Abstract
Aims:
The study aimed to explore the antioxidant activity of ethanolic leaf extract of Manihot esculenta Crantz leaves (MEC) in wistar rats.
Materials and Methods:
Ethanolic extract of MEC leaves in the doses of 50 mg/kg, 100 mg/kg, and 200 mg/kg were used in wistar rats of either sex. The oxidative stress was produced by overdose of acetaminophen and estimation of serum concentration of various enzymes such as malonaldehyde (MDA), superoxide dismutase (SOD), reduced glutathione (GSH), and catalase (CAT) were measured by standard biochemical methods. Silymarin (100 mg/kg) was used as a standard drug for assessment of antioxidant status.
Statistical Analysis Used:
Results were analyzed by one-way analysis of variance followed by Student's unpaired t-test.
Results:
When compared with the standard antioxidant silymarin, MEC extract did not exhibit antioxidant activity in terms of MDA level reduction. However, it significantly increased serum levels of the antioxidant enzymes (SOD, GSH, and CAT) exerting a potent antioxidant effect in a graded manner.
Conclusions:
The observed results suggest that MEC could be a potential source of antioxidants. However, further studies are required to explore this therapeutic property of plant.
KEY WORDS: Flavonoids, Manihot esculenta Crantz, lipid peroxidation, silymarin
Since the beginning of human civilization, medicinal plants are local heritage with global importance providing starting materials for isolation or synthesis of many conventional drugs and the process is still ongoing. Currently, approximately 25% of drugs are derived from plants, and many others are synthetic analogues built on prototype compounds isolated from plant species in the modern pharmacopoeia.[1] In the last few decades, there has been a tremendous growth in research on herbal medicines and various new species have been evaluated for their medicinal properties. Manihot esculenta Crantz (MEC) is one such species which is being actively researched recently for various indications.
MEC, popularly known as cassava or tapioca is a woody shrub belonging to the family Euphorbiaceae, is one of the major staple food crops cultivated in tropical and subtropical regions that is, Africa, Asia, and Latin America.[2] Roots are generally used for nutritional importance, third largest source of carbohydrates in the meal.[3] MEC leaves have been mentioned in various literature for their possible medicinal properties such as fever, diarrhoea, headache, and rheumatism.[4] In Nigeria, the plant is used for the treatment of ringworm, conjunctivitis, sores, and abscess.[5] Apart from these, various literature have mentioned the use of plant leaves in hypertension, irritable bowel syndrome, aches, cancerous affections, affective excrescences of the eyes and tumors, antiseptic, cyanogenetic, demulcent, diuretic, dysentery, flu, marasmus, snake bites, prostatitis, and spasms, etc.[6,7,8]
Apart from these medicinal properties of MEC leaves, few studies have been conducted to reveal the presence of various antioxidant and antiradical substances and hence to study its possible antioxidant potential. A study conducted by Tsumbu et al. revealed the first insights into the antioxidant and antiradical properties in a model of a complete lipid peroxidation and cellular “inflammation like” conditions. In this model, leaf extracts significantly decreased the formation of typical markers of an advanced lipid peroxidation such as lipid hydroperoxides and ethylene. Moreover, the extract solutions showed the inhibition of the formation of transient free radicals at an early stage of lipid peroxidation.[5]
Suresh et al. investigated MEC leaves extract for total phenols, anthocyanins, ascorbic acid and their ability to reduce many free radicals along with superoxide radical scavenging activity.[9] Yi et al. identified 10 antioxidant compounds from the plant by an activity-guided isolation such as coniferaldehyde, isovanillin, 6-deoxyjacareubin, scopoletin, syringaldehyde, pinoresinol, p-coumaric acid, ficusol, balanophonin, and ethamivan. All these compounds were found to have 2,2-diphenylpicrylhydrazyl (DPPH) scavenging capacity and 2,2'-azinobis 3-ethylbenzthiazoline-6-sulfonic acid-free radical scavenging ability.[10] Gómez-Vásquez et al. reported phenolic compounds in MEC leave such as coumarin, scopolin, aglycone scopoletin, and the flavonoids like kaempferol-3-O-rutinoside and rutin supporting the possible antioxidant activity of the plant leaves.[11] Al-Rofaai et al. investigated the total tannin compounds of leaf extract of MEC using tannin quantification assay and antioxidant activity using the DPPH radical scavenging assay. Total tannin was estimated to 254.44 TAE/mg of extract suggesting that extract may be potentially utilized as a source of natural antioxidants.[12] Additionally, the plant contains various micro and macro nutrients and antioxidants such as â-carotene (around 23–86 mg/100 g), Vitamin C (1.7–419 mg/100 g), Vitamin A, saponins, steroids, and glycosides.[2,13]
From the perusal of literature, it appears that antioxidant activities of this plant have been less investigated. Therefore, it is of interest to evaluate this unexplored biochemical activity by the standard experimental models in this important but relatively neglected medicinal plant.
Materials and Methods
Animals
Healthy adult wistar rats of either sex weighing 150–250 g were used for the study. They were caged in polyvinyl wire mesh cages in the animal house of department. They were maintained under standard laboratory conditions (12 h light and dark cycle and temperature of 27°C ± 2°C and humidity (60 ± 10%) with free access to food and water ad libitum. Animals were acclimatized to laboratory conditions before the experiment for 14 days.
Collection of plant material
Fresh leaves of MEC were collected from the farms and authenticated by a botanist. The leaves were shade-dried in the department, finely powdered and stored in an air tight container.
Preparation of plant extract
The powder was extracted with 90% ethanol using Soxhlet apparatus at 50–55°C for 3 days. The extract was concentrated in a ventilated oven at 45°C for 24 h. Fifty grams powder yielded 10 g of extract after drying and concentrating. It was dissolved in 2% gum acacia before administering to the experimental animals. The extract was freshly prepared each time before using in the experiment.
Drugs and chemicals
Various drugs such as silymarin - Microlabs Ltd., acetaminophen - GSK and chemicals such as trichloroacetic acid (TCA) - India thiobarbituric acid (TBA), tris-HCL buffer, hydrogen peroxide (H2O2) - SRL, 5,5'-dithiobis (2-nitrobenzoic acid) (DTNB) and glutathione (GSH) standard - SRL NaOH - HiMedia, pyragallol - Ranbaxy, ethylene di-amine tetraacetic acid (EDTA) - SD Fines Chem. Ltd., diethlene triamine pentaacetic acid (DTPA) - Sigma-Aldrich, Germany were purchased from local medical store and kept under appropriate storage conditions.
Ethical clearance
Ethical clearance was taken from Institutional Animal Ethics Committee of Institute.
Methods
Acute oral toxicity study
Acute oral toxicity study for the test extract of the plant was carried out as per the guidelines set by Organization for Economic Co-operation and, revised draft guidelines 425 and by the Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Social Justice and Empowerment, Government of India.[14] The study revealed that the administration of ethanolic leaf extract of MEC was safe up to a dose of 2000 mg/kg. No death was observed up to this dose, and the experimental animals were physically active. By keeping 1/10th (200 mg/kg) dose as highest, the doses of 50 mg/kg; 100 mg/kg, and 200 mg/kg were selected as working doses for the present study.
Methods for evaluation of antioxidant activity in rats
Antioxidant activity of ethanolic leaf extract of MEC was evaluated by measurement of oxidative stress markers and antioxidant enzymes in serum of albino rats after the induction of oxidative stress by the procedure described by Galal et al.[15] The animals were divided into five groups of six animals each. Group I received normal saline orally and served as control group. Group II received silymarin (100 mg/kg) orally and served as a standard group. Group III, IV, and V received ethanolic leaf extracts of MEC at 50 mg/kg, 100 mg/kg, and 200 mg/kg orally and served as test groups. The animals were orally treated with respective drugs for 7 successive days. On the 6th day (i.e., 1-day before the last treatment) animals of all groups were fasted for 18 h. On the 7th day, 1 h after the last dose of agents given, all the animals were given acetaminophen 2 g/kg per os for the induction of oxidative stress. After 24 h of administration, all the animals were sacrificed by ether overdose. Blood samples were collected by cardiac puncture and used for further laboratory investigations.
Estimation of malondialdehyde[16]
Malondialdehyde in plasma is one of the aldehyde products of lipid peroxidation which react with TBA to form a colored product, the absorbance of which is measured spectro-photometrically at 530 nm. In the test tubes 0.5 ml of serum from test samples were taken, and 3 ml of 10% TCA was added to it, mixed well and the tubes were left to stand for 10 min at room temperature, and then centrifuged for 15 min at 5000 rpm. Two sets of test tubes were taken marked as blank and test. For a test sample, 2 ml of supernatant fluid was taken and added to 1.5 ml of 0.67% TBA. For a blank sample, 2 ml of distilled was added in 2 ml of 0.67% TBA. After mixing well and keeping in the boiling water bath for 10 min, they were cooled under tap water. A pale pink color developed, the color intensity was measured at 530 nm by colorimeter. Using the molar extension coefficient (1.5 × 105) and result was expressed as n moles of malonaldehyde (MDA)/100 ml of serum.
1.5 = 100 µmol/L (here, 100 is for conversion from ml to dl).
Then MDA = 100 × O.D. of unknown/1.5.
Estimation of superoxide dismutase[17]
This method utilizes the inhibition of auto-oxidation of pyrogallol by superoxide dismutase (SOD) enzyme. The assay mixture in a 3 ml volume consisted of 100 μL each of 0.2 mM pyrogallol, 1 mM EDTA, 1 mM DTPA, and varying concentrations of standard SOD enzyme or 100 μL of serum in air equilibrated tris-HCl buffer (50 mM; pH 8.2). The reaction mixture prepared in 3 sets includes standard, test and control. Pyrogallol was added after the addition of all other reagents to start the reaction. Initial 10 s period was considered as induction period of the enzyme. So after 10 s, change in absorbance at 420 nm at 10 s intervals was recorded to a period of 4 min. The average change in the absorbance per minute was calculated.
One unit of enzyme SOD was defined as the amount of enzyme received to cause 50% inhibition of pyrogallol auto-oxidation. Accordingly, the activity of the enzyme in different standards was expressed in units/ml.
Estimation of reduced glutathione[18]
The method described is based on the development of a yellow color when DTNB (Ellman's Reagent) is added to sulphydryl compounds due to redox reaction between GSH and DTNB. The color which develops is fairly stable for about 10 min, and the reaction is little affected by variation in temperature. The reaction is read at 412 nm. GSH in red cells is relatively stable and venous blood samples anticoagulated with ACD maintain GSH levels up to 3 weeks at 4°C GSH is slowly oxidized in solution, so only fresh lysates should be used for the assay.
Whole blood (200 µl) is mixed thoroughly with 1.8 ml of distilled water and 3 ml of PPT solution and allows standing for 5 min and filtered. Two test tube marked test and blank were taken. In test marked test tube, 2 ml of clear filtrate added from the above mixture to 8 ml of disodium phosphate buffer and 1 ml of DTNB reagent added to it, and mixed well. The color developed rapidly, stable for 10 min. A reagent blank was made using 2 ml of distilled water, 8 ml of phosphate buffer, and 1 ml of DTNB reagent. Readings were taken at 412 nm in the spectrophotometer. Reading was taken; the curve was plotted taking absorbance at 412 nm on the Y-axis and concentration on the X-axis. The concentration of the test samples was calculated by using standard curve. Reduced GSH concentration in blood sample expressed in mg/dl.
Estimation of catalase[19]
The method is based on the fact that dichromate in acetic acid gets reduces to chromic acetate when heated in the presence of H2O2 with the formation of perchromic acid as an unstable intermediate. The chromic acetate thus produced is measured colorimetrically at 570 nm. The catalase (CAT) preparation allows splitting of H2O2 for different periods of time. The reaction is stopped at a particular time by the addition of dichromate/acetic acid mixture, and the remaining H2O2 is determined by measuring chromic acid colorimetrically after heating the reaction mixture.
Three sets of tubes were arranged and labeled as blank, test (0 s) and test (60 s), and proper reagent additions were made to them: The tubes were boiled for 10 min, cooled to room temperature and readings were taken at 570 nm. Different concentrations of H2O2 ranging from 10 to 160 µmoles were taken in tubes and preceded. The activity of CAT was expressed as units/ml of the serum sample. One unit of CAT activity represents the amount of enzyme that destroys 1 µmole H2O2/min.
Statistical analysis
The data are presented as a mean ± standard error mean. The data were analyzed by one-way analysis of variance followed by Student's unpaired t-test by using Graph Pad Prism 6.03 version. P values of <0.05, <0.0,1 and <0.001 were considered to be significant, very significant, and highly significant, respectively.
Results
In this method, antioxidant activity was measured by the estimation of serum concentrations of various enzymes by standard biochemical methods. Silymarin (100 mg/kg) was used as a standard drug for assessment of antioxidant status. Silymarin is obtained from the plant sliybon marinum, and it has already proven as an antioxidant and antifibrotic.[20]
Serum MDA level was lowered in the standard group (2.93 ± 0.29) but that was statistically not significant when compared with control (3.84 ± 0.37). Serum MDA levels were also found to be reduced in MEC (100 mg/kg) and MEC (200 mg/kg) groups, but they were also not statistically significant in comparison with control group. While, in MEC (50 mg/kg) group, there was an increase in serum MDA level in comparison with control group, but that was also not significant[Table 1].
Table 1.
Effect of ethanolic leaf extract of Manihot esculenta Crantz on serum antioxidant enzyme levels
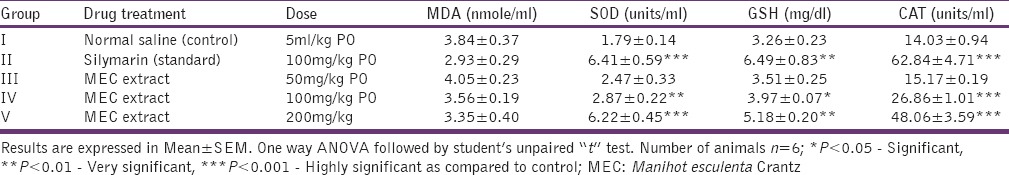
Standard increased the serum SOD level, and the difference was highly significant (P < 0.001) when compared with control group indicating potent antioxidant capacity. Oral administration of graded doses of MEC extracts also increased serum SOD levels when compared with control group. The highest increase was found in MEC (200 mg/kg) group (P < 0.001), while MEC (100 mg/kg) showed very significant increase (P < 0.01) in comparison with control group. In MEC (50 mg/kg) group, serum SOD level was increased but the difference was statistically nonsignificant in comparison with control group. In this aspect, MEC (200 mg/kg) was comparable with the standard silymarin [Table 1].
Serum reduced GSH level was found to be increased in standard and all three extract groups in a graded manner in comparison with control group. Both standard group and MEC (200 mg/kg) showed a very significant increase (P < 0.01) as compared with control group. MEC (100 mg/kg) group showed a significant increase (P < 0.05) in comparison with control group. MEC (50 mg/kg) group also increased serum GSH level, but this was statistically nonsignificant in comparison with control group. In this aspect, MEC (200 mg/kg) was comparable with the standard silymarin [Table 1].
Serum CAT level was found to be increased in standard and all three extract groups in a graded manner in comparison with control group. This increase was statistically very significant (P < 0.001) in the standard group, MEC (100 mg/kg) and MEC (200 mg/kg) groups in comparison with control groups. The increase in MEC (50 mg/kg) groups was statistically nonsignificant in comparison with control group. In this aspect, MEC (200 mg/kg) was relatively comparable with the standard silymarin [Table 1].
Discussion
For assessing antioxidant activity of ethanolic leaf extract of MEC, the oxidative stress was produced in rats by an overdose of acetaminophen and estimation of serum concentration of various enzymes by standard biochemical methods. Serum levels of MDA, SOD, reduced GSH, and CAT were measured. Our results showed that standard drug silymarin lowered serum MDA level in comparison with control. The graded extract doses increased serum MDA levels but not significantly as compared to control. This shows that the extract does not have antioxidant activity in terms of MDA level in the reduction of peroxidation level. Standard increased serum levels of all the antioxidant enzymes (SOD, GSH and CAT) significantly exerting a potent antioxidant effect. MEC (100 mg/kg) and MEC (200 mg/kg) also showed similar results in a graded manner and thus exerted a potent antioxidant effect. However, MEC (50 mg/kg) was totally ineffective in this regard. Hence, it can be concluded that plant exert potent antioxidant activity at higher doses. Especially, MEC (200 mg/kg) is comparable with standard silymarin in this aspect.
MDA is a stable secondary aldehyde degeneration product of lipid peroxidation and is used as a biological marker for the assessment of lipid peroxidation.[21] The relative increase in serum MDA levels in all extract groups proved that extract was not as an effective antioxidant as standard.
An antioxidant works by retarding the process of oxidation by free radicals and further damage. Increased levels of measured antioxidant enzymes clearly envisaged the antioxidant potential of this plant. SOD is an important endogenous antioxidant enzyme acting as the first line defense system against reactive oxygen species (ROS) which scavenges superoxide radicals to H2O2 and thus provide protection against the deleterious effects of radicals.[22,23] H2O2 accumulated by this reaction leads to the formation of hydroxyl radicals which can be harmful too. GSH and CAT work as antioxidant enzymes by virtue of scavenging these hydroxyl radicals. GSH is a tripeptide and a powerful antioxidant present in the cytosol of cells and is the major intracellular nonprotein thiol compound. SH groups present in GSH reacts with H2O2 and the hydroxyl radical and prevent tissue damage, and it is also capable of scavenging ROS directly or enzymatically.[22]
The antioxidant effects observed in our study can be attributed to many phytochemicals in the experimental plant leaves as they are reported to possess the antioxidant activity. Among these, flavonoids have been very frequently correlated with the antioxidant potential of any plant extract. It has been proposed by Ye et al.[24] that flavonoids have the very strong capacity to eliminate free radicals in the blood and promotes the activities of antioxidant enzymes such as SOD, GSH, and CAT. These actions of flavonoids are also dose dependent. Hence, an increase in the serum concentrations of antioxidant enzymes in our study can be explained by this background. A low dose of MEC extract 50 mg/kg) was unable to increase these enzyme levels, hence failed to exert antioxidant activity.
Antioxidant potential of the flavonoids has also been suggested by various other researchers in past.[2,4,5,25,26] They are effective scavengers of various types of free radicals. Anthocyanins are also a group of flavonoids which can be helpful as antioxidants.[9,13] Yi et al.[10] and Gómez-Vásquez et al.[11] reported the presence of ten important antioxidant substances in MEC leaves. Apart from these, the plant leaves have shown the presence of other constituents such as polyphenols, tannins, anthocyanins, alkaloids, glycosides, saponins, steroids, iron, and vitamins such as A, C, E which have been linked with the antioxidant potential by many researchers. Tsumbu et al.[5] also suggested the possible antioxidant activity of tannins. Ghani suggested the antioxidant potential of alkaloids, glycosides.[27] Vitamins such as A, C, and E are very much known as antioxidants since long time as supported by various literature.[13,25,26,28] Thus, the antioxidant activity of MEC can be explained on the basis of the presence of these antioxidant chemical constituents which are known to reduce oxidative stress by various mechanisms. Miladiyah et al. also have proposed in their study that MEC leaves are capable of counteracting the harmful effects of free radicals because of various antioxidant compounds.[4] Nassar et al.[29] have provided very strong correlation of flavonoids with analgesic, anti-inflammatory, antipyretic, antidiarrhoeal, antioxidant, and many other biological properties indicating the utmost importance of this phytoconstituents.
Conclusion
The ethanolic extract of MEC leaves demonstrated significant antioxidant properties in experimental animals in this study. These activities may be attributed to the various phytoconstituents of MEC leaves such as flavonoids, tannins, saponins, alkaloids, anthocyanins, glycosides, polyphenols, steroids, iron, and vitamins such as A, C, E. However, further experimental studies are required to explore the exact mechanism of actions and next level of clinical trials to generate novel drugs. This might prove helpful to use its immense therapeutic efficacy as a potent antioxidant phytomedicine.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rao MR, Palada MC, Becker BN. Medicinal and aromatic plants in agro-forestry systems. Agroforestry Syst. 2004;61:107–22. [Google Scholar]
- 2.Fasuyi AO. Nutrient composition and processing effects on cassava Leaf (Manihot esculenta, Crantz) antinutrients. Pak J Nutr. 2005;4:37–42. [Google Scholar]
- 3.Fauquet C, Fargette D. African cassava mosaic virus: Etiology, epidemiology, and control. Plant Dis. 1990;74:404–11. [Google Scholar]
- 4.Miladiyah I, Dayi F, Desrini S. Analgesic activity of ethanolic extract of Manihot esculenta Crantz leaves in mice. Univ Med. 2011;30:3–10. [Google Scholar]
- 5.Tsumbu CN, Deby-Dupont G, Tits M, Angenot L, Franck T, Serteyn D, et al. Antioxidant and antiradical activities of Manihot esculenta Crantz (Euphorbiaceae) leaves and other selected tropical green vegetables investigated on lipoperoxidation and phorbol-12-myristate-13-acetate (PMA) activated monocytes. Nutrients. 2011;3:818–38. doi: 10.3390/nu3090818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abd Aziz SM, Low CN, Chai LC, Abd Razak SS, Selamat J, Son R, et al. Screening of selected Malaysian plants against several food borne pathogen bacteria. Int Food Res J. 2011;18:1195–201. [Google Scholar]
- 7.Hartwell JL. Plants used against cancer. A survey. Lloydia. 1971;34:386–425. [PubMed] [Google Scholar]
- 8.Duke JA, Wain KK. Vol. 3. UK: Longman Group Ltd; 1981. Medicinal Plants of the World: Computer Index with More Than 85,000 Entries. [Google Scholar]
- 9.Suresh R, Saravanakumar M, Suganyadev P. Anthocyanins from Indian cassava (Manihot esculenta Crantz) and its antioxidant properties. Int J Pharm Sci Res. 2011;2:1819–28. [Google Scholar]
- 10.Yi B, Hu L, Mei W, Zhou K, Wang H, Luo Y, et al. Antioxidant phenolic compounds of cassava (Manihot esculenta) from Hainan. Molecules. 2011;16:10157–67. doi: 10.3390/molecules161210157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gómez-Vásquez R, Day R, Buschmann H, Randles S, Beeching JR, Cooper RM. Phenylpropanoids, phenylalanine ammonia lyase and peroxidases in elicitor-challenged cassava (Manihot esculenta) suspension cells and leaves. Ann Bot. 2004;94:87–97. doi: 10.1093/aob/mch107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Rofaai A, Rahman WA, Sulaiman SF, Yahaya ZS. In vitro ovicidal and larvicidal activity of methanolic leaf extract of Manihot esculenta (cassava) on susceptible and resistant strains of Trichostrongylus colubriformis. Vet Parasitol. 2012;190:127–35. doi: 10.1016/j.vetpar.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 13.Okeke CU, Iweala E. Antioxidant profile of Dioscorea rotundata, Manihot esculenta, Ipoemea batatas, Vernonia amygdalina and Aloe vera. J Med Res Technol. 2007;4:4–10. [Google Scholar]
- 14.OECD Guidelines for the Testing of Chemicals (Acute Oral Toxicity - Up and Down Procedure) [Last cited on 2015 Jan 21]. Available from: http://www.oecd.org .
- 15.Galal RM, Zaki HF, Seif El-Nasr MM, Agha AM. Potential protective effect of honey against paracetamol-induced hepatotoxicity. Arch Iran Med. 2012;15:674–80. [PubMed] [Google Scholar]
- 16.Slater TF, Sawyer BC. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. General features of the systems used. Biochem J. 1971;123:805–14. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 18.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 19.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 20.Schuppan D, Hahn EG. Clinical studies with silymarin: Fibrosis progression is the end point. Hepatology. 2001;33:483–4. doi: 10.1002/hep.510330230. [DOI] [PubMed] [Google Scholar]
- 21.Comporti M. Lipid peroxidation and cellular damage in toxic liver injury. Lab Invest. 1985;53:599–623. [PubMed] [Google Scholar]
- 22.Mahantesh SP, Gangawane AK, Patil CS. Free radicals, antioxidants, diseases and phytomedicines in human health: Future perspects. World Res J Med Aromat Plants. 2012;1:6–10. [Google Scholar]
- 23.Olawale O, Ikechukwu NE, Grace TO, Chidiebere EU. Oxidative stress and superoxide dismutase activity in brain of rats fed with diet containing permethrin. Biokemistri. 2008;20:93–8. [Google Scholar]
- 24.Ye Y, Guo Y, Luo YT. Anti-inflammatory and analgesic activities of a novel biflavonoid from shells of Camellia oleifera. Int J Mol Sci. 2012;13:12401–11. doi: 10.3390/ijms131012401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noori S. An overview of oxidative stress and antioxidant defense system. Sci Rep. 2012;1:413. [Google Scholar]
- 26.Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–62. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- 27.Ghani A. Introduction to Pharmacognosy. Vol. 45. Zaria, Nigeria: Ahmadu Bello University Press, Ltd; 1990. pp. 187–97. [Google Scholar]
- 28.Thomas DR. Vitamins in health and aging. Clin Geriatr Med. 2004;20:259–74. doi: 10.1016/j.cger.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Nassar MI, Aboutabl ESA, Eskander DM, Grace MH, El-Khrisy ED, Sleem AA. Flavonoid glycosides and pharmacological activity of Amphilophium paniculatum. Pharmacognosy Res. 2013;5:17–21. doi: 10.4103/0974-8490.105643. [DOI] [PMC free article] [PubMed] [Google Scholar]


