Abstract
Bisretinoid fluorophores of retinal pigment epithelial (RPE) lipofuscin have been shown to undergo degradation in two ways, the first involving photofragmentation following photooxidation of their polyene structure and the second being enzyme-mediated and limited, thus far, to in vitro models employing horseradish peroxidase (HRP). Here we show that both of these processes impact the ubiquitin-proteasome system (UPS) of the RPE cell. By measuring the consumption of A2E and all-trans-retinal dimer by HPLC, we confirmed that both HRP-mediated and photodegradation of the compounds occurred and that in both cases the chymotrypsin-like and trypsin-like activities of the proteasome system were decreased. With HRP-mediated degradation of A2E, there was a small negative impact on cell viability that was not mitigated by elevating gluthathione in the cell.
Keywords: Bisretinoid, Lipofuscin, Proteasome, Retinal pigment epithelium, Enzyme degradation
75.1 Introduction
Proteasomes are protease assemblies responsible for the degradation and recycling of proteins which have been previously tagged with ubiquitin. Proteins damaged by postsynthetic alterations are important substrates for degradation by the ubiquitin– proteasome system (UPS) [1], and one such modification is the advanced glycation end product (AGEs). We have uncovered a unique source of AGEs that form in retinal pigment epithelial (RPE) cells. Specifically, we demonstrated that methylglyoxal (MG) and glyoxal (GO), two small dicarbonyls known to form AGEs, are released upon photodegradation of A2E and all-trans-retinal dimer, two bisretinoids that accumulate with age as lipofuscin in RPE. This cleavage of bisretinoid occurs upon exposure to wavelengths of light that reach the retina. Significantly, we have also observed that the processes of photooxidation and photodegradation of intracellular bisretinoid in RPE cells alters the function of the ubiquitin–proteasome system (UPS) [2].
In work designed to test the possibility that exogenous enzymes can be delivered so as to degrade the bisretinoid constituents of RPE lipofuscin and protect against their accumulation, we previously demonstrated that the oxo-iron heme-based enzyme horseradish peroxide (HRP) can cleave the bisretinoid A2E [3]. We also identified aldehyde-bearing fragments that were products of this activity. Here we have examined for whether the products of enzyme-mediated cleavage of A2E cause cellular stress by impacting proteasome activity and whether reduced glutathione is protective. We also compared the proteasome activity to that in cells undergoing A2E photodegradation.
75.2 Materials and Methods
75.2.1 Cell-Associated A2E
Confluent ARPE-19 cells (ATCC, Manassas, VA) (35 mm dishes) that lack endogenous lipofuscin accumulated A2E in lysosomes as described [3, 4].
75.2.2 Photodegradation of Cell-Associated A2E
For 430 nm irradiation, cultures were transferred to PBS with calcium, magnesium, and glucose and were exposed as previously reported [5] and then incubated for an additional 6 h before harvesting.
75.2.3 Enzymatic Degradation of Cell Associated A2E
HRP was delivered to the cells using the Bioporter reagent (Sigma-Aldrich Corp., St. Louis, MO) as previously described [3].
75.2.4 Proteasome Activity Assays
Trypsin-like proteasome activity was measured in a live-cell assay using a luminogenic substrate (Z-LRR-aminoluciferin; Proteasome-Glo, Promega Corporation Madison, WI). Briefly, to cells released into a suspension (10,000 cells/100 μL) 100 μL of Proteasome-Glo cell-based reagent was added. After mixing and incubating (6 min, room temperature) luminescence was measured using the SoftMax Pro 5 microplate reader (Molecular Devices, Inc. Sunnyvale, CA). Samples were assayed in duplicate.
Chymotrypsin-like proteasome activity was measured using a fluorescence assay that employs an AMC-tagged peptide substrate (Succ-LLVY-AMC; BioVision Incorporated, Mountain View, CA) and cells homogenized in 0.5 % NP-40 (Sigma-Aldrich Corp., St. Louis, MO). Fluorescence (excitation/emission, 350/440 nm) was measured in the microplate reader (37 °C from 30–60 min). Nonspecific (non-proteasome) fluorescence measured in the presence of a proteasome inhibitor was subtracted, values were adjusted to protein concentration and proteasome activity was determined from a standard curve of AMC fluorescence. Samples were assayed in duplicate.
75.2.5 HPLC Quantitation of A2E
Chromatographic separation and A2E quantification was performed by reversephase HPLC as previously described [6]. An Atlantis® dC18 (3 μm, 4.6 × 150 mm, Waters Corp, Milford MA) column was employed with an acetonitrile and water gradient and 0.1 % trifluoroacetic acid (85–100 %, 0.8 mL/min 15 min; 100 % acetonitrile, 0.8–1.2 mL/min 15–20 min; monitoring at 430 nm; 30 μL injection volume).
75.2.6 Sulforaphane Treatment
Cells were treated with sulforaphane (1-isothiocyanato-4-(methyl sulfinyl)butane; 5 μM, 48 h; LKT Laboratories, St. Paul, MN) in DMEM and Ham's F-12 medium (1:1) with 10 % FBS that had been heat- (90 min at 55 °C) and charcoal-treated (1 % w/v) to reduce the presence of endogenous NQO1 inducers.
75.2.7 GSH Measurement
Supernatants from cell lysates containing 1 % 5-sulfosalicylic acid were submitted to GSH colorimetric assay (405 nm absorbance) in the presence of GSH reductase, NADPH, and DTNB (BioVision Research Products). GSH concentration was determined using a calibration curve and protein was measured by Bio-Rad assay (Bio-Rad, Hercules, CA).
75.2.8 Cell Viability Assay
Cytotoxicity was assayed by MTT (4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) assay (Roche Diagnostics, Basel, Switzerland).
75.3 Results
75.3.1 HRP Degrades Bisretinoids in a Cell-Free Assay
We have previously shown in non-cellular assays that HRP can degrade A2E [3]. Here we compared the degradation of A2E to another bisretinoid having a polyene structure, all- trans-retinal dimer. After a 3 h incubation period, A2E levels were decreased by 35 % in the presence of HRP and H2O2(Fig. 75.1). The effect of HRP on all-trans-retinal dimer was even more robust, with levels of the latter compound being reduced by 85 % within 3 h.
Fig. 75.1.
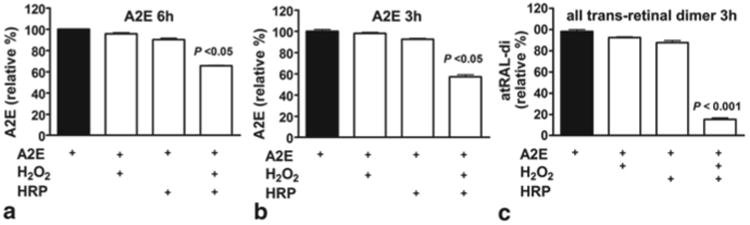
HPLC quantitation of the bisretinoids A2E (a, b) and all-trans-retinal dimer (atRAL-di) (c) after incubation in the presence (+) and absence (−) of horseradish peroxidase and hydrogen peroxide (HRP/H2O2) for time periods (hours) indicated. Mean ± SEM, 3 experiments. One-way ANOVA and Newman Keul Multiple Comparison test
75.3.2 Reduced Proteasome Activity with HRP-Mediated Degradation of Intracellular A2E
We also previously showed that HRP, when delivered to cultured RPE via the Bioporter® system, becomes located in lysosomes [3].
In the current experiments, we quantified A2E by integrating HPLC peak areas 3 days after introducing HRP to the cells. A2E levels were reduced by ∼40 % as compared to starting levels. BioPorter in the absence of HRP conferred no changes in A2E levels (Fig. 75.1c), indicating that HRP is exclusively responsible for the oxidation and degradation of A2E.
We next assayed for proteasome activity using luminogenic and fluorogenic substrates that generate a signal upon proteasome cleavage. We found that HRP-mediated degradation of intracellular A2E reduced chymotrypsin-like and trypsin-like proteasome activity by 36 % and 18 % respectively (Fig.75.2). This level of interference in proteasome activity by HRP-mediated degradation of intracellular A2E was similar to that observed in RPE cells following 430 nm-irradiation of A2E-containing ARPE-19 cells.
Fig. 75.2.
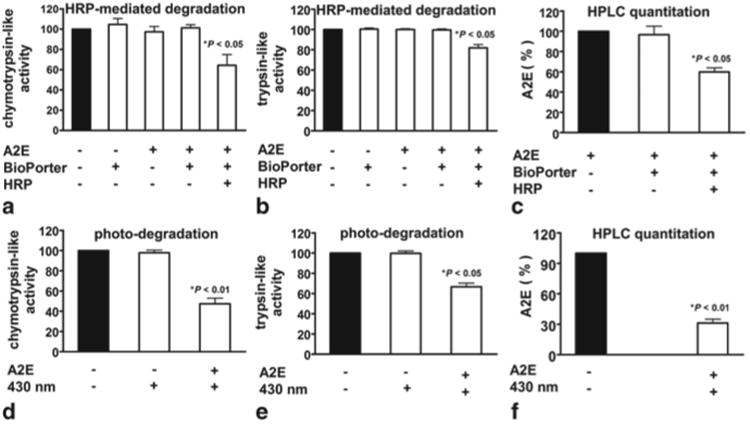
Enzyme-mediated degradation and photo-degradation of A2E impacts proteasome activity in RPE cells. HRP was delivered to A2E-containing RPE by BioPorter vehicle. a–c HRP-mediated degradation of A2E followed by measurement of chymotrypsin- (a) and trypsin-like (b) proteasome activity. (c). HPLC quantitation of A2E confirms A2E degradation by HRP. Controls include absence of A2E; BioPorter in absence of HRP. d–e Photodegradation (430 nm light) of A2E followed by measurement of chymotrypsin- (d) and trypsin-like (e) proteasome activity. f HPLC quantitation of A2E and A2E isomers confirms A2E photodegradation. Controls include absence of A2E and A2E in absence of 430 nm exposure. Values normalized to untreated cells; mean ± SEM, 3 experiments; one-way ANOVA and Newman Keul Multiple Comparison test
75.3.3 GSH Effects on HRP-Mediated Degradation of Intracellular A2E
We also sought to determine whether reduced glutathione (GSH), a cellular source of reducing equivalents, could protect the RPE cell in the presence of HRP-mediated A2E cleavage. To this end we treated the cells with sulphoraphane, a phytochemical that increases cellular GSH levels [7, 8]. With sulphoraphane, cellular GSH levels were increased by 58 % (as compared to untreated RPE) (Fig. 75.3). HRP-mediated degradation of A2E caused a 25 % decline in GSH levels measured 3 days after HRP was delivered. Seven (7) and 14 days after HRP delivery, cell viability was diminished by 14 % and 19 %, respectively (as compared to A2E-containing cells in the absence of HRP; p < 0.05)) and protection by sulphoraphane pretreatment was not detectable (p > 0.05) (Fig. 75.3).
Fig. 75.3.
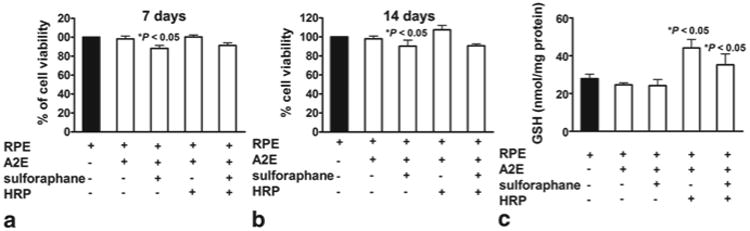
Cell viability and GSH levels in RPE cells pre-treated with sulforaphane 24 h before HRP was delivered to the cells to degrade A2E. a, b Cell viability was determined after 24 h by MTT assay, a decrease in the absorbance (570 nm) of reduced MTT being indicative of diminished cell viability. Values were normalized to untreated cells. c GSH quantitation. Total GSH expressed as nanomoles per milligram of cytosolic protein. Means ± SEM, 4 experiments; one-way ANOVA and Newman Keul Multiple Comparison test
75.4 Discussion
Autofluorescent bisretinoid pigments such as A2E and all-trans-retinal dimer accumulate with age as the lipofuscin of retinal pigment epithelial (RPE) cells in the eye. These pigments originate in photoreceptor outer segments from reactions of visual cycle vitamin A aldehyde and are deposited in the RPE secondarily. The bisretinoids of RPE lipofuscin likely accumulate because they are refractory to lysosomal proteolysis. RPE lipofuscin is implicated in a number of macular diseases [9]. Pre-clinical therapeutic strategies aimed at preventing vision loss in ABCA4-associated disease have focused on viral vector mediated delivery of the wild-type gene or systemic administration of compounds that limit the retinoid cycle [10–14]. These approaches, however, cannot reverse the accumulation of lipofuscin bisretinoids, once it has already occurred. Thus we have been exploring an additional line of attack that would involve delivery of exogenous enzyme having the capability to cleave the bisretinoids of RPE lipofuscin. As proof of principle, we have experimented with a well-known enzyme, HRP. As shown here and previously, HRP can bring about the degradation of A2E [3]; HRP can even more effectively cleave the bisretinoid all-trans-retinal dimer. Interestingly, all-trans-retinal dimer is also more susceptible to photooxidation [15].
As shown here, one effect of this form of enzyme degradation could be perturbation of the UPS. The mechanism by which the UPS is impacted is not clear. Perhaps by engaging the proteasome, the products of HRP-mediated A2E degradation overwhelm other UPS substrates, thereby competitively inhibiting proteasome function. Alternatively, the degradation fragments could react with, and thus attenuate proteasome enzyme activity. In the future we will test additional enzyme candidates for their ability to safely degrade the bisretinoids of RPE lipofuscin.
Acknowledgments
This study was supported by the Edward N. and Della L. Thome Memorial Foundation, National Institutes of Health grant P30EY019007, and a grant from Research to Prevent Blindness to the Department of Ophthalmology, Columbia University.
Contributor Information
Janet R. Sparrow, Email: jrs88@columbia.edu, Department of Ophthalmology and Cell Biology, Columbia University, 630 W. 168 Street, New York, NY 10032, USA, Department of Pathology and Cell Biology, Columbia University, New York, NY, USA.
Jilin Zhou, Email: jz219@columbia.edu, Department of Ophthalmology and Cell Biology, Columbia University, 630 W. 168 Street, New York, NY 10032, USA.
Shanti Kaligotla Ghosh, Email: shanti111us@gmail.com, Department of Ophthalmology and Cell Biology, Columbia University, 630 W. 168 Street, New York, NY 10032, USA.
Zhao Liu, Department of Ophthalmology and Cell Biology, Columbia University, 630 W. 168 Street, New York, NY 10032, USA.
References
- 1.Dudek EJ, Shang F, Valverde P, Liu Q, Hobbs M, Taylor A. Selectivity of the ubiquitin pathway for oxidatively modified proteins: relevance to protein precipitation diseases. FASEB J. 2005;19(12):1707–1709. doi: 10.1096/fj.05-4049fje. [DOI] [PubMed] [Google Scholar]
- 2.Fernandes AF, Zhou J, Zhang X, Bian Q, Sparrow JR, Taylor A, Pereira P, Shang F. Oxidative inactivation of the proteasome in retinal pigment epithelial cells. A potential link between oxidative stress and up-regulation of interleukin-8. J Biol Chem. 2008;283:20745–20753. doi: 10.1074/jbc.M800268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y, Zhou J, Fishkin N, Rittmann BE, Sparrow JR. Enzymatic degradation of A2E, a retinal pigment epithelial lipofuscin bisretinoid. J Am Chem Soc. 2011;133:849–857. doi: 10.1021/ja107195u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sparrow JR, Parish CA, Hashimoto M, Nakanishi K. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest Ophthalmol Vis Sci. 1999;40(12):2988–2995. [PubMed] [Google Scholar]
- 5.Sparrow JR, Zhou J, Ben-Shabat S, Vollmer H, Itagaki Y, Nakanishi K. Involvement of oxidative mechanisms in blue light induced damage to A2E-laden RPE. Invest Ophthalmol Vis Sci. 2002;43(4):1222–1227. [PubMed] [Google Scholar]
- 6.Kim SR, Jang YP, Jockusch S, Fishkin NE, Turro NJ, Sparrow JR. The all-trans-retinal dimer series of lipofuscin pigments in retinal pigment epithelial cells in a recessive Stargardt disease model. Proc Natl Acad Sci U S A. 2007;104:19273–19278. doi: 10.1073/pnas.0708714104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, Gao X, Cai B, Sparrow JR. Indirect antioxidant protection against photooxidative processes initiated in retinal pigment epithelial cells by a lipofuscin pigment. Rejuven Res. 2006;9(2):256–263. doi: 10.1089/rej.2006.9.256. [DOI] [PubMed] [Google Scholar]
- 9.Sparrow JR, Gregory-Roberts E, Yamamoto K, Blonska A, Ghosh SK, Ueda K, Zhou J. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res. 2012;31:121–135. doi: 10.1016/j.preteyeres.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allocca M, Doria M, Petrillo M, Colella P, Garcia-Hoyos M, Gibbs D, Kim SR, Maguire AM, Rex TS, Di Vicino U, Cutillo L, Sparrow JR, Williams DS, Bennett J, Auricchio A. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest. 2008;118:1955–1964. doi: 10.1172/JCI34316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong J, Kim SR, Binley K, Pata, Doi K, Mannik J, Zernant-Rajang J, Kan O, Iqball S, Naylor S, Sparrow JR, Gouras P, Allikmets R. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 2008;15(19):1311–1320. doi: 10.1038/gt.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radu RA, Han Y, Bui TV, Nusinowitz S, Bok D, Lichter J, Widder K, Travis GH, Mata NL. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci. 2005;46:4393–4401. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- 13.Maiti P, Kong J, Kim SR, Sparrow JR, Allikmets R, Rando RR. Small molecule RPE65 antagonists limit the visual cycle and prevent lipofuscin formation. Biochem. 2006;45:852–860. doi: 10.1021/bi0518545. [DOI] [PubMed] [Google Scholar]
- 14.Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon KD, Yamamoto K, Zhou J, Sparrow JR. Photo-products of retinal pigment epithelial bisretinoids react with cellular thiols. Mol Vis. 2011;17:1839–1849. [PMC free article] [PubMed] [Google Scholar]


