Abstract
Background
Although a number of exercise systems have been developed to mitigate the physiological deconditioning that occurs in microgravity, few have the capacity to positively impact multiple physiological systems and still meet the volume/mass requirements needed for missions beyond low earth orbit. The purpose of this study was to test the gravity-independent Multi-Mode Exercise Device (M-MED) for both resistance (RE) and aerobic (AE) training stimuli.
Methods
Eight men and nine women (mean age 22.0±0.4 years) completed five weeks of training on the M-MED: RE 4×7 squats two days a week, and AE 4×4-min rowing bouts at ~90% VO2max three days a week. Pre- and post-training data collection included an aerobic capacity test, MR imaging, strength testing, and vastus lateralis muscle biopsy.
Results
VO2max increased 8%, 3RM strength 18%, and quadriceps femoris cross-sectional area (CSA) 10%. Knee extensor strength increased at all isokinetic speeds tested. Subjects also demonstrated improved resistance to fatigue in knee extension. At the cellular and molecular level, the biopsy revealed increases in mixed myofiber CSA (13%), citrate synthase activity (26%), total RNA concentration (24%), IGF-I mRNA (77%), Type IIa Myosin Heavy Chain (MHC) mRNA (8%), and concomitant decrease in Type IIx MHC mRNA (−23%). None of the changes were gender-specific.
Discussion
Both the functional outcomes and biomarker changes indicate that a very low volume of M-MED exercise results in robust adaptation in the cardiovascular and musculoskeletal systems. The M-MED has the potential to provide a wide range of countermeasure exercises and should be considered for testing in ground-based spaceflight simulation.
Keywords: countermeasure, endurance, muscle, space flight, strength
INTRODUCTION
The National Aeronautics and Space Administration (NASA) has identified a critical need for both equipment and training protocols that act as robust countermeasures to microgravity-induced de-conditioning while meeting critical parameters related to vehicle space constraints and crew time. NASA has stated that “… it is clear that severe limitations of stowage weight and volume during these missions will only allow for one or perhaps two small (~20 lb) exercise devices, and that TVIS (Treadmill Vibration Isolation System), CEVIS (Crew Exercise Vibration Isolation System) and RED (Resistive Exercise Device) as currently flown on ISS will not be available” (JSC Small Assessment Team Report 12/2006). The report notes that no single gravity independent device, that meets anticipated size requirements, has been developed and tested as a countermeasure to both cardiovascular and muscular de-conditioning, and to loss of muscle mass that results from unmitigated exposure to microgravity. Given this background, we explored the use of a modified YoYo device recently described by Tesch et al. (23). The unique aspects of this Multi-Mode Exercise Device (M-MED) are that it: i) can be used to impose high loading conditions (concentric and eccentric) on skeletal muscle thereby potentially mitigating the losses of muscle mass and function that occur in microgravity; ii) can be quickly configured to provide a spectrum of both resistance and aerobic training effects; iii) is gravity-independent; and iv) has a low volume requirement.
The current report summarizes the findings of a proof-of-principle study that had two primary objectives. The first was to test the hypothesis that a concurrent resistance (RE) and aerobic (AE) exercise training program using the M-MED would enhance muscle strength and endurance performance. The second objective was to examine underlying cellular and molecular mechanisms known to be associated with key functional measures made in this study (e.g., muscle strength and aerobic performance).
MATERIALS & METHODS
Subjects
Seventeen subjects (eight men, nine women) with a mean (±s.e.m.) age of 22.0±0.4 years completed this study. Mean body mass of the subjects was 68±3kg (men 75±4kg, women 61±3kg) with a mean body mass index of 23.9±0.8 (men 24.2±1.5, women 23.6±0.8). Subjects had not participated in any athletic or other programmed physical activities for at least six months. This study protocol was approved in advance by the Institutional Review Board at the University of California, Irvine. Subjects were recruited via posters and written informed consent was obtained.
Equipment
All training was conducted using a single Multi-Mode Exercise Device (YoYo™ Technology AB, Stockholm, Sweden), a modified version of the RAD device described by Tesch et al. (23). This device was configured for either low-repetition/high-resistance (squatting; RE) or high-repetition/low-resistance (rowing; AE) exercise. The acronym M-MED is employed in this manuscript only to indicate the multimodality of this device, is not a brand name, and is not commercially advertised as such.
Procedure
The study design consisted of three phases. Phase I consisted of a familiarization session and pre-training (PRE) testing, during which time subjects were familiarized with all required tasks, muscle biopsies were collected and performance measurements obtained. Phase II consisted of five weeks of training, during which time subjects trained for five days a week, alternating RE and AE workouts. The third phase involved post-training (POST) testing, during which time all subjects underwent muscle biopsies and performance measurements in the same sequence as during PRE testing.
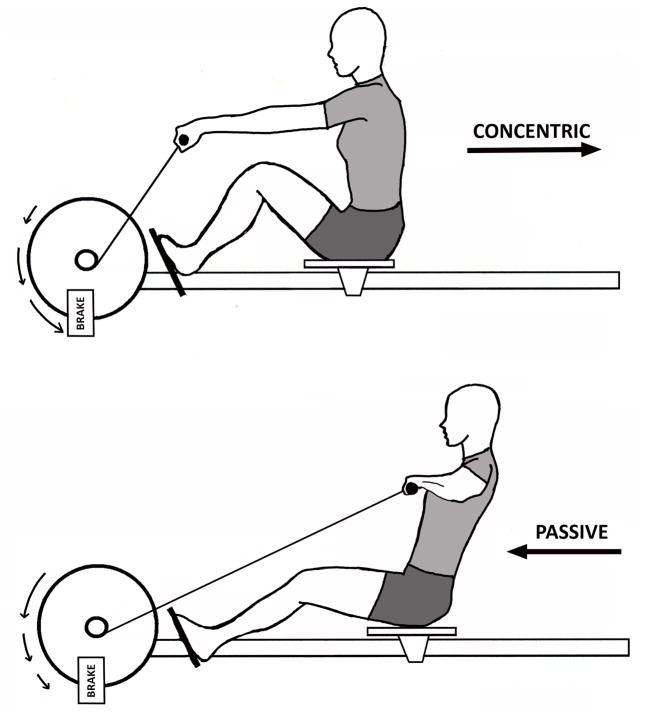
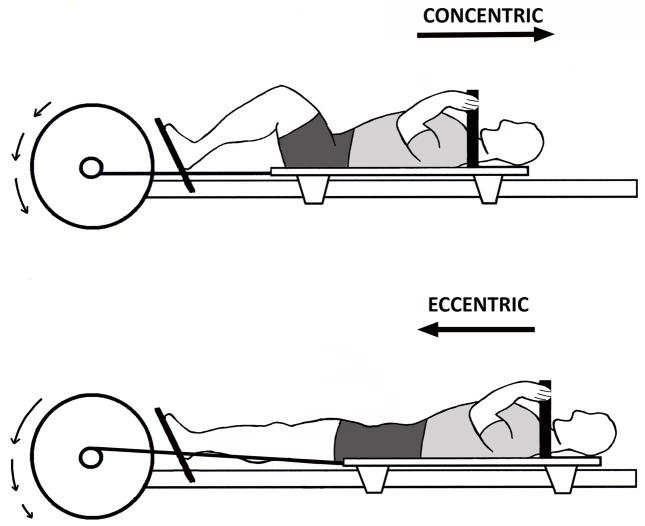
Aerobic training occurred three days a week with the M-MED configured for rowing (Fig. 1A). Resistance training occurred twice a week with the M-MED configured for a modified supine squat (Fig. 1B). RE and AE workouts occurred on alternating days. The last (15th) AE workout was completed four days and the last (11th) RE workout was three days before the POST biopsy.
Figure 1.
M-MED can operate in both aerobic (AE) and resistance (RE) modes. (a) AE mode training approximates rowing exercise. (b) RE mode training (as shown) approximates supine squats. In AE mode, the flywheel is accelerated by concentric contraction of knee extensors (KE), and decelerated by a magnetic brake (23). In RE mode, the flywheel is accelerated by concentric KE contraction and decelerated by eccentric KE contraction. In this study, efficacy of the M-MED was tested strictly on KE, but the device can be reconfigured to target other muscle groups. Straight arrows show linear translation of the subject with respect to the flywheel. Arced arrows show acceleration (arrows getting longer) and deceleration (arrows getting shorter) of the flywheel.
Each AE-mode session consisted of four 4min intervals of high-intensity rowing. Exercise intensity during each interval targeted the subject’s specific heart rate equivalent of ≥ 90% aerobic capacity (VO2max), as determined in PRE maximal exercise tests. Real-time feedback from a chest-strapped Polar™ heart rate monitor RS800 (Polar Electro Oy, Kempele, Finland) was provided to the subject to allow the modulation of the workout intensity. Between-interval exercise was maintained at a heart rate equivalent to ~50% VO2max. Warm-up and cool-down consisted of 5min rowing or cycling, respectively, at heart rate equivalent to ~50% VO2max.
Each RE-mode session consisted of four sets of a squat-type exercise, with each set involving seven integrated shortening (CON) and lengthening (ECC) repetitions. Subjects cycled lightly on a stationary bicycle ergometer for 5min before and between sets. Custom-built LabVIEW instrumentation (National Instruments, Austin, Texas) provided real-time visual feedback on power output to subjects on a large computer screen. This included a target indicator showing previous session performance to provide progressive increments in performance.
The various tests/measurements made on each subject included: i) magnetic resonance imaging (MRI) of the thigh for determination of muscle cross-sectional area; ii) measurements of muscle strength and fatigability; iii) muscle biopsies; and iv) measurements of maximal oxygen uptake.
All MRI scans were performed in early morning (0700-1000) to minimize gravity-driven fluid shifts, which occur during upright posture. Subjects were expressly instructed to minimize time standing and not exercise prior to the scans. On arrival at the MR facility, each subject was positioned in supine recumbency on a gurney for 30 minutes. The subject was then transferred (while recumbent) onto the sliding table of the MR imager and remained in this position for the duration of the scan. Subject’s feet were placed in adjustable stirrups suspended toes-up in a custom-built foot restraint. The foot restraint was adjustable and indexed to ensure fidelity of subject alignment at the pre- and post-treatment imaging sessions.
Images were obtained using a 3Tesla magnetic resonance imager (Philips), with 300mm (anteroposterior) × 250mm (superoinferior) × 529mm (right-left) field of view, and linear resolution of 1.38mm/pixel. Each scan acquired 50 images of the thighs in the transverse plane, with slice thickness of 5mm. Scans were centered at mid-thigh, so as to image the bulk of knee extensor musculature, and proceeded in the distal-to-proximal direction. The total scan time was 13min (2min survey, 11min scan).
Alignment of PRE and POST scans was achieved using the PRE biopsy incision site as reference. The PRE mid-thigh position had been indelibly marked prior to imaging (Day 0) and its distance to the PRE biopsy site (Day 2) recorded. The POST scan could thus be centered using the clearly visible PRE biopsy skin scar as reference.
MR images were acquired in PAR format, converted to TIFF in MIPAV 5.0 software, and analyzed with ImageJ 1.36b software (rsb.info.nih.gov/ij). To ensure correspondence between the PRE and POST scans, additional verification of image registration included identification of anatomical features (e.g., shape of femoral cross-sections, blood vessel topography). Five successive slices at mid-thigh level were selected for analysis. Using computerized planimetry (Intuos2 Graphic Tablet, Wacom Technology, Vancouver, WA, USA), the perimeter of the quadriceps complex muscles was manually circumscribed in each image, and the cross-sectional area (CSA) calculated. All subject data were coded and operators blinded to treatment.
Leg press three-repetition maximum (3RM) strength was determined using a plate-loaded leg press machine (Hoist Fitness, San Diego, CA). Testing sessions consisted of a 5min warm-up on a cycle ergometer and a low-weight leg press warm up on the testing machine. Subjects completed repetitions over a fixed range of motion using successive 9.09 kg increments of weight, starting with category 6 on Borg’s CR10 scale of perceived exertion as determined from previous familiarization sessions for each subject. The 3RM weight was recorded as the greatest weight the subjects could move through the full range of motion three times. Testing was conducted on three (PRE) or two (POST) separate days to ensure that a true 3RM had been recorded.
Muscle strength was also assessed by making measurements of torque-velocity relationships for the right knee extensors using a Biodex™ System 3 dynamometer (Biodex Medical Systems, Shirley, New York) (6). Subjects were familiarized twice with the Biodex system approximately one week before PRE testing. Subjects were seated on a chair with a slightly reclined backrest with the right thigh resting on and securely strapped to the seat. Subjects were encouraged to make maximal efforts throughout the range of motion (−100° to 0°; where 0° = horizontal plane). For CON actions, subjects performed ve trials at each test speed (30°, 60°, 90°, 120°, 180°, 240°, 300° s−1) with 1min rest between efforts. For ECC contractions, two speeds (30°, 120° s−1) were used, with 1min rest between efforts. For isometric contractions, subjects completed three 5s trials, with 5s rest between efforts. The highest torque at each speed was used for data analysis. Calibration of the dynamometer was checked before each test session, including a correction for the gravitational moment of the limb-lever system.
In order to examine fatigue characteristics of the knee extensors, subjects were required to complete three sets of 30 repetitions of integrated maximal knee extension (180° s−1) and knee flexion (120° s−1) movements (total of 90), with 1min rest between sets. During each movement subjects were encouraged to make maximal efforts throughout the range of motion while visual feedback was provided via the Biodex™ computer screen. Data were averaged in five-contraction bins.
Three biopsy samples were taken from the vastus lateralis muscle (VL) using the percutaneous technique with a modified Bergström needle attached to a vacuum pump, as reported previously (4). One sample was mounted on cork and quickly frozen in isopentane pre-cooled with liquid nitrogen. The other two samples were immediately frozen in liquid nitrogen, stored at −80°C, and later used for analyses of cellular and molecular biomarkers.
The first sample was serially sectioned in a −20°C cryostat at 10μm thickness. Tissue sections were placed on glass slides, allowed to air-dry overnight and subsequently stained using the haematoxylin/eosin protocol. Sections were examined under a Leica DMLS microscope, and images digitally captured at 10× magnification for analyses of muscle cross-sectional area. For each subject, five fields were randomly selected and 10 muscle fibers measured in each field (i.e., n=50 bers per muscle sample) using ImageJ software.
Muscle total DNA concentration was based on total DNA concentration in the total homogenate, as determined by a fluorometric assay with calf thymus DNA standard (19). Muscle total protein concentration was based on protein concentration of the whole-muscle homogenate, as determined by the Bio-Rad Protein Assay with bovine γ-globulin as a standard. The whole homogenate was diluted to a final protein concentration of 1mg/ml in a storage buffer containing 50% glycerol, 50mM Na4P2O7, 2.5mM EDTA, and 1mM 2-mercaptoethanol (pH=8.8) and stored at −20°C until subsequent analyses for myosin heavy chain (MHC) isoform and actin protein content.
MHC and actin protein contents were determined by densitometry and image analysis of stained protein bands following MHC and actin protein separation, using the SDS-PAGE technique described in detail previously (14). MHC protein isoform distribution was determined using SDS-PAGE on 2.5μg of the stored total protein (22). Only three adult isoforms (types I, IIa, IIx) were identified, whereby type I is the fastest-migrating and the IIx is the slowest-migrating band. Type IIb or developmental isoforms were not detected. The relative proportions of MHC isoforms were determined by densitometry of the digital image using ImageQuant software (GE Healthcare).
A pre-weighed portion of muscle tissue was homogenized in 30 volumes of ice-cold PBS. An aliquot of the total homogenate was stored at −80°C for subsequent use to determine citrate synthase (CS) activity. Whole-muscle homogenates were frozen and thawed three times to disrupt the mitochondria. CS activity was estimated as the reduction of 5,5 dithiobis-(2-nitrobenzoic) acid (DTNB) at 412nm over 4min using a spectrophotometer.
RNA Extraction occurred from pre-weighed frozen muscle samples using the Tri-Reagent (Molecular Research Center, Cincinnati, Ohio). The extracted RNA pellet was suspended in nuclease-free water (1μl/mg tissue). RNA concentration was determined by OD260 in a Nanodrop spectrophotometer (conversion factor 40μg/ml per unit OD260). RNA samples were stored frozen at −80°C for subsequent analyses for specific gene expression using an endpoint RT-PCR approach. Prior to cDNA synthesis, RNA integrity was checked by electrophoresis of 400ng total RNA on 1% agarose gel stained with Gelgreen stain (Biotium). Reverse transcription of total RNA (1μg) into cDNA used the SuperScript II RT (Invitrogen, Grand Island, NY) in a 20μl total reaction volume following manufacturer’s guidelines.
Specific PCR primers were designed using PrimerSelect software (Lasergene, DNAStar) and the reference mRNA sequence from NCBI GenBank. Forward and reverse primers (Operon Biotechnologies, Huntsville, Alabama) were designed on different exons separated by large introns, so that genomic DNA product would separate from the cDNA PCR product. For each primer set, PCR conditions (cDNA dilution and PCR cycle number) were set to optimal conditions, so that the target mRNA product yields were in the linear range of the semilog plot when the yield was expressed as a function of the number of PCR cycles. Target mRNA PCR yields were tightly correlated to input cDNA (5). At the end of amplifications, PCR products were separated on a 2.5% agarose gel by electrophoresis and stained with SYBR Green. The UV-induced fluorescence of stained DNA bands was captured by a digital camera. Band intensities were quantified by densitometry with ImageQuant (6). Each specific mRNA signal was expressed in arbitrary units (AU) per ng of total RNA. Each PCR signal representing specific mRNA expression was normalized to tissue mass using total RNA concentration. Reporting RNA expression per unit muscle mass is preferred, since total RNA concentration may vary in muscle tissue in response to training or other activity paradigms, whereas traditional internal controls (GAPDH, large ribosomal proteins) often vary in muscle subjected to different activity paradigms (18).
MHC mRNA isoform distribution was determined by competitive PCR on cDNA using a synthetic DNA fragment as control, a common forward primer for all the MHC mRNAs, and an isoform-specific reverse primer. Synthetic DNA fragment was built using the same base DNA backbone as previously (3, 8). An overlapping PCR approach was used to extend the DNA backbone with specific primers for human MHC mRNA. The general PCR method was performed as described previously for determining rodent MHC mRNA composition (3, 8). In this method, MHC mRNA expression is reported as relative percent of the total MHC isoform expression (I+IIa+IIx).
Three candidate mRNA biomarkers were assessed:
Insulin-like growth factor-I (IGF-I) is a highly anabolic peptide expressed by skeletal muscle in response to changes in loading state. Primers were designed from common sequences for both IGF-I and MGF. IGF-I is the major isoform expressed in skeletal muscle. The amplification product for MGF is 301bp. Under our PCR conditions, only IGF-I could be amplified; MGF was not detected in any of the samples.
Insulin-like growth factor-binding protein 4 (IGFBP4) is a member of the insulin-like growth factor binding protein (IGFBP) family and encodes a protein that binds both IGF-I and -II, and circulates in plasma. The binding protein prolongs the half-life of IGFs and alters their interaction with cell surface receptors.
Collagen, type III, alpha 1 (Col3A1) gene encodes the pro-alpha1 chains of type III collagen, a fibrillar collagen that is found in extensible connective tissues. Collagen III is a major component of tendon and extracellular matrix of skeletal muscle. Collagens are important for muscle function, especially in relation to force transmission. Col3A1 mRNA expression is very responsive to increased loading state of the muscle (17).
Aerobic capacity was assessed by measuring maximal oxygen uptake (VO2max) during a standard incremental exercise protocol designed to last 8–12 minutes. Using an electronically-braked bicycle ergometer, work rate was incrementally increased by 25W (men) or 15W (women) every minute. Subjects were asked to pedal at 60–80 rpm. Gas exchange was measured breath-by-breath with an Encore VMax metabolic cart (SensorMedics, Yorba Linda, California). A four-lead electrocardiogram and heart rate were obtained throughout exercise. Ratings of perceived exertion (Borg CR 10 scale) were assessed every three minutes. The value used for VO2max was the highest O2 consumption measured at the point when the respiratory quotient was ≥ 1.1.
Statistical Analysis
Subject information was coded and data analysis was conducted by researchers blinded to treatment. We tested the effects of training and gender, and their interaction, using a repeated-measures analysis of variance (RM-ANOVA), with significance determined when p ≤ 0.05.
RESULTS
One of the objectives of this study was to contrast the response of male and female subjects to the overall training paradigm. As noted in Tables I–IV, there were no gender specific differences with respect to the training response.
Table I.
Anthropometric Characteristics
| Variable | MEN | WOMEN | Training effect | Gender effect | Interaction effect | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| PRE | POST | PRE | POST | ||||
| Body mass (kg) | 75.3±4.3 | 74.8±4.5 | 61.3±3.1 | 61.0±3.1 | F1,15=4.4562 NS |
F1,15=7.8315 p=0.0135 |
F1,15=0.4745 NS |
| BMI (kg.m−2) | 24.3±1.5 | 24.1±1.5 | 23.6±0.9 | 23.5±0.9 | F1,15=4.4511 NS |
F1,15=0.1466 NS |
F1,15=0.2721 NS |
| DXA lean body mass (g) | 57157±2518 | 57686±2583 | 39471±1569 | 40167±1609 | F1,15=7.8493 p=0.0134 |
F1,15=41.247 p<0.0001 |
F1,15=0.1475 NS |
| DXA lean arm mass (g) | 6920±392 | 6981±420 | 3654±161 | 3713±148 | F1,15=2.5049 NS |
F1,15=71.323 p<0.0001 |
F1,15=0.0008 NS |
| DXA lean leg mass (g) | 19111±845 | 19372±843 | 12907±571 | 13223±535 | F1,15=10.577 p=0.0054 |
F1,15=44.895 p<0.0001 |
F1,15=0.0976 NS |
Data are mean ± standard error. Statistical significance was determined by RM-ANOVA, and is reported for p < 0.05. NS, not significant.
Table IV.
Molecular Biomarkers
| Variable | MEN | WOMEN | Training effect | Gender effect | Interaction effect | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| PRE | POST | PRE | POST | ||||
| IGF-I mRNA (AU.mg−1) | 20.6±1.9 | 34.1±2.6 | 18.8±2.6 | 35.5±6.3 | F1,15=29.325 p<0.0001 |
F1,15=0.0024 NS |
F1,15=0.3226 NS |
| IGFBP4 mRNA (AU.mg−1) | 16.5±1.6 | 26.5±2.0 | 11.2±1.6 | 25.6±3.4 | F1,15=26.310 p=0.0001 |
F1,15=2.3621 NS |
F1,15=0.8706 NS |
| Collagen α1 mRNA (AU.mg−1) | 15.4±2.5 | 46.1±8.9 | 13.0±2.0 | 44.0±11.8 | F1,15=22.820 p=0.0002 |
F1,15=0.0817 NS |
F1,15=0.0005 NS |
| Citrate synthase mRNA (AU.mg−1) | 14.4±1.9 | 17.7±2.1 | 12.7±1.1 | 15.8±1.5 | F1,14=9.5233 p=0.0081 |
F1,14=0.9178 NS |
F1,14=0.0035 NS |
Data are mean ± standard error. Statistical significance was determined by RM-ANOVA, and is reported for p < 0.05. NS, not significant.
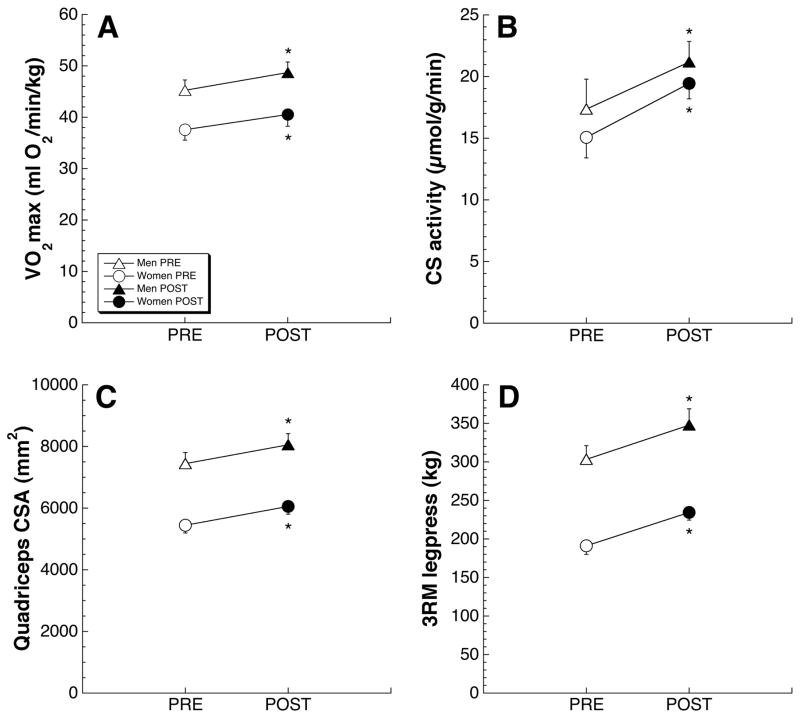
Significant increases (~7–8%) in aerobic capacity (measured as maximal rate of oxygen consumption, mlO2/kg/min) were seen (Fig. 2A) and these corresponded to a 26% increase in the mean activity of CS (Fig. 2B) in the VL.
Figure 2.
Effects of five weeks of concurrent AE and RE mode training.
In both men and women subjects, M-MED-based training increased VO2max (a), citrate synthase activity (b), quadriceps CSA (c), and muscle strength (d). PRE (open symbols) and POST (filled symbols) data points show mean±s.e.m. All differences (POST vs. PRE) significant at p<0.05 by RM-ANOVA.
As a result of the 5-week training protocol, the mean cross-sectional area of the quadriceps femoris muscle group increased by 10% (Fig. 2C). There was also a commensurate increase (+13%) in mean cross-sectional area of VL myofibers (Table II).
Table II.
Indicators of Hypertrophy
| Variable | MEN | WOMEN | Training effect | Gender effect | Interaction effect | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| PRE | POST | PRE | POST | ||||
| Mixed myofibre CSA (μm2) | 6362 ±290 | 7234 ±300 | 4708 ±277 | 5245 ±427 | F1,14=12.008 p=0.0038 |
F1,14=21.664 p=0.0004 |
F1,14=0.6783 NS |
| Total protein (μm.mg−1) | 231 ±12 | 229 ±13 | 234 ±13 | 232 ±10 | F1,15=0.0663 NS |
F1,15=0.0547 NS |
F1,15=0.0025 NS |
| MHC protein (AU.mg−1 protein) | 3.13 ±0.14 | 3.06 ±0.17 | 3.29 ±0.20 | 3.43 ±0.14 | F1,15=0.1201 NS |
F1,15=2.0253 NS |
F1,15=0.8638 NS |
| Actin protein (AU.mg−1 protein) | 1.18 ±0.08 | 1.21 ±0.12 | 1.16 ±0.11 | 1.18 ±0.10 | F1,15=0.1746 NS |
F1,15=0.0300 NS |
F1,15=0.0342 NS |
| RNA (mg.g−1 tissue) | 0.480 ±0.015 | 0.583 ±0.030 | 0.441 ±0.021 | 0.557 ±0.017 | F1,15=41.468 p<0.0001 |
F1,15=2.0404 NS |
F1,15=0.1390 NS |
| DNA (mg.g−1 tissue) | 0.842 ±0.022 | 0.867 ±0.030 | 0.881 ±0.026 | 1.004 ±0.036 | F1,15=12.851 p=0.0027 |
F1,15=6.9585 p=0.0186 |
F1,15=5.4989 p=0.0332 |
| RNA/DNA ratio | 0.57 ±0.02 | 0.67 ±0.03 | 0.50 ±0.02 | 0.56 ±0.02 | F1,15=13.674 p=0.0021 |
F1,15=18.223 p=0.0007 |
F1,15=1.0164 NS |
| DNA/total protein ratio (x10−3) | 3.73 ±0.27 | 3.88 ±0.30 | 3.85 ±0.26 | 4.43 ±0.36 | F1,15=3.5996 NS |
F1,15=0.9078 NS |
F1,15=1.2898 NS |
| DNA/MHC protein ratio | 0.27 ±0.01 | 0.29 ±0.02 | 0.27 ±0.01 | 0.30 ±0.02 | F1,15=3.1392 NS |
F1,15=0.1049 NS |
F1,15=0.0951 NS |
Data are mean ± standard error. Statistical significance was determined by RM-ANOVA, and is reported for p < 0.05. NS, not significant.
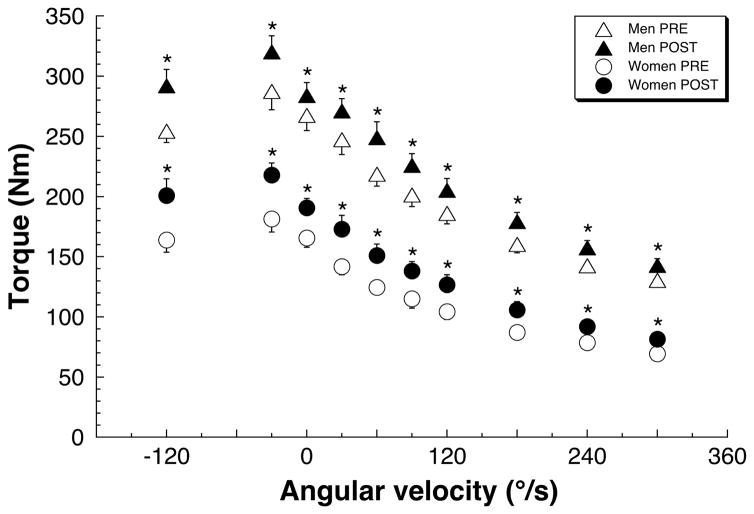
Subjects experienced 18% (44kg) increase in leg press performance as measured by 3RM testing (Fig. 2D). Improvements in knee extensor strength were significant at all isokinetic speeds (Fig. 3).
Figure 3.
Speed-dependent muscle strength increased in men and women subjects under eccentric, isometric and concentric conditions following the five-week training on the M-MED. PRE (open symbols) and POST (filled symbols) data points show mean±s.e.m. All differences (POST vs. PRE) significant at p<0.05 by RM-ANOVA.
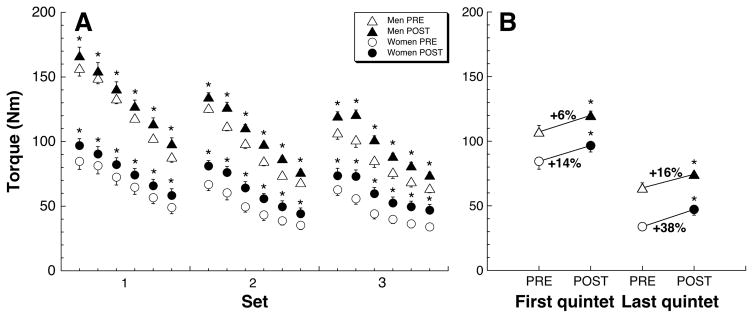
Resistance to knee extensor fatigue was significantly enhanced by training (Fig. 4A). More importantly, the difference in force generation widened with successive repetitive actions. Thus, force generation improved by 9% for the first quintet of actions, and by 24% for the last quintet (Fig. 4B).
Figure 4.
M-MED training improved knee extensor endurance.
Subjects completed three sets of 30 maximum knee extension actions, at 180°/s, separated by 1min rest. (a) While fatigue ensued with duration of exercise, improvements in muscle strength and fatigue resistance of both men and women subjects are evident in POST measurements. Each datum depicts a quintet of maximum contraction repetitions. (b) Relative improvement in muscle strength and fatigue resistance due to M-MED training was greater in women than men, and more pronounced with duration of exercise (last vs. first quintet). PRE (open symbols) and POST (filled symbols) data points show mean±s.e.m. All differences (POST vs. PRE) significant at p<0.05 by RM-ANOVA.
Concentration of total protein, MHC protein, and actin protein in the VL biopsy samples did not change significantly (Table II). Concentrations of total RNA and DNA increased significantly (+24% and +9%, respectively) after training, as did the ratio of RNA:DNA (+15%). There was a trend (p=0.0772) of increased DNA:protein content. The training program produced a downregulation of the Type IIx MHC protein isoform (−23%) and a concomitant increase (+8%) in the Type IIa MHC protein isoform. No significant changes in Type I MHC protein were observed (Table III). Alterations in the amount of mRNA for the MHC isoforms tended to parallel changes in protein expression. Abundance of the mRNA for IGF-I, IGFBP4 and collagenα1 in the VL increased 77%, 90%, 318%, respectively, as a result of the training protocol (Table IV).
Table III.
Indicators of Muscle Phenotype
| Variable | MEN | WOMEN | Training effect | Gender effect | Interaction effect | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| PRE | POST | PRE | POST | ||||
| Type I | 30.7±4.0 | 30.7±3.6 | 33.9±2.9 | 33.9±3.4 | F1,15=0.0001 NS |
F1,15=0.6191 NS |
F1,15=0.0003 NS |
| Type IIa | 53.2±2.8 | 55.7±3.6 | 48.6±2.2 | 53.7±2.4 | F1,15=4.8433 p=0.0438 |
F1,15=0.9971 NS |
F1,15=0.5628 NS |
| Type IIx | 16.2±3.2 | 13.6±3.6 | 17.5±3.3 | 12.4±2.2 | F1,15=5.0362 p=0.0403 |
F1,15=0.0001 NS |
F1,15=0.5444 NS |
| Type I mRNA | 31.2±3.3 | 30.6±3.1 | 32.2±2.3 | 33.9±2.8 | F1,15=0.1100 NS |
F1,15=0.3871 NS |
F1,15=0.5623 NS |
| Type IIa mRNA | 53.0±3.2 | 60.5±2.7 | 50.3±2.8 | 58.4±2.7 | F1,15=19.1588 p=0.0005 |
F1,15=0.5357 NS |
F1,15=0.0250 NS |
| Type IIx mRNA | 15.7±4.0 | 8.9±3.5 | 17.5±4.4 | 7.7±2.2 | F1,15=10.1120 p=0.0062 |
F1,15=0.0071 NS |
F1,15=0.3403 NS |
Data are mean ± standard error. Statistical significance was determined by RM-ANOVA, and is reported for p < 0.05. NS, not significant.
DISCUSSION
The challenges of transitioning the human space program from low earth orbit to planetary exploration (e.g., Mars) will be considerable, from both the technical and physiologic perspective. The fundamental challenge is ensuring crew health and safety within the constraints imposed by costs, mission objectives, transit time, vehicle design, upload mass, and volume. Within this context, it is widely recognized that such missions need to incorporate appropriate countermeasures to the well-known deconditioning that occurs across a broad spectrum of physiological systems in microgravity. In particular, countermeasure systems that are capable of impacting multiple physiological systems will be essential given the practical limitations noted above. Herein lies an important advantage of the M-MED system, which potentially can favorably impact the cardiovascular system, muscle, and bone. Additionally, the M-MED system can be used to impose a broad array of resistance, aerobic, and anaerobic training paradigms.
As noted in the INTRODUCTION, this study was designed as a proof-of-principle and focused on determining whether the M-MED device could effectively produce concomitant gains in both muscle and cardiovascular performance in both male and female subjects. Three important findings emerged from this study. First, we observed that the combined training program was effective in producing beneficial effects in muscle mass, muscle function, and cellular/molecular adaptations. Second, the combined training program was effective in producing increases in maximal oxygen uptake and markers of cellular respiration. Finally, we observed that both male and female subjects responded similarly to the combined training program. The following discussion addresses these three observations in more detail.
It has been well established that selective atrophy of specific skeletal muscles occurs during prolonged space flight (2, 11). It is important to emphasize that the observed muscle atrophy appears to be confined primarily to weight-bearing extensor muscles, which are unloaded in microgravity conditions, as opposed to a more generalized muscle loss from illness-induced cachexia or nutritional restriction. The selective nature of this adaptation also demonstrates that unloading-induced atrophy is not a result of changes in circulating factors, such as hormones, and it will most likely not be amenable to attenuation solely via some non-specific (e.g., systemic) treatment.
In a previous review we described in detail the loss of strength and muscle size that has been reported to occur with space flight or ground-based analogues thereof (2). It is important to note that the decrements in muscle size and strength which have been reported following space flight have occurred despite the invariable inclusion of some level of countermeasure exercise activity (2). This indicates that, in a number of space flight missions, the previous countermeasures employed were not completely effective. More recently, the provision of improved exercise equipment and nutritional support has yielded results that suggest that lean body mass can be protected during extended space flight (21). However, the current generation of countermeasure hardware is not expected to meet the engineering requirements for the next generation of spacecraft.
In the current study, muscle function was increased in ambulatory subjects using the M-MED exercise device (Fig. 2–4). This provides a compelling justification for further evaluation of the M-MED in a space-flight analogue setting. In addition to strength improvement under 3RM testing, results of isokinetic dynamometry indicate that the strength gains occurred over a wide range of speeds (−120° to 300°/s). This is particularly important, as it suggests that strength gains seen with M-MED-based training can occur under diverse functional settings.
In parallel to increases in strength, endurance of knee extensors was significantly improved by M-MED training. Post-training, subjects were capable of maintaining a relatively greater torque generation over repetitions (Fig. 4). Whereas training improved strength by 9% for the first quintet of the endurance test, it resulted in a 24% torque increase for the last quintet, suggesting a much improved fatigue resistance component (Fig. 4B).
In addition to changes in function, the de-conditioning of skeletal muscle during space flight can be expected to manifest as a loss in cross-sectional area of whole muscles and of the constituent myofibers. In the current study, the M-MED-based training resulted in increases in muscle size of the quadriceps femoris complex (Fig. 2C). At the cellular level, there was an increase in the mean mixed myofiber cross-sectional area of the VL (Table II). An increase in total muscle and myofiber CSA in the presence of unchanged concentrations of total, MHC and actin proteins (Table II) indicates that the hypertrophic changes seen with training were functionally relevant in that the amount of muscle protein in general, and of motor proteins specifically, increased in parallel with the changes in muscle and myofiber size.
It is well established that unloading results in a relative increase in the proportional area of myofibers expressing “fast” (e.g. Type IIx/b) myosin heavy chain (MHC) isoforms (10, 24). Such adaptations can result in a decrease in overall muscle strength and diminished muscular endurance. In the current study, the training protocols produced a reverse shift. In this study, the POST muscle phenotype shifted towards greater expression of the “slower” MHC isoform Type IIa and away from the “faster” isoform Type IIx (Table III).
A shift from slow to fast myofiber phenotype can impact work capacity in a number of ways. First, Type IIx expressing myofibers have a lower energetic economy with regard to cross bridge turnover (15). Furthermore, the metabolic phenotype of fast myofibers generally involves an intrinsically lower level of expression in the metabolic pathways that support oxidative phosphorylation. As a result, the slow-to-fast shift in phenotype can contribute significantly to a decline in the oxidative capacity of skeletal muscle, which, in turn, is a major determinant of the reduced demand placed on the cardiovascular system during work. In the current study, M-MED-based training resulted in concurrent shifts towards the Type IIa phenotype (noted above), and a 26% increase in citrate synthase activity.
Significant changes in the loading state of skeletal muscles results in adaptations that are regulated by pre-translational, translational and post-translational mechanisms. In particular, alterations in the balance between protein synthesis and degradation will ultimately determine how muscle structure and function are affected by altered loading. One of the more sensitive steps in the adaptive process is the regulation of translational capacity, i.e., the amount of ribosomal RNA (rRNA) present in cells. For example, we have previously reported that muscle total RNA decreased by 13% as a result of five weeks of single limb muscle unloading (13). In skeletal muscle the majority of RNA present is rRNA, thus shifts in total RNA primarily represent changes in translational capacity. Conversely, we have shown that as few as two bouts of resistance exercise can increase total RNA levels in ambulatory individuals (4). In the current study, the training protocol significantly increased the concentration of RNA in VL muscle samples demonstrating the ongoing enhancement of anabolic potential in these muscles (Table II). Skeletal muscle hypertrophy is also thought to include a component involving cell proliferation, most likely amongst satellite cells (1). In the current study, an increase in DNA suggests that this process was stimulated by the training program (Table II).
Several key loading-sensitive regulatory pathways have been characterized by our group, as well as other investigators. For example, a number of components of the insulin-like growth factor system/pathway respond to increases in loading and appear to be required for a hypertrophy response (5, 12). In the current study, M-MED-based training resulted in up-regulation of the IGF-I system (Table IV).
Although the primary focus on this study was on skeletal muscle adaptations, it was also essential to establish that the concurrent training program was effective in producing beneficial aerobic effects as measured by whole-body VO2max. Via rowing exercise, the M-MED offers the unique benefit of recruiting, and conditioning, a large number of muscle groups (25). In the current study, rowing exercise, conducted three days per week, increased the aerobic capacity of subjects by 8% (Fig. 2A). Consistent with these observations, we also found that this training program produced an increase of ~20–25% in the activity of citrate synthase (Fig. 2B), which is thought to be the rate limiting enzyme of the Krebs cycle and reflective of mitochondrial content. Both of these adaptations are very encouraging given the relative brevity of the actual aerobic portion of the total training paradigm. Over the entire 35 days of training only four hours were spent in the target heart rate range (~90% VO2max). This exercise protocol potentially represents significant economies with regard to crew time.
In the current study, we observed that the same M-MED training program produced similar outcomes in both male and female subjects. This was true for both functional and cellular/molecular adaptations. Importantly, however, these observations should be interpreted within the constraints of this study. The M-MED produced significant improvements in muscle strength and endurance in relatively young and sedentary subjects; whether the M-MED can induce similar improvements in older and rigorously-trained astronauts, or can only prevent muscle atrophy and aerobic deconditioning, remains to be tested. Further, these findings should not be extrapolated to provide definitive insight regarding the responses that might be seen under other environmental conditions, e.g., altered gravitational field. Rather, these findings provide a basis for hypothesizing that similar protocols would produce similar effects in both men and women exposed to microgravity. Harm et al. (16) concluded that in most instances there are no significant gender-specific responses to microgravity, except in relation to orthostatic tolerance. Others, such as Lemoine et al. (20), have proposed that there exists a gender-specific response to microgravity with respect to the loss of key sarcomeric proteins in specific muscle groups. Unfortunately, few studies have directly compared muscle responses of both genders to loading under simulated microgravity. In light of our results on ambulatory subjects, the M-MED offers unique opportunity to rigorously test the hypothesis of the gender versus individual variability response to countermeasures from both RE and AE training perspective (16).
The initial evaluation of a potential countermeasure exercise is most often conducted in ambulatory subjects. In this context, an increase in performance is generally taken to suggest that a given countermeasure is appropriate for further testing via ground-based analogues of space flight. The results reported herein clearly indicate that the M-MED modality is effective in increasing cardiovascular and musculoskeletal fitness, and inducing positive adaptations at the cellular and molecular level in ambulatory subjects. This, in turn, suggests this modality should be considered for further investigation as a potential platform for countermeasure exercise during long-term space flight.
Acknowledgments
The authors wish to thank the subjects who enthusiastically participated in the project. Erick Maravilla designed and implemented the real-time power output module for the M-MED. Rudy Limburg (RIP), machinist extraordinaire, was indispensable in customizing mechanical modifications to the M-MED. Dr. Stuart S. Sumida illustrated the operating principle of M-MED in Fig.1. Support of the UC Irvine’s Institute for Clinical and Translational Research (ICTS), and especially of Barb Bodenhoffer, RN, was critical to the successful completion of this work. Dr. Clay Pandorf and Mike Baker assisted with muscle biopsies. Funding was provided by the National Space Biology Research Institute (NSBRI, NCC 9-58-70, MA01601) to GRA, and the National Institutes of Health (NIH, UCI CTSA UL1 TR000153) to ICTS. TO and JAC were supported by the NIH training grant 2T32AR047752 to VJC and the Multidisciplinary Exercise Science Program at UC Irvine. PAT received support from the Swedish National Space Board. Author Per A. Tesch (PAT) disagrees over use of the acronym M-MED to portray the device employed in this study. Instead, PAT prefers the acronym “RAD (YoYo Multigym)”, as described previously (see reference 23). PAT is part owner of YoYo Technology AB, which controls the material rights for the patented (U.S. Patent No. US8,162,802 B2) flywheel technology. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Contributor Information
Tomasz Owerkowicz, Email: towerkow@csusb.edu.
Joshua A. Cotter, Email: joshua.cotter@csulb.edu.
Fadia Haddad, Email: fhaddad@uci.edu.
Alvin M. Yu, Email: yuam@uci.edu.
Marinelle L. Camilon, Email: camilonm@gmail.com.
Theresa Hoang, Email: theresa.hoang7@gmail.com.
Daniel Jimenez, Email: danjimenez@mednet.ucla.edu.
Arthur Kreitenberg, Email: akreit@msn.com.
Per A. Tesch, Email: Per.Tesch@ki.se.
Vincent J. Caiozzo, Email: vjcaiozz@uci.edu.
Gregory R. Adams, Email: gradams@uci.edu.
REFERENCES CITED
- 1.Adams GR, Bamman MM. Characterization and regulation of mechanical loading-induced compensatory muscle hypertrophy. Comprehensive Physiol. 2012:2829–2870. doi: 10.1002/cphy.c110066. [DOI] [PubMed] [Google Scholar]
- 2.Adams GR, Caiozzo VJ, Baldwin KM. Invited review: Skeletal muscle unweighting: Space flight and ground based models. J Appl Physiol. 2003;95:2185–2201. doi: 10.1152/japplphysiol.00346.2003. [DOI] [PubMed] [Google Scholar]
- 3.Adams GR, Haddad F, McCue SA, Bodell PW, Zeng M, Qin A, Qin X, Baldwin KM. Effects of spaceflight and thyroid deficiency on rat hindlimb development: II. Expression of MHC isoforms. (PMID: 10710385 ) J Appl Physiol. 2000;88:904–916. doi: 10.1152/jappl.2000.88.3.904. [DOI] [PubMed] [Google Scholar]
- 4.Bickel CS, Slade J, Mahoney E, Haddad F, Dudley GA, Adams GR. Time course of molecular responses of human skeletal muscle to acute bouts of resistance exercise. J Appl Physiol. 2005;98:482–488. doi: 10.1152/japplphysiol.00895.2004. [DOI] [PubMed] [Google Scholar]
- 5.Bickel CS, Slade JM, Haddad F, Adams GR, Dudley GA. Acute molecular responses of skeletal muscle to resistance exercise in able-bodied and spinal cord-injured subjects. J Appl Physiol. 2003;94:2255–2262. doi: 10.1152/japplphysiol.00014.2003. [DOI] [PubMed] [Google Scholar]
- 6.Caiozzo VJ, Haddad F, Lee S, Baker M, Paloski W, Baldwin KM. Artificial gravity as a countermeasure to microgravity: a pilot study examining the effects on knee extensor and plantar flexor muscle groups. J Appl Physiol. 2009;107:39–46. doi: 10.1152/japplphysiol.91130.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colliander EB, Tesch PA. Effects of eccentric and concentric muscle actions in resistance training. Acta Physiol Scand. 1990;140:31–39. doi: 10.1111/j.1748-1716.1990.tb08973.x. [DOI] [PubMed] [Google Scholar]
- 8.di Maso NA, Haddad F, Zeng M, McCue SA, Baldwin KM. Role of denervation in modulating IIb MHC gene expression in response to T3 plus unloading state. J Appl Physiol. 2000;88:682–689. doi: 10.1152/jappl.2000.88.2.682. [DOI] [PubMed] [Google Scholar]
- 9.Dudley GA, Tesch PA, Miller BJ, Buchanan P. Importance of eccentric actions in performance adaptations to resistance training. Avait Space Environ Med. 1991;62:543–550. [PubMed] [Google Scholar]
- 10.Edgerton VR, Zhou MY, Ohira Y, Klitgaard H, Jinag B, Bell G, Harris B, Saltin B, Gollnick PD, Roy RR. Human fiber size and enzymatic properties after 5 and 11 days of space flight. J Appl Physiol. 1995;78:1733–1739. doi: 10.1152/jappl.1995.78.5.1733. [DOI] [PubMed] [Google Scholar]
- 11.Fitts RH, Riley DR, Widrick JJ. Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J Appl Physiol. 2000;89:823–839. doi: 10.1152/jappl.2000.89.2.823. [DOI] [PubMed] [Google Scholar]
- 12.Haddad F, Adams GR. Selected contribution: Acute cellular and molecular responses to resistance exercise. J Appl Physiol. 2002;93:394–403. doi: 10.1152/japplphysiol.01153.2001. [DOI] [PubMed] [Google Scholar]
- 13.Haddad F, Baldwin KM, Tesch PA. Pretranslational markers of contractile protein expression in human skeletal muscle: Effect of limb unloading plus resistance exercise. J Appl Physiol. 2005;98:46–52. doi: 10.1152/japplphysiol.00553.2004. [DOI] [PubMed] [Google Scholar]
- 14.Haddad F, Roy RR, Zhong H, Edgerton VR, Baldwin KM. Atrophy responses to muscle inactivity. I. Cellular markers of protein deficits. J Appl Physiol. 2003;95:781–790. doi: 10.1152/japplphysiol.00317.2003. [DOI] [PubMed] [Google Scholar]
- 15.Han Y-S, Geiger PC, Cody MJ, Macken RL, Sieck GC. ATP consumption rate per cross bridge depends on myosin heavy chain isoform. J Appl Physiol. 2003;94:2188–2196. doi: 10.1152/japplphysiol.00618.2002. [DOI] [PubMed] [Google Scholar]
- 16.Harm DL, Jennings RT, Meck JV, Powell MR, Putcha L, Sams CP, Schneider SM, Shackelford LC, Smith SM, Whitson PA. Invited review: Gender issues related to spaceflight: a NASA perspective. J Appl Physiol. 2001;91:2374–2383. doi: 10.1152/jappl.2001.91.5.2374. [DOI] [PubMed] [Google Scholar]
- 17.Heinemeier KM, Olesen JL, Haddad F, Langberg H, Kjaer M, Baldwin KM, Schjerling P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol. 2007;582:1303–1316. doi: 10.1113/jphysiol.2007.127639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, Kjaer M. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol. 2007;102:573–581. doi: 10.1152/japplphysiol.00866.2006. [DOI] [PubMed] [Google Scholar]
- 19.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 20.Lemoine JK, Haus JM, Trappe SW, Trappe TA. Muscle proteins during 60-day bedrest in women: impact of exercise or nutrition. Muscle Nerve. 2009;39:463–471. doi: 10.1002/mus.21189. [DOI] [PubMed] [Google Scholar]
- 21.Smith SM, Heer MA, Shackelford LC, Sibonga JD, Ploutz-Snyder L, Zwart SR. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J Bone Miner Res. 2012;27:1896–1906. doi: 10.1002/jbmr.1647. [DOI] [PubMed] [Google Scholar]
- 22.Talmadge RJ, Roy RR. Electrophoretic seperation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol. 1993;75:2337–2340. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- 23.Tesch P, Pozzo M, Ainegren M, Swarén M, Linnehan R. Cardiovascular responses to rowing on a novel ergometer designed for both resistance and aerobic training in space. Aviat Space Environ Med. 2013;84:516–521. doi: 10.3357/asem.3552.2013. [DOI] [PubMed] [Google Scholar]
- 24.Trappe S, Costill D, Gallagher P, Creer A, Peters JR, Evans H, Riley DA, Fitts RH. Exercise in space: human skeletal muscle after 6 months aboard the International Space Station. (PMID: 19150852 ) J Appl Physiol. 2009;106:1159–1168. doi: 10.1152/japplphysiol.91578.2008. [DOI] [PubMed] [Google Scholar]
- 25.Yoshiga CC, Higuchi M. Oxygen uptake and ventilation during rowing and running in females and males. Scand J Med Sci Sports. 2003;13:359–363. doi: 10.1046/j.1600-0838.2003.00324.x. [DOI] [PubMed] [Google Scholar]