Abstract
Disease ailments are changing the patterns, and the new diseases are emerging due to changing environments. The enormous growth of world population has overburdened the existing resources for the drugs. And hence, the drug manufacturers are always on the lookout for new resources to develop effective and safe drugs for the increasing demands of the world population. Seventy-five percentage of earth's surface is covered by water but research into the pharmacology of marine organisms is limited, and most of it still remains unexplored. Marine environment represents countless and diverse resource for new drugs to combat major diseases such as cancer or malaria. It also offers an ecological resource comprising a variety of aquatic plants and animals. These aquatic organisms are screened for antibacterial, immunomodulator, anti-fungal, anti-inflammatory, anticancer, antimicrobial, neuroprotective, analgesic, and antimalarial properties. They are used for new drug developments extensively across the world. Marine pharmacology offers the scope for research on these drugs of marine origin. Few institutes in India offer such opportunities which can help us in the quest for new drugs. This is an extensive review of the drugs developed and the potential new drug candidates from marine origin along with the opportunities for research on marine derived products. It also gives the information about the institutes in India which offer marine pharmacology related courses.
KEY WORDS: Anticancer, bryostatin, cytarabine, keyhole limpet hemocyanin, mariculture, sponge, ziconotide
Ocean represents a source of a varied type of organisms due to the diversified environment offered by different oceanic zones. The enormous ecological resources of the sea have been exploited since ancient times and included the use of marine animals like fish and preparations from algae as the sources of medicine. Fish oils are the classic example of marine-derived product in use since ages. Marine pharmacology is a branch of pharmaceutical sciences which focuses on the substances with active pharmacological properties present in marine species of plants and animals. Marine environment is an exceptional store house of novel bioactive natural products, with structural and chemical features generally not found in terrestrial natural products. The marine organisms also provide a rich source of nutraceuticals and potential candidates for the treatment of several human diseases. The modern day focus of marine pharmacology is on microbes.[1] This includes the discovery of new pharmaceutical candidates from marine microbes.[2] The ocean provides enormous opportunities to discover new compounds as it has more than 13,000 molecules described out of which 3000 are having active properties.[3] Marine natural products are generally secondary metabolites. They are not generated by biological or regular metabolic pathways and have no primary function associated with the development, growth, or propagation of a species.[4] Sixty-three percentage of the new drugs are classified as naturally derived (i.e., modified natural product, unmodified natural product or synthetic compound with a natural product as pharmacophore). Covering the period from 1981 to 2008, around 68% of all the drugs used to curb infection (including antibacterial, antiviral, antiparasitic, and antifungal compounds) and 63% of anti-cancer drugs were naturally derived.[5]
Biodiversity of Marine Environment
Marine environment is a natural habitat for a broad variety of living organisms having different physiology and capacity to adapt their environment. Out of over 33 animal phyla known today, a total of 32 phyla are embodied in the marine environment out of which 15 varieties are exclusively present in the marine environment.[6] Such genetic diversity renders chemical diversity which is promising for new drug development.
Oceans contain more than 80% of diverse plant and animal species in the world. Marine organisms such as sponges, tunicates, fishes, soft corals, nudibranchs, sea hares, opisthobranch Molluscs, echinoderms, bryozoans, prawns, shells, sea slugs, and marine microorganisms are sources of bioactive compounds (viz. oils and cosmetics).[7] The first biologically active marine natural product was formally reported in late 1950 by Bergmann.[8] In late 1970, it was established that marine plants and animals are genetically and biochemically unique. Around 15,000 such unique natural compounds have been described and out of them 30% products have been isolated from sponges.[9] The remarkable discovery of unusual arabino-or ribo-pentosyl nucleosides in marine sponges was the first illustration that is naturally occurring nucleosides could contain sugars other than ribose and deoxyribose.[10] It was also observed that molecules of marine origin can be accepted by humans with minimal manipulation.[3]
There are some reports on the characterization of the antimicrobial activity of marine macroorganisms collected from the Indian coastline have appeared. Streptomyces sp. has been the most widely studied microbial species from the Indian coastal waters as a source of antibiotics.[11] In a study, 75 bacterial strains from 4 species of marine sponges were isolated, out of which 21% of the isolates have shown good antibacterial activity, with some of the strains showing species specificity.[11] The study indicated the diversity of antibiotic producing marine bacteria and also established that sponges are a rich source of bacteria capable of producing novel pharmacologically active molecules.
Marine Pharmacology in India
India has over 8000 km of coastline with clusters of marine habitats like inter-tidal rocky, muddy and sandy shores, coral reefs, and mangrove forests. The potential of Indian marine habitat has remained largely unexplored for their potential of new drugs and biotechnological programs. Some of the selected institutes such as National Institute of Oceanology, Goa; Central Drug Research Institute, Lucknow; Bose Institute, Kolkata; Central Institute of Fisheries Education, Mumbai; Regional Research Laboratory, Bhubaneswar of Council for Scientific and Industrial Research are presently working for exploration of life saving drugs from marine sources. Many other Indian institutes, universities, and pharmaceutical companies have also recognized the significance of this subject.[12]
Marine pharmacology has been reviewed extensively in the past all over the world as well as in India, but still there is a need to review the potential of the oceans as source for the development of new drugs, considering the advantage of their abundance in nature and large scale production. At present, the drug industry is working on screening and isolation of novel molecules with unreported pharmacological properties that can be exploited for the development of new therapeutic agents for commercial use. This review has largely focused on different classes of marine drugs currently in use and at different stages of trials for approval and marketing in future. The review has also tried to delve into the limitations and future trends of the drugs from marine sources.
Classification of Marine Pharmacology
Marine pharmacology can be classified on the basis of source of the candidate drug[9]
Genetically engineered marine organisms
Manufacture of pharmaceuticals and nutraceuticals of marine origin
Chemicals produced by or found in marine organisms shown to have a wide variety of applications as pharmaceuticals.
Marine drugs can be broadly classified based on their actions as follows:
Antibacterial
Eicosapentaenoic acid, a polyunsaturated fatty acid, isolated from a diatom of marine origin Phaeodactylum tricornutum which has shown activity against an array of Gram-positive and Gram-negative bacteria, which also includes a multidrug-resistant variety of Staphylococcus aureus.[13]
Anti-inflammatory
The anti-inflammatory function of extracts and other parts of a Mediterranean sponge species Spongia officinalis in the in vivo study on rat model of carrageenan-induced paw edema assay.[14]
Neuroprotective
The extracts of South Indian green seaweed Ulva reticulata has shown neuroprotection by inhibiting acetyl-and butyryl-cholinesterases, efficacy comparable to agents currently approved for Alzheimer's disease treatment.[15]
Antiparasitic
Extracts of Sarcotragus sp. known as Tunisian sponge prepared in dichloromethane has demonstrated in-vitro anti-leishmanial activity by demonstrating the associated morphological alterations in promastigotes of leishmania major.[16]
Antiviral agents
Anti-herpes simplex virus-1 (HSV) activity found in high molecular weight exo-polysaccharides extracted from the Celtodoryx girardae (French marine sponge) and its associated symbiotic bacteria has been reported.[17]
Anticancer
Bryostatin, primarily obtained from the Bryozoan, Bugula neritina, although some forms have been extracted from sponges and tunicates. Sorbicillin-derived alkaloids sorbicillactone A and its 2', 3'-dihydro analog sorbicillactone-B has shown activity against leukemia cells free from any noteworthy cytotoxicity. Sorbicillactone-B has been derived from a salt-water culture of a bacterial strain Penicillium chrysogenum which has been isolated from a sponge Ircinia fasciculata, a Mediterranean sponge specimen.[18]
Another promising anticancer drug used as an immunotherapeutic agent is keyhole limpet hemocyanin (KLH). KLH is a copper containing extracellular respiratory protein present in Megathura crenulata, a marine Gastropod species found in large numbers at the Pacific coast of California and Mexico. KLH is found in two isoforms KLH1 and KLH2.[19] KLH is reported to possess remarkable immunostimulatory properties in experimental animals and human, used in experimental immunology and also clinically as an immunotherapeutic agent.[20] KLH is specifically used in clinical setup for the treatment of bladder carcinoma, and its efficacy is perhaps due to a cross-reacting carbohydrate epitope. KLH may also have significant potential for the treatment of other types of cancers, particularly the adenocarciomas derived from the epithelium, by using it as a carrier for gangliosides of carcinoma and mucin-like epitopes.[19]
KLH is intravesically administered to patients with bladder carcinoma. Its clinical success in carcinoma patients is attributed to the presence of the disaccharide epitope Gal (β1-3), Ga1NAc.[21] This epitope of KLH is believed to be cross-reactive with an equivalent epitope on the urinary bladder tumor cell surface. The cumulative cellular and humoral immunological responses to KLH can result in a cytolytic reduction of tumor growth.[19]
In addition to tumor immunotherapy, KLH is also prescribed in the following conditions:[19]
As a generalized vaccine component for antigen presentation, alone or in adjuvant cocktail
For diagnosis of schistosomiasis because of cross-reactivity to one of the epitopes on larval schistosomes
In drug assays
Treatment of drug addiction by immunoassay for abused drugs
For immune competence testing
Assessment of stress and inflammation.
Analgesic
Ziconotide was the first drug of marine origin to obtain approval from the U.S. Food and Drug Administration (USFDA) in 2004 to treat pain. It is also known as Prialt, and it was originally extracted from the marine snail Conus magus. Results from animal studies suggested the role of ziconotide in blocking of N-type calcium channels on the primary nociceptive nerves of the spinal cord.[22]
Antimicrobial
The cephalosporins are well-known antimicrobial agents with a marine source of origin. Cephalosporin C was firstly extracted and purified from a marine fungus, Cephalosporium acremonium.[9]
Antimalarial activity
Isonitrile containing antimalarial molecules have been extracted from the Acanthella sp., a Japanese sponge. The isolated molecules belong to kalihinane diterpenoids class, which also contains antifungal, anthelmintic, and antifouling compounds.[23]
Evolution of Marine Pharmacology
The recent global marine, pharmaceutical pipeline consists of only 3 USFDA approved drugs, and one European Union (EU) registered drug. Currently, marine drugs in the clinical lineup have 13 compounds that are at different stages of clinical trials, with a very large number of marine-derived compounds/molecules in the preclinical testing pipeline as well.[2]
The three Food and Drug Administration (FDA) approved the marine-derived drugs currently used in the United States are, cytarabine (Cytosar-UW, DepocytW), vidarabine (Vira-AW), and ziconotide (PrialtW). Various marine drugs in different phases of clinical trials are summarized in Table 1.[2]
Table 1.
A perspective of pipeline of marine drugs
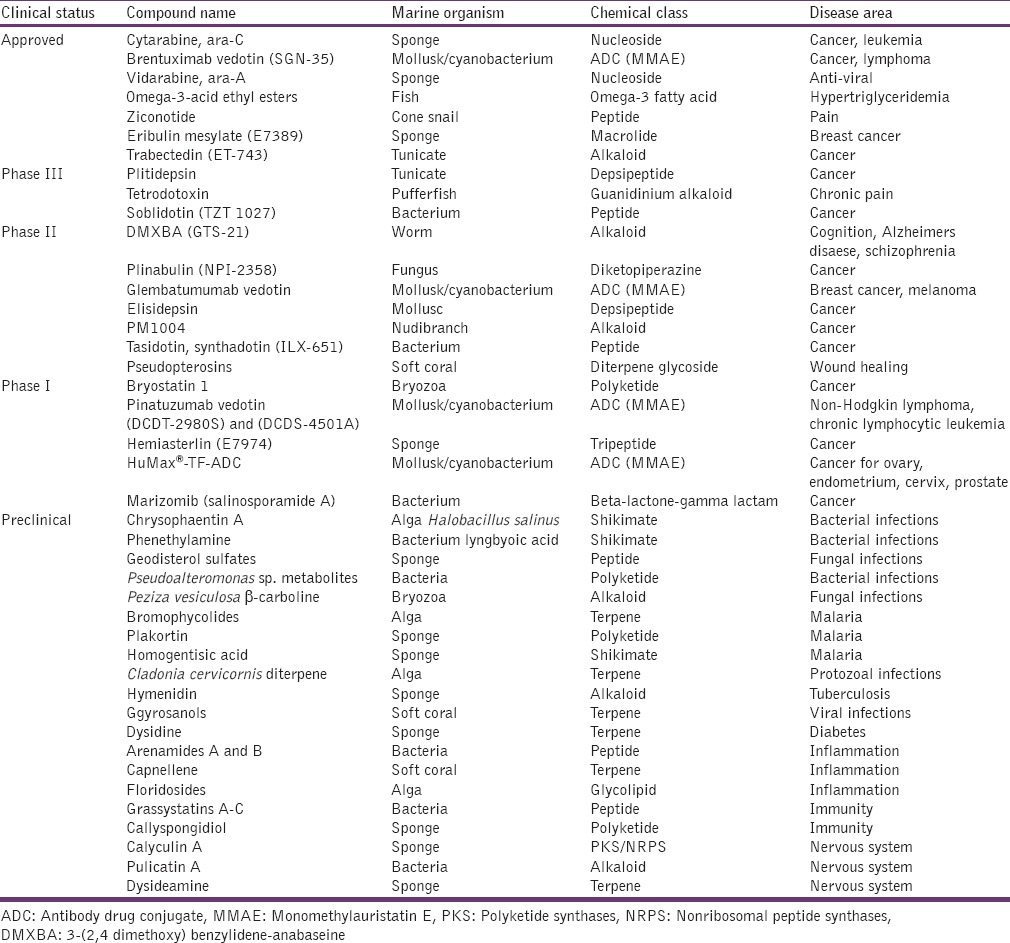
Approved drugs of marine origin
Some of the drugs of marine origin approved for human use in different parts of the world are as follows:
Cytarabine (cytosine arabinoside or arabinosyl cytosine, ara-C)
Cytarabine is a synthetic pyrimidine nucleoside derived from spongothymidine and primarily isolated from a Caribbean sponge species Tethya crypta. It is FDA approved and mainly used in different types of leukemia, including acute myelocytic leukemia, lymphocytic leukemia, meningeal leukemia, and blast crisis phase of chronic myelogenous leukemia.[2]
Vidarabine (adenine arabinoside, ara-A or arabinofuranosyladenine)
Vidarabine is a synthetic purine nucleoside isolated from the Caribbean sponge T. crypta and developed from spongouridine is currently obtained from Streptomyces antibioticus. It is approved by FDA for use in recurrent epithelial keratitis caused by HSV) type 1 and 2, acute kerato-conjunctivitis, and also for superficial keratitis.[2]
Ziconotide
Ziconotide is a synthetic molecule, equivalent to a natural 25-amino acid peptide, v-conotoxin MVIIA. It is originally extracted and purified from the venom of marine snail C. magus, which is a fish-hunting species. Ziconotide has shown potential as an analgesic with a novel mechanism of action.[2] It is approved as an analgesic by FDA.
Trabectedin
A marine natural product extracted from a tunicate species Ecteinascidia turbinata generally inhabitant of Mediterranean and Caribbean Sea. Trabectedin molecule is an alkaloid of tetrahydroisoquinoline class, and it was the first anticancer molecule of marine origin got approval in EU for use in the treatment of soft-tissue sarcoma and in relapsed cases of platinum-sensitive ovarian cancer.[2]
Marine Drugs in Clinical Phase III Trial
Eribulin mesylate (E7389) or halichondrin B
It is a polyether macrolide natural molecule originally extracted from marine sponges, with potent anticancer activity reported in preclinical animal models. Eribulin is a potent molecule which produces irreversible antimitotic activity leading to cell death by apoptotic pathway.[2] On-going Phase III studies are evaluating the comparative clinical efficacy of eribulin versus capecitabine and eribulin versus other preferred treatment choice.
Soblidotin (auristatin PE or TZT-1027)
Is a synthetic derivative of the dolastatin backbone from dolastatin 10. It is a vascular disrupting agent causing the collapse of the vasculature inside the tumor, in addition to its tubulin inhibitory activity. This drug is undergoing trials in clinical Phases I, II, and III with different companies who are trying to use it as a weapon to specific monoclonal antibodies linked via customized peptides.[2]
Tetrodotoxin
A very well known “marine toxin,” and highly substituted guanidine-derivative.[24] It is not an anti-tumor agent, currently in Phase III trials as analgesic against inadequately controlled pain related to the cancer. A Phase II trial is ongoing to assess the efficacy of tetrodotoxin against the neuropathic pain related to chemotherapy-induced peripheral neuropathy.[25]
Marine-Derived Compounds in Clinical Phase II Trial
DMXBA (GTS-21) [3-(2,4-dimethoxybenzylidene)-anabaseine; GTS-21]
It is a synthetic imitative of anabaseine, which is an alkaloid found in many species of aquatic worms of phylum nemertea. DMXBA is reported to be beneficial for the central nervous system, improves cognition and sensory gating deficiency in a variety of laboratory animals.[2] A recent clinical trial of the molecule in Phase II with schizophrenic patients has shown noteworthy improvement in cognitive functions.[2]
Plitidepsin
It is a natural marine depsipeptide, currently obtained by total synthesis. It was primarily isolated from a tunicate Aplidium albicans found in the Mediterranean Sea. Plitidepsin is a highly potent apoptosis inducer with low nanomolar (nM) range of IC50 values. The major toxicity found with most schedules of plitidepsin were muscle toxicity, an increase of transaminases, general fatigue, diarrhea, and cutaneous rash.[2]
Elisidepsin (PM02734)
It is a novel cyclic peptide derived from marine sources and belongs to the Kahalalide family of compounds. It is currently undergoing development in Phase II with primary evidence of antitumor potency with encouraging therapeutic index.[2] It has shown potent in vitro cytotoxic action against diverse human tumor cell lines, which may be because of oncolytic cell death induction instead of apoptotic cell death.[2]
PM00104 (Zalypsis)
It is a novel alkaloid with DNA-binding capacity. It is linked to jorumycin extracted from the Pacific nudibranch's (Jorunna funebris) skin and mucus as well as from renieramiycins extracted from varieties of sponges and tunicates. Preclinical in vivo studies done earlier with these molecules indicated considerably high antitumor activity in cells of breast, prostate and renal cancers with a modest antitumor action on colon cancer cells. Reversible hematological disorders or liver enzymes imbalance were the main toxicities found to be associated with Zalypsis treatment during the Phase I trials.[2]
Plinabulin (NPI-2358)
It is a fully laboratory made analog of the natural product halimide originally derived from marine Aspergillus sp. CNC-139 (from Halimeda lacrimosa) and phenylahistin extracted from Aspergillus ustus.[2] It functions by inhibiting the polymerization of tubulin, which leads to destabilization of the vascular endothelial architecture of the tumor.[26]
ILX-651 (tasidotin or synthadotin)
Its a synthetic derivative of dolastatin-15 and it inhibits assembly of tubulin.[2] It is an orally active drug and has progressed to Phase II trials in different types of cancer. ILX-651 is also under study in preclinical stages for exploring the routes and targets of its actions, including its use in advanced stage refractory neoplasms.[2]
Pseudopterosins
A leading class of diterpene glycosides primarily extracted from the octocoral Pseudopterogorgia elisabethae.[2] Pseudopterosin A is a strong phorbol myristate acetate inhibitor, reported to cause topical inflammation in a mouse model with degranulation of human polymorphonuclear leukocytes and prevents phagosome creation in tetrahymena cells. In a double-blind, Phase II clinical trial, pseudopterosins were found to augment re-epithelialization process with qualitative enhancement in the early wound repair process.[2]
Marine Drugs in Clinical Phase I Trial
Leconotide (AM-336, ω-conotoxin CVID)
It is a peptide having 27 residues containing 3 CYS-CYS bonds. It is similar to Ziconotide and is undergoing Phase I trials for the treatment of cancer. Although, in initial studies, it is used through intrathecal route (as ziconotide),[27] currently the systemic administration is done.[28,29]
Enfortumabvedotin
It is used in immunotherapy and it is a combination of a fully human IgG1k antibody and monomethyl auristatin E. It is also known under the code names of AGS-22MSE and AGS-22ME and reported to be currently in a Phase I trial.[23]
Vorsetuzumab mafdotin (SGN-75)
An antibody-drug conjugate, with monomethyl-auristatin F attached to the anti-CD70 monoclonal humanized antibody 1F6.[30] This molecule is currently being evaluated for its efficacy in relapsed and refractory non-Hodgkin's lymphoma in Phase I clinical trials and also in metastatic renal cancer with CD70 epitope expressing cancer cells.[31]
Bryostatin 1
It has in vivo biological active molecule bryostatin 3 (one of the 20 known varieties) isolated from the bryozoan B. neritina. Since the year 2007, at least four Phase I and eight Phase II trials with bryostatin against multiple carcinomas have been reported, with all the studies using a combination with biologicals or cytotoxins. Bryostatin is currently undergoing two Phase I trials for assessment as a treatment for Alzheimer's disease.[2]
Hemiasterlin (E7974)
It's a cytotoxic tripeptide molecule, originally isolated from marine sponges.[2] Dose-limiting toxicities such as neutropenia/febrile neutropenia along with some other adverse events such as general fatigue, nausea, vomiting sensation, and constipation, were reported in the Phase I studies.[2]
Marizomib (NPI-0052, salinosporamide A)
It is a natural compound derived from marine actinomycete Salinispora tropica. Marizomib is a potent and selective inhibitor of the proteasome; a multicatalytic enzyme complex found to be associated with the degradation of nonlysosomal proteins found in cells making it a proven target for the treatment of cancer.[2] Translational biology studies established marizomab activity as a single therapeutic compound against the solid tumor and hematological malignancies (e.g., multiple myelomas). Later studies confirmed the potential of marizomib use in combination with chemotherapy and/or biologics.[2]
Preclinical Pipeline
The preclinical drug pipeline plays an important role and currently supplying hundreds of novel marine natural compounds postsafety screening every year and continue to support the clinical pipeline with potentially valuable compounds. Since 1974 (when FDA approved 3 marine drugs), it required over 30 years for any other natural product of marine origin to get approved for clinical use.
In the last 5 years, preclinical pharmacology of 262 marine compounds has remained under various stages of study spreading over 35 countries including the USA, they are now part of the preclinical pharmaceutical pipeline.[32] Promising antibacterial, antifungal, antiprotozoal, antitubercular, and antiviral activities have been reported for 102 natural marine compounds.[32] As reported by Mayer et al., around 60 marine compounds have been found to have a potency of immune and nervous systems, some anti-diabetic and anti-inflammatory effects have also been observed. Finally, 68 promising molecules extracted from marine sources were found to interact with an array of molecular targets and receptors, which may probably contribute to develop various pharmacologically active classes of drugs upon more focused studies for confirmation of their mechanism of action.[32] The important agents from the preclinical pipeline are also summarized in Table 1.
Anticancer Drugs Developed from Marine Origin: Hope for Future
Targeting the various signal transduction pathways involved in carcinogenesis is one of the best treatment strategies for cancer. Some inhibitors of these pathways derived from the marine organisms have been re-designed, and further studies were done to offset tumor progression and curtail carcinogenesis.[33] Modern anticancer drugs discovery is looking for cytotoxic agents with improved accuracy, efficacy with target specificity and sensitivity.
According to an estimate by Sawadogo et al., in 2011 available promising anticancer compounds of marine origin can be divided into different classes of chemicals majority represented by terpenes and terpenoids (40.5%) following by peptides (19%), macrolides 14.3%), and alkaloids (12%). Among them, 50% are 1st time looked upon as anticancer agents. The majority of these compounds are chemotherapeutic agents (92.7%), and only 7.3% are chemopreventives, which are known as nutraceuticals available in vegetables and fruits.[34] The biological mechanisms involved in the anti-cancer properties of the investigated compounds in Sawadogo et al. study are mainly cell cycle arrest through tubulin inhibitory effect; apoptosis through the various mechanisms such as caspases 3, 8, 7, and 9 activation, MMP depolarization, bib truncation, Bcl-xL, Bax and poly(ADP-ribose) polymerase cleavages; anti-migratory effect through specific inhibitory effect on TRPM7 channels, anti-angiogenic property by inhibition of vascular endothelial growth factor-A secretion; anti-inflammatory effect through the inhibition of COX-2 and iNOS expression.[34] Looking at the possibility of a large number of therapeutic compounds, it may be possible to find out drugs of marine origin for targeted therapy of cancer which is not possible until now with much success.
Challenges and Future Trends
There are certain major challenges to derive the drugs from marine sources. The variable environmental conditions could result in the production of different metabolite every time from the same organism. A major challenge sometimes faced is that the microorganisms residing in the marine animal, and not the invertebrate marine hosts actually produces the bioactive molecules.[4] Sustainable supply of isolated and identified lead compounds sometimes pose a problem because the lead compound is present only in low quantity and/or technically it becomes very difficult to isolate such compound.[35] For any of intended use (drug, cosmetic, etc.,) of the compound, the required quantity may vary from few grams needed for preclinical drug development and safety studies in different setup; to quantities in kilogram required for clinical study in different phases and many of tons of cosmetics.[4] And the availability of lead compound in such abundance can be a key issue.
The limiting factors for the marine drug developments have been summarized in Table 2. Lack of sustainable supply of the candidate compound has sometimes held back further research and development of many extremely potent marine novel compounds. Attempts have been made to beat this hurdle by increased development of synthetic or hemisynthetic analogues derivatives with desired and customized properties, or designing a pharmacophore of lower complexity with easier synthesis method.[36] Identification of a bioactive compound synthesized or hemisynthesized must be done with the reference to the compound derived from the biological source. The structural complexity of the isolated compound and meager yield which is generally faced with marine compounds, may lead to wrong assignment of chemical formula of the compound, its real constitution (planar connectivity), configuration of intramolecular bonds, configuration entirity, and incorrectly assigned one or multiple stereocenters.[37] To overcome the issue of regular supply, the use of natural resources should be under control and need to favor the growth of marine organisms in its natural environment by farming which is also known as “Mariculture.”[37,38] Another option is to culture the marine organisms under artificial conditions by the process called as “aquaculture” [Table 2].[37,38]
Table 2.
Limiting factors for the marine drug developments

Martins et al. has very well elucidated the commercial and market issues that are relevant and mostly overlooked in the developmental process of new natural products.[4] Some of the points that need to be addressed from the very early development phase are as follows: (i) What are the potential industrial use of the product and need of that particular activity of the compound in the market? (ii) What will be the final cost per kg for the final bioactive material? (iii) The desired formulation and preferred route of administration of the compound; (iv) What process of manufacture is being used and whether the supply is sustainable? and finally (v) How will the product reach the market chain?
Some limitations of the marine drug development includes the development of universal expression systems for biosynthesis of small molecules with high-yield, development of genetic tools to access the in vivo potential of cultured marine microorganisms, and the regulatory arousing of silent biosynthetic pathways for small molecule discovery.[38]
The subsequent levels of development of drugs comprises in vivo evaluations of safety and efficacy in animal models, determination of the mechanism and site of action, development of structure-activity relationships, formulation and characterization of pharmacokinetics parameters and pharmaceutical properties including improvements through the use of medicinal chemistry.[38]
Initial efforts in marine natural products chemistry have largely focused on collecting metabolites from most easily collected species.[30] Minor metabolites present in very small quantities are a challenge for analytical and biological evaluations. In silico screening programs can be useful to understand the natural scaffolding of these minor drug candidates better.[30] Scientists are making efforts to improve the access to minor metabolites through technological advancements, such as increasingly widespread use of NMR microcryogenic and capillary flow-probes, biological assays in increasingly smaller volumes such as in 384- and 1534-well plate formats and enhancements of the methods as well as informatics and logistics associated with mass spectrometry.[39] Another area of improvement in marine drug discovery programs is the biological assay methods of extracts, fractions, and pure compounds. Assay-based isolation design for marine natural products has the potential for automation that may result in dramatic improvement in the way by which different classes of natural products are discovered in nature.[38]
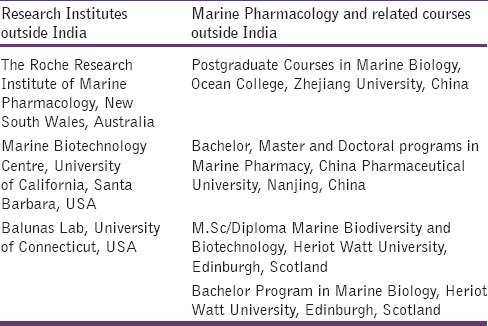
Looking at the vast potential and leads, there are several Institutes in India as well as all over the world, concentrating on research and training in marine pharmacology field. Most of the research institutes are concentrating on the discovery of potential novel compounds from marine organisms, extraction/isolation, their safety and efficacy assessment and large-scale commercial production. Some of the institutes doing research and universities imparting training on marine pharmacology in and [Tables 3 and 4] outside India are mentioned in respectively.
Table 3.
Institutes/universities offering training or research on marine drugs in India

Table 4.
Institutes/Universities offering training/research on marine drugs outside India
Conclusions
Marine environment has become a promising source of natural products, molecules, and drugs of therapeutic use. Having enormous varieties with a great diversity of organism and virgin areas of marine life, the prospects of yielding more novel products from the sea is enormous. The curiosity of science and industry has established the oceans as a prospective source for new potential drug leads. Scientists have come up with drugs of various categories out of which anticancer, anti-inflammatory, analgesics, and antivirals are the most important to mention. These lead molecules are in different stages of preclinical and clinical testing stages around the world. Many drugs from marine sources have a promising effect on several chronic and unbeatable diseases like cancer. They may prove to open up a new chapter of making the treatment of chronic diseases cheaper and successful.
After identification, extraction, and large scale production of promising marine natural products of therapeutic uses, their marketing and commercial exploitation of potential is dependent on the results of preclinical and clinical data. The current screening for active natural products should be increased along with a large and rapid random screening method. Several research institutes and universities are working in this field to develop new moieties and train people to work in this area. The technology should be targeted optimally for drug research, approvals, and launches. The medical pharmacologist from India should consider taking up the further research in marine pharmacology to help our country in new drug developments. The importance of Marine Pharmacology is summarized in Figure 1. The evolution of marine pharmacology as a specialty in India will help us optimizing the use of rich marine resources around our beautiful country gifted with a vast coastline [Figure 1].
Figure 1.

Scope of marine pharmacology
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Author acknowledge the support received from Dr. Namrata Singh for reviewing and editing the manuscript.
References
- 1.Kijjoa A, Sawangwong P. Drugs and cosmetics from the sea. Mar Drugs. 2004;2:73–82. [Google Scholar]
- 2.Mayer AM, Glaser KB, Cuevas C, Jacobs RS, Kem W, Little RD, et al. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol Sci. 2010;31:255–65. doi: 10.1016/j.tips.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Vignesh S, Raja A, James RA. Marine drugs: Implication and future studies. Int J Pharmacol. 2011;7:22–30. [Google Scholar]
- 4.Martins A, Vieira H, Gaspar H, Santos S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar Drugs. 2014;12:1066–101. doi: 10.3390/md12021066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chem Rev. 2009;109:3012–43. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- 6.Margulis L, Schwartz KV. 3rd ed. New York, USA: W.H. Freeman and Company; 1998. Five Kingdoms – An Illustrated Guide to the Phyla of Life on Earth. [Google Scholar]
- 7.Donia M, Hamann MT. Marine natural products and their potential applications as anti-infective agents. Lancet Infect Dis. 2003;3:338–48. doi: 10.1016/S1473-3099(03)00655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergmann W, Stempien MF. Contributions to the study of marine products. XLIII. The nucleosides of sponges V The synthesis of spongosine. J Org Chem. 1957;2:1557–75. [Google Scholar]
- 9.Murti Y, Agarwal T. Marine derived pharmaceuticals-development of natural health products from marine biodiversity. Int J ChemTech Res. 2010;2:2198–217. [Google Scholar]
- 10.Imhoff JF, Labes A, Wiese J. Bio-mining the microbial treasures of the ocean: New natural products. Biotechnol Adv. 2011;29:468–82. doi: 10.1016/j.biotechadv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Anand TP, Bhat AW, Shouche YS, Roy U, Siddharth J, Sarma SP. Antimicrobial activity of marine bacteria associated with sponges from the waters off the coast of South East India. Microbiol Res. 2006;161:252–62. doi: 10.1016/j.micres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Thakur NL, Thakur AN, Muller WEG. Marine natural products in drug discovery. Natural Product Radiance. 2005;4:471–7. [Google Scholar]
- 13.Desbois AP, Mearns-Spragg A, Smith VJ. A fatty acid from the diatom Phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA) Mar Biotechnol (NY) 2009;11:45–52. doi: 10.1007/s10126-008-9118-5. [DOI] [PubMed] [Google Scholar]
- 14.Dellai A, Laroche-Clary A, Mhadhebi L, Robert J, Bouraoui A. Anti-inflammatory and antiproliferative activities of crude extract and its fractions of the defensive secretion from the mediterranean sponge. Spongia officinalis. Drug Dev Res. 2010;71:412–8. [Google Scholar]
- 15.Suganthy N, Karutha Pandian S, Pandima Devi K. Neuroprotective effect of seaweeds inhabiting South Indian coastal area (Hare Island, Gulf of Mannar Marine Biosphere Reserve): Cholinesterase inhibitory effect of Hypnea valentiae and Ulva reticulata. Neurosci Lett. 2010;468:216–9. doi: 10.1016/j.neulet.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Ben Kahla-Nakbi A, Haouas N, El Ouaer A, Guerbej H, Ben Mustapha K, Babba H. Screening of antileishmanial activity from marine sponge extracts collected off the Tunisian coast. Parasitol Res. 2010;106:1281–6. doi: 10.1007/s00436-010-1818-x. [DOI] [PubMed] [Google Scholar]
- 17.Rashid ZM, Lahaye E, Defer D, Douzenel P, Perrin B, Bourgougnon N, et al. Isolation of a sulphated polysaccharide from a recently discovered sponge species (Celtodoryx girardae) and determination of its anti-herpetic activity. Int J Biol Macromol. 2009;44:286–93. doi: 10.1016/j.ijbiomac.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Bringmann G, Gulder TA, Lang G, Schmitt S, Stöhr R, Wiese J, et al. Large-scale biotechnological production of the antileukemic marine natural product sorbicillactone A. Mar Drugs. 2007;5:23–30. doi: 10.3390/md502023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris JR, Markl J. Keyhole limpet hemocyanin (KLH): A biomedical review. Micron. 1999;30:597–623. doi: 10.1016/s0968-4328(99)00036-0. [DOI] [PubMed] [Google Scholar]
- 20.Curtis JE, Hersh EM, Butler WT, Rossen RD. Antigen dose in the human immune response. Dose-relationships in the human immune response to Keyhole limpet hemocyanin. J Lab Clin Med. 1971;78:61–9. [PubMed] [Google Scholar]
- 21.Wirguin I, Suturkova-Milosevic L, Briani C, Latov N. Keyhole limpet hemocyanin contains Gal(beta 1-3)-GalNAc determinants that are cross-reactive with the T antigen. Cancer Immunol Immunother. 1995;40:307–10. doi: 10.1007/BF01519630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skov MJ, Beck JC, de Kater AW, Shopp GM. Nonclinical safety of ziconotide: An intrathecal analgesic of a new pharmaceutical class. Int J Toxicol. 2007;26:411–21. doi: 10.1080/10915810701582970. [DOI] [PubMed] [Google Scholar]
- 23.Miyaoka H, Shimomura M, Kimura H, Yamada Y, Kim HS, Yusuke W. Antimalarial activity of kalihinol A and new relative diterpenoids from the Okinawan sponge, Acanthella sp. Tetrahedron. 1998;54:13467–74. [Google Scholar]
- 24.Moczydlowski EG. The molecular mystique of tetrodotoxin. Toxicon. 2013;63:165–83. doi: 10.1016/j.toxicon.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 25.Chau R, Kalaitzis JA, Neilan BA. On the origins and biosynthesis of tetrodotoxin. Aquat Toxicol. 2011;104:61–72. doi: 10.1016/j.aquatox.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Kanoh K, Kohno S, Katada J, Hayashi Y, Muramatsu M, Uno I. Antitumor activity of phenylahistin in vitro and in vivo. Biosci Biotechnol Biochem. 1999;63:1130–3. doi: 10.1271/bbb.63.1130. [DOI] [PubMed] [Google Scholar]
- 27.Jayamanne A, Jeong HJ, Schroeder CI, Lewis RJ, Christie MJ, Vaughan CW. Spinal actions of omega-conotoxins, CVID, MVIIA and related peptides in a rat neuropathic pain model. Br J Pharmacol. 2013;170:245–54. doi: 10.1111/bph.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daly NL, Craik DJ. Conopeptides as novel options for pain management. Drugs Future. 2011;36:25–32. [Google Scholar]
- 29.Yanagita Y, Takenaka T. Astellas' drug discovery strategy: Focus on oncology. Jpn J Clin Oncol. 2012;42:241–6. doi: 10.1093/jjco/hys014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iyer U, Kadambi VJ. Antibody drug conjugates – Trojan horses in the war on cancer. J Pharmacol Toxicol Methods. 2011;64:207–12. doi: 10.1016/j.vascn.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Newman DJ, Cragg GM. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar Drugs. 2014;12:255–78. doi: 10.3390/md12010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer AM, Rodríguez AD, Taglialatela-Scafati O, Fusetani N. Marine pharmacology in 2009-2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar Drugs. 2013;11:2510–73. doi: 10.3390/md11072510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhatnagar I, Kim SK. Marine antitumor drugs: Status, shortfalls and strategies. Mar Drugs. 2010;8:2702–20. doi: 10.3390/md8102702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawadogo WR, Schumacher M, Teiten MH, Cerella C, Dicato M, Diederich M. A survey of marine natural compounds and their derivatives with anti-cancer activity reported in 2011. Molecules. 2013;18:3641–73. doi: 10.3390/molecules18043641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molinski TF, Dalisay DS, Lievens SL, Saludes JP. Drug development from marine natural products. Nat Rev Drug Discov. 2009;8:69–85. doi: 10.1038/nrd2487. [DOI] [PubMed] [Google Scholar]
- 36.Radjasa OK, Vaske YM, Navarro G, Vervoort HC, Tenney K, Linington RG, et al. Highlights of marine invertebrate-derived biosynthetic products: Their biomedical potential and possible production by microbial associants. Bioorg Med Chem. 2011;19:6658–74. doi: 10.1016/j.bmc.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maier ME. Structural revisions of natural products by total synthesis. Nat Prod Rep. 2009;26:1105–24. doi: 10.1039/b809658a. [DOI] [PubMed] [Google Scholar]
- 38.Gerwick WH, Moore BS. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem Biol. 2012;19:85–98. doi: 10.1016/j.chembiol.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerwick WH, Fenner AM. Drug discovery from marine microbes. Microb Ecol. 2013;65:800–6. doi: 10.1007/s00248-012-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


