Abstract
Background:
The cause of the adjacent segment degeneration (ASD) after fusion remains unknown. It is reported that adjacent facet joint stresses increase after anterior cervical discectomy and fusion. This increase of stress rate may lead to tissue injury. Thus far, the load rate of the adjacent segment facet joint after fusion remains unclear.
Methods:
Six C2–C7 cadaveric spine specimens were loaded under four motion modes: Flexion, extension, rotation, and lateral bending, with a pure moment using a 6° robot arm combined with an optical motion analysis system. The Tecscan pressure test system was used for testing facet joint pressure.
Results:
The contact mode of the facet joints and distributions of the force center during different motions were recorded. The adjacent segment facet joint forces increased faster after fusion, compared with intact conditions. While the magnitude of pressures increased, there was no difference in distribution modes before and after fusion. No pressures were detected during flexion. The average growth velocity during extension was the fastest and was significantly faster than lateral bending.
Conclusions:
One of the reasons for cartilage injury was the increasing stress rate of loading. This implies that ASD after fusion may be related to habitual movement before and after fusion. More and faster extension is disadvantageous for the facet joints and should be reduced as much as possible.
Keywords: Action Related, Adjacent Segment Degeneration, Facet Joints Load, Fusion, Load Rate
INTRODUCTION
Spinal intervertebral fusion remains the gold standard for treating spinal disease. Since the surgery changes the original structure of the spine, the mechanical environment can also be changed, and concomitant symptoms will occur, such as adjacent segment degeneration (ASD). Hilibrand et al. found the incidence of ASD to be 3% per year, of 300 cases in 10 years of follow-up.[1] Goffin et al. reported the incidence of ASD to be up to 92% based on imaging diagnosis.[2] Beyond doubt, the mechanical changes in the environment are the primary reason for ASD, but the exact mechanism remains unclear.
Some studies indicate that facet joint pressure of the adjacent segment increases after fusion.[3,4,5] Based on this, some researchers believe that load increase on the facet joint is the initiating factor for ASD. Faizan et al. reported that the pressure increased significantly after fusion; double segment fusion makes the pressure three times greater than in an intact joint.[4] Jaumard et al. found that facet joint load (FJL) increased ipsilaterally during lateral bending, and contralaterally during axial rotation after disc prosthesis implantation.[6] Due to limitations in measurement conditions, no distribution or growth mode for FJL were reported in vitro until now.
Bauman reported that there was no significant impact on the FJL after ProDisc-C implantation, during spinal bending.[7] Jaumard et al. found that during loading motions such as lateral bending and rotation, there were no significant differences after prosthesis implantation as compared with the intact joint.[6] The mechanisms of action were different during various motions, as well as facet joint contact mode and load. Thus far, no such studies were reported for the change in adjacent segment facet joints with motion after intervertebral fusion.
In this study, human cadaveric cervical vertebra samples were used for studying the load rate increase and the force distribution of the adjacent segment facet joint after single and double segment fusion. The hypothesis is that the loading rate of adjacent segmental facet joint after fusion will be different in various motions and will be increased with number of fusion segments.
METHODS
Three groups of six cadaveric cervical vertebrae (C2–C7) were used for biomechanical testing. No clinical degeneration or osteoporosis was found on radiographic examination. Samples were thawed from −20° C to 4° C for 4 h, and defrosted for 20 h at room temperature in normal saline. All muscles were removed; ligaments and joint capsules were kept intact. All samples were obtained under a body donation agreement, and informed consent was obtained from relatives.
Each sample was placed in three groups in the following order: The intact group, the one segment fusion group (C4–5; FUS1), and the two segments fusion group (C4–5 and C5–6; FUS2; Figure 1). Anterior cervical discectomy and fusion were performed on the C4–5 level in the FUS1 group and the C4–6 levels for the FUS2 group. The operative procedure is shown in the attachment (titanium alloy internal fixators, Shandong Weigao, China), and the adjacent segment is C3–4. The cranial and caudal vertebrae of each specimen were embedded in an upright tensionless position using self-curing denture acrylic (Shanghai New Century Dental Material Co. Ltd., Shanghai, China) in a framed clamp construction. The spine testing device [Figure 2] consisted of four parts. A 6° of freedom robot arm (Yaskawa Motoman, Tokyo, Japan) which enabled execution of complex motion patterns was used to manipulate the specimen via predefined motion or load on the cranial vertebrae. A sensitive Force/Torque Sensor (Mini 45, ATI, USA) was mounted at the robot's end-effector, which enabled simultaneous measurement of applied forces and torques during load-controlled robot movement. Motion of each vertebra was acquired using an optical motion capture system (Optotrak-Certus/NDI, Waterloo/Ontario, Canada) with active infrared light-emitting diode markers. The pressure sensor I-scan 6900R (Tecscan Inc., Boston, MA, USA) was used for pressure measurement embedded in the facet joint.
Figure 1.
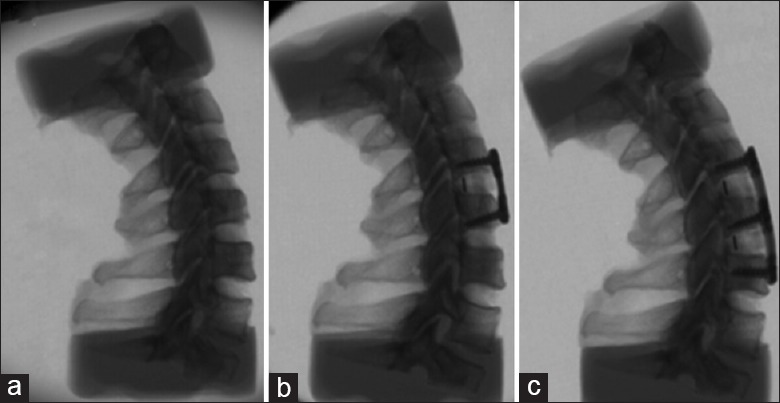
Three groups of test (a) intact, (b) C4-5 fusion (c) C4-6 fusion.
Figure 2.
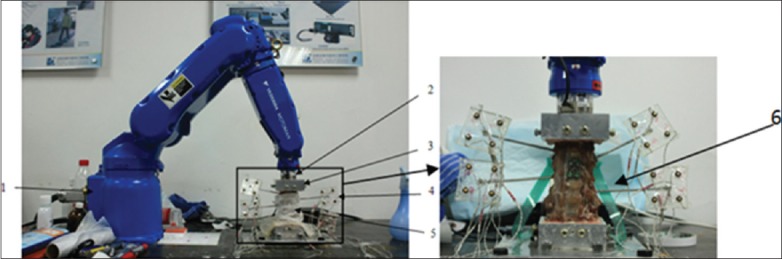
Testing facility: 1. Robot arm; 2. 6-axis sensor; 3. Clamps; 4. LED Marks; 5. Samples; 6. Pressure sensors.
Biomechanical testing
Accurate positioning of the upper vertebra relative to the clamp was essential to avoid misalignment of anatomical planes of the specimen and robot end-effector plane. All groups were tested in flexion, extension, bending, and rotation in the intact and fusion groups, respectively. The loading protocol of Panjabi[8] was used and was specialized for studying adjacent segment problems. This hybrid method was based on the pure moment loading mode, with the intact one loaded first with force-control, and the fused mode of the same sample with a position-control method. According to the method of Woo et al.,[9,10] disperse pure moment points were found first, and then the automatic interpolation function of the robot was used to fulfill continuous pure moment loading. The interval angle was 2° per point. The loading procedure was as follows: Left bending–right bending–flexion–extension–left rotation–right rotation. These procedures were controlled to finish in one session using robot-embedded programming. Three motion cycles were applied to each specimen starting in an unloaded upright position. The test speed was 1°/s. The intact group was loaded to 2 Nm, and the fused group was loaded with the same range of motion (ROM) with the intact group at 2 Nm. All operative procedures were performed by the same surgeon.
Paired comparisons between treatment groups were made using Student's paired t-test. A P < 0.05 was considered as statistically significant. Values are presented as the mean/standard deviation of the mean. All data were analyzed by SPSS 17.0 software (SPSS Inc., USA).
RESULTS
The ROM of operative levels before and after fusion is shown in Table 1. The adjacent FJLs (C3–4) were measured under the three states: Intact, single fused, and double fused. FJL during flexion in the three groups was zero. The FJL of fusion groups was larger than the intact groups, of which the maximum FJL in the fusions were significantly higher than the intact groups during extension (P < 0.05) [Figures 3 and 4].
Table 1.
ROM of operation levels
| Items | Left bending | Flexion | Extension | Left rotation | ||||
|---|---|---|---|---|---|---|---|---|
| C4-5 | C4-6 | C4-5 | C4-6 | C4-5 | C4-6 | C4-5 | C4-6 | |
| INT | 4.783/0.6129 | 8.746/1.9288 | 2.549/0.8626 | 6.690/2.8186 | 2.282/0.4184 | 6.481/1.3396 | 4.472/2.3786 | 7.768-2.8402 |
| FUS1 | 1.708/0.2784 | – | 0.713/0.2496 | – | 0.783/0.4753 | – | 1.689/0.7009 | – |
| FUS2 | – | 1.766/0.6934 | – | 2.854/1.0796 | – | 1.519/1.3148 | – | 1.316/0.7801 |
| t-test | * | * | * | * | * | * | * | * |
*P < 0.05. ROM: Range of motion. All data were shown as mean/standard deviation.
Figure 3.
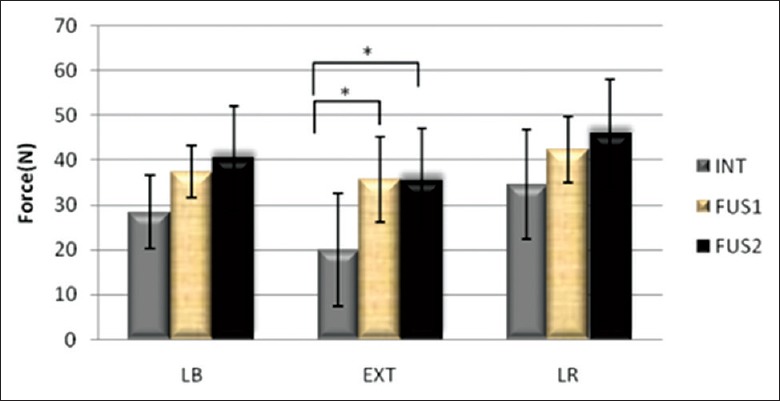
Max force of C3 –4 facet joints.
Figure 4.
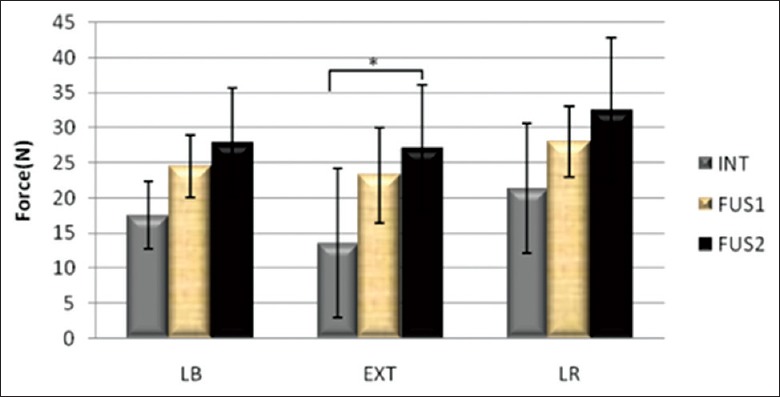
Average force of C3 –4 facet joints.
Facet joint load increased after fusion and positively correlated with the number of fusion segments. Moreover, as shown in Figures 5 and 6, the growth speed of the FJL also increased with the number of fusion segments, in which extension was the fastest of the motions (P < 0.05). The response pressure-time curve of the joints of one sample is shown in Figure 7; the pressure difference under continuous loading can be seen, with the following magnitude sequence: Rotation, extension, lateral bending, and flexion.
Figure 5.
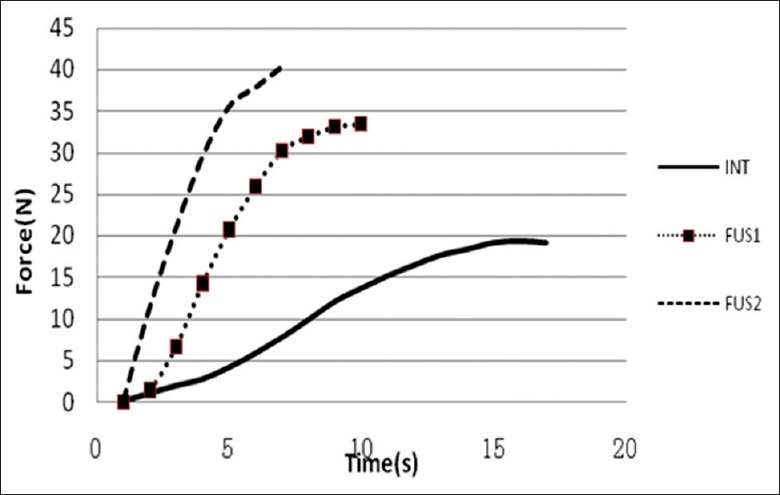
Force-time curves.
Figure 6.
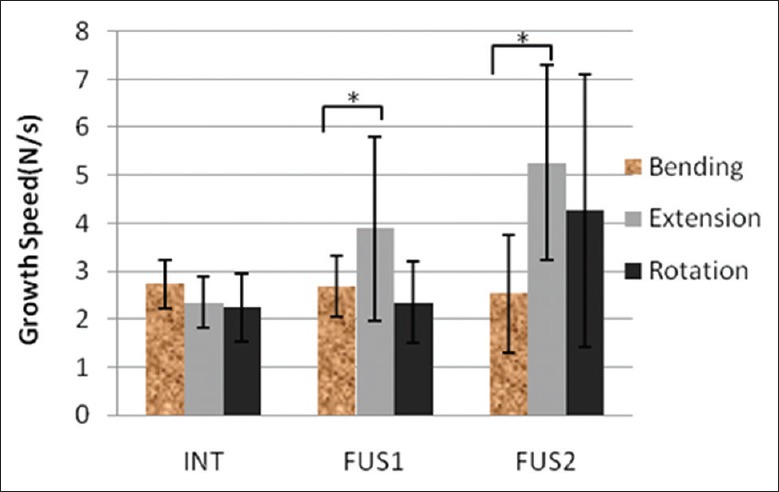
C3 –4 facet load growth speed of different motions.
Figure 7.
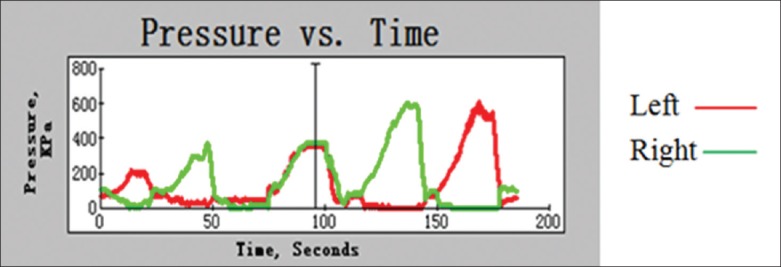
Pressure-time curve of left and right sides of facet joints.
Force distribution patterns of the four motions were different. There was no pressure signal when loading with flexion for the facet joints were open during this motion. During extension, the force was symmetric on the left and right facet joints. The center of force extended from the distal tip to the proximal roots of the facet joints. During lateral bending, pressure center transfer from the bending side to the other side and the center appeared to be of the banding type. During rotation, FJL increased contralaterally, and the center of force spread from the contralateral part of the forced articular side to the entire area. No changes of distribution regularity occurred after fusion on the adjacent facet joint. The distribution clouds are shown in Figure 8.
Figure 8.
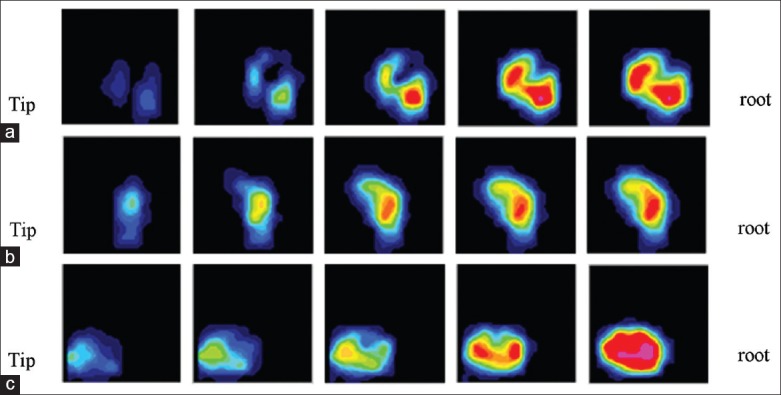
Transformation of facet joints for three motions: (a) Lateral bending (b) Extension (c) Rotation.
DISCUSSION
Adjacent segment degeneration after fusion is becoming increasingly popular in both clinical and research fields. The mechanism for ASD is complicated and not uniform at present. Some researchers suggest that mechanical change of the adjacent segment facet joints is the initial factor for the ASD. The intervertebral disc and the two facet joints make up the constrained three balance points during spine finishing movements. The instantaneous center of rotation transfers in the area is surrounded by these three points.[11] Any mechanical changes of the facet joints may affect the structural stability and health of the joints, especially the occurrence of spinal degeneration and osteoarthritis (OA).[11,12,13,14,15,16,17,18]
There are certain difficulties in studying facet joints. First, facet joints are deeply located in the inner spine and wrapped by capsules, so it is impossible to obtain pressure information without destroying the capsules for in vitro testing. As discussed in the cited literature, damage to the capsule can incur an abnormal change in the facet joints.[7] The cutting and suture methods used in our study can measure pressure data with the sensor slice inside the joint, with minimal damage to the capsules. Second, the Tecscan 6900R pressure sensor used for testing is optimal for facet joints. The plastic package can be used in a damp environment, and the flexional properties can ensure real-time measurement during the entire movement process of the test. These advantages overcome other limitations of traditional methods, such as Fuji pressure sensitive paper, and greatly reduce the influence of environmental factors.
The pressure during flexion is zero, which is consistent with the finite element method (FEM) computation reported by Faizan et al.[4] This is due to the upper and lower joints being far apart during flexion. The FEM results show that the pressure of the adjacent joint increased by three times after double-segment fusion,[19] while our results show a 50–80% increase. The reasons may be that the degree of freedom of the fusion segment could be completely constrained while using FEM; however, for the in vitro test, the motion cannot be completely removed, so the operative segment can move to some extent after fusion. Second, the same samples were used for testing in the three groups for comparison; each sample required being encountered twice for surgery and three times for testing. The compensatory effects were therefore weakened. Rotation results were identified with the FEM, at approximately 50 N.
Schmorl's nodes are associated with heavy labor occupation factors.[20] Lumbar disc herniation has been shown to be significantly associated with cumulative exposures during a physical workload.[21] The mechanisms for these spinal diseases are related to different types of cumulative motions and postures. We found that the load rates of the facet joints are different for different motions and that there may be a good explanation for this.
It has been shown that the articular cartilage is very sensitive to the loads that occur during daily normal or specialized activities. Fast or impact loading may diminish proteoglycan synthesis and damage tissue through necrosis. The initial failure of subchondral bone is suggested to be the cause of OA and joint degeneration.[22] A quick change in stress rate may lead to very fast crack propagation, which may lower the toughness of the articular surface.[23] Failure of the joints may lead to accumulation of damage in other joint components such as the bone, muscles, ligaments, tendons, and nerves, which participate in load transmission.[22,23,24] In this study, the adjacent segment FJL rates were found be faster after fusion. This may be a mechanism for ASD. Further in vivo experiments should be performed to determine the etiology of ASD.
Adjacent segment degeneration is always concomitant with facet joint degeneration, although it remains inconclusive whether degeneration of the facet joint or disc occurs first. We found that different motions may affect the force and force velocity of the facet joint, which may incur facet joint degeneration. This finding suggests that ASD may be related to the exercise habit before and after surgery; more studies are required to confirm this hypothesis.
ACKNOWLEDGEMENTS
The authors would like to thank the Department of Orthopedics of Beijing Chao-Yang Hospital for technical support.
Footnotes
Edited by: Yi Cui
Source of Support: This study was supported by grants from the National Science Foundation of China (No. 11372029) and the Beijing Municipal Science and Technology Plan Projects (No.Z111100074911005) and the National Science and Technology Support Plan (No. 2012BAI22B00 and No. 2012BAI18B07).
Conflict of Interest: None declared.
REFERENECES
- 1.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–28. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Goffin J, Geusens E, Vantomme N, Quintens E, Waerzeggers Y, Depreitere B, et al. Long-term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech. 2004;17:79–85. doi: 10.1097/00024720-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Chang UK, Kim DH, Lee MC, Willenberg R, Kim SH, Lim J. Changes in adjacent-level disc pressure and facet joint force after cervical arthroplasty compared with cervical discectomy and fusion. J Neurosurg Spine. 2007;7:33–9. doi: 10.3171/SPI-07/07/033. [DOI] [PubMed] [Google Scholar]
- 4.Faizan A, Goel VK, Biyani A, Garfin SR, Bono CM. Adjacent level effects of bi level disc replacement, bi level fusion and disc replacement plus fusion in cervical spine – A finite element based study. Clin Biomech (Bristol, Avon) 2012;27:226–33. doi: 10.1016/j.clinbiomech.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Kim D, Limm J, Lee M, Park J. P92. Adjacent disc pressure and facet force comparison for cervical spine arthroplasty with spinal kinetics, Prodisc-C, prestige artificial discs, and anterior cervical discectomy and fusion. Spine J. 2005;5(4 Suppl):S154–6. [Google Scholar]
- 6.Jaumard NV, Bauman JA, Guarino BB, Gokhale AJ, Lipschutz DE, Weisshaar CL, et al. ProDisc cervical arthroplasty does not alter facet joint contact pressure during lateral bending or axial torsion. Spine (Phila Pa 1976) 2013;38:E84–93. doi: 10.1097/BRS.0b013e31827b8a2d. [DOI] [PubMed] [Google Scholar]
- 7.Bauman JA, Jaumard NV, Guarino BB, Weisshaar CL, Lipschutz DE, Welch WC, et al. Facet joint contact pressure is not significantly affected by ProDisc cervical disc arthroplasty in sagittal bending: A single-level cadaveric study. Spine J. 2012;12:949–59. doi: 10.1016/j.spinee.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Panjabi MM. Hybrid multidirectional test method to evaluate spinal adjacent-level effects. Clin Biomech (Bristol, Avon) 2007;22:257–65. doi: 10.1016/j.clinbiomech.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Rudy TW, Livesay GA, Woo SL, Fu FH. A combined robotic/universal force sensor approach to determine in situ forces of knee ligaments. J Biomech. 1996;29:1357–60. doi: 10.1016/0021-9290(96)00056-5. [DOI] [PubMed] [Google Scholar]
- 10.Woo SL, Debski RE, Wong EK, Yagi M, Tarinelli D. Use of robotic technology for diathrodial joint research. J Sci Med Sport. 1999;2:283–97. doi: 10.1016/s1440-2440(99)80002-4. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt H, Heuer F, Claes L, Wilke HJ. The relation between the instantaneous center of rotation and facet joint forces - A finite element analysis. Clin Biomech (Bristol, Avon) 2008;23:270–8. doi: 10.1016/j.clinbiomech.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Liu YK, Goel VK, Dejong A, Njus G, Nishiyama K, Buckwalter J. Torsional fatigue of the lumbar intervertebral joints. Spine (Phila Pa 1976) 1985;10:894–900. doi: 10.1097/00007632-198512000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Jeffrey JE, Gregory DW, Aspden RM. Matrix damage and chondrocyte viability following a single impact load on articular cartilage. Arch Biochem Biophys. 1995;322:87–96. doi: 10.1006/abbi.1995.1439. [DOI] [PubMed] [Google Scholar]
- 14.Kurz B, Jin M, Patwari P, Cheng DM, Lark MW, Grodzinsky AJ. Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res. 2001;19:1140–6. doi: 10.1016/S0736-0266(01)00033-X. [DOI] [PubMed] [Google Scholar]
- 15.Morel V, Merçay A, Quinn TM. Prestrain decreases cartilage susceptibility to injury by ramp compression in vitro. Osteoarthritis Cartilage. 2005;13:964–70. doi: 10.1016/j.joca.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Knecht S, Vanwanseele B, Stüssi E. A review on the mechanical quality of articular cartilage - Implications for the diagnosis of osteoarthritis. Clin Biomech (Bristol, Avon) 2006;21:999–1012. doi: 10.1016/j.clinbiomech.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Isaac DI, Meyer EG, Haut RC. Chondrocyte damage and contact pressures following impact on the rabbit tibiofemoral joint. J Biomech Eng. 2008;130:041018. doi: 10.1115/1.2948403. [DOI] [PubMed] [Google Scholar]
- 18.Natoli RM, Scott CC, Athanasiou KA. Temporal effects of impact on articular cartilage cell death, gene expression, matrix biochemistry, and biomechanics. Ann Biomed Eng. 2008;36:780–92. doi: 10.1007/s10439-008-9472-5. [DOI] [PubMed] [Google Scholar]
- 19.Stieber JR, Quirno M, Kang M, Valdevit A, Errico TJ. The facet joint loading profile of a cervical intervertebral disc replacement incorporating a novel saddle-shaped articulation. J Spinal Disord Tech. 2011;24:432–6. doi: 10.1097/BSD.0b013e3182027297. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Videman T, Battié MC. ISSLS prize winner: Lumbar vertebral endplate lesions: Associations with disc degeneration and back pain history. Spine (Phila Pa 1976) 2012;37:1490–6. doi: 10.1097/BRS.0b013e3182608ac4. [DOI] [PubMed] [Google Scholar]
- 21.Ahsan MK, Matin T, Ali MI, Ali MY, Awwal MA, Sakeb N. Relationship between physical work load and lumbar disc herniation. Mymensingh Med J. 2013;22:533–40. [PubMed] [Google Scholar]
- 22.Arokoski JP, Jurvelin JS, Väätäinen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading. Scand J Med Sci Sports. 2000;10:186–98. doi: 10.1034/j.1600-0838.2000.010004186.x. [DOI] [PubMed] [Google Scholar]
- 23.Stok K, Oloyede A. A qualitative analysis of crack propagation in articular cartilage at varying rates of tensile loading. Connect Tissue Res. 2003;44:109–20. [PubMed] [Google Scholar]
- 24.Maffulli N, King JB. Effects of physical activity on some components of the skeletal system. Sports Med. 1992;13:393–407. doi: 10.2165/00007256-199213060-00003. [DOI] [PubMed] [Google Scholar]


