-3-carboxylic acid treated with nitrite in the presence of ethanol. Mutat Res. 1998;415:219–26. doi: 10.1016/s1383-5718(98)00079-5. [DOI] [PubMed] [Google Scholar]
- 8.Le Marchand L, Murphy SP, Hankin JH, Wilkens LR, Kolonel LN. Intake of flavonoids and lung cancer. J Natl Cancer Inst. 2000;92:154–60. doi: 10.1093/jnci/92.2.154. [DOI] [PubMed] [Google Scholar]
- 9.Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol. 1999;126:673–80. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bok SH, Shin YW, Bae KH. Effects of naringin and lovastatin on plasma and hepatic lipids in high-fat and high-cholesterol fed rats. Nutr Res. 2000;20(Suppl 7):1007–15. [Google Scholar]
- 11.Shin YW, Bok SH, Jeong TS, Bae KH, Jeoung NH, Choi MS, et al. Hypocholesterolemic effect of naringin associated with hepatic cholesterol regulating enzyme changes in rats. Int J Vitam Nutr Res. 1999;69:341–7. doi: 10.1024/0300-9831.69.5.341. [DOI] [PubMed] [Google Scholar]
- 12.Jeon SM, Park YB, Choi MS. Antihypercholesterolemic property of naringin alters plasma and tissue lipids, cholesterol-regulating enzymes, fecal sterol and tissue morphology in rabbits. Clin Nutr. 2004;23:1025–34. doi: 10.1016/j.clnu.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Mateljan G. The World's Healthiest Foods Grapefruit. [Last accessed on 2013 Dec]. Available from: http://www.whfoods.com/genpage.php?tname=foodspice and dbid25#healthbenefits .
- 14.Cerda JJ, Robbins FL, Burgin CW, Baumgartner TG, Rice RW. The effects of grapefruit pectin on patients at risk for coronary heart disease without altering diet or lifestyle. Clin Cardiol. 1988;11:589–94. doi: 10.1002/clc.4960110902. [DOI] [PubMed] [Google Scholar]
- 15.Fujioka K, Greenway F, Sheard J, Ying Y. The effects of grapefruit on weight and insulin resistance: Relationship to the metabolic syndrome. J Med Food. 2006;9:49–54. doi: 10.1089/jmf.2006.9.49. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94:391–8. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- 17.Armando C, Maythe S, Beatriz NP. Antioxidant activity of grapefruit seed extract on vegetable oils. J Sci Food Agric. 1998;77:463–7. [Google Scholar]
- 18.Gorinstein S, Caspi A, Libman I, Lerner HT, Huang D, Leontowicz H, et al. Red grapefruit positively influences serum triglyceride level in patients suffering from coronary atherosclerosis: Studies in vitro and in humans. J Agric Food Chem. 2006;54:1887–92. doi: 10.1021/jf058171g. [DOI] [PubMed] [Google Scholar]
- 19.Gao K, Henning SM, Niu Y, Youssefian AA, Seeram NP, Xu A, et al. The citrus flavonoid naringenin stimulates DNA repair in prostate cancer cells. J Nutr Biochem. 2006;17:89–95. doi: 10.1016/j.jnutbio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Holzapfel NP, Holzapfel BM, Champ S, Feldthusen J, Clements J, Hutmacher DW. The potential role of lycopene for the prevention and therapy of prostate cancer: From molecular mechanisms to clinical evidence. Int J Mol Sci. 2013;14:14620–46. doi: 10.3390/ijms140714620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown ND, Steven F, Lori G. Grapefruit and drugs interaction. J Altern Complement Med. 2002;8:521. [Google Scholar]
- 22.Liu Y, Heying E, Tanumihardjo SA. History, global distribution, and nutritional importance of citrus fruits. Compr Rev Food Sci Food Saf. 2012;11:530–45. [Google Scholar]
- 23.Peluso MR. Flavonoids attenuate cardiovascular disease, inhibit phosphodiesterase, and modulate lipid homeostasis in adipose tissue and liver. Exp Biol Med (Maywood) 2006;231:1287–99. doi: 10.1177/153537020623100802. [DOI] [PubMed] [Google Scholar]
- 24.Chiba H, Uehara M, Wu J, Wang X, Masuyama R, Suzuki K, et al. Hesperidin, a citrus flavonoid, inhibits bone loss and decreases serum and hepatic lipids in ovariectomized mice. J Nutr. 2003;133:1892–7. doi: 10.1093/jn/133.6.1892. [DOI] [PubMed] [Google Scholar]
- 25.Graciela OR, Fredy Y, Betzabe SF, Lilibeth C. Volatile fraction composition of Venezuelan sweet orange essential oil (Citrus sinensis (L.) Osbeck) Ciencia. 2003;11:55–60. [Google Scholar]
- 26.Kurowska EM, Borradaile NM, Spence JD, Carroll KK. Hypocholesterolemic effects of dietary citrus juices in rabbits. Nutr Res. 2000;20:121–9. [Google Scholar]
- 27.Roza JM, Xian-Liu Z, Guthrie N. Effect of citrus flavonoids and tocotrienols on serum cholesterol levels in hypercholesterolemic subjects. Altern Ther Health Med. 2007;13:44–8. [PubMed] [Google Scholar]
- 28.Buscemi S, Rosafio G, Arcoleo G, Mattina A, Canino B, Montana M, et al. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am J Clin Nutr. 2012;95:1089–95. doi: 10.3945/ajcn.111.031088. [DOI] [PubMed] [Google Scholar]
- 29.Ghanim H, Sia CL, Upadhyay M, Korzeniewski K, Viswanathan P, Abuaysheh S, et al. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am J Clin Nutr. 2010;91:940–9. doi: 10.3945/ajcn.2009.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawalha S, Arráez-Román D, Segura-Carretero A, Fernández-Gutiérrez A. Quantification of main phenolic compounds in sweet and bitter orange peel using CE–MS/MS. Food Chem. 2009;116:567–74. [Google Scholar]
- 31.Manach C, Morand C, Gil-Izquierdo A, Bouteloup-Demange C, Rémésy C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur J Clin Nutr. 2003;57:235–42. doi: 10.1038/sj.ejcn.1601547. [DOI] [PubMed] [Google Scholar]
- 32.Ha SK, Park HY, Eom H, Kim Y, Choi I. Narirutin fraction from citrus peels attenuates LPS-stimulated inflammatory response through inhibition of NF-κB and MAPKs activation. Food Chem Toxicol. 2012;50:3498–504. doi: 10.1016/j.fct.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Funaguchi N, Ohno Y, La BL, Asai T, Yuhgetsu H, Sawada M, et al. Narirutin inhibits airway inflammation in an allergic mouse model. Clin Exp Pharmacol Physiol. 2007;34:766–70. doi: 10.1111/j.1440-1681.2007.04636.x. [DOI] [PubMed] [Google Scholar]
- 34.Rogerio AP, Sá-Nunes A, Faccioli LH. The activity of medicinal plants and secondary metabolites on eosinophilic inflammation. Pharmacol Res. 2010;62:298–307. doi: 10.1016/j.phrs.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Council NR. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: The National Academic Press; 2011. [Google Scholar]
- 36.Sumbul S, Ahmed SI. Anti-hyperlipidemic activity of Carissa carandas (Auct.) leaves extract in egg yolk induced hyperlipidemic rats. J Basic Appl Sci. 2012;8:124–34. [Google Scholar]
- 37.Sanguino E, Roglans N, Alegret M, Sánchez RM, Vázquez-Carrera M, Laguna JC. Atorvastatin reverses age-related reduction in rat hepatic PPARalpha and HNF-4. Br J Pharmacol. 2005;145:853–61. doi: 10.1038/sj.bjp.0706260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trinder P. Enzymatic calorimetric determination of triglycerides by GOP- PAP method. Ann Clin Biochem. 1969;6:24–7. [Google Scholar]
- 39.Warnick GR, Wood PD. National Cholesterol Education Program recommendations for measurement of high-density lipoprotein cholesterol: Executive summary. The National Cholesterol Education Program Working Group on Lipoprotein Measurement. Clin Chem. 1995;41:1427–33. [PubMed] [Google Scholar]
- 40.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 41.Brisson GJ. Netherlands: Springer; 1981. Lipids in Human Nutrition. The Enigma of the Trans Fatty Acids, An Appraisal of Some Dietary Concepts; pp. 41–71. [Google Scholar]
- 42.Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol. 1983;32:1141–8. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- 43.Choi JS, Yokozawa T, Oura H. Antihyperlipidemic effect of flavonoids from Prunus davidiana. J Nat Prod. 1991;54:218–24. doi: 10.1021/np50073a022. [DOI] [PubMed] [Google Scholar]
- 44.Kurowska EM, Spence JD, Jordan J, Wetmore S, Freeman DJ, Piché LA, et al. HDL-cholesterol-raising effect of orange juice in subjects with hypercholesterolemia. Am J Clin Nutr. 2000;72:1095–100. doi: 10.1093/ajcn/72.5.1095. [DOI] [PubMed] [Google Scholar]
- 45.Verlecar XN, Jena KB, Chainy GB. Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chem Biol Interact. 2007;167:219–26. doi: 10.1016/j.cbi.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 46.Zulet MA, Barber A, Garcin H, Higueret P, Martínez JA. Alterations in carbohydrate and lipid metabolism induced by a diet rich in coconut oil and cholesterol in a rat model. J Am Coll Nutr. 1999;18:36–42. doi: 10.1080/07315724.1999.10718825. [DOI] [PubMed] [Google Scholar]
- 47.Kanungo SK, Panda DS, Swain SR, Barik BB, Tripathi DK. comparative evaluation of hypolipidemic activity of some marketed herbal formulations in triton induced hyperlipidemic rats. Pharmacol Online. 2007;3:211–21. [Google Scholar]
- 48.Patel DK, Patel KA, Patel UK, Thounaoja MC, Jadeja RN, Ansarullah, et al. Assessment of lipid lowering effect of Sida rhomboidea. Roxb methanolic extract in experimentally induced hyperlipidemia. J Young Pharma. 2009;1:233–8. [Google Scholar]
- 49.Matsuo N, Osada K, Kodama T, Lim BO, Nakao A, Yamada K, et al. Effects of gamma-linolenic acid and its positional isomer pinolenic acid on immune parameters of brown-Norway rats. Prostaglandins Leukot Essent Fatty Acids. 1996;55:223–9. doi: 10.1016/s0952-3278(96)90002-2. [DOI] [PubMed] [Google Scholar]
- 50.Wilcox LJ, Borradaile NM, Kurowska E, Telford DE, Huff MW. Naringenin, a citrus flavonoid, markedly decreases apoB secretion in HepG2 cells and inhibits acyl CoA: Cholesterol acyltransferase. Circulation. 1998;98:531–7. [Google Scholar]
- 51.Wilcox LJ, Borradaile NM, Huff MW. Antiatherogenic properties of naringenin, a citrus flavonoid. Cardiovasc Drug Rev. 1999;17:160–78. [Google Scholar]
- 52.Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Uelmen PJ, Oka K, Sullivan M, Chang CC, Chang TY, Chan L. Tissue-specific expression and cholesterol regulation of acylcoenzyme A: Cholesterol acyltransferase (ACAT) in mice. Molecular cloning of mouse ACAT cDNA, chromosomal localization, and regulation of ACAT in vivo and in vitro . J Biol Chem. 1995;270:26192–201. doi: 10.1074/jbc.270.44.26192. [DOI] [PubMed] [Google Scholar]
- 54.Grogan WM, Bailey ML, Heuman DM, Vlahcevic ZR. Effects of perturbations in hepatic free and esterified cholesterol pools on bile acid synthesis, cholesterol 7 alpha-hydroxylase, HMG-CoA reductase, acyl-CoA: Cholesterol acyltransferase and cytosolic cholesteryl ester hydrolase. Lipids. 1991;26:907–14. doi: 10.1007/BF02535976. [DOI] [PubMed] [Google Scholar]
- 55.Mortensen A, Breinholt V, Dalsgaard T, Frandsen H, Lauridsen ST, Laigaard J, et al. 17beta-Estradiol but not the phytoestrogen naringenin attenuates aortic cholesterol accumulation in WHHL rabbits. J Lipid Res. 2001;42:834–43. [PubMed] [Google Scholar]
- 56.Nielsen LB, Stender S, Kjeldsen K. Effect of lovastatin on cholesterol absorption in cholesterol-fed rabbits. Pharmacol Toxicol. 1993;72:148–51. doi: 10.1111/j.1600-0773.1993.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 57.Zeiher AM, Drexler H, Saurbier B, Just H. Endothelium-mediated coronary blood flow modulation in humans. Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J Clin Invest. 1993;92:652–62. doi: 10.1172/JCI116634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quyyumi A. Effect of atherosclerosis on endothelium dependent inhibition of platelet activation in humans. Circulation. 1998;98:17–24. doi: 10.1161/01.cir.98.1.17. [DOI] [PubMed] [Google Scholar]
- 59.Anoosh E, Mojtaba E, Fatemeh S. Study the effect of juice of two variety of pomegranate on decreasing plasma LDL cholesterol. Procedia Soc Behav Sci. 2010;2:620–3. [Google Scholar]


