Abstract
Purpose
The process of assessing patient symptoms and functionality using patient-reported outcomes (PROs) and functional performance status (FPS) is an essential aspect of patient-centered oncology research and care. However, PRO and FPS measures are often employed separately or inconsistently combined. Thus, the purpose of this study was to conduct a systematic review of the level of association between PRO and FPS measures to determine their differential or combined utility.
Methods
A systematic search was conducted using five databases (1966 to February 2014) to identify studies that described an association between PRO and FPS. Studies were excluded if they were non-cancer specific, did not include adults aged 18 or older, or were review articles. Publications were selected for review by consensus among two authors, with a third author arbitrating as needed.
Results
A total of 18 studies met inclusion criteria. FPS was primarily assessed by clinicians using the ECOG Performance Status or Karnofsky Performance Status measures. PROs were captured using a variety of measures, with numerous domains assessed (e.g., pain, fatigue, and general health status). Concordance between PROs and FPS measures was widely variable, falling in the low to moderate range (0.09–0.72).
Conclusions
Despite consistency in the method of capture of PROs or FPS, domain capture varied considerably across reviewed studies. Irrespective of the method of capturing PROs or FPS, the quantified level of association between these two areas was moderate at best, providing evidence that FPS and PRO assessments offer unique information to assist clinicians in their decision-making.
Keywords: Patient outcome assessment, Health status, Neoplasms, Karnofsky performance status
The process of assessing patient symptoms, quality of life (QoL), and treatment-related side effects is important in the study of cancer treatment efficacy, as well as to ensure high-quality, patient-centered care in oncology settings [1]. Two primary methods are utilized to assess these effects, physician-rated functional performance status (FPS) and patient-reported outcomes (PROs).
FPS refers to a patient’s ability to perform activities and functions that meet basic physical needs in the face of illness [2]. Although several FPS measures exist, the two most widely used in clinical and research settings are the Eastern Cooperative Oncology Group (ECOG PS) [3] and Karnofsky Performance Scale (KPS) [4]. The ECOG PS is used by physicians to report on severity of toxicity experienced by patients on oncology treatment trials. The ECOG measures toxicity effects on a five-point scale with 0 being “fully active, able to carry on all pre-disease performance without restriction” and 5 indicating that the patient is deceased. Similarly, the KPS is a physician-reported indicator of a patient’s ability to engage in normal daily activities, as well as dependence on medical care to function. The KPS is on a scale from 100 to 0, 100 meaning that the patient has “no complaints; no evidence of disease” and 0 meaning the patient is deceased. Basch et al. [5] indicate that clinician reports of FPS are particularly useful as strong predictors of death and emergency room visits, although they may be less accurate in assessing patient symptoms.
PROs are considered to be the “gold standard” for the reporting of the patient symptomatic experience and are defined as any report of the status of a patient’s health condition that comes directly from the patient, without interpretation or filtration of this response by a clinician or anyone else [6]. Although PROs are often used in clinical research trials, their utility can extend to multiple settings, including determining product safety and effectiveness and promoting patient-centered care (i.e., health care that incorporates active involvement of the patient in the decision-making process) that may improve patient quality of life, communication with providers, patient satisfaction, and symptom management [7–9]. PROs come in many forms but typically assess patient symptoms, side effects, and/or quality of life concerns through a self-report, quantitative scale and are commonly used in clinical cancer research [10]. The timing of these self-report assessments differs depending on a number of factors, including the study/trial protocol, the established psychometric properties of the measurement tools for optimally avoiding recall bias, the outcome being assessed, and the setting. For example, in their study of patient-reported chemotherapy toxicity, Judson et al. [11] found that a monthly PRO assessment was more feasible than weekly.
Although these measures are often employed separately and not consistently combined, there has been a broader movement toward patient-centered care [12] with FDA recommendations encouraging researchers to integrate patient report measures into clinical trials of medication [13]. Thus, it is important to identify the level of association between FPS and PROs as an important step to determine their differential or combined utility [12]. Identifying whether PROs systematically perform better or equal to physician-rated FPS could potentially reduce staffing burden; improve the accuracy of identifying patient symptoms, treatment side effects, concerns with treatments; and promote a patient-centered care environment. Further, the use of PROs to measure performance status is increasingly becoming an area of interest in medicine, as evidenced by the National Quality Forum’s (NQF) recent recommendations to create a PRO performance measure [7]. Thus, the purpose of this study was to systematically review and thus better characterize the level of association between FPS and PROs.
Method
Search strategy
A comprehensive electronic literature search for articles was conducted in the following databases: PubMed, PsycINFO (Psychological Abstracts) via OVID, Health and Psychosocial Instruments (HAPI) via OVID, Cochrane via Wiley, and EMBASE provided by Elsevier. There were no date or language restrictions; each database was searched in its entirety, through February 2014. Controlled vocabulary (Medical Subject Headings [MeSH], PsycINFO Subject Headings, EMTREE) and keywords were applied. Grey literature references (meeting abstracts) identified in BIOSIS Previews®.
Four broad-concept categories were searched (i.e., cancer, patient-reported outcomes, quality of life, and daily living), and results were combined using the appropriate Boolean operators (AND, OR). The MeSH search terms were as follows: (“Neoplasms”[MeSH] AND (patient-reported outcomes OR PRO OR PROs OR patient-reported outcomes) AND (“Quality of Life”[MeSH] OR “Personal Satisfaction”[MeSH] OR “Patient Satisfaction”[MeSH] OR “Patient Compliance”[MeSH] OR “Pain”[MeSH] OR “Body Image”[MeSH] OR “Social Adjustment”[MeSH] OR “Social Behavior”[MeSH] OR “Shyness”[MeSH] OR “Social Distance”[MeSH] AND “Social Isolation”[MeSH] OR “Fear”[MeSH] OR “Frustration”[MeSH] OR “Personal Autonomy”[MeSH] OR “Self Concept”[MeSH] OR “Adaptat ion, Psychological”[MeSH] OR “Social Adjustment”[MeSH] OR “Stress, Psychological”[MeSH] OR “Emotions”[MeSH] OR “Fatigue”[MeSH] OR “Depression ”[MeSH] OR “Sleep”[MeSH] OR “Spirituality”[MeSH]) AND (“Quality-Adjusted Life Years”[MeSH] OR “Health Status”[MeSH] OR “Activities of Daily Living”[MeSH]). Keyword terms used were as follows: (cancer OR neoplasm) AND (patient-reported outcomes OR PRO OR PROs OR patient-reported outcomes) AND (quality of life OR QOL OR HRQL OR HRQOL OR personal satisfaction OR patient satisfaction OR patient compliance OR pain OR disability OR disabilities OR disabled OR body image OR social function OR social behavior OR social behavior OR shyness OR social distance OR social isolation OR fear OR frustration OR autonomy OR self-concept OR adaptation OR adjustment OR coping OR stress OR emotion OR fatigue OR depression OR sleep OR spiritual OR spirituality) AND (quality adjusted life years OR QALY OR health status OR well-being OR functional status OR functional capacity OR functional performance OR daily activities OR daily living). Related terms were also incorporated into the search strategy to ensure that all relevant papers were retrieved.
Selection strategy
Studies were deemed eligible for inclusion if they included an original characterization of the level of association between physician-rated FPS and PROs in adult (i.e., age ≥18) patients with cancer.
Screening process
Initially, titles were independently reviewed for eligibility by two co-authors. Second, each potentially eligible article was randomly assigned to a pair of co-authors for full abstract screening. Articles moved forward for full-text review if both co-authors reached concensus on eligibility. In instances of disagreement, a third co-author arbitrated the article. For the full-text review phase, the randomly assigned author teams consisted of a primary reviewer and a secondary reviewer for the purposes of verification and quality assurance. Both reviewers independently completed standardized coding forms to extract the pre-determined information from each potentially eligible article. All reviewers then met as a group and compared full-text article reviews to resolve any potential discrepancies and make final decisions regarding article inclusion. Each author searched references from the included full-text articles to determine whether they should be also considered for inclusion. Study quality was assessed using a modified version of the Downs and Black Study Quality Checklist [14].
Results
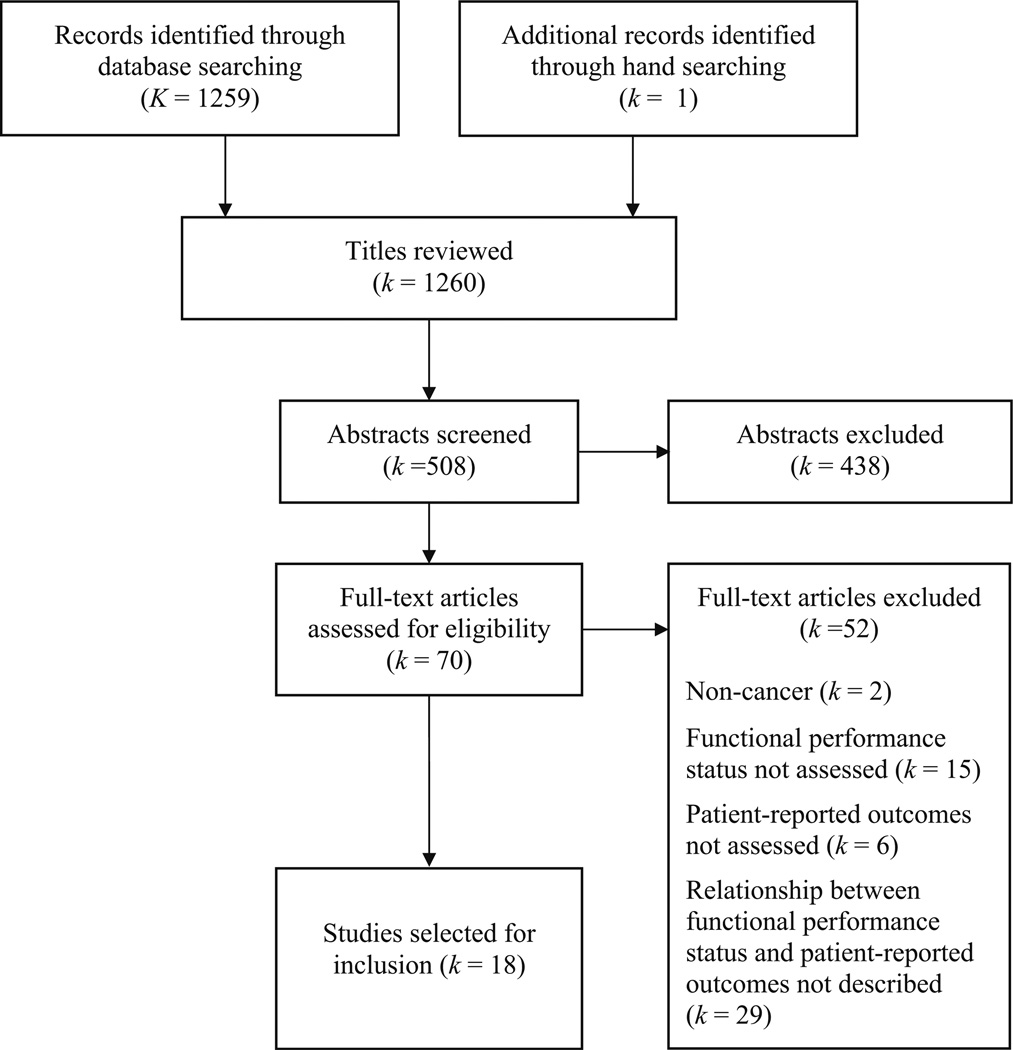
The initial electronic literature search yielded a total of 1259 titles. A single additional title was added through hand searching. Following the process of title screening, two of the primary authors independently reviewed each of the 508 unique article abstracts; 70 (60 full-text articles, 10 conference proceeding abstracts) were retained for the full-text review. Reasons for article exclusion during the full-text review phase included the following: non-cancer (k=1), FPS was not assessed (k=14), PROs were not assessed (k=5), and the level of association between FPS and PROs was not captured/described (k=28). A total of 18 articles met eligibility criteria and were included in this review (Fig. 1). All but 1 of the 18 articles possessed at least half of the relevant quality indicators from a modified version of the Downs and Black Study Quality Checklist [14].
Fig. 1.
PRISMA flow chart
Study characteristics
Table 1 provides study demographics and clinical characteristics of the included studies. Patients were of mixed cancer types, including breast, colorectal, non-Hodgkins lymphoma, pancreatic, kidney, and non-small-cell lung cancer. Eleven studies reported race and six studies reported ethnicity, with the majority of patients in these studies identified as White and non-Hispanic.
Table 1.
Demographics and clinical characteristics for included studies (k=18)
| Study | Number | % Female | Age | Race | Ethnicity | Cancer type | FPS measure | PRO measure | Reported association |
|---|---|---|---|---|---|---|---|---|---|
| Basch et al. 2009 [5] | 163 | 55 % | M=63 | N/A | N/A | 83 % NSCLC, 9 % SCLC, 4 % Thymoma, 4 % Mesothelioma |
KPS | EQ-5D, Patient-KPS |
Higher levels of concordance between patient reports of FPS and health status than between physician reported KPS and health status |
| Bergman et al. 1991 [15] | 62 | 29 % | M=66 | N/A | N/A | SCLC on active chemotherapy |
ECOG PS | SIP; HADS |
SIP indices were strongly related to ECOG PS |
| Bock et al. 2012 [25] | 106 | 100 % | M=57 | 87 % White | 91 % Non-Hispanic | Breast Cancer Stage I–III | KPS | Online Symptom Assessment | The number of self-reported symptoms increases with perceived functional impairment as measured by KPS |
| Butt et al. 2013 [30] | 2000 | 50 % | Equal age bands of 18–29, 30–44, 45–59, 60–74, >75 |
86 % White | 94.5 % Non-Hispanic | N/A | NFKSI | SF-36; PROMIS-29 |
Symptom reports on the NFKSI scales were significantly correlated with the SF-36 and PROMIS-29 scales |
| Butt et al. 2013 [16] | 297 | 64 % | M=58 | 83 % White | N/A | 34 % Breast cancer, 12.5 % colorectal, 8.5 % non-Hodgkins lymphoma, 7.1 % ovarian, 6.5 % lung, 5.1 % prostate |
ECOG PS | FACIT-Fatigue | Fatigue was significantly, negatively correlated with ECOG PS |
| Carter et al. 2008 [17] | 59 | 30 % | Mdn=66 | N/A | N/A | Relapsed/ refractory mantle cell lymphoma |
ECOG PS | FACT-Lym | The FACT-Lym was able to differentiate patients based on ECOG PS and measure changes in QoL associated with worsening health status |
| Cella et al. 2006 [18] | 40 patients with Thromb; 43 without |
Thromb group: 35 % Non-Thromb: 60 % |
Thromb group: M=52.5; Non-Thromb: M=61.6 |
80 % White | Thromb group: 97 % and Non-Thromb: 95 % Non-Hispanic |
Cancer patients with Thromb |
ECOG PS | FACT-Th | The FACT-Th differentiated patients with good FPS from those with poor FPSs |
| Colwell et al. 2010 [19] | 391 | 34 % | <65 (60 %) ≥65 (40 %) >75 (10 %) |
99 % White | N/A | Metastatic colorectal | ECOG PS | FCSI-9; EQ-5D |
Subjects with better FPS scores reported significantly higher FCSI-9 scores than those with lower FPS scores at both baseline and week 8 |
| Jones et al. 2014 [20] | 320 | 0 % | Mdn=72 | 83 % White | 77.5 % non-Hispanic | Prostate | ECOG PS | MDASI | MDASI had strong ability to differentiate between clinically different patient groups based lon FPS |
| Kamal et al. 2013 [29] | 460 | 56 % | <65 (34 %) ≥65 (66 %) |
84 % White | N/A | Cancer patients receiving palliative care |
PPS | 4-level categorical scale of QoL |
PPS was a significant predictor of high QoL |
| Karnell et al. 1999 [32] | 68 | 19 % | M=59 | N/A | N/A | Head and Neck | PSS-HNC | Head and Neck Health Status Assessment Inventory |
Patients’ QoL and FPS ratings were significantly associated |
| Robinson et al. 2008 [26] | 86 | 46 % | M=65 | 99 % White | N/A | Pancreatic | KPS | FACIT-F, FAACT, BPI, SF-36 |
KPS correlated with several PROs in the weak to moderate range |
| Rothrock et al. 2013 [21] | 50 | 36 % | M=59 | 94 % White | 96 % Non- Hispanic |
Kidney Cancer stage III–IV |
ECOG PS | NFKSI-19 | NFKSI-19 significantly differentiated between the three categories of FPS |
| Singh et al. 2014 [33] | 9295 | 62 % Female; 31 % missing |
Missing (N=2898); <50; (N=910); 50–64 (N=2378); 65–71 (N=1444); 72+ (N=1665) |
N/A | N/A | Cancer patients and healthy volunteers |
N/A | LASA | Overall QoL was weakly related to FPS |
| Velanovich and Wollner 2011 [27] | 80 | 33 % | M=61 | N/A | N/A | Pancreatic & periampullary tumor patients being screened for surgical treatment |
KPS | SF-36 | KPS was significantly associated with all domains of the SF-36; appeared to be independent of tumor pathology and stage. Physical functioning domain most closely associated with KPS |
| Walker et al. 2011 [22] | 102 | 100 % | M=57 | 72 % White | N/A | HER-2 negative metastatic breast cancer |
ECOG PS | PCM | Lower FPS related to higher patient-reported symptom burden in both psychological and physical domains |
| Yost et al. 2005 [23] | 175 | 61 % | Mdn=52 | N/A | N/A | Chronic phase chronic myelogenous leukemia |
ECOG PS | FACT-BRM, GRC |
Overall agreement between FPS change and GRC as measured by weighted kappa statistic was poor; better at 1 month assessment than at months 3 and 6 |
| Yount et al. 2002 [24] | 59 | 49 % | M=61 | 88 % White | 90 % Non-Hispanic | Hepatobiliary cancer: Colon with liver metastases, hepatocellular, pancreatic, gallbladder. |
ECOG PS | FHSI-8 | FHSI-8 scores significantly differentiated two of the three levels of FPS |
M mean, Mdn median, N/A not available, FPS functional performance status, PS performance status, PRO patient-reported outcome, NSCLC non-small-cell lung cancer, SCLC small cell lung cancer, Throm thrombocytopenia, KPS Karnofsky Performance Status, ECOG PS Eastern Cooperative Oncology Group Performance Status, PSS-HNC Performance Status Scale–Head and Neck Cancer, PPS Palliative Performance Status, NFKSI National Comprehensive Cancer Network-Functional Assessment of Cancer Therapy–Kidney Symptom Index (NFKSI), SIP Sickness Impact Profile, HADS Hospital Anxiety and Depression Scale, SF-36 Short Form Health Survey 36, PROMIS Patient-Reported Outcomes Measurement Information System, FACIT Functional Assessment of Chronic Illness Therapy, FACIT-F FACIT-Fatigue Module, FACT Functional Assessment of Cancer Therapy, FACT-Th FACT-Thrombocytopenia Subscale, FSHI-8 FACT-Hepatobiliary Symptom Index-8, FACT-BRM FACT-Biological Response Modifiers, BPI Brief Pain Inventory, FAACT Functional Assessment Of Anorexia/Cachexia Therapy, QoL quality of life, FCSI-9 Functional Assessment Of Cancer Therapy/National Comprehensive Cancer Network Colorectal Cancer Symptom Index, MDASI MD Anderson Symptom Inventory, LASA linear analogue self-assessment, PCM patient care monitor, GRC Patient Global Rating of Change
Over half of the studies (k=10) used ECOG PS to assess FPS [15–24], with four using KPS [5, 25–27], one using a modified version of KPS known as the Palliative Performance Status (PPS; [28]) [29], one making use of the patient-reported National Comprehensive Cancer Network-Functional Assessment of Cancer Therapy–Kidney Symptom Index (NFKSI) [30], one using the patient-reported Performance Status Scale–Head and Neck Cancer (PSS-HNC; [31]) [32], and one cooperative group study that used a variety of FPS measures, including ECOG PS and KPS [33]. Basch and colleagues [5], in addition to using KPS to assess patient FPS, administered a patient-reported version of KPS scaled from 0 to 100.
The use of PRO measures in these studies was much more variable, with only the Medical Outcomes Study Short Form-36 (SF-36; [34]), European Quality of Life (EQ-5D; [35]), Functional Assessment of Cancer Therapy (FACT-G [36]), and Functional Assessment of Chronic Illness Therapy (FACIT; [37]) instruments used in multiple studies. Other PRO measures administered included the Hospital Anxiety and Depression Scale (HADS; [38]), the MD Anderson Symptom Assessment Inventory (MDASI; [39]), the Brief Pain Inventory (BPI; [40, 41]), linear analogue self assessment (LASA; [33]), and Patient-Reported Outcomes Measurement Information System-29 (PROMIS-29 [42]). The SF-36 and FACT-G/FACIT assessment tools are widely used measures of global QoL, including emotional, physical, social, and functional well-being. The FACT/FACIT family of measures includes a number of disease/symptom specific modules for patients with cancer, including the FACT-Lym (lymphoma), FACT-KSI (kidney symptom), FACT-Th (thrombocytopenia), FHSI-8 (hepatobiliary symptom), and FACIT-F (fatigue). EQ-5D is a brief, commonly used instrument to characterize patient health status. The HADS and BPI are well-validated measures of anxiety and depression, and pain, respectively. MDASI is a psychometrically sound assessment of QoL and symptom impact with daily activities. LASA is a single-item measure of overall QoL that uses a 0–10 numeric rating scale. PROMIS-29 captures patient-reported physical function, anxiety, depression, fatigue, sleep disturbance, and satisfaction with social role, as well as pain intensity and interference.
Associations between FPS and PRO measures
In the majority of studies (k=14), FPS was reported as being significantly positively correlated with PRO measures. This included all three studies that utilized a patient-reported assessment of FPS [5, 21, 32]. Five studies made use of PRO measures to successfully differentiate between various levels of FPS. As part of a study that validated the MDASI in patients with prostate cancer, it was found that the overall score of this measure is sensitive to differentiating between patients with good (0) or poor (≥1) ECOG PS [20]. In the validation studies for the FACT-KSI, FACT-Th FHSI-8, and FACT-Lym, the individual domain subscores for these patient-reported measures were shown to be sensitive to differentiation between patients who were rated by physicians as having ECOG PS 0, 1, or ≥2 [17, 18, 21, 24].
There were four instances of negative and/or weak levels of association between FPS and PRO measures. These included two studies that specifically captured patient-reported fatigue via the FACIT-F (i.e., r=0.43 [26] and r=−0.55 [16]), as well as a study that examined the level of association between FPS and PROs in patients undergoing treatment with biological response modifiers (i.e., ECOG PS vs. patient global rating of change for emotional well-being [r range 0.11–0.24], emotional-well being [r range 0.09–0.23], and change in physical functioning and well being [r range 0.25–0.37] [23]. A multicenter study that consisted of healthy volunteers and cancer trial patients, as well as hospice patients and their caregivers (N=9295) reported a significantly negative association between FPS and overall QoL, as measured by LASA (r=−0.29, p<0.0001) [33].
Discussion
The assessment of patient outcomes is an essential component of providing patient-centered, effective care [1]. However, there is wide variation in not only measurement tools but also how the outcomes are measured, whether through clinician report in FPS measures or PROs. Thus, the current systematic review investigated the level of association between FPS and PROs in order to determine if they similarly capture patient outcomes. The findings of this systematic review were consistent with literature on clinician versus PROs that suggests that, while there is often overlap, there is also some variation in clinician and patient reports [43, 44]. This indicates that FPS and PROs provide unique information not consistently captured by one form of assessment or another. Despite strong evidence that PROs provide valuable information about patient symptoms that may not be captured by physician assessment of FPS, they are not well-integrated into clinical practice outside of research. A reliance on clinician assessment through FPS rather than PROs may be due to potential challenges in implementing PRO programs, issues with missing data, and the need to promote standardization and electronic reporting potions [12].
When compared to FPS measures, PROs appear to offer many potential benefits to patients, treatment teams, drug developers, regulatory bodies, and insurance payment companies [45]. Through their critical assessment of the Common Terminology Criteria for Adverse Events (CTCAE), a clinician-based system for reporting adverse events [46–48], Bruner et al. [49] argue that the CTCAE leaves room for error because the patient symptom information must go through many channels, including the physician, medical records, data entry, and final interpretation. Given that adverse events are usually reported by clinicians, this could contribute to underreporting or inaccurately reporting symptoms [45]. It has been suggested that receiving the information directly from the patient and using that information as the symptom measure, rather than going through the clinician, is one way to make this process more direct and potentially more accurate [49]. Basch and colleagues have proposed that one way to address this issue and more accurately inform treatment and clinical research would be to encourage medical institutions to embrace collaborative reporting, such that both clinician- and patient-reported outcomes are utilized [45].
Another potential avenue of reconciling the gap between FPS and PRO information is through the use of wearable activity tracking devices (e.g., FitBit®, Garmin®, Jawbone®, and Apple Watch®) to monitor movement-related activities (e.g., steps walked, distance ran/cycled, and flights of stairs climbed) by a patient over a given time period (e.g., daily, since last visit). There has been a recent push toward the use of such technology as an objective measure of patient physical activity and quality of life in cancer survivors [50–52]. Data collected from these devices could provide important between-visit contextual information to physicians when assigning FPS scores to patients, while allowing patients to consciously monitor their activity in real time.
The findings of this review were largely limited by the limitations of the studies themselves. In particular, we were unable to conduct a meta-analysis of the findings given effect sizes were not uniformly provided across studies. Additionally, our findings indicate that there is a lack of consistency across studies investigating both FPS and PROs in how these outcomes are measured. The administration of a standard battery of FPS and PROs would enable a better assessment of the differential utility of FPS and PROs. Lastly, a direct comparison between FPS and the various physical functioning domains of the PRO measures would have greatly assisted in better understanding the association between FPS and PROs. Unfortunately none of the included articles described such a comparison, other than to illustrate that PROs could differentiate between various levels of FPS [17, 18, 21, 24]. Future studies should explicitly quantify this relationship to assist with describing how patient-reported physical functioning and FPS are associated.
Our findings suggest that FPS and PRO may provide unique information and, when combined, may facilitate more effective care for patients. This study serves as a step toward investigating the possibility of a PRO performance measure by demonstrating the need for a measure that captures both FPS and PROs.
Acknowledgments
This project was supported by a National Institutes of Health Research Training Grant (T32 CA009461-25); as well as a National Institutes of Health Support Grant (P30 CA08748-48), which provides partial support for the Behavioral Research Methods Core Facility used in conducting this investigation.
Footnotes
A portion of these results were presented at the 2015 Meeting of the Society of Behavioral Medicine in San Antonio, TX.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Kotronoulas G, Kearney N, Maguire R, et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32:1480–1501. doi: 10.1200/JCO.2013.53.5948. [DOI] [PubMed] [Google Scholar]
- 2.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
- 3.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 4.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod CM, editor. Evaluation of chemotherapeutic agents. New York: Columbia University Press; 1949. pp. 199–205. [Google Scholar]
- 5.Basch E, Jia X, Heller G, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101:1624–1632. doi: 10.1093/jnci/djp386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362:865–869. doi: 10.1056/NEJMp0911494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basch E, Snyder C, McNiff K, et al. Patient-reported outcome performance measures in oncology. J Oncol Pract. 2014;10:209–211. doi: 10.1200/JOP.2014.001423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basch E. Beyond the FDA PRO guidance: steps toward integrating meaningful patient-reported outcomes into regulatory trials and US drug labels. Value Health. 2012;15:401–403. doi: 10.1016/j.jval.2012.03.1385. [DOI] [PubMed] [Google Scholar]
- 9.Bruner DW, Hanisch LJ, Reeve BB, et al. Stakeholder perspectives on implementing the national cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) Behav Med Pract Policy Res. 2011;1:110–122. doi: 10.1007/s13142-011-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30:4249–4255. doi: 10.1200/JCO.2012.42.5967. [DOI] [PubMed] [Google Scholar]
- 11.Judson TJ, Bennett AV, Rogak LJ, et al. Feasibility of long-term patient self-reporting of toxicities from home via the internet during routine chemotherapy. J Clin Oncol. 2013;31:2580–2585. doi: 10.1200/JCO.2012.47.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basch E. New frontiers in patient-reported outcomes: adverse event reporting, comparative effectiveness, and quality assessment. Annu Rev Med. 2014;65:307–317. doi: 10.1146/annurev-med-010713-141500. [DOI] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services. Guidance for industry. [Accessed 27 Jul 2015];Patient-reported outcome measures: Use in medical development to support labeling claims December 2009. 2009 Available from: http://www.fda.gov/downlods/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf.
- 14.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergman B, Sullivan M, Sörenson S. Quality of life during chemotherapy for small cell lung cancer. I. An evaluation with generic health measures. Acta Oncol. 1991;30:947–957. doi: 10.3109/02841869109088248. [DOI] [PubMed] [Google Scholar]
- 16.Butt Z, Lai JS, Rao D, et al. Measurement of fatigue in cancer, stroke, and HIV using the functional assessment of chronic illness therapy - fatigue (FACIT-F) scale. J Psychosom Res. 2013;74:64–68. doi: 10.1016/j.jpsychores.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter GC, Liepa AM, Zimmermann AH, Morschhauser F. Validation of the functional assessment of cancer therapy - lymphoma (FACT-LYM) in patients with relapsed/refractory mantle cell lymphoma. Paper presented at: 50th American society of hematology annual meeting and exposition; December 7; San Francisco, CA. 2008. [Google Scholar]
- 18.Cella D, Beaumont JL, Webster KA, Lai JS, Elting L. Measuring the concerns of cancer patients with low platelet counts: the functional assessment of cancer therapy—thrombocytopenia (FACT-Th) questionnaire. Support Care Cancer. 2006;14:1220–1231. doi: 10.1007/s00520-006-0102-1. [DOI] [PubMed] [Google Scholar]
- 19.Colwell HH, Mathias SD, Turner MP, et al. Psychometric evaluation of the FACT colorectal cancer symptom index (FCSI-9): reliability, validity, responsiveness, and clinical meaningfulness. Oncologist. 2010;15:308–316. doi: 10.1634/theoncologist.2009-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones D, Zhao F, Fisch MJ, et al. The validity and utility of the MD Anderson symptom inventory in patients with prostate cancer: evidence from the symptom outcomes and practice patterns (SOAPP) data from the eastern cooperative oncology group. Clin Genitourin Cancer. 2014;12:41–49. doi: 10.1016/j.clgc.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothrock NE, Jensen SE, Beaumont JL, et al. Development and initial validation of the NCCN/FACT symptom index for advanced kidney cancer. Value Health. 2013;16:789–796. doi: 10.1016/j.jval.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker MS, Hasan M, Yim YM, et al. Retrospective study of the effect of disease progression on patient reported outcomes in HER-2 negative metastatic breast cancer patients. Health Qual Life Outcomes. 2011;9:46. doi: 10.1186/1477-7525-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yost KJ, Sorensen MV, Hahn EA, et al. Using multiple anchor- and distribution-based estimates to evaluate clinically meaningful change on the functional assessment of cancer therapy-biologic response modifiers (FACT-BRM) instrument. Value Health. 2005;8:117–127. doi: 10.1111/j.1524-4733.2005.08202.x. [DOI] [PubMed] [Google Scholar]
- 24.Yount S, Cella D, Webster K, et al. Assessment of patient-reported clinical outcome in pancreatic and other Hepatobiliary cancers: the FACT Hepatobiliary symptom index. J Pain Symptom Manag. 2002;24:32–44. doi: 10.1016/s0885-3924(02)00422-0. [DOI] [PubMed] [Google Scholar]
- 25.Bock M, Moore D, Hwang J, et al. The impact of an electronic health questionnaire on symptom management and behavior reporting for breast cancer survivors. Breast Cancer Res Treat. 2012;134:1327–1335. doi: 10.1007/s10549-012-2150-1. [DOI] [PubMed] [Google Scholar]
- 26.Robinson DW, Jr, Eisenberg DF, Cella D, et al. The prognostic significance of patient-reported outcomes in pancreatic cancer cachexia. J Support Oncol. 2008;6:283–290. [PubMed] [Google Scholar]
- 27.Velanovich V, Wollner I. Quality of life and performance status in patients with pancreatic and periampullary tumors. Int J Clin Oncol. 2011;16:401–407. doi: 10.1007/s10147-011-0200-z. [DOI] [PubMed] [Google Scholar]
- 28.Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): a new tool. J Palliat Care. 1996;12:5–11. [PubMed] [Google Scholar]
- 29.Kamal AH, Bull J, Stinson CS, Blue DL, Abernethy AP. Conformance with supportive care quality measures is associated with better quality of life in patients with cancer receiving palliative care. J Oncol Pract. 2013;9:e73–e76. doi: 10.1200/JOP.2013.000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butt Z, Peipert J, Webster K, Chen C, Cella D. General population norms for the functional assessment of cancer therapy-kidney symptom index (FKSI) Cancer. 2013;119:429–437. doi: 10.1002/cncr.27688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.List MA, Ritter-Sterr C, Lansky SB. A performance status scale for head and neck cancer patients. Cancer. 1990;66:564–569. doi: 10.1002/1097-0142(19900801)66:3<564::aid-cncr2820660326>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 32.Karnell LH, Funk GF, Tomblin JB, Hoffman HT. Quality of life measurements of speech in the head and neck cancer patient population. Head Neck. 1999;21:229–238. doi: 10.1002/(sici)1097-0347(199905)21:3<229::aid-hed8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 33.Singh JA, Satele D, Pattabasavaiah S, Buckner JC, Sloan JA. Normative data and clinically significant effect sizes for single-item numerical linear analogue self-assessment (LASA) scales. Health Qual Life Outcomes. 2014;12:187. doi: 10.1186/s12955-014-0187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 health survey: manual and interpretation guide. Boston, MA: Health Institute, New England Medical Centre; 1993. [Google Scholar]
- 35.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 37.Webster K, Cella D, Yost K. The functional assessment of chronic illness therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 39.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson symptom inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 40.Daut RL, Cleeland CS. The prevalence and severity of pain in cancer. Cancer. 1982;50:1913–1918. doi: 10.1002/1097-0142(19821101)50:9<1913::aid-cncr2820500944>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 41.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin brief pain questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 42.Cella D, Riley W, Stone A, et al. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao C, Polomano R, Bruner DW. Comparison between patient-reported and clinician-observed symptoms in oncology. Cancer Nurs. 2013;36:E1–E16. doi: 10.1097/NCC.0b013e318269040f. [DOI] [PubMed] [Google Scholar]
- 44.Ryan S, Atkinson TM, Bennett AV, et al. Concordance between symptomatic adverse event ratings by clinicians and patients: a systematic review. Paper presented at: 20th annual meeting for the international society for quality of life research; October 10; Miami, FL. 2013. [Google Scholar]
- 45.Basch E, Bennett A, Pietanza MC. Use of patient-reported outcomes to improve the predictive accuracy of clinician-reported adverse events. J Natl Cancer Inst. 2011;103:1808–1810. doi: 10.1093/jnci/djr493. [DOI] [PubMed] [Google Scholar]
- 46.National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) Version 4.0. [Accessed 7 Jun 2015];2010 Published May 28, 2009; Revised Version 4.03 June 14, 2010 Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 47.Basch E, Reeve BB, Mitchell SA, et al. Development of the national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) J Natl Cancer Inst. 2014;106(9):21. doi: 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hay JL, Atkinson TM, Reeve BB, et al. Cognitive interviewing of the US national cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) Qual Life Res. 2014;23:257–269. doi: 10.1007/s11136-013-0470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruner DW, Movsas B, Basch E. Capturing the patient perspective: patient-reported outcomes as clinical trial endpoints. Am Soc Clin Oncol Educ Book. 2012;2012:139–144. doi: 10.14694/EdBook_AM.2012.32.80. [DOI] [PubMed] [Google Scholar]
- 50.Blaauwbroek R, Bouma MJ, Tuinier W, et al. The effect of exercise counselling with feedback from a pedometer on fatigue in adult survivors of childhood cancer: a pilot study. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2009;17:1041–1048. doi: 10.1007/s00520-008-0533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vallance JK, Courneya KS, Plotnikoff RC, Yasui Y, Mackey JR. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25:2352–2359. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- 52.Bourke L, Homer KE, Thaha MA, et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev. 2013;9:Cd010192. doi: 10.1002/14651858.CD010192.pub2. [DOI] [PubMed] [Google Scholar]