Abstract
Background:
Gastric cancer (GC) is one of the most prevalent malignancies in the world today, with a high mortality rate. CDX2 is a Drosophila caudal-related homeobox transcription factor that plays an important role in GC. Phosphatase and tensin homologue deleted from chromosome 10 (PTEN) is an important tumor suppressor which is widely expressed in normal human tissues. The aim of the study was to determine the relationship and mechanism between CDX2 and PTEN in invasion and migration of GC cells.
Methods:
pcDNA3-CDX2 plasmids were transfected into MGC-803 cells to up-regulate CDX2 protein, and small interfering RNA-CDX2 was transfected to down-regulate CDX2. The influence of CDX2 or PTEN on cell migration and invasion was measured by invasion, migration and wound healing assays. Western blotting assay and immunofluorescence were used to detect the expression of CDX2, PTEN, phosphorylation of Akt, E-cadherin and N-cadherin. Statistical significance was determined by one-way analysis of variance.
Results:
The results showed that CDX2 reduced the migration and invasion of GC cells (P < 0.05), and inhibited the activity of Akt through down-regulating PTEN expression (P < 0.05). CDX2 also restrained epithelial-mesenchymal transition of GC cells.
Conclusions:
CDX2 inhibited invasion and migration of GC cells by PTEN/Akt signaling pathway, and that may be used for potential therapeutic target.
Keywords: CDX2, Epithelial-mesenchymal Transition, Gastric Cancer, PTEN
INTRODUCTION
Gastric cancer (GC) is the fourth most common malignancy worldwide and the second most common cause of cancer-related deaths each year (10.4% of cancer deaths).[1] A total of 989,600 new stomach cancer cases and 738,000 deaths are estimated to have occurred in 2008, and over 70% of new cases and deaths occur in developing countries such as China.[2] Although the detailed mechanisms of gastric carcinogenesis are not yet fully understood, several associated environmental and genetic factors have been reported to play an important role in promoting GC, such as Helicobacter pylori infection, and mutation in the E-cadherin/CDH1 gene. GC has been classified histologically into either intestinal or diffuse types by the Lauren classification system.[3] The intestinal type cancer has been identified to evolve in apparently sequential steps that progress through chronic gastritis, atrophy, intestinal metaplasia, and dysplasia.[4] CDX2 is specifically expressed in the intestine, which makes it important for intestinal metaplasia of the stomach.
CDX2 is a Drosophila caudal-related homeobox transcription factor that regulates cellular processes such as cell differentiation, proliferation, cell adhesion, migration, and tumor genesis.[5] CDX2 is specifically expressed in the intestine, colon and intestinal metaplasia of the stomach, but not in the normal epithelium of the esophagus and stomach in adults.[6] CDX2 is important for the proliferation and differentiation of intestinal epithelial cells and maintenance of the intestinal phenotype. CDX2 is involved in the pathogenesis of gastric intestinal metaplasia and GC. According to a study, CDX2 expression is detected in 87.1% of intestinal metaplasia, 48.2% of GC.[7] Immunohistochemical analysis demonstrates that the expression of CDX2 was significantly higher in intestinal-type cancer than in diffuse type. The patients with CDX2-positive expression showed a lower proliferation rate and a higher survival rate than those with CDX2-negative expression.[8] Therefore, CDX2 is connected with intestinal metaplasia and the intestinal-type GC. CDX2 is a useful marker for the prognosis of GC.
A phosphatase and tensin homologue deleted from chromosome 10 (PTEN) is an important tumor suppressor gene that is widely expressed in normal human tissues. PTEN is involved in the control of cell growth, proliferation, migration and apoptosis.[9] The PTEN gene is mutated in a wide kind of human cancers including glioblastoma, hysterocarcinoma, breast cancer, prostate cancer and GC prognosis.[10,11] Several researches indicated that PTEN plays a critical role in apoptosis, proliferation and metastasis of tumor cells, and promotes intestinal differentiation.[12,13] The PTEN protein antagonizes PI3K activity and inhibits the downstream signaling pathway through Akt. Inhibition of Akt protein leads to activation of Caspase-9, which is associated with cell apoptosis. The intestinal CDX2 homeobox gene is a target of PTEN/phosphatidylinositol 3-kinase signaling and tumor necrosis factor α signaling via Nuclear Factor κB–dependent pathways.[14] CDX2 plays an important role in the differentiation, inhibiting proliferation, and increasing sensitivity to apoptosis of GC, and PTEN is involved in tumor invasion and migration.[15]
This study aimed to investigate the possible role of CDX2 and PTEN in GC, and to discover its involvement to the invasion and migration of cancer. We overexpressed CDX2 and PTEN in MGC-803 cells, and suppressed CDX2 and PTEN in NCI-N87 cells to determine if CDX2 and PTEN contributed to tumor epithelial-mesenchymal transition (EMT).
METHODS
Reagents
MGC-803 and NCI-N87 cells were purchased from American Type Culture Collection. Dulbeco's Modified Eagle's Medium (DMEM) was purchased from Hyclone (Logan, UT, USA), fetal bovine serum, Lipofectamine 2000, and transwell chambers were purchased from Invitrogen (Carlsbad, CA, USA), Matrigel was purchased from BD Biosciences (NY, USA), CDX2, PTEN, E-cadherin, N-cadherin, GAPDH, and immunoglobulin G (IgG) antibodies were purchased from Santa Cruz Biotechnology (CA, USA), phosphorylation of Akt (pAkt) was purchased from Abcam (Cambridge, MA USA), immobilon western chemilumihescent horseradish peroxidase (HRP) substrate was purchased from Millipore (Billerica, MA, USA), and DAPI was purchased from Sigma (St. Louis, MO, USA).
Plasmid construction
The coding sequence (CDS) region of human CDX2 gene was amplified from cDNA, digested with BamHI/HindIII and cloned into pcDNA3 vector. Similarly, human PTEN CDS was digested with XhoI/BamHI, and cloned into pcDNA3 vector. The resulting constructs were confirmed by DNA sequencing.
Cell culture and transfection
MGC-803 and NCI-N87 cells were cultured in DMEM supplemented with 10% fetal bovine serum at 37°C in a humidified atmosphere containing 5% CO2. The CDX2-expressing vectors (pcDNA3-CDX2) were transfected into MGC-803 cells by using Lipofectamine 2000 transfection reagent.
CDX2 gene silencing
The small interfering RNA (siRNA) was synthesized from Invitrogen. The target sequence (5’-AACCAGGACGAAAGACAAAUA-3’) was used to down-regulate CDX2 in vitro. A nontarget siRNA sequence was used as a negative control. Lipofectamine 2000 transfection reagent was used for transfection of siRNAs.
Targeted and nontargeted siRNA was transfected into cells at a final concentration of 25 nmol/L and cells were incubated for 24 h before the medium was exchanged. The total time of incubation with siRNA was 72 h.
Invasion and migration assay
Cell invasion and migration assays were performed using Transwell chambers. 2 × 105 cells were seeded into the upper chamber in a serum-free DMEM. For invasion assay, the upper chamber was coated with Matrigel and incubated overnight before starting the invasion assay. The lower chamber was filled with 10% serum DMEM, which acted as a chemo-attractant. Cells were further incubated at 37°C in 5% CO2 for 24 h. The passed cells on the lower surface of the membrane were fixed and stained with crystal violet solution. Cells was counted under the light microscope (×10 objective) from 10 random fields for each well. Migration assay was performed as for the invasion assay described above with no Matrigel precoated.
Wound healing assay
In wound healing assay, the cells were cultured to 80–90% confluence in six-well plates. A 200 μl sterile pipet tip was used to make a central linear wound, and the plates were washed with PBS to remove the floating cells. Micrographs were taken at 0 and 48 h. The speed of wound closure was analyzed by measuring the distance of the wound edge to the original wound site. Each experiment was performed in triplicate.
Western blotting analysis
Cells were lysed at 4°C with RIPA lysis buffer containing 1 μmol/L PMSF. Equal amounts of protein were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and electrotransferred onto polyvinylidene fluoride membrane. The membrane was sealed with PBS containing 5% Skimmed milk powder for 1 h and incubated with primary antibodies (CDX2, PTEN, E-cadherin, N-cadherin, GAPDH, and pAkt) at 4°C overnight. The membrane was washed with PBST (PBS, pH 7.5, containing 0.1% Tween-20) and incubated with a secondary antibody for 1 h. Anti-mouse or anti-rabbit antibodies against IgG conjugated with HRP were adopted as the secondary antibodies. Immobilon western chemilumihescent HRP substrate was used to detect peroxidase activity.
Immunofluorescence
Cells adhered to chamber slides and grew to 50–60% confluence, then fixed with cold methanol. The slides were incubated 1 h at 37°C with the primary antibodies (E-cadherin and N-cadherin) and secondary antibodies. The FITC- and TRITC-conjugated mouse and rabbit IgG antibody were used as labels for immunofluorescence assay. After immunolabeling, cells were washed, stained with DAPI, mounted, and then viewed with fluorescent microscopy (Nikon, Japan).
Statistical analysis
Results were expressed as mean ± standard deviation (SD). All data analyses were performed by one-way analysis of variance (ANOVA) using SPSS version 19 software (IBM, Armonk, NY, USA). Differences were considered statistically significant at values of P < 0.05.
RESULTS
CDX2 inhibits migration and invasion of gastric cancer cells
Given the up-regulated cell model in MGC-803 and knockdown cell model in NCI-N87, we studied the invasion and migration ability of MGC-803 and NCI-N87 after performing CDX2 over-expression or knockdown, respectively. As presented in Figure 1a and b, significant decrease of cell invasion and migration was observed in the CDX2-transfected MGC-803 cells compared with the negative vector control (P < 0.05). We also observed an obvious increase in CDX2-siRNA–transfected NCI-N87 cells compared with the negative vector control (P < 0.05, Figure 1e and f).
Figure 1.
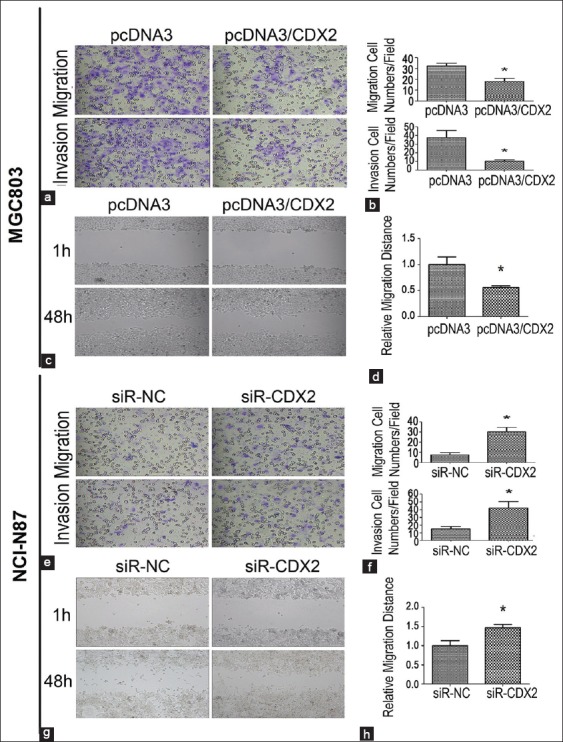
Effect of CDX2 overexpression and knockdown on invasion and migration abilities in gastric cancer cells. (a and b) The CDX2 overexpression reduced the migration and invasion abilities of MGC-803 cells; (c and d) In the wound healing assay, the MGC-803/CDX2 cells exhibited decreased migration ability; (e and f) The down-regulated CDX2 expression significantly increased the migration and invasion abilities; (g and h) The results of wound healing assay revealed the NCI-N87/siR-CDX2 cells decreased the migration ability. Results were presented as mean ± standard deviation of three independent experiments, *P < 0.05.
As shown in Figure 1c and d, following a wound healing assay, the CDX2-transfected MGC-803 cells showed about a two-fold decreased migration distance than the negative control (P < 0.05). The analysis also suggested a three-fold increase in the speed of wound healing in CDX2-siRNA–transfected NCI-N87 cells compared with the negative vector control (P < 0.05, Figure 1g and h). The results proved that CDX2 inhibits the migration and invasion ability of GC cells.
CDX2 impacts the expression of PTEN and the activity of Akt
Transfection of pcDNA3/CDX2 plasmid revealed that CDX2 was overexpressed in MGC-803 cells (P < 0.05). Compared with the negative control, PTEN expression significantly increased in MGC-803/CDX2 cells (P < 0.05, Figure 2a and b). The expression of the pAkt decreased in the transfection of CDX2 in MGC-803 cells compared with the negative vector control (P < 0.05, Figure 2a and b). Obviously, the overexpression of CDX2 induced PTEN expression and repressed Akt activation in MGC-803 cells. Similarly, Western blotting analysis in CDX2-siRNA–transfected NCI-N87 cells showed the corresponding experimental results [Figure 2c and d].
Figure 2.
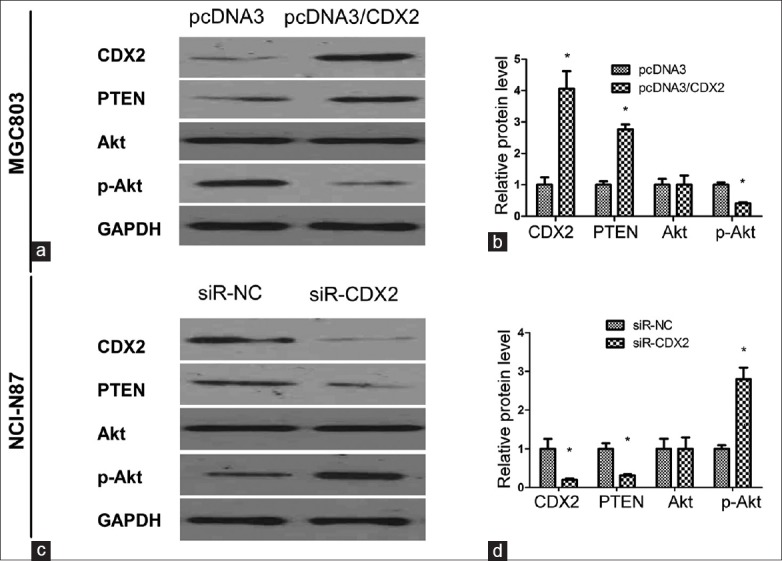
Detection of PTEN and phosphorylation of Akt (pAkt) expression with up-regulated and down-regulated CDX2 by Western blotting assay. (a and b) Detection of expressed CDX2, PTEN, Akt, and pAkt in MGC-803/CDX2 cells. The increased levels of CDX2 led to increased PTEN expression and decreased Akt activation; (c and d) The expression of CDX2, PTEN, Akt, and pAkt in NCI-N87/siR-CDX2 cells were detected. The repressed CDX2 expression down-regulated the PTEN protein and up-regulated the phosphorylation of Akt. Results were presented as mean ± standard deviation, n = 3, *P < 0.05.
CDX2 determines the epithelial phenotype of gastric cancer
Epithelial cells can convert into mesenchymal cells through a process known as EMT. EMT contributes to cancer invasion and metastasis. EMT markers include E-cadherin, cytokeratin and occludin (down-regulating during EMT); and N-cadherin, vimentin, and fibronectin (up-regulating during EMT). Immunofluorescence and Western blotting assay revealed increased E-cadherin and decreased N-cadherin in MGC-803/CDX2 cells compared with the negative control (P < 0.05, Figure 3a–c). For NCI-N87 cells, knockdown CDX2 by siRNA decreased E-cadherin expression and increased N-cadherin expression detected with immunofluorescence and Western blotting (P < 0.05, Figure 3d–f).
Figure 3.
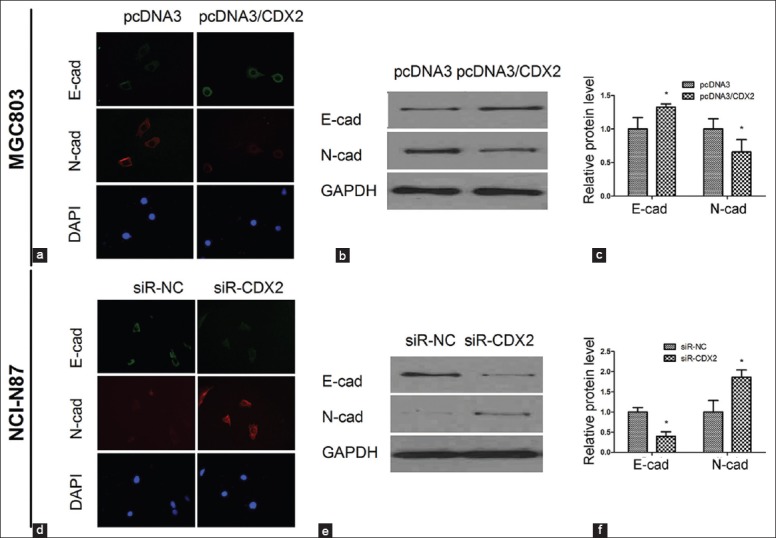
The expression of epithelial-mesenchymal transition markers was impacted by CDX2. (a) Immunofluorescence images of E-cadherin and N-cadherin in MGC-803/CDX2 cells; (b and c) Western blotting assay presented that E-cadherin expression up-regulated and N-cadherin expression down-regulated by CDX2 overexpression; (d) Immunofluorescence images of E-cadherin and N-cadherin in NCI-N87/siR-CDX2 cells; (e and f) With CDX2 down-regulated in NCI-N87/siR-CDX2 cells, the expression of E-cadherin was decreased, while the expression of N-cadherin was increased. Results were presented as mean ± standard deviation, n = 3, *P < 0.05.
CDX2 inhibits invasion, migration and epithelial-mesenchymal transition of gastric cancer by targeting PTEN
We used MGC-803/PTEN cells and NCI-N87/siR-PTEN cells to investigate the effect of PTEN on cell invasion and migration. The migration and invasion cell numbers per field were about two-fold and 1.9-fold less in MGC-803/PTEN cells than that of the negative control cells, respectively (P < 0.05, Figure 4a and b). We also observed an obvious increase of invasion and migration in PTEN-siRNA–transfected NCI-N87 cells compared with the negative vector control (P < 0.05, Figure 4e and f). In the wound healing assay, cell migration rate of MGC-803/PTEN cells was significantly lower than the negative control (P < 0.05, Figure 4c and d). Moreover, the cell migration rate of NCI-N87/siR-PTEN cells was about 1.5-fold higher than the negative control (P < 0.05, Figure 4g and h).
Figure 4.
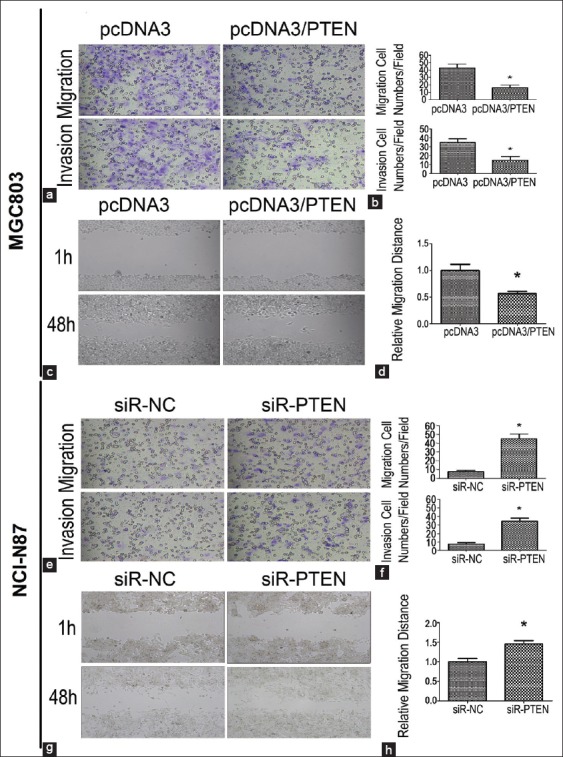
Effect of phosphatase and tensin homologue deleted from chromosome 10 (PTEN) on invasion and migration abilities in gastric cancer cells. (a and b) With overexpression of PTEN in MGC-803 cells, the migration and invasion abilities were repressed compared with the negative control; (c and d) The migration distance was lower in MGC-803/PTEN cells than the negative control; (e and f) The down-regulated PTEN in NCI-N87/siR-PTEN cells significantly increased the migration and invasion abilities compared with the negative control; (g and h) With the down-regulated PTEN protein, the higher migration distance was detected. Results were presented as mean ± standard deviation, n = 3, *P < 0.05.
In the Western blotting assay, overexpression or knockdown the PTEN protein had no effect on the CDX2 expression [Figure 5]. We detected the expression of EMT marker E-cadherin and N-cadherin in GC cells with PTEN up- or down-regulated. Figure 5 showed that the overexpression of PTEN resulted in an increased expression of E-cadherin and a decreased expression of N-cadherin (P < 0.05, Figure 5a and b), indicative of EMT. While we down-regulated PTEN expression in NCI-N87/siR-PTEN cells, E-cadherin expression decreased and N-cadherin expression increased compared with the negative control (P < 0.05, Figure 5c and d).
Figure 5.
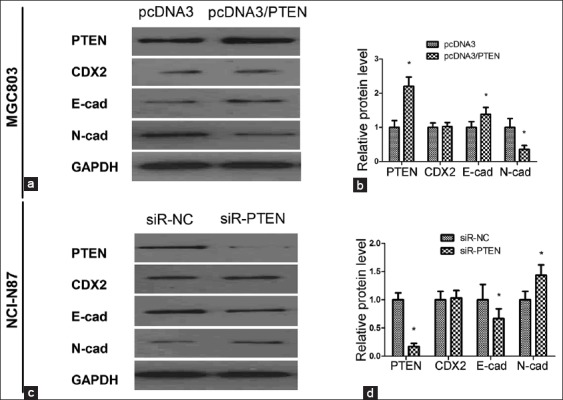
Western blotting assay of CDX2, E-cadherin and N-cadherin in MGC-803/phosphatase and tensin homologue deleted from chromosome 10 (PTEN) cells and NCI-N87/siR-CDX2 cells; (a and b) The up-regulated PTEN had no effect on CDX2 expression, but increased the expression of E-cadherin, and meanwhile decreased the N-cadherin compared with the negative control; (c and d) The down-regulated PTEN in NCI-N87/siR-CDX2 cells led to the decrease of E-cadherin and increase of N-cadherin compared with the negative control. Results were presented as mean ± standard deviation, n = 3, *P < 0.05.
DISCUSSION
In the present study, we constructed recombinant pcDNA3-CDX2 plasmids and successfully transfected these plasmids into human GC MGC-803 cells. Our results clearly showed that CDX2 overexpression in MGC-803 cells inhibited GC cell migration and invasion. We found that the transfection of NCI-N87 cells with CDX2 siRNA could knock down the expression of CDX2 and promote cell invasion and migration in vitro. These data showed that CDX2 played an important role in GC cell invasion and migration.
We found that expression of PTEN was increased when CDX2 was transfected into MGC-803 cells. And also, when CDX2 gene was knocked down, the expression of PTEN was decreased. So, we transfected pcDNA3-PTEN into MGC-803 cells, and found that the abilities of invasion and migration were inhibited, but the expression of CDX2 was not changed. Thus, we concluded that CDX2 acted as a potential tumor suppressor in GC, whose function was connected with PTEN.
Phosphatase and tensin homologue deleted from chromosome 10 is a tumor suppressor gene with phosphatase activity, in which mutations can often promote tumorigenicity.[15] Our study revealed that when CDX2 protein was overexpressed, expression of PTEN increased, while pAkt expression declined. Furthermore, if CDX2 was down-regulated, PTEN expression was decreased, while the expression of pAkt increased. PI3K/Akt pathway played an important role in tumor cell invasion. In PI3K/Akt pathway, Rac1 acted to modulate cell adhesion properties, Akt induced MMP-9 production, and both Rac1 and Akt promote cell migration and invasion.[16] PTEN can dephosphorylate PIP3 to reduce the level of phosphorylation Akt, and then inhibited the invasion and migration of cancer cells. This suggested that CDX2 inhibited the invasion and migration through the PTEN-PI3K/Akt pathway in the GC cells.
Epithelial-mesenchymal transition is a key reversible step that facilitates tumor migration, invasion and metastasis.[17,18] And inhibition of EMT is an attractive therapeutic approach that can significantly alter disease outcome.[19] Our studies examined the E-cadherin and N-cadherin expression with respect to CDX2 up-regulation or knockdown in MGC-803 and NCI-N87 cells. Data showed that E-cadherin expression was up-regulated and N-cadherin was down-regulated following CDX2 expression up-regulation; down-regulated CDX2 also resulted in decreased E-cadherin and increased N-cadherin. Concomitantly, PTEN overexpression revealed up-expression of E-cadherin and down-expression of N-cadherin, all of which are consistent with EMT.
In the present study, we found that in GC, up-regulated CDX2 restrained EMT and down-regulated CDX2 promoted EMT, while the overexpression of PTEN inhibited EMT. CDX2 may regulate GC cell EMT via PTEN. In general, CDX2 was identified as an inhibitor of EMT.
In conclusion, CDX2 was up-regulated leading to inhibited migration and invasion of GC cells, and, up-regulation of CDX2 restrained the development of EMT in GC via inhibition of PTEN expression. In conclusion, our study showed that CDX2 can decrease migratory, invasive and EMT behaviors by increasing the expression of PTEN and attenuating the activity of Akt. Our findings indicated that the restoration of the tumor suppressor CDX2 might be useful in the anti-GC therapy.
Footnotes
Edited by: Yuan-Yuan Ji
Source of Support: This study was supported by grants from the Beijing Natural Science Foundation (No. 7112034 and No. 7122052).
Conflict of Interest: None declared.
REFERENECES
- 1.Parkin DM. International variation. Oncogene. 2004;23:6329–40. doi: 10.1038/sj.onc.1207726. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Lauren P. the two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 4.Correa P. Human gastric carcinogenesis: A multistep and multifactorial process – First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–40. [PubMed] [Google Scholar]
- 5.Coskun M, Troelsen JT, Nielsen OH. The role of CDX2 in intestinal homeostasis and inflammation. Biochim Biophys Acta. 2011;1812:283–9. doi: 10.1016/j.bbadis.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Hryniuk A, Grainger S, Savory JG, Lohnes D. Cdx1 and Cdx2 function as tumor suppressors. J Biol Chem. 2014;289:33343–54. doi: 10.1074/jbc.M114.583823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Y, Li L, Wang X, Qin Y, Qian Q, Yuan X, et al. Overexpression of Cdx2 inhibits progression of gastric cancer in vitro. Int J Oncol. 2010;36:509–16. [PubMed] [Google Scholar]
- 8.Qin R, Wang NN, Chu J, Wang X. Expression and significance of homeodomain protein Cdx2 in gastric carcinoma and precancerous lesions. World J Gastroenterol. 2012;18:3296–302. doi: 10.3748/wjg.v18.i25.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gil A, Andrés-Pons A, Fernández E, Valiente M, Torres J, Cervera J, et al. Nuclear localization of PTEN by a Ran-dependent mechanism enhances apoptosis: Involvement of an N-terfsecal nuclear localization domain and multiple nuclear exclusion motifs. Mol Biol Cell. 2006;17:4002–13. doi: 10.1091/mbc.E06-05-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–70. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 11.Bai Z, Ye Y, Chen D, Shen D, Xu F, Cui Z, et al. Homeoprotein Cdx2 and nuclear PTEN expression profiles are related to gastric cancer prognosis. APMIS. 2007;115:1383–90. doi: 10.1111/j.1600-0463.2007.00654.x. [DOI] [PubMed] [Google Scholar]
- 12.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–63. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 13.Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–7. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Domon-Dell C, Wang Q, Chung DH, Di Cristofano A, Pandolfi PP, et al. PTEN and TNF-alpha regulation of the intestinal-specific Cdx-2 homeobox gene through a PI3K, PKB/Akt, and NF-kappaB-dependent pathway. Gastroenterology. 2002;123:1163–78. doi: 10.1053/gast.2002.36043. [DOI] [PubMed] [Google Scholar]
- 15.Bai ZG, Ye YJ, Shen DH, Lu YY, Zhang ZT, Wang S. PTEN expression and suppression of proliferation are associated with Cdx2 overexpression in gastric cancer cells. Int J Oncol. 2013;42:1682–91. doi: 10.3892/ijo.2013.1875. [DOI] [PubMed] [Google Scholar]
- 16.Kim D, Kim S, Koh H, Yoon SO, Chung AS, Cho KS, et al. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 2001;15:1953–62. doi: 10.1096/fj.01-0198com. [DOI] [PubMed] [Google Scholar]
- 17.Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–56. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funakoshi S, Kong J, Crissey MA, Dang L, Dang D, Lynch JP. Intestine-specific transcription factor Cdx2 induces E-cadherin function by enhancing the trafficking of E-cadherin to the cell membrane. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1054–67. doi: 10.1152/ajpgi.00297.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ezaki T, Guo RJ, Li H, Reynolds AB, Lynch JP. The homeodomain transcription factors Cdx1 and Cdx2 induce E-cadherin adhesion activity by reducing beta- and p120-catenin tyrosine phosphorylation. Am J Physiol Gastrointest Liver Physiol. 2007;293:G54–65. doi: 10.1152/ajpgi.00533.2006. [DOI] [PubMed] [Google Scholar]


