Abstract
Aim:
Citrullus colocynthis plant was traditionally used for the treatment of diabetes in Sabzevar city, Iran. This study aimed to investigate the effects of C. colocythis on fasting blood sugar (FBS) and HbA1c in patients with type II diabetes.
Materials and Methods:
Totally 70 patients with type II diabetes attending the diabetes clinic in Sabzevar city were investigated. Patients were divided into two groups of intervention and placebo randomly and were studied for 2 months. Mean serum level of FBS and HbA1c was compared within and between groups at the end of the study. Data were analyzed using student and paired t-tests via SPSS software version 16.
Results:
A significant difference was revealed between before and after intervention for HbA1c and FBS levels in the intervention group (P = 0.01 and P = 0.04, respectively). The difference between before and after intervention for FBS and HbA1c levels in the placebo group were not significant (P = 0.8, P = 0.68 respectively). This study showed a negative relationship between either the mean ± standard deviation serum level of FBS or HbA1c and body mass index in the treatment group significantly (P = 0.03, 0.008, respectively). The present study did not identify any side effects during the study period among the treatment group.
Conclusion:
According to the findings of this study, application of 125 mg C. colocynthis once per day for 2 months can lead to considerable decrease in the mean levels of HbA1c and FBS among the patients with type II diabetes without any side effects.
KEY WORDS: Citrullus colocynthis, diabetes, fasting blood sugar, HbA1c
Diabetes mellitus (DM) is a chronic progressive metabolic disorder characterized by hyperglycemia mainly due to absolute (type I DM) or relative (type II DM) deficiency of insulin hormone or body resistance to insulin. Insulin dysfunction causes chronic hyperglycemia, irregularities in the metabolism of carbohydrates, fat, and proteins.[1] Systemic effects of diabetes may lead to a variety of complications including impaired vision,[2] impaired function of kidneys, heart, blood vessels, nerves, and diabetic wounds that may result in the amputation of limbs.[3] Global prevalence of diabetes has dramatically increased over the past two decades from 30 million in 1985 to 285 million in 2010.[4] According to the International Federation of Diabetes prediction, the current figures of the diabetic population will increase to 438 million patients with diabetes by 2030.[5] The US Prevention and Control Diseases Center reported that about 25.8 million people (8.3%) were living with diabetes in 2010 in the USA.[4] In Iran, only among the adults population (25–64 years old), 2 million people were living with diabetes, and 16.8% of this population had also impaired fasting glycemia.[5] Reduction of the serum levels of glucose among patients with diabetes, particularly by medications with minimum side effects, has been investigated widely in recent decades. There are numerous plants worldwide, which have beneficial effects on the treatment of diabetes. Several studies have shown reassurance to the traditional medicine and natural therapies in the treatment of diabetes.[4,5] Also, WHO emphasizes on diabetes therapy with traditional treatments, particularly by natural hypoglycemic plants as a useful source of oral medications.[6] Application of chemical treatments such as sulfonylurea agents and metformin should be limited, due to their pharmacokinetic properties and different side effects. Citrullus colocynthis plant has a strong laxative property and application of high dosages of this herbal drug can cause diarrhea, painful cramps, and bloody diarrhea in humans.[7,8] Application of very high doses of this plant in laboratory animals has caused heart attack and death.[9,10,11,12] C. colocynthis is currently prescribed, as a dried fruit, for patients with diabetes in several cities of Iran, by traditional healers, in different dosages ranged from 300 to 800 mg/day. There is no monitoring for the evaluation of effectiveness and/or toxicity rates of this plant among patients using the treatment.[13,14] Although, experimental studies have confirmed the blood glucose lowering properties of C. colocynthis plant, to our knowledge, only a few clinical studies have been performed on its effectiveness or relevant side effects among patients so far.[14] The present study was designed to investigate the efficacy of C. colocynthis fruit on fasting blood glucose and glycosylated hemoglobin as well as its possible side effects among the patients with type II diabetes in Sabzevar city in the Northeast of Iran.
Materials and Methods
This study followed the guidelines of The Declaration of Helsinki and Tokyo for humans and was approved by Research Ethics Committee of Ilam University of Medical Sciences, Ilam-Iran and was dedicated the IRB approval of EC/92/H/187. All patients completed an informed consent form before starting the study.
In this double-blind clinical trial study, among those with inclusion criteria, a total of 70 consecutive patients with type II diabetes attended to the Diabetes Clinic of Sabzevar Medical University in Northeast of Iran, during 2 months period in 2014, were enrolled. A written informed consent was obtained from the each patient. All the participants had previously confirmed diabetes and were under treatment for type II diabetes. Inclusion criteria were: Having diabetes type II, mean glycosylated hemoglobin more than 7 in the three recent consecutive testing, age group of 40–65 years old, at least 1-year duration of diabetes, and filling an informed consent form. Exclusion criteria were: Being on insulin therapy, cardiovascular diseases, infectious diseases, pregnancy, breastfeeding, digestive tract diseases, liver and kidney diseases, impaired liver function tests, and history of surgery on the digestive system.
Participants were randomly divided into an intervention group (receiving capsules of C. colocynthis) and a control group (receiving placebo: Starch powder). Demographic characteristics of patients were collected by a prepared validated questionnaire. C. colocynthis (125 mg whole dried fruit powder per capsule), and placebo in the same shape and color were prepared in the Institute of Medicinal Plants in Tehran, Iran. All the patients with diabetes in both groups continued metformin and glibenclamide drugs as usual. Two blood samples were taken at the beginning and end of the 2 months study to compare the mean fasting blood sugar (FBS) and glycosylated hemoglobin levels in each group before and after intervention, as well as, between the two groups of intervention and placebo. Blood samples were taken after an overnight (12 h) fasting. Patients in the treatment group took one capsule of C. colocynthis (125 mg) once per day just before lunch meal, and controls received placebo at the same time. Patients were advised to measure their fasting blood glucose by glucometer, during the first 3 days of taking treatments, and inform the researcher if their blood glucose level was sharply decreased and then continue testing and recording their FBS weekly to the end of study. Diet regimens and patients lifestyles were continued as before Table 1.
Table 1.
Flowchart of grouping and random allocations of participants in the diabetes clinical trial
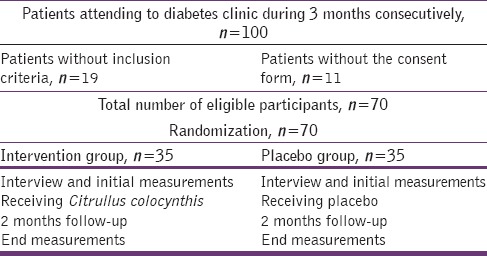
All the patients were required to report any possible side effects by telephone. In addition, all the patients were visited in the clinic, by general practitioner twice monthly and by a specialist once monthly, during the study period, to control any possible complications. The relevant data were entered into the SPSS 16 (SPSS Inc., 233 South Wacker Drive, 11th Floor, Chicago, USA) software and analyzed using independent and paired t-tests. Analysis of covariance (ANCOVA) was used to adjust possible confounding variables and test for heterogeneity. P <0.05 was considered as statistically significant.
Results
General characteristics of all participants are summarized in Table 2. The mean ± standard deviation (SD) of fasting blood glucose level in the intervention group was 170.6 ± 46 mg/dl at the beginning of the study which was significantly decreased to 159 ± 53mg/dl at the end of the study (P = 0.04). Although not significant (P = 0.8), the average fasting blood glucose levels of the participants in the placebo group were even increased during the study. Moreover, the average levels of the glycosylated hemoglobin (HbA1c) was significantly reduced in the intervention group (P = 0.01), at the end of the study [Table 3].
Table 2.
General characteristics of participants
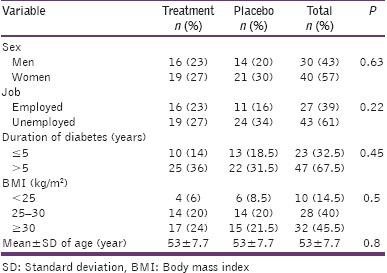
Table 3.
Comparison of mean serum level of 2 measured variables between treatment and placebo groups before and after intervention
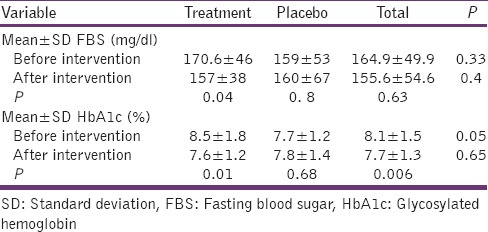
Inversely, the average levels of the glycosylated hemoglobin (HbA1c) for the placebo group did not show any reduction, and although not significant (P = 0.68), it was even slightly increased. Independent t-test analysis revealed a significant relationship between the average glycosylated hemoglobin at the beginning of the study in the two groups (P = 0.05). In addition, no significant relation was observed in both groups at the end of the study for this marker (P = 0.65). Application of C. colocynthis by patients with higher fasting blood glucose or higher glycosylated hemoglobin levels, showed greater reducing the effect on these variables.
According to the results of this study, both mean ± SD serum level of FBS and HbA1c showed a significantly negative relationship with body mass index (BMI) in the treatment group (P = 0.03, 0.008, respectively) [Tables 4 and 5].
Table 4.
Comparison of the mean±SD serum level of FBS between treatment and placebo groups after intervention according to different variables
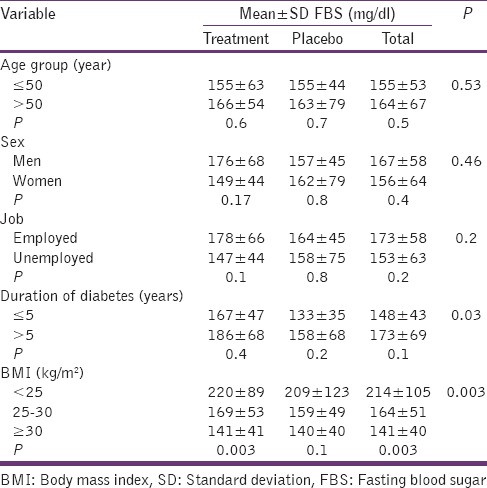
Table 5.
Comparison of the mean±SD serum level of HbA1c between treatment and placebo groups after intervention according to different variables

The results of covariance analysis showed that the mean serum levels of HbA1c were reduced significantly within groups, but nonsignificantly between groups (P < 0.001, P = 0.369, respectively) Figure 1.
Figure 1.
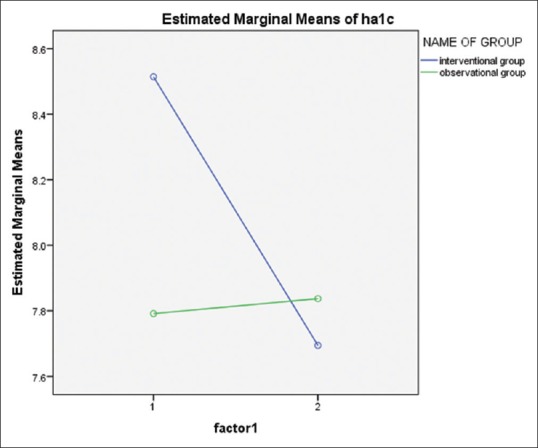
Result of covariance analysis of HbA1c for intervention and placebo groups
Discussion
The present study showed that C. colocynthis fruit has a considerable effect on reduction in the mean serum level of HbA1c and FBS in patients with the type II diabetes. Although the hypoglycemic effect of C. colocynthis has been reported by experimental studies,[14] its relevant mechanism is not still clear. One study indicated experimentally that C. colocynthis had a therapeutic effect on isolated pancreatic islets similar to insulin.[15] Another study showed that C. colocynthis inhibited the toxic effects of streptozocin on pancreatic cells in rats.[16] In type II diabetes, the increasing rate of serum glucose and free fatty acid levels causes oxidative stress and the release of reactive oxygen species.[17] These metabolic abnormalities lead to chronic complications of diabetes and insulin resistance, β-cell dysfunction, and impaired insulin secretion.[16,18,19] The favorable effects of antioxidants on the oxidative metabolic disorder, caused by hyperglycemia, have been reported by other studies.[14] The toxicity of high doses of C. colocynthis has been reported by experimental studies on both animal and human samples.[7] Abdel-Hassan et al. reported saponine as the main component of C. colocynthis, involved with the blood glucose lowering effects of this plant,[14] while Kumar et al. suggested that hypoglycemic properties of C. colocynthis were due to the presence of phenolic antioxidants and flavonoids components.[20] Sebbagh and colleagues have reported that even the oil of C. colocynthis can cause a significant decrease in the blood glucose levels of diabetic rats.[21] We used the fleshy part of C. colocynthis plant without skin and seed to evaluate the effects of this plant on fasting blood glucose in patients with type II diabetes. Several studies on animals and humans have reported different side effects for C. colocynthis including: Liver disorders, gastrointestinal disorders such as diarrhea, painful cramp, and bloody diarrhea or even death particularly after its high dose application. However, in the current study, consumption of 125 mg of this plant, once per day, did not show any side effects. Our findings indicate that 2 months consumption of 125 mg capsule of C. colocynthis, once per day, is safe for patients with diabetes. This trial was the first study in which the effects of C. colocynthis with a dosage of less than 125 mg in combined with a powder (including tragacanth, starch, Arabic gum, and fennel all equal to 25 mg) was applied. Based on our findings, the average blood levels of the investigated variables among intervention group, before and after the study, were significantly reduced. Therefore, consumption of C. colocynthis capsules in the intervention group significantly reduced the mean fasting blood glucose and glycosylated hemoglobin levels and did not show any side effects at the dosage of 125 mg or less during study period. This result was in accordance with several other studies[14,22,23] which reported that the aqueous extract of the outer shell of C. colocynthis has hypoglycemic effects in animals with diabetes which was associated with its saponins and glycosidic components. Considering the notable hypoglycemic effects of different parts of C. colocynthis, its toxic effects, which appear mainly in its high doses, cannot be regarded as an inhibiting factor for application of this useful plant, particularly among patients with diabetes.
Compared to other studies in Iran, we used a lower dosage of C. colocynthis in our study, and the same effectiveness without any side effects was obtained. Though, there were no significant side effects during the study period (2 months), the long-term side effects of this medication are unknown and should be investigated via the longitudinal studies in the future. Although, there was a significant reduction in the mean level of FBS in the treatment group, at the end of the study compared to the beginning, there was no statistically significant difference for this marker between placebo and intervention groups at the end of study. It seems that this discrepancy was due to a higher level of mean FBS in the intervention group (170.6 ± 46 mg/dl), compared to that in the control group (159 ± 53 mg/dl) at the beginning of the study. One of the limitations of this study was the slight heterogeneity of the mean FBS, between intervention and placebo groups, at the start of the study. We performed the ANCOVA to adjust the possible confounding variables and heterogeneity to reduce this limitation.
Conducting future studies to differentiate the therapeutic and toxic effects of this hypoglycemic plant is recommended. The duration of diabetes and the body mass index of the patients are important factors on the effectiveness of taken drugs. The average duration of diabetes for participants in this study was 7.5 years. Therefore, for the better efficacy of C. colocynthis plant among patients with diabetes, it is necessary to keep in mind the patients' weights and duration of diabetes, before starting the application of this herbal drug. Further experimental and animal studies are recommended to evaluate the possible effects of C. colocynthis on insulin production by pancreatic islets cells via increasing the dosage or duration of the therapy taking also into account the toxicity of this plant.
According to the results of the present study, 2 months application of 125 mg C. colocynthis, once per day can significantly decrease the mean serum levels of HbA1c and FBS among patients with type II diabetes. Application of this herbal drug at the defined dosage did not show any side effects and patients with higher fasting blood glucose and glycosylated hemoglobin and even higher BMI showed greater reduction rate. The toxicity of the high doses of C. colocynthis has been reported by experimental and trial studies on both animal and human samples; however, the lower dosage (125 mg once per day) applied in this study showed similar efficacy without any side effects.
Financial support and sponsorship
This work was supported by Faculty of Medicine, Ilam university of Medical Sciences, Ilam, Iran.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 2.Bearse MA, Jr, Han Y, Schneck ME, Barez S, Jacobsen C, Adams AJ. Local multifocal oscillatory potential abnormalities in diabetes and early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45:3259–65. doi: 10.1167/iovs.04-0308. [DOI] [PubMed] [Google Scholar]
- 3.Wallace C, Reiber GE, LeMaster J, Smith DG, Sullivan K, Hayes S, et al. Incidence of falls, risk factors for falls, and fall-related fractures in individuals with diabetes and a prior foot ulcer. Diabetes Care. 2002;25:1983–6. doi: 10.2337/diacare.25.11.1983. [DOI] [PubMed] [Google Scholar]
- 4.Power AC. Diabetes mellitus. In: Faucy AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, et al., editors. Harrison Principle of Internal Medicine. 17th ed. New York: The McGraw Hill Companies; 2012. pp. 2274–91. [Google Scholar]
- 5.Esteghamati A, Gouya MM, Abbasi M, Delavari A, Alikhani S, Alaedini F, et al. Prevalence of diabetes and impaired fasting glucose in the adult population of Iran: National Survey of Risk Factors for Non-Communicable Diseases of Iran. Diabetes Care. 2008;31:96–8. doi: 10.2337/dc07-0959. [DOI] [PubMed] [Google Scholar]
- 6.McCune LM, Johns T. Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American boreal forest. J Ethnopharmacol. 2002;82:197–205. doi: 10.1016/s0378-8741(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 7.Goldfain D, Lavergne A, Galian A, Chauveinc L, Prudhomme F. Peculiar acute toxic colitis after ingestion of colocynth: A clinicopathological study of three cases. Gut. 1989;30:1412–8. doi: 10.1136/gut.30.10.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Faraj S. Haemorrhagic colitis induced by Citrullus colocynthis. Ann Trop Med Parasitol. 1995;89:695–6. doi: 10.1080/00034983.1995.11813006. [DOI] [PubMed] [Google Scholar]
- 9.Al-Yahya MA, AL-Farhan AH, Adam SE. Preliminary toxicity study on the individual and combined effects of Citrullus colocynthis and Nerium oleander in rats. Fitoterapia. 2000;71:385–91. doi: 10.1016/s0367-326x(00)00135-0. [DOI] [PubMed] [Google Scholar]
- 10.Diwan FH, Abdel-Hassan IA, Mohammed ST. Effect of saponin on mortality and histopathological changes in mice. East Mediterr Health J. 2000;6:345–51. [PubMed] [Google Scholar]
- 11.Bakhiet AO, Adam SE. An estimation of Citrullus colocynthis toxicity for chicks. Vet Hum Toxicol. 1995;37:356–8. [PubMed] [Google Scholar]
- 12.Elawad AA, Abdel Bari EM, Mahmoud OM, Adam SE. The effect of Citrullus colocynthis on sheep. Vet Hum Toxicol. 1984;26:481–5. [PubMed] [Google Scholar]
- 13.Ziyyat A, Legssyer A, Mekhfi H, Dassouli A, Serhrouchni M, Benjelloun W. Phytotherapy of hypertension and diabetes in oriental Morocco. J Ethnopharmacol. 1997;58:45–54. doi: 10.1016/s0378-8741(97)00077-9. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Hassan IA, Abdel-Barry JA, Tariq Mohammeda S. The hypoglycaemic and antihyperglycaemic effect of Citrullus colocynthis fruit aqueous extract in normal and alloxan diabetic rabbits. J Ethnopharmacol. 2000;71:325–30. doi: 10.1016/s0378-8741(99)00215-9. [DOI] [PubMed] [Google Scholar]
- 15.Nmila R, Gross R, Rchid H, Roye M, Manteghetti M, Petit P, et al. Insulinotropic effect of Citrullus colocynthis fruit extracts. Planta Med. 2000;66:418–23. doi: 10.1055/s-2000-8586. [DOI] [PubMed] [Google Scholar]
- 16.Paolisso G, Giugliano D. Oxidative stress and insulin action: Is there a relationship? Diabetologia. 1996;39:357–63. doi: 10.1007/BF00418354. [DOI] [PubMed] [Google Scholar]
- 17.McGarry JD. Banting lecture 2001: Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 18.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 19.Rösen P, Nawroth PP, King G, Möller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: A summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Kumar D, Manjusha, Saroha K, Singh N, Vashishta B. Antioxidant and free radical scavenging potential of Citrullus colocynthis (L.) Schrad. methanolic fruit extract. Acta Pharm. 2008;58:215–20. doi: 10.2478/v10007-008-0008-1. [DOI] [PubMed] [Google Scholar]
- 21.Sebbagh N, Cruciani-Guglielmacci C, Ouali F, Berthault MF, Rouch C, Sari DC, et al. Comparative effects of Citrullus colocynthis, sunflower and olive oil-enriched diet in streptozotocin-induced diabetes in rats. Diabetes Metab. 2009;35:178–84. doi: 10.1016/j.diabet.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Huseini HF, Darvishzadeh F, Heshmat R, Jafariazar Z, Raza M, Larijani B. The clinical investigation of Citrullus colocynthis (L.) Schrad fruit in treatment of type II diabetic patients: A randomized, double-blind, placebo controlled study. J Med Plants. 2006;5:31–5. doi: 10.1002/ptr.2754. [DOI] [PubMed] [Google Scholar]
- 23.Al-Ghaithi F, El-Ridi MR, Adeghate E, Amiri MH. Biochemical effects of Citrullus colocynthis in normal and diabetic rats. Mol Cell Biochem. 2004;261:143–9. doi: 10.1023/b:mcbi.0000028749.63101.cc. [DOI] [PubMed] [Google Scholar]


