Abstract
Background:
Calpain, a calcium-dependent cysteine protease, has been demonstrated to regulate osteoclastogenesis, which is considered one of the major reasons for cancer-induced bone pain (CIBP). In the present study, calpain inhibitor was applied in a rat CIBP model to determine whether it could reduce CIBP through regulation of osteoclastogenesis activity.
Methods:
A rat CIBP model was established with intratibial injection of Walker 256 cells. Then, the efficacy of intraperitoneal administered calpain inhibitor III (MDL28170, 1 mg/kg) on mechanical withdrawal threshold (MWT) of bilateral hind paws was examined on postoperative days (PODs) 2, 5, 8, 11, and 14. On POD 14, the calpain inhibitor's effect on tumor bone tartrate-resistant acid phosphatase (TRAP) stain and radiology was also carefully investigated.
Results:
Pain behavioral tests in rats showed that the calpain inhibitor effectively attenuated MWTs of both the surgical side and contralateral side hind paws on POD 5, 8, and 11 (P < 0.05). TRAP-positive cell count of the surgical side bone was significantly decreased in the calpain inhibitor group compared with the vehicle group (P < 0.05). However, bone resorption and destruction measured by radiographs showed no difference between the two groups.
Conclusions:
Calpain inhibitor can effectively reduce CIBP of both the surgical side and nonsurgical side after tumor injection in a rat CIBP model. It may be due to the inhibition of receptor activator of nuclear factor-kappa B ligand-induced osteoclastogenesis. Whether a calpain inhibitor could be a novel therapeutic target to treat CIBP needs further investigation.
Keywords: Calpain, Cancer-induced Bone Pain, Inhibitor, Osteoclastogenesis
INTRODUCTION
One of the most common causes of pain in patients with malignant disease is cancer-induced bone pain (CIBP), affecting patients with primary bone sarcomas and those with bone metastases.[1,2] The pain consists of a triad of states; background pain, spontaneous pain, and movement-induced (incident) pain.[3] Current interventions including antiinflammatory drugs, opioids, bisphosphonates and radiation are often effective in controlling tonic pain, but not incident pain, calling for new therapeutic methods to be developed.[4,5]
The mechanism of CIBP still remains unclear. Bone remodeling and its homeostasis are delicately maintained via bone resorption by osteoclasts and bone formation by osteoblasts.[6] It is suggested that bone destruction caused by unbalanced osteoclastogenesis in the tumor bone microenvironment could be a major reason for CIBP.[7,8,9] Besides the classic antiresorptive agent bisphosphonate, other therapies targeting osteoclasts are also under extensive investigation for alleviating tumor-induced bone remodeling. It is proven that the receptor activator of nuclear factor-kappaB ligand (RANKL) is the main driver of osteoclast formation, function, and survival.[8,9] Sequestration of RANKL with OPG attenuates CIBP, bone remodeling, and tumor growth within the bone.[10] Moreover, currently phase III trials have already been completed and have proven that denosumab, a humanized RANKL monoclonal antibody, can effectively reduce bone absorption and skeletal-related events, including CIBP.[11,12,13]
Calpains are a family of calcium-dependent intracellular cysteine protease that catalyze the limited cleavage of specific proteins during regulatory signaling. They play a major role in various cellular processes, such as signal transduction, cell growth, differentiation and fusion, apoptosis, necrosis, etc.[12] Among them, μ-calpain is known to degrade various substrates, particularly IκBα, c-Jun, and c-Fos, all of which are essential molecular players in osteoclastogenesis.[13,14,15,16] Our previous study has already shown that a calpain inhibitor can significantly attenuate RANKL-induced osteoclastogenesis in murine RAW264.7 cells in vitro.[14] Therefore, calpain might play a pivotal role in the regulation of osteoclastogenesis. However, whether such an effect exists in an in vivo animal model and its mechanisms are yet to be tested. The aim of this study was to determine the efficacy of a calpain inhibitor on bone resorption and behavioral responses to pain in vivo in intratibial tumor injected CIBP rats.
METHODS
All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Ethical Issues of the IASP and were approved by the Chinese Academy of Medical Sciences/Peking Union Medical College Hospital Committee on Animal Care.
Induction of bone cancer
Adult male Sprague-Dawley rats (240–270 g) used for the study were housed under a 12-h light/dark cycle in a pathogen-free area with ad libitum access to water and food. Walker 256 breast cancer cells were kindly provided by the Department Pharmacology, Institute of Pharmacology of Chinese Academy of Medical Sciences. The surgical procedure was the same as described previously.[15] Briefly, anesthesia was induced and maintained via a nose cone with halothane (1.5–2.0%). The right hind leg was shaved and disinfected with 70% (v/v) ethanol. The medullary canal of the tibia was approached from a 1-cm long skin incision, by inserting a 23-gauge needle through a drilled hole. A 10-μl volume of Walker 256 cells (5 × 105 cells) was injected into the bone cavity. The hole was sealed using bone wax, and the wound was irrigated with Gentamicin saline and was closed with 1–0 silk threads. The procedure was identical for the sham group except for injection with saline alone. The rats were allowed unrestricted movement in their cages after recovery and carefully monitored.
Drug administration
Immediately after the operation, calpain inhibitor III (MDL 28170, Calbiochem Merck, Darmstadt, Germany) dissolved in 0.1% dimethyl sulfoxide (DSMO) was given at 1 mg/kg (in a volume of 8 ml/kg body weight) intraperitoneally for the inhibitor group, and 0.1% DSMO solution only was given at 10 ml/kg to the vehicle group. Rats were divided into seven groups: (1) Sarcoma group (n = 9): Received intratibial injection of Walker 256 cells; (2) sarcoma + inhibitor group (n = 9): Received intratibial injection of Walker 256 cells and intraperitoneal injection of calpain inhibitor III every day postoperatively; (3) sarcoma + vehicle group (n = 9): Received intratibial injection of Walker 256 cells and intraperitoneal injection of 0.1% DSMO every day postoperatively; (4) sham group (n = 9): Received intratibial injection of saline; (5) sham + inhibitor group (n = 9): Received intratibial injection of saline and intraperitoneal injection of calpain inhibitor III every day postoperatively; (6) sham + vehicle group (n = 9): Received intratibial injection of saline and intraperitoneal injection of 0.1% DSMO every day postoperatively; (7) naïve group (n = 9): No inoculation and no treatment.
Behavioral testing
Animals were left to acclimatize to the area for 30 min before testing. Behavioral signs of mechanical hyperalgesia and cold allodynia were assessed preoperatively and on postoperative days (PODs) 2, 5, 8, 11 and 14. Mechanical sensitivity was assessed by application of four von Frey filaments with increasing bending forces of 1, 5, 9, and 15 g (electronic von Frey anesthesiometer; IITC, Woodland Hills, CA, USA). The nociceptive stimulus was applied perpendicularly to the medial surface of the hind paw with increasing forces. The endpoint was taken as nocifensive paw withdrawal accompanied by head turning, biting and/or licking, and the required pressure was considered the mechanical withdrawal threshold (MWT) value. Bilateral hind paws of each rat were tested in triplicate at each time point, and the average for the three measurements was then calculated. Cold allodynia was assessed by five applications of 100 μl of acetone to the plantar surface of the hind paw. As with mechanical testing, this was carried out on bilateral hind paws, with each application separated by at least 5 min. The number of lifts observed of the maximum total of five was expressed as percentage response.
Calpain activity assay
Bone powder was used to measure calpain activity using a calpain activity assay kit (Biovision, Mountain View, CA, USA). Tumor bone tissue was carefully ground into powder in liquid nitrogen. Then the bone powder was lysed with extraction buffer in the kit on ice for 30 min. Tissue lysates were centrifuged at 12,000 × g at 4°C for 15 min, and the supernatants were collected. After quantification of protein in the supernatant with the biobarcode assay kit (Qcbio S and T, Beijing, China), 100 μg of protein was used for the calpain activity assay with Ac-LLY-AFC, a fluorescent calpain substrate, in an all-dark fluorescent microplate at 37°C for 1 h. The samples were read in a fluorometer equipped with 400 nm excitation and 505 nm emission filters.
Tartrate-resistant acid phosphatase staining
Formalin-fixed and paraffin-embedded tumor bone tissue was prepared. Tissue sections were then fixed with a solution of formaldehyde, acetone and citrate for 30 s, and incubated for 40 min at 37°C in a staining solution provided in the tartrate-resistant acid phosphatase (TRAP) staining kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer's instructions. TRAP-positive cells with three or more nuclei were counted as multinucleated osteoclasts under ×40 light microscope.
Radiology
The extent of sarcoma induced bone destruction was examined by X-ray assessment of bilateral hind paws of anesthetized rats on POD 14. The conditions of X-ray exposure were 56 kV, 200 mA, and 20 ms. According to the bone destruction standard reported by Honore et al.,[16] radiographs of tumor-bearing tibia revealed loss of bone that was quantified on a 0–5 scale: 0 – normal bone; 1 – 1–3 spotted bone resorption sites; 2 – 4–6 spotted bone resorption sites with minor loss of bone in the medullary canal; 3 – substantial loss of bone in the medullary canal with some destruction of the distal cortical bone; 4 – total destruction and discontinuity of cortical bone; 5 – total destruction and discontinuity of cortical bone with displaced fractures. The scale of the bilateral tibia bone of each group was examined and recorded by the same investigator.
Statistical analysis
Data are presented as mean ± standard deviation unless otherwise stated. For quantitative data, differences between treated and control groups were analyzed with one-way ANOVA test using the Statistical Package for the Social Sciences (SPSS) software (version 17.0, SPSS Inc., USA). For semi-quantitative data, statistical analyses were performed using the Kruskal–Wallis H-test. A P < 0.05 was set as the level of statistical significance.
RESULTS
Calpain activity was successfully suppressed with calpain inhibitor in cancer-induced bone pain rats in vivo
Whether calpain activity was inhibited after intraperitoneal injection of calpain inhibitor in the established CIBP model was essential to this study. Therefore, total protein of the tumor injected tibia was extracted, and calpain activity was measured in all groups. The results showed that the calpain activity in the sarcoma + inhibitor group and sham + inhibitor group were significantly decreased compared with the other groups (P < 0.05) [Figure 1], providing the foundation for further explaining the effect of calpain inhibition.
Figure 1.
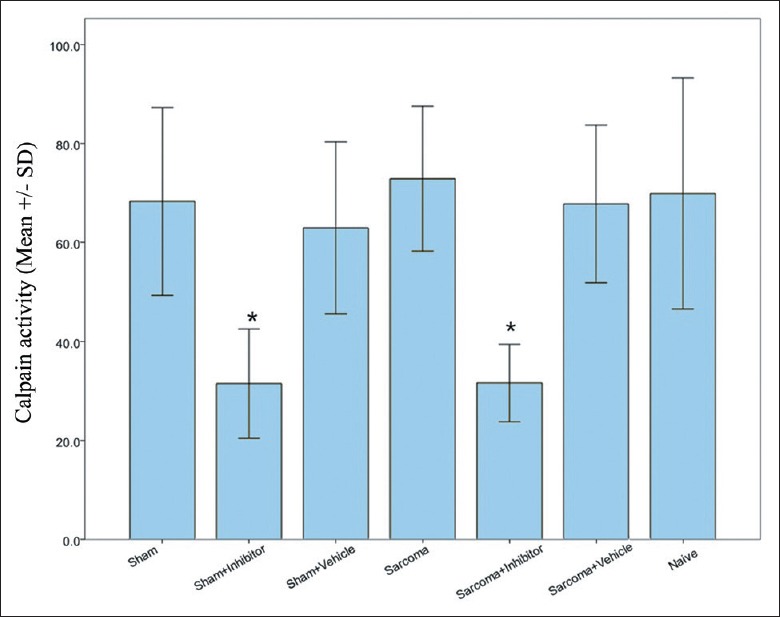
Calpain activity in the surgical hind paws of each group. *P < 0.05 for both sham + inhibitor group and sarcoma + inhibitor group versus naïve group.
Mechanical withdrawal thresholds recovered after calpain inhibition in both the surgical and nonsurgical hind paws in cancer-induced bone pain rats in vivo
Since activated osteoclastogenesis is one of the reasons for CIBP, the effect of calpain inhibitor on the pain behavioral level in vivo was tested. After establishing the rat CIBP model, the MWTs of both the tumor injected side and the contralateral side hind paws of all groups were recorded before surgery and on POD 2, 5, 8, 11 and 14. For the surgical side, after calpain inhibition, the MWT of the sarcoma + inhibitor group recovered and was significantly higher than that of the sarcoma + vehicle group on POD 5, 8 and 11 (P < 0.05), but it was not normalized to the level of the naïve group (P < 0.05) [Figure 2a]. For the nonsurgical side, the result was surprisingly similar. The MWT of the sarcoma + inhibitor group was also increased compared with the sarcoma + vehicle group (P < 0.05) but was still lower than the naïve group on POD 8, 11 and 14 (P < 0.05) [Figure 2b]. The behavioral score of cold allodynia was also examined on bilateral hind paws. However, there was no difference between the sarcoma + inhibitor group and the sarcoma + vehicle group from POD 2 to 14 (results not shown).
Figure 2.
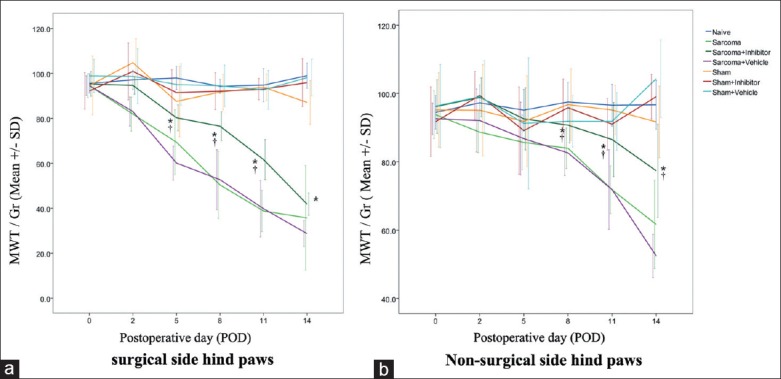
Mechanical withdrawal threshold of surgical side (a) and nonsurgical side (b) hind paws of each group. *P < 0.05 for sarcoma + inhibitor versus naïve. †P < 0.05 for sarcoma + inhibitor versus sarcoma + vehicle.
Tartrate-resistant acid phosphatase positive cell count was decreased after calpain inhibition in cancer-induced bone pain rats in vivo
To determine whether the calpain inhibitor can suppress RANL-induced osteoclastogenesis in vivo, the tibia bone tissue of all groups were subjected to TRAP staining. The results of the Sarcoma group and sarcoma + vehicle group clearly showed more fused multi-nucleated cells, meaning the osteoclastogenesis process was activated after tumor injection [Figure 3a]. However, after calpain inhibition, the TRAP-positive cell count was significantly decreased in the sarcoma + inhibitor group comparing with the sarcoma + vehicle group (P < 0.05) [Figure 3b], indicating that the osteoclastogenesis process could be blocked with calpain inhibitor in vivo in the CIBP rats.
Figure 3.
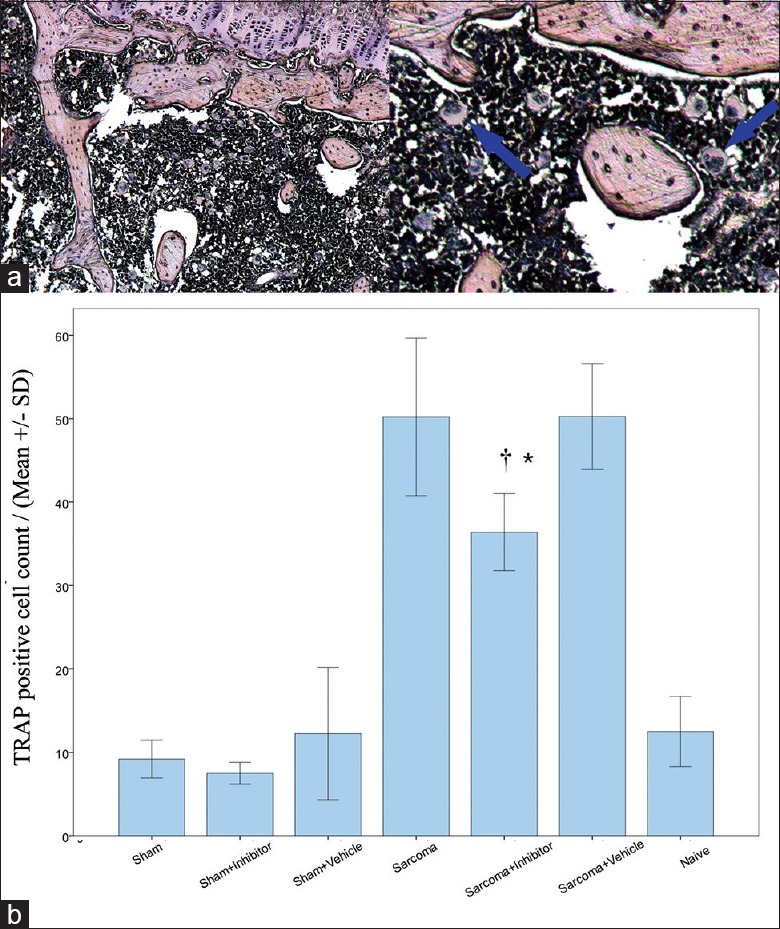
(a) Tartrate-resistant acid phosphatase (TRAP) stain of matured osteoclasts in tumor bone site (left: Original magnification, ×100, right: Original magnification, ×200); (b) TRAP positive cell count in tumor injected tibia bone of each group. *P < 0.05 for sarcoma + inhibitor versus naïve. †P < 0.05 for sarcoma + inhibitor versus sarcoma + vehicle.
Calpain inhibition could not reduce tumor induced bone resorption and destruction in cancer-induced bone pain rats
On POD 14, CIBP rats of all groups were subjected to the radiology assessment. As shown in the tibia X-ray radiographs in Figure 4a, the tibia bones of the “sarcoma groups,” including the Sarcoma, sarcoma + inhibitor and sarcoma + vehicle groups, exhibited pathological bone characteristics such as high density mass or low density defects in the bone and cortical bone discontinuity. On the contrary, tibia bones of the sham group, naïve group, and nonsurgical side legs of all groups appeared normal. After examining the scale of the radiographs, the “sarcoma groups” had higher scores than the naïve group and a sham group (P < 0.01) [Figure 4b]. However, there was no significant difference between the sarcoma + inhibitor group and sarcoma + vehicle group, indicating no anti-bone destruction effect of calpain inhibition in CIBP rats in vivo.
Figure 4.
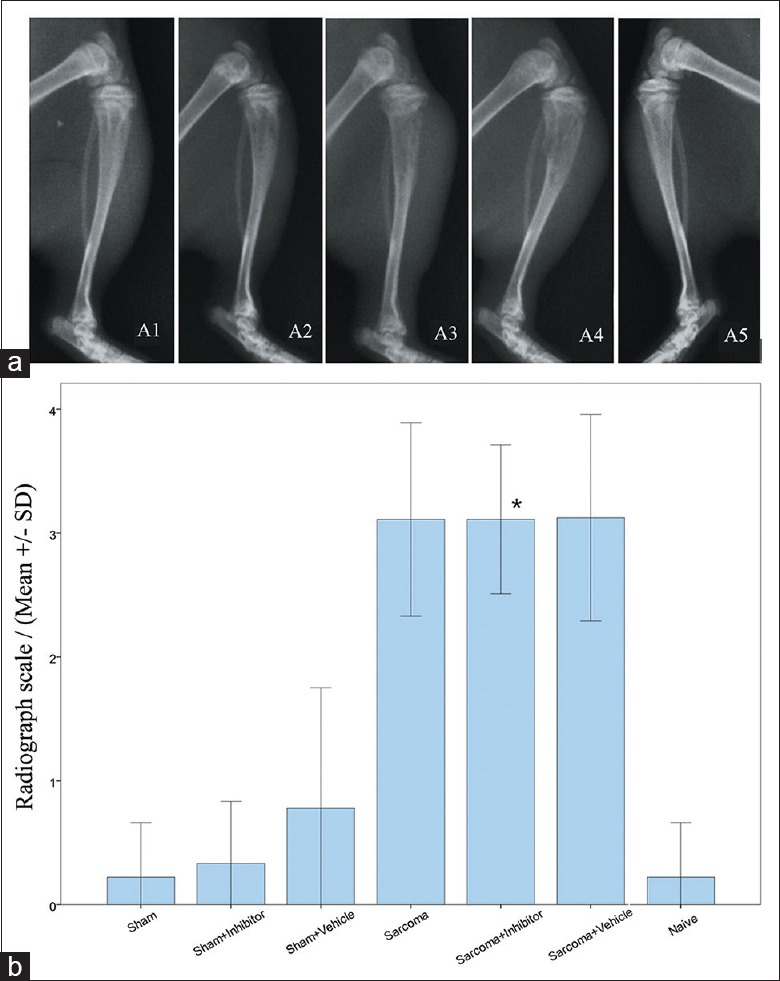
Radiographs and radiology scale of surgical side tibia of each group. (a) Radiographs of different levels of bone destruction. A1: Normal bone; A2: Spotted bone resorption sites; A3: Loss of bone in medullary canal with destruction of distal cortical bone; A4: Total destruction and discontinuity of cortical bone; A5: Non-surgical side hind paw; (b) Radiology scale of surgical side hind paws. *P < 0.05 for sarcoma + inhibitor versus naïve group.
DISCUSSION
This study shows that the calpain inhibitor can alleviate mechanical hyperalgesia of bilateral hind paws determined by MWT testing in CIBP rats in vivo. This might be mainly due to the suppression of the osteoclastogenesis process in the tumor bone microenvironment proved by the significant decrease of the TRAP-positive cell count. Since IκBα is one of calpain's hydrolysis substrates and also the inhibitor of nuclear factor-kappaB (NF-κB), the suppressed osteoclastogenesis after calpain inhibition could be due to the reduction of IκBα hydrolysis, leading to the inhibition of RANKL-induced NF-κB activation, as proved by Lee et al.[17] Similar with our previous in vitro study, TRAP-positive cells in the tibia were significantly decreased after calpain inhibition, indicating that the osteoclastogenesis process can also be interfered with by a calpain inhibitor in vivo. However, after the radiology assessment, there was no statistical significance demonstrated of the bone resorption between the calpain inhibitor group and the control group, which was contrary to the existing evidence. The reason for metastatic bone resorption is that tumor cells destroy the balance between osteoblasts and osteoclasts by alteration of the microenvironment.[18] Several human clinical trials have already proven that anti-RANKL antibody can reduce metastatic cancer induced bone resorption through inhibition of the RANK/RANKL/OPG pathway.[13,14,15] The result of this study could be attributable to the fact that tumor bone destruction was too serious for the amount of calpain inhibitor we used to take effect, or that the X-ray radiology measurement was not sensitive enough to detect the already decreased bone resorption. The dosage of calpain inhibitor III, 1 mg/kg, administered in the present study was set according to the previous research by Yu and Geddes,[19] who investigated the same agent in a rat spinal cord injury model. Unfortunately, this is among the very few studies that have ever explored the effect of a calpain inhibitor in pain studies in vivo.[20,21] Until date, there has not been any study that reported focusing on the CIBP model and osteoclastogenesis, therefore, we had very few references to consult. Whether an increased dosage of calpain inhibitor or a more sensitive method, such as MRI analysis, is effective can be addressed in future studies.
Our study also showed that the MWTs of not only the surgical side but also the nonsurgical side hind paws were decreased after bone cancer induction in the sarcoma groups. Since the nonsurgical side hind paws did not exhibit signs of bone resorption under radiology testing, bone cancer metastasis was not the reason. Therefore, bilateral hyperalgesia, or “mirror-image pain,” was revealed in CIBP rats. Koltzenburg et al.[22] suggested that trans-median sprouting of the commissural interneurons present in the spinal cord and brainstem could be the reason, whereas Milligan et al.[23] believed that mirror-image allodynia is created through glial and proinflammatory cytokine actions. Aside from the CIBP models, mirror-image pain can be seen in many types of neuropathic pain models.[24] Several classic neuropathic pain indicators, such as spinal microglial cell activation and up-regulation of postsynaptic receptor proteins in dorsal root (DR) ganglion, were proven to exist in CIBP models.[25,26] Moreover, Donovan-Rodriguez et al.[27] reported that Gabapentin, the well-accepted neuropathic pain inhibitor, can normalize CIBP induced dorsal horn neuronal changes and attenuated pain behavior. Therefore, it is consistent with the fact that CIBP has a neuropathic pain component.
Interestingly, our study demonstrates that a calpain inhibitor can also ameliorate mirror-image pain through increase of MWT of the nonsurgical side hind paws in CIBP rats, suggesting that calpain might somehow be involved in the neuropathic pain mechanism. Xie et al.[28] reported that a calpain inhibitor significantly blocked nerve injury-induced myelin-associated glycoprotein down-regulation in spinal DR and lysophosphatidic acid-induced neuropathic pain. However, there are still very few studies focused on the mechanism of calpain and neuropathic pain. The correlation and mechanism need further investigation.
Footnotes
Edited by: De Wang
Source of Support: This study was funded by Beijing Natural Science Foundation (No. 7092083).
Conflict of Interest: None declared.
REFERENECES
- 1.Bruera E, Kim HN. Cancer pain. JAMA. 2003;290:2476–9. doi: 10.1001/jama.290.18.2476. [DOI] [PubMed] [Google Scholar]
- 2.Colvin L, Fallon M. Challenges in cancer pain management – Bone pain. Eur J Cancer. 2008;44:1083–90. doi: 10.1016/j.ejca.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: Characteristics and impact in patients with cancer pain. Pain. 1999;81:129–34. doi: 10.1016/s0304-3959(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 4.Bruera E, Schoeller T, Wenk R, MacEachern T, Marcelino S, Hanson J, et al. A prospective multicenter assessment of the Edmonton staging system for cancer pain. J Pain Symptom Manage. 1995;10:348–55. doi: 10.1016/0885-3924(95)00052-z. [DOI] [PubMed] [Google Scholar]
- 5.Clohisy DR, Mantyh PW. Bone cancer pain and the role of RANKL/OPG. J Musculoskelet Neuronal Interact. 2004;4:293–300. [PubMed] [Google Scholar]
- 6.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–49. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 7.Coleman RE, Lipton A, Roodman GD, Guise TA, Boyce BF, Brufsky AM, et al. Metastasis and bone loss: Advancing treatment and prevention. Cancer Treat Rev. 2010;36:615–20. doi: 10.1016/j.ctrv.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 9.Dougall WC, Chaisson M. The RANK/RANKL/OPG triad in cancer-induced bone diseases. Cancer Metastasis Rev. 2006;25:541–9. doi: 10.1007/s10555-006-9021-3. [DOI] [PubMed] [Google Scholar]
- 10.Honore P, Luger NM, Sabino MA, Schwei MJ, Rogers SD, Mach DB, et al. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat Med. 2000;6:521–8. doi: 10.1038/74999. [DOI] [PubMed] [Google Scholar]
- 11.Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet. 2011;377:813–22. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki K, Hata S, Kawabata Y, Sorimachi H. Structure, activation, and biology of calpain. Diabetes. 2004;53(Suppl 1):S12–8. doi: 10.2337/diabetes.53.2007.s12. [DOI] [PubMed] [Google Scholar]
- 13.Zenz R, Eferl R, Scheinecker C, Redlich K, Smolen J, Schonthaler HB, et al. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res Ther. 2008;10:201. doi: 10.1186/ar2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu JY, Liu W, Huang YG. The effect of calpain and its function of inhibiting murine osteoclastogenesis. Chin J Osteoporos Bone Miner Res. 2011;4:101–7. [Google Scholar]
- 15.Medhurst SJ, Walker K, Bowes M, Kidd BL, Glatt M, Muller M, et al. A rat model of bone cancer pain. Pain. 2002;96:129–40. doi: 10.1016/s0304-3959(01)00437-7. [DOI] [PubMed] [Google Scholar]
- 16.Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–98. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 17.Lee FY, Kim DW, Karmin JA, Hong D, Chang SS, Fujisawa M, et al. Mu-Calpain regulates receptor activator of NF-kappaB ligand (RANKL)-supported osteoclastogenesis via NF-kappaB activation in RAW 264. 7 cells. J Biol Chem. 2005;280:29929–36. doi: 10.1074/jbc.M414600200. [DOI] [PubMed] [Google Scholar]
- 18.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 19.Yu CG, Geddes JW. Sustained calpain inhibition improves locomotor function and tissue sparing following contusive spinal cord injury. Neurochem Res. 2007;32:2046–53. doi: 10.1007/s11064-007-9347-4. [DOI] [PubMed] [Google Scholar]
- 20.Uçeyler N, Biko L, Sommer C. MDL-28170 has no analgesic effect on CCI induced neuropathic pain in mice. Molecules. 2010;15:3038–47. doi: 10.3390/molecules15053038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czeiter E, Büki A, Bukovics P, Farkas O, Pál J, Kövesdi E, et al. Calpain inhibition reduces axolemmal leakage in traumatic axonal injury. Molecules. 2009;14:5115–23. doi: 10.3390/molecules14125115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koltzenburg M, Wall PD, McMahon SB. Does the right side know what the left is doing? Trends Neurosci. 1999;22:122–7. doi: 10.1016/s0166-2236(98)01302-2. [DOI] [PubMed] [Google Scholar]
- 23.Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, et al. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–40. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, et al. A new model of sciatic inflammatory neuritis (SIN): Induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–44. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- 25.Baron R, Binder A, Wasner G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–19. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 26.Finnerup NB, Jensen TS. Mechanisms of disease: Mechanism-based classification of neuropathic pain-a critical analysis. Nat Clin Pract Neurol. 2006;2:107–15. doi: 10.1038/ncpneuro0118. [DOI] [PubMed] [Google Scholar]
- 27.Donovan-Rodriguez T, Dickenson AH, Urch CE. Gabapentin normalizes spinal neuronal responses that correlate with behavior in a rat model of cancer-induced bone pain. Anesthesiology. 2005;102:132–40. doi: 10.1097/00000542-200501000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Xie W, Uchida H, Nagai J, Ueda M, Chun J, Ueda H. Calpain-mediated down-regulation of myelin-associated glycoprotein in lysophosphatidic acid-induced neuropathic pain. J Neurochem. 2010;113:1002–11. doi: 10.1111/j.1471-4159.2010.06664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


