Abstract
Background:
Pulmonary embolism (PE) can be difficult to diagnose in elderly patients because of the coexistent diseases and the combination of drugs that they have taken. We aimed to compare the clinical diagnostic values of the Wells score, the revised Geneva score and each of them combined with D-dimer for suspected PE in elderly patients.
Methods:
Three hundred and thirty-six patients who were admitted for suspected PE were enrolled retrospectively and divided into two groups based on age (≥65 or <65 years old). The Wells and revised Geneva scores were applied to evaluate the clinical probability of PE, and the positive predictive values of both scores were calculated using computed tomography pulmonary arteriography as a gold standard; overall accuracy was evaluated by the area under the curve (AUC) of receiver operator characteristic curve; the negative predictive values of D-dimer, the Wells score combined with D-dimer, and the revised Geneva score combined with D-dimer were calculated.
Results:
Ninety-six cases (28.6%) were definitely diagnosed as PE among 336 cases, among them 56 cases (58.3%) were ≥65 years old. The positive predictive values of Wells and revised Geneva scores were 65.8% and 32.4%, respectively (P < 0.05) in the elderly patients; the AUC for the Wells score and the revised Geneva score in elderly was 0.682 (95% confidence interval [CI]: 0.612–0.746) and 0.655 (95% CI: 0.584–0.722), respectively (P = 0.389). The negative predictive values of D-dimer, the Wells score combined with D-dimer, and the revised Geneva score combined with D-dimer were 93.7%, 100%, and 100% in the elderly, respectively.
Conclusions:
The diagnostic value of the Wells score was higher than the revised Geneva score for the elderly cases with suspected PE. The combination of either the Wells score or the revised Geneva score with a normal D-dimer concentration is a safe strategy to rule out PE.
Keywords: D-dimer, Elderly, Pulmonary Embolism, Revised Geneva Score, Wells Score
INTRODUCTION
Pulmonary embolism (PE) is a common cardiopulmonary emergency, whose mortality is 30% if untreated,[1] but it would drop to 2–8% if treated appropriately.[2] The incidence of venous thromboembolism increases markedly with age,[3] and the mortality in elderly patients with PE is greater than in non-elderly patients.[4] The increasing prevalence of other cardiopulmonary diseases, such as chronic obstructive pulmonary disease or congestive heart failure, leads to rising rate of misdiagnosis and missed diagnosis of PE. Thus, it is necessary to explore suitable and noninvasive approaches for PE diagnosis in elderly patients.
Recently, several prediction rules make it possible to discriminate suspected PE patients in categories of clinical or pretest probability.[5,6,7] Two of the most extensively validated and widely used scores, the Wells and revised Geneva scores,[8,9] stratify patients into low, moderate, and high clinical probability groups. The two scores were validated and have become important strategies for accurate diagnosis, but there are few studies in elderly patients.[10] Therefore, this study aims to explore diagnostic values of the Wells and revised Geneva scores and their combination with D-dimer, for suspected PE in elderly patients.
METHODS
Patients
Three hundred and thirty-six consecutive patients with suspected PE due to chest pain, dyspnea, and hemoptysis who visited Peking University People's Hospital, between March 2006 and April 2011, were enrolled in this study. The elderly group was defined as ≥65 years of age.[3,10,11]
Assessment of clinical probability
For each suspected PE patient, the characteristics were recorded retrospectively, including gender, age, main symptoms (dyspnea, chest pain, hemoptysis, syncope, unilateral lower limb pain), signs (heart rate, respiration rate, pain on lower limb deep vein at palpation and unilateral edema), and risk factors (previous deep vein thrombosis or PE, cancer, fracture within 4 weeks, immobilization or surgery within 4 weeks). The Wells and revised Geneva scores were applied to evaluate the probability of PE and the patients were classified into categories of clinical probability [Table 1]. The diagnosis of PE was ruled out if D-dimer test was normal in patients with Wells score ≤4 or revised Geneva score ≤3.[12]
Table 1.
The Wells and the revised Geneva score
| Wells score | Revised Geneva score | ||
|---|---|---|---|
| Variable | Points | Variable | Points |
| Previous DVT or PE | 1.5 | Age >65 years | 1 |
| Recent surgery or immobilization | 1.5 | Previous DVT or PE | 3 |
| Cancer | 1 | Surgery or fracture within 1-month | 2 |
| Hemoptysis | 1 | Active malignancy | 2 |
| Heart rate >100 beats/min | 1.5 | Unilateral lower limb pain | 3 |
| Clinical signs of DVT | 3 | Hemoptysis | 2 |
| Alternative diagnosis less likely than PE | 3 | Heart rate 75–94 beats/min | 3 |
| Heart rate ≥95 beats/min | 5 | ||
| Pain on lower limb deep vein at palpation and unilateral edema | 4 | ||
| Clinical probability (3 levels) | Total | Clinical probability (3 levels) | Total |
| Low | 0–1 | Low | 0–3 |
| Intermediate | 2–6 | Intermediate | 4–10 |
| High | ≥7 | High | ≥11 |
| Clinical probability (2 levels) | Clinical probability (2 levels) | ||
| PE unlikely | 0–4 | PE unlikely | 0–3 |
| PE likely | >4 | PE likely | >3 |
DVT: Deep vein thrombosis; PE: Pulmonary embolism.
Computed tomography pulmonary arteriography
Computed tomography pulmonary arteriography (CTPA) was used for PE diagnosis. Pulmonary artery signs of PE were central filling defects or eccentric partial filling defects surrounded by contrast medium, filling defects occupying the total vessel section and mural defects. CTPA was performed with a 64-row multidetector CT scanner (Lightspeed VCT, GE Healthcare). Scanning parameters were set as follows: Tube voltage of 120 kV and current of 300 mA, collimation of 0.6 mm, gantry rotation time of 0.4 s, and reconstruction increment of 1.25 mm. A mechanical injector was used for intravenous bolus injection of iopromide (Ultravist, 370 mg/ml; Bayer Schering Pharma, Germany) at a flow rate of 3.0–3.5 ml/s.
D-dimer test
D-dimer was measured by a high sensitive turbidimetric immunoassay (D-Dimer-0020008500, HemosIL™, Italy). The D-dimer kit consists of the latex reagent, reaction buffer, and D-dimer calibrator. The level of D-dimer is defined as normal if <500 ng/ml, abnormal if ≥500 ng/ml.
Statistical analysis
Chi-square test was used for comparison of categorical data, and Mann–Whitney U-test was used for comparison of continuous variables with a skewed distribution. The comprehensive degree of accuracy of both scores was compared using area under the curve (AUC) of receiver operating characteristic (ROC) curves. SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) and MedCalc version 11.5.0.0 (MedCalc Software, Mariakerke, Belgium) were used for statistical analysis. Statistical significance was accepted at P < 0.05.
RESULTS
General characteristics
There were 196 elderly people (100 males and 96 females) with mean (standard deviation [SD]) age of 76.1 (6.2), and 140 nonelderly patients (81 males and 59 females) with mean (SD) age of 48.0 (11.4). By CTPA, PE was diagnosed in 96 (56 elderly patients, 40 nonelderly) of 336 patients, and the other 240 patients (49 respiratory failure, 48 chronic obstructive pulmonary disease, 39 pneumonia and bronchitis, 36 heart failure, 30 coronary atherosclerotic heart disease, 11 arrhythmia, 7 acute cerebral infarction, 20 unknown causes) were excluded PE. Table 2 shows the clinical characteristics of PE patients. For the symptoms, the incidence of dyspnea was significantly higher in elderly patients than in the nonelderly (75% vs. 48%, P = 0.006), while incidences of chest pain and hemoptysis were lower in elderly (chest pain: 16% vs. 38%, P = 0.017; hemoptysis: 2% vs. 18%, P =0.018) patients. For signs, heart rate and respiration rate of elderly patients were higher than the non-elderly (heart rate: 95.0 ± 19.0 vs. 85.9 ± 13.7 beats/min, P < 0.05; respiration rate: 20.5 ± 3.7 vs. 18.9 ± 2.1 breaths/min, P < 0.05). And the arterial oxygen saturation was lower in elderly patients than the nonelderly (92.7 ± 6.2% vs. 95.6 ± 4.0%, P < 0.05).
Table 2.
Clinical presentation in elderly and nonelderly patients with PE
| Clinical presentation | ≥65 years n = 56 | <65 years n = 40 | χ2/u | P |
|---|---|---|---|---|
| Cancer, n (%) | 8 (14) | 9 (23) | 1.08 | 0.299 |
| Previous DVT/PE, n (%) | 4 (7) | 4 (10) | 0.02 | 0.901 |
| Recent surgery or immobilization, n (%) | 17 (30) | 7 (18) | 2.06 | 0.151 |
| Dyspnea, n (%) | 42 (75) | 19 (48) | 7.62 | 0.006* |
| Chest pain, n (%) | 9 (16) | 15 (38) | 5.71 | 0.017 |
| Hemoptysis, n (%) | 1 (2) | 7 (18) | 5.63 | 0.018 |
| Syncope, n (%) | 4 (7) | 5 (13) | 0.28 | 0.594 |
| Unilateral lower limb pain, n (%) | 6 (11) | 10 (25) | 3.43 | 0.064 |
| Temperature (°C) | 36.7 ± 0.7 | 36.9 ± 0.4 | 776.00 | 0.041 |
| Heart rate (beats/min) | 95.0 ± 19.0 | 85.9 ± 13.7 | 780.50 | 0.012 |
| Respiration rate (breaths/min) | 20.5 ± 3.7 | 18.9 ± 2.1 | 833.50 | 0.028 |
| Blood pressure (mmHg) | ||||
| Systolic pressure | 128.5 ± 17.5 | 126.0 ± 18.6 | 991.00 | 0.335 |
| Diastolic pressure | 73.1 ± 9.9 | 77.3 ± 12.0 | 927.50 | 0.144 |
| Arterial oxygen saturation (%) | 92.7 ± 6.2 | 95.6 ± 4.0 | 622.50 | 0.011 |
DVT: Deep vein thrombosis; PE: Pulmonary embolism. *P < 0.05.
The predictive values of the Wells score and the revised Geneva score in the elderly group
Proportion of patients ultimately diagnosed with PE for the Wells and revised Geneva group in the elderly, and non-elderly patients are presented in Figure 1a and b.
Figure 1.
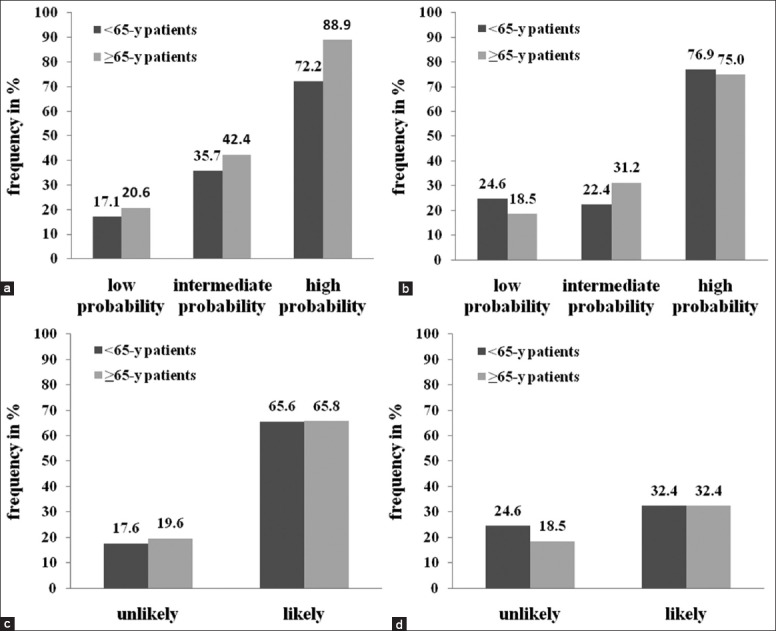
Frequency of pulmonary embolism in patients with different clinical probability according to age. (a) Frequency of pulmonary embolism (PE) in 3-level clinical probability categories of Wells score; (b) Frequency of PE in 3-level clinical probability categories of revised Geneva score; (c) Frequency of PE in 2-level clinical probability categories of Wells score; (d) Frequency of PE in 2-level clinical probability categories of revised Geneva score.
For the elderly people, the positive predictive value of the Wells score was significantly higher than that of the revised Geneva score (65.8% vs. 32.4%, P < 0.01). It was also similar for non-elderly patients: 65.6% and 32.4% (Mantel–Haezel test: P < 0.01) respectively [Figure 1c, d]. For the elderly people, there was no significant difference between the overall accuracies of the Wells (0.682, 95% confidence interval [CI]: 0.612–0.746) and revised Geneva scores (0.655, 95% CI: 0.584–0.772) as determined by the area under ROC curves (P = 0.389) [Figure 2]. The AUC for the Wells score and the revised Geneva score in nonelderly patients was 0.732 (95% CI: 0.650–0.803) and 0.633 (95% CI: 0.548–0.713) respectively, with statistically significant difference (P = 0.010) [Figure 3].
Figure 2.
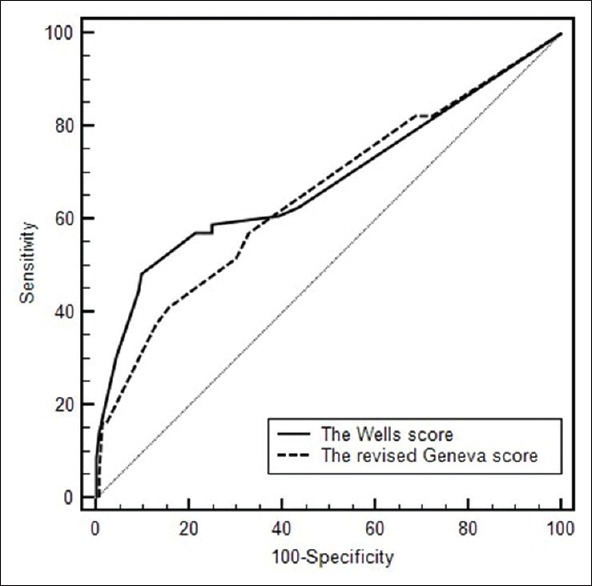
Receiver operating characteristic curves of Wells score and revised Geneva score for the elderly patients.
Figure 3.
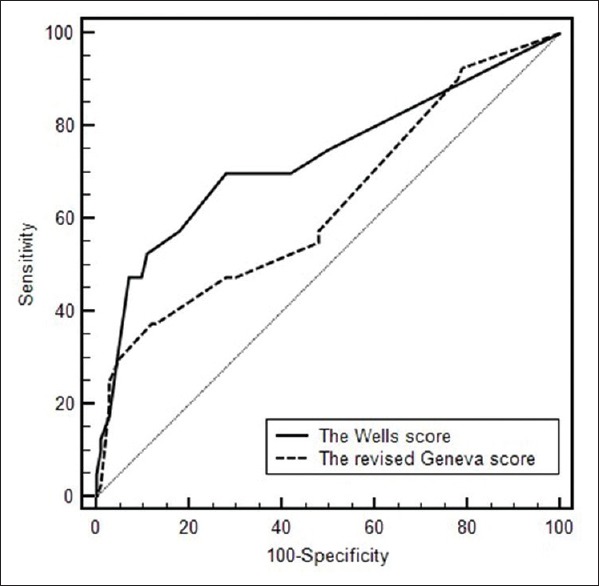
Receiver operating characteristic curves of Wells score and revised Geneva score for the nonelderly patients.
Negative predictive values for D-dimer only or combined with either the Wells score or the revised Geneva score
D-dimer
There were 63 cases with D-dimer <500 ng/ml in the elderly patients, 59 cases of whom were ruled out PE by CTPA, thus the negative predictive value was 93.7%; while there were 45 cases of D-dimer <500 ng/ml in the nonelderly patients, 42 cases of whom were finally ruled out by CTPA, with the negative predictive value of 93.3% [Table 3]. The negative values were similar, with no statistically significant difference (P = 1.000).
Table 3.
PPV and NPV for the patients (%)
| Items | ≥65 years | <65 years | ||
|---|---|---|---|---|
| PPV | NPV | PPV | NPV | |
| Wells score | 65.8 | 80.4 | 65.6 | 82.4 |
| Revised Geneva score | 32.4 | 81.5 | 32.4 | 75.4 |
| D-dimer | 39.0 | 93.7 | 41.2 | 93.3 |
| Wells score with D-dimer | 40.6 | 100 | 43.2 | 100 |
| Revised Geneva score with D-dimer | 32.3 | 100 | 38.8 | 100 |
PPV: Positive predictive value; NPV: Negative predictive value.
The Wells score combined with D-dimer
There were 58 elderly patients whose Wells scores ≤4 and D-dimer <500 ng/ml, and all of them were excluded PE by CTPA; while 42 cases were all excluded PE by CTPA in the non-elderly. The negative predictive values of the Wells score ≤4 combined with normal D-dimer were 100% in both groups [Table 3].
The revised Geneva score combined with D-dimer
There were 25 elderly patients whose Geneva scores ≤3 and D-dimer <500 ng/ml, and all of them were ruled out PE by CTPA; while 32 cases were also fully ruled out PE by CTPA in the other group. The negative predictive values of the revised Geneva score ≤3 combined with normal D-dimer were 100% in both groups [Table 3].
Comparison of negative predictive values for D-dimer only or combined with either the Wells score or the revised Geneva score
There was no significant difference for negative predictive value between normal D-dimer and the Wells score ≤4 combined with normal D-dimer (93.7% vs. 100%, P = 0.149) in elderly patients. The negative values of normal D-dimer and revised Geneva score ≤3 combined with normal D-dimer were also similar (93.7% vs. 100%, P = 0.470) in elderly patients.
DISCUSSION
In this study, the positive predictive value of the Wells score was higher than that of the revised Geneva score among the elderly group. The negative predictive values of D-dimer combined with either the Wells score or revised Geneva score were both 100% in the elderly patients, which is more specific of negative prediction for PE than D-dimer only.
We found that heart rate and respiration rate in elderly PE patients were higher than those of the nonelderly, which was similar to the previous report.[13] The reason might be as followings: Most elderly patients with PE also had cardiopulmonary comorbidities such as chronic obstructive pulmonary disease or congestive heart failure, which impairs cardiopulmonary function, so that they have poor hypoxic tolerance. The manifestations of patients with hypoxia would be dyspnea or tachycardia. So if there are unknown causes of the signs above, clinicians should have a high index of suspicion of PE.
Some researchers applied the Wells score[8] and the revised Geneva score[9] for diagnosis of patients with suspected PE. Although it has been proved that the Wells score is used more generally and has a higher predictive value,[14] one item of the score “alternative diagnosis less likely than PE” is highly subjective and influenced by other factors in the rule,[15] which reduces its diagnostic value and reproducibility.[16] While the revised Geneva score consists entirely of objective clinical items, which would be less influenced by the clinical experience. However, Penaloza et al.[17] reported that compared with the revised Geneva score, the Wells score appeared to be more convenient and accurate. The same conclusion was obtained by Calisir et al.[18] But there were few studies focused on the elderly PE patients. It was shown in this study that the positive predictive value of the Wells score was higher than that of the revised Geneva score among the elderly group. The possible reason of the result may be as follows: In 2-level scoring systems, 4 points was the cut-off value in Wells score to categorized patients as “PE unlikely” or “PE likely,” while 3 points was used in revised Geneva score. This may lead more cases categorized as “likely PE” evaluated by revised Geneva score, and thus decrease the positive predictive value of it. It is necessary to find an appropriate cut-off value in 2-level scoring system of revised Geneva score. The AUCs were similar in both rules for the elderly patients while the AUC of the Wells score was larger than revised Geneva score for the nonelderly group. This is likely due to a variety of other diseases, which the elderly patients were often concomitant with. The individual symptoms were complicated or atypical, so it was difficult for the subjective item in the Wells score to assess the elderly patients. Unlike nonelderly, the clinical diagnostic value of the Wells score was not superior to the revised Geneva score in elderly patients.
There was a high percentage (71.4%) of suspected patients who were finally excluded from PE diagnosis by CTPA, similar to the previous report.[19] Consequently, it is necessary to find a method with high negative predictive value so that unnecessary diagnostic tests could be avoided. Plasma D-dimer is a degradation product of cross-linked fibrin, levels of which are elevated in plasma in the presence of an acute clot because of simultaneous activation of coagulation and fibrinolysis. It is available for excluding PE.[20,21] However, the negative predictive value of D-dimer is about 94% in all subjects.[22] In the elderly group of this study, the negative predictive value of D-dimer was 93.7%. When a normal D-dimer combined with the Wells score ≤4, it reached to 100%, which is similar as a meta-analysis result.[23] Similarly, when a normal D-dimer combined with the Geneva score ≤3, the negative predictive value reached to 100%. The combination of using either Wells or revised Geneva score with a normal D-dimer was a safe strategy to rule out PE for elderly cases. Through chi-square tests, negative predictive values for D-dimer only or combined with either the Wells score or the revised Geneva score were similar. This may be due to the small number of patients in this study. Further studies with more cases are needed.
Admittedly, this study also has some limitations: Limited samples and follow-up. In addition, the cardiac troponin T, NT-proBNP or echocardiography of the patients which were required for risk stratification were not analyzed. However, with high diagnostic value, either the Wells score or the revised Geneva score was a simple and applicable method for risk stratification without using the items mentioned above.
In conclusion, for elderly cases, the Wells score appeared to be a more accurate rule, with which could reduce the rate of misdiagnosis and avoid unnecessary tests; D-dimer is of greater value in excluding PE when combined with Wells score or revised Geneva score.
Footnotes
Edited by: De Wang
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENECES
- 1.Donato AA, Scheirer JJ, Atwell MS, Gramp J, Duszak R., Jr Clinical outcomes in patients with suspected acute pulmonary embolism and negative helical computed tomographic results in whom anticoagulation was withheld. Arch Intern Med. 2003;163:2033–8. doi: 10.1001/archinte.163.17.2033. [DOI] [PubMed] [Google Scholar]
- 2.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: Clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–9. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 3.Busby W, Bayer A, Pathy J. Pulmonary embolism in the elderly. Age Ageing. 1988;17:205–9. doi: 10.1093/ageing/17.3.205. [DOI] [PubMed] [Google Scholar]
- 4.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Predictors of survival after deep vein thrombosis and pulmonary embolism: A population-based, cohort study. Arch Intern Med. 1999;159:445–53. doi: 10.1001/archinte.159.5.445. [DOI] [PubMed] [Google Scholar]
- 5.Wicki J, Perneger TV, Junod AF, Bounameaux H, Perrier A. Assessing clinical probability of pulmonary embolism in the emergency ward: A simple score. Arch Intern Med. 2001;161:92–7. doi: 10.1001/archinte.161.1.92. [DOI] [PubMed] [Google Scholar]
- 6.Miniati M, Monti S, Bottai M. A structured clinical model for predicting the probability of pulmonary embolism. Am J Med. 2003;114:173–9. doi: 10.1016/s0002-9343(02)01478-x. [DOI] [PubMed] [Google Scholar]
- 7.Stöllberger C, Finsterer J, Lutz W, Stöberl C, Kroiss A, Valentin A, et al. Multivariate analysis-based prediction rule for pulmonary embolism. Thromb Res. 2000;97:267–73. doi: 10.1016/s0049-3848(99)00180-2. [DOI] [PubMed] [Google Scholar]
- 8.Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: Increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83:416–20. [PubMed] [Google Scholar]
- 9.Le Gal G, Righini M, Roy PM, Sanchez O, Aujesky D, Bounameaux H, et al. Prediction of pulmonary embolism in the emergency department: The revised Geneva score. Ann Intern Med. 2006;144:165–71. doi: 10.7326/0003-4819-144-3-200602070-00004. [DOI] [PubMed] [Google Scholar]
- 10.Masotti L, Ceccarelli E, Cappelli R, Guerrini M, Forconi S. Pulmonary embolism in the elderly: Clinical, instrumental and laboratory aspects. Gerontology. 2000;46:205–11. doi: 10.1159/000022161. [DOI] [PubMed] [Google Scholar]
- 11.Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2011;123:2434–506. doi: 10.1161/CIR.0b013e31821daaf6. [DOI] [PubMed] [Google Scholar]
- 12.Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Eur Heart J. 2008;29:2276–315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 13.Masotti L, Ray P, Righini M, Le Gal G, Antonelli F, Landini G, et al. Pulmonary embolism in the elderly: A review on clinical, instrumental and laboratory presentation. Vasc Health Risk Manag. 2008;4:629–36. doi: 10.2147/vhrm.s2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klok FA, Karami Djurabi R, Nijkeuter M, Huisman MV. Alternative diagnosis other than pulmonary embolism as a subjective variable in the Wells clinical decision rule: Not so bad after all. J Thromb Haemost. 2007;5:1079–80. doi: 10.1111/j.1538-7836.2007.02475.x. [DOI] [PubMed] [Google Scholar]
- 15.Klok FA, Zidane M, Djurabi RK, Nijkeuter M, Huisman MV. The physician's estimation ‘alternative diagnosis is less likely than pulmonary embolism’ in the Wells rule is dependent on the presence of other required items. Thromb Haemost. 2008;99:244–5. doi: 10.1160/TH07-09-0560. [DOI] [PubMed] [Google Scholar]
- 16.Wolf SJ, McCubbin TR, Feldhaus KM, Faragher JP, Adcock DM. Prospective validation of Wells Criteria in the evaluation of patients with suspected pulmonary embolism. Ann Emerg Med. 2004;44:503–10. doi: 10.1016/j.annemergmed.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Penaloza A, Melot C, Motte S. Comparison of the Wells score with the simplified revised Geneva score for assessing pretest probability of pulmonary embolism. Thromb Res. 2011;127:81–4. doi: 10.1016/j.thromres.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Calisir C, Yavas US, Ozkan IR, Alatas F, Cevik A, Ergun N, et al. Performance of the Wells and Revised Geneva scores for predicting pulmonary embolism. Eur J Emerg Med. 2009;16:49–52. doi: 10.1097/MEJ.0b013e328304ae6d. [DOI] [PubMed] [Google Scholar]
- 19.Ceriani E, Combescure C, Le Gal G, Nendaz M, Perneger T, Bounameaux H, et al. Clinical prediction rules for pulmonary embolism: A systematic review and meta-analysis. J Thromb Haemost. 2010;8:957–70. doi: 10.1111/j.1538-7836.2010.03801.x. [DOI] [PubMed] [Google Scholar]
- 20.Stein PD, Hull RD, Patel KC, Olson RE, Ghali WA, Brant R, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: A systematic review. Ann Intern Med. 2004;140:589–602. doi: 10.7326/0003-4819-140-8-200404200-00005. [DOI] [PubMed] [Google Scholar]
- 21.Parent F, Maître S, Meyer G, Raherison C, Mal H, Lancar R, et al. Diagnostic value of D-dimer in patients with suspected pulmonary embolism: Results from a multicentre outcome study. Thromb Res. 2007;120:195–200. doi: 10.1016/j.thromres.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Liu ZH, Zhang HL, Luo Q, Zhao ZH, Zhao Q. Predictive value of D-dimer test for recurrent venous thromboembolism at hospital discharge in patients with acute pulmonary embolism. J Thromb Thrombolysis. 2011;32:410–6. doi: 10.1007/s11239-011-0625-2. [DOI] [PubMed] [Google Scholar]
- 23.Pasha SM, Klok FA, Snoep JD, Mos IC, Goekoop RJ, Rodger MA, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: A meta-analysis. Thromb Res. 2010;125:e123–7. doi: 10.1016/j.thromres.2009.11.009. [DOI] [PubMed] [Google Scholar]


