Abstract
Background:
Endothelial dysfunction is considered as the initiating process and pathological basis of cardiovascular disease. Cyclooxygenase-2 (COX-2) and prostacyclin synthase (PGIS), inducible nitric oxide synthase (iNOS) and endothelial NOS (eNOS) are key enzymes with opposing actions in inflammation and oxidative stress, which are believed to be the major driver of endothelial dysfunction. And in hypoxia (Hx), Hx-inducible factor (HIF)-1α and HIF-2α are predominantly induced to activate vascular endothelial growth factor (VEGF), resulting in abnormal proliferation. Whether and how Tongxinluo (TXL) modulates COX-2, PGIS, iNOS, eNOS, HIF-1α, HIF-2α, and VEGF in Hx-stimulated human cardiac microvascular endothelial cells (HCMECs) have not been clarified.
Methods:
HCMEC were treated with CoCl2 to mimic Hx and the mRNA expressions of COX-2, PGIS, iNOS, eNOS, HIF-1α, HIF-2α, and VEGF were first confirmed, and then their mRNA expression and protein content as well as the cell pathological alterations were evaluated for TXL treatment with different concentrations. In addition, the effector molecular of inflammation prostaglandin E2 (PGE2) and the oxidative marker nitrotyrosine (NT) was adopted to reflect HCMEC injury.
Results:
Hx could induce time-dependent increase of COX-2, iNOS, HIF-2α, and VEGF in HCMEC. Based on the Hx-induced increase, TXL could mainly decrease COX-2, iNOS, HIF-2α, and VEGF in a concentration-dependent manner, with limited effect on the increase of PGIS and eNOS. Their protein contents verified the mRNA expression changes, which was consistent with the cell morphological alterations. Furthermore, high dose TXL could inhibit the Hx-induced increase of PGE2 and NT contents, attenuating the inflammatory and oxidative injury.
Conclusions:
TXL could inhibit inflammation-related COX-2, oxidative stress-related iNOS, and HIF-2α/VEGF to antagonize Hx-induced HCMEC injury.
Keywords: Cyclooxygenase-2, Hypoxia-inducible Factor-2α, Hypoxia, Inducible Nitric Oxide Synthase, Tongxinluo, Vascular Endothelial Growth Factor
INTRODUCTION
Cardiovascular disease is the leading cause of deaths worldwide, especially atherosclerosis, and the subsequent vessel obliterations are the primary cause of ischemic disease (stroke, coronary heart disease).[1,2] Endothelial, especially microvascular endothelial dysfunction is believed to be the initiating process and pathological basis of cardiovascular disease.[3,4,5]
Inflammation and oxidative stress have been widely accepted to be the key pathological process of cardiac microvascular endothelial cells.[6,7] Cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS) are key enzymes in inflammation and oxidative stress, both of which are inducible enzymes and can be induced to mediate pathological damages.[8,9] When COX-2 is induced, excess prostaglandin E2 (PGE2) can be formed to mediate inflammation injury. While induced iNOS leads to superfluous peroxynitrite formation, which causes extensive protein tyrosine nitration, and nitrotyrosine (NT) is believed to be a marker of oxidative stress injury. In contrast to COX-2, prostacyclin synthase (PGIS) antagonize inflammation to play protective roles.[10,11] And in contrary to inducible iNOS, constitutive expression of endothelial NOS (eNOS) is necessary for physiological vasodilatation.[12]
Hypoxia (Hx)-inducible factors (HIFs) are transcription factors that respond to Hx. Mammalian HIFs function as heterodimers composed of either HIF-1α or HIF-2α bound to HIF-1β. HIF-1α appears to be ubiquitously expressed, whereas HIF-2α is more restricted to vascular endothelial cells.[13,14] HIFs control a serial of genes to adapt to Hx, and vascular endothelial growth factor (VEGF), a major target gene of HIFs, promotes abnormal proliferation in Hx condition.[15] Although VEGF has the ability to promote angiogenesis, it may stimulate abnormal basement membrane thickening and smooth muscle cell proliferation, which result in vessel obliterations.[16] Besides VEGF, HIFs also contribute to the transactivation of COX-2 and iNOS, which leads to pathological processes.[17,18]
Tongxinluo (TXL) was registered in the State Food and Drug Administration of China for treatment of angina pectoris in 1996. It is extracted, concentrated, and freeze-dried from a group of herbal medicines, such as ginseng, radix paeoniae rubra, borneol, and spiny jujuba seed, which contains multiple active components that may be responsible for its antianginal effects.[19,20,21] Our previous study revealed that ginsenoside, the main active component of TXL, could inhibit COX-2 and iNOS in L-methionine-induced rat vascular lesion.[8] Although TXL is reported to inhibit vascular inflammation, its effects on COX-2 and PGIS have not been explored.[22] Several studies revealed that TXL could modulate vascular endothelial function by inducing eNOS expression,[19,23] however, what effect on iNOS is unknown. Moreover, TXL is found to have positive effects on myocardial HIF-1α and VEGF, but no effect on HIF-2α was found.[24]
As the effect target of TXL is in microcirculation,[25,26] the human cardiac microvascular endothelial cells (HCMECs) were adopted in this study to investigate whether TXL modulate COX-2, PGIS, iNOS, eNOS, HIF-1α, HIF-2α and VEGF expressions in Hx condition. After the cells were exposed to Hx and treated with TXL, the mRNA expressions of COX-2, PGIS, iNOS, eNOS, HIF-1α, HIF-2α, and VEGF were detected by real-time reverse transcription-polymerase chain reaction (RT-PCR), their protein contents was evaluated by Western blotting, the cell morphological alterations were observed under light microscope, PGE2 content was analyzed by ELISA and NT content was evaluated by immunofluorescence to reveal the antagonizing mechanism of TXL against the Hx-induced HCMEC injury.
METHODS
Cells and treatment
Human cardiac microvascular endothelial cell were purchased from ScienCell and cultured in Endothelial Cell Medium (ScienCell, USA). After the cells were treated with CoCl2 (dissolved in phosphate buffered saline (PBS), with final concentration 100 μmol/L),[27] the gene expressions of COX-2, PGIS, iNOS, eNOS, HIF-1α, HIF-2α, and VEGF were detected at different time to verify the optimal Hx stimulation time. Then the cells were divided into control (Con), Hx, Hx plus low dose TXL (300 μg/ml), Hx plus middle dose TXL (400 μg/ml) and Hx plus high dose TXL (500 μg/ml) groups.[22] The mRNA expressions of COX-2, PGIS, iNOS, eNOS, HIF-1α, HIF-2α, and VEGF were measured at 6 h treatment, and their protein contents were evaluated, the cell morphological alterations were observed under light microscope, NT distribution and content was evaluated and the supernatant was collected to analyze PGE2 content at 24 h treatment.
Real-time reverse transcription-polymerase chain reaction
The mRNA expressions of COX-2, PGIS, iNOS, eNOS, HIF-1α, HIF-2α, and VEGF were quantified by real-time RT-PCR. Total RNA was isolated using Trizol reagent (Takara, China) and reverse transcribed into cDNA using RevertAid First Strand cDNA synthesis Kit (Fermentas, USA), followed by real-time PCR amplification using specific primers [Table 1]. Actin primers were used as an internal standard.
Table 1.
Primers used for real time PCR detection
| Items | Primers (5’−3’) | Annealing temperature (°C) | Product size (bp) |
|---|---|---|---|
| COX-2 | |||
| Sense | CTTTGCCCAGCACTTCACGCATCAG | 65 | 269 |
| Anti-sense | CCTGCCCCACAGCAAACCGTAGATG | ||
| PGIS | |||
| Sense | GAGACGGAGTTTCACGCTTATTGC | 60 | 228 |
| Anti-sense | AGGCAAATCACGAGGTCAGGAG | ||
| iNOS | |||
| Sense | CCAGCCTCAAGTCTTATTTCCTC | 55 | 177 |
| Anti-sense | GCAAGTTCCATCTTTCACCCAC | ||
| eNOS | |||
| Sense | ACGAGACGCTGGTGCTGGTGGTAAC | 62 | 274 |
| Anti-sense | GAGCCGAGCCCGAACACACAGAAC | ||
| HIF-1 | |||
| Sense | CCTGCTTGGTGCTGATTTGTG | 55 | 265 |
| Anti-sense | CTGTACTGTCCTGTGGTGACTTGTC | ||
| HIF-2 | |||
| Sense | GAAATGGAATCCTGCTCACAAAATC | 60 | 287 |
| Anti-sense | TGAAAGACCATCCGAGTCACATAGC | ||
| VEGF | |||
| Sense | TAGACACACCCACCCACATACATAC | 55 | 173 |
| Anti-sense | CCCAACTCAAGTCCACAGCAG | ||
| β-actin | |||
| Sense | CTCCATCCTGGCCTCGCTGT | 60 | 268 |
| Anti-sense | GCTGTCACCTTCACCGTTCC |
PCR: Polymerase chain reaction.
Western blotting
The protein contents of COX-2, PGIS, iNOS, eNOS, HIF-1α, HIF-2α, and VEGF were detected by Western blotting, with the protocol previously described.[28] Briefly, cells were lysed in lysis buffer (RIPA, Solarbio, China) to extract whole proteins. Protein content was determined by NanoDrop-1000 (Thermo Scientific, USA). Then samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to the polyvinylidene fluoride membrane (Millipore, USA). After the membrane was blocked with milk, anti-COX-2 (Santa Cruz, USA), anti-PGIS (Santa Cruz), anti-iNOS (Santa Cruz), anti-eNOS (Santa Cruz), anti-HIF-1α (Sangon Biotech), anti-HIF-2α (Sangon Biotech), anti-VEGF antibodies (Sangon Biotech, China) or anti-beta actin (Sangon Biotech) were used. Band intensity was quantified and calculated.
ELISA
After treated, the culture medium was collected to analyze PGE2 by ELISA (Jiancheng, Nanjing, China). Standards and samples were added to wells of the plate and incubated for 1 h. After the wells were washed with the ELISA wash buffer, the conjugated antibody was added and incubated for 1 h. Then the wells were again washed with the ELISA wash buffer. The substrate was added in the wells and incubated for 15 min. Then the stop solution was added, and absorption was measured with an ELISA reader at 450 nm. The tests were carried out in duplicate.
Immunofluorescence
The distribution and contents of NT were measured by immunofluorescence with the procedure previously reported.[28] After incubated with the anti-NT antibody (Abcam, UK) and then the secondary antibody (fluorescein isothiocyanate, Zhongshan, China), cells were observed under a fluorescence microscope (Olympus IX51). To get exact results, controls (PBS instead of the first antibody or the second antibody) were designed.
Statistical analysis
Statistical analysis of the data was performed using SPSS software (Chicago, IL, USA) and presented as means ± standard deviation. The experiments were repeated twice, and comparisons between two groups were performed using Student's t-test. P < 0.05 were considered statistically significant.
RESULTS
Hypoxia-induced time-dependent changes of gene expressions
After the cells were stimulated by Hx for different time, the gene expressions of COX-2, PGIS, iNOS, eNOS, HIF-1α, HIF-2α, and VEGF were detected by real-time RT-PCR [Figure 1]. The inflammation-related COX-2 increased at 4 h, 6 h and 8 h, while the protective PGIS decreased at 8 h [Figure 1a]. The oxidative stress-related iNOS elevated sharply at 4 h, 6 h and 8 h, with no significant changes of eNOS [Figure 1b]. The Hx-induced HIF-1α increased at 4 h and 6 h, but HIF-2α and VEGF rose obviously at 4 h, 6 h and 8 h [Figure 1c]. The results indicated that Hx mainly triggered COX-2, iNOS, and HIF-2α/VEGF in a time-dependent manner in HCMEC.
Figure 1.
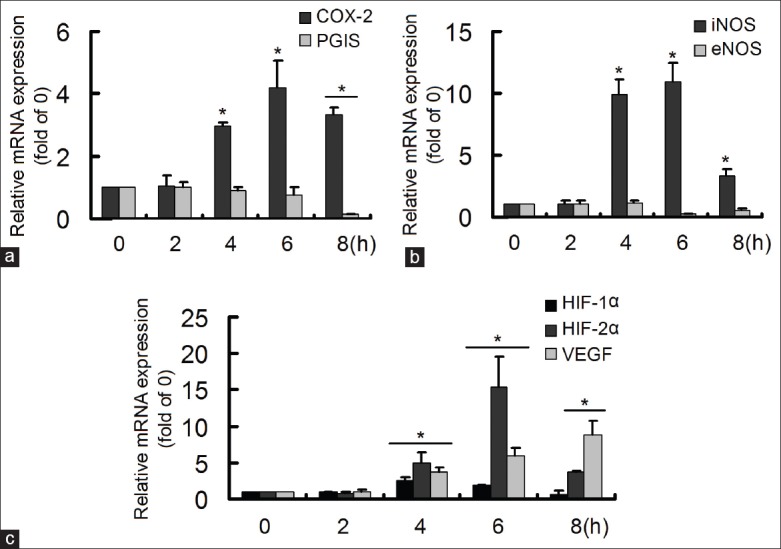
Hypoxia (Hx) induced time-dependent changes of gene expressions. The gene expressions were detected by real-time reverse transcription-polymerase chain reaction. (a) Hx induced increase of cyclooxygenase-2 and decrease of prostacyclin synthase; (b) Hx induced increase of inducible nitric oxide synthase; (c) Hx induced increase of Hx-inducible factor (HIF)-1α, HIF-2α and vascular endothelial growth factor *P < 0.05, compared with 0 h group. The data were repeated twice and represented as mean ± standard division.
Tongxinluo modulated the hypoxia-induced gene expressions
The cells were treated with low-, middle- and high-dose TXL for 6 h, and then the gene expressions of COX-2, PGIS, iNOS, eNOS, HIF-1α, HIF-2α, and VEGF were detected by real-time RT-PCR [Figure 2]. At first, it was found that middle dose TXL had no obvious effect on the gene expressions. The Hx-induced COX-2 and iNOS could be reduced by middle- and high-dose-TXL, and PGIS and eNOS increased significantly only in high-dose TXL group [Figure 2a and b]. The Hx-induced HIF-2α and VEGF were also decreased in the middle- and high-dose TXL groups, although HIF-1α showed no significant changes among groups [Figure 2c]. The results manifested that TXL, especially in high dose, could suppress Hx-induced COX-2, iNOS, and HIF-2α/VEGF to exhibit protective roles in HCMEC.
Figure 2.
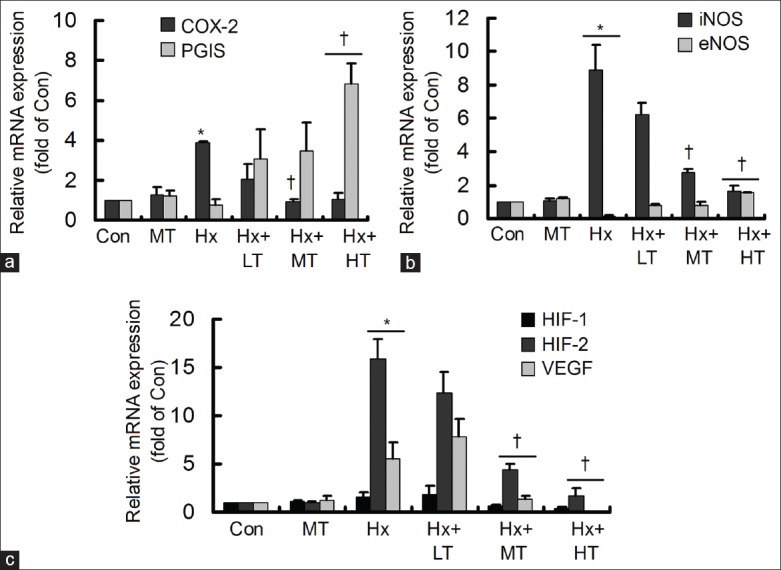
Tongxinluo (TXL) affected the hypoxia-induced gene expression changes. The gene expressions were detected by real-time reverse transcription-polymerase chain reaction. (a) TXL decreased the hypoxia-induced cyclooxygenase-2, with increase of prostacyclin synthase; (b) TXL decreased the hypoxia-induced inducible nitric oxide synthase, with increase of endothelial NOS; (c) TXL decreased the hypoxia-induced factor-2α and vascular endothelial growth factor *P < 0.05, compared with control (Con) group; †P < 0.05, compared with hypoxia group. The data were repeated twice and represented as mean ± standard division. LT: Low dose TXL; MT: Middle dose TXL; HT: High-dose TXL.
Tongxinluo adjusted the hypoxia-induced protein contents
The cells were treated with low-, middle- and high-dose TXL for 24 h, and then the protein contents of COX-2, PGIS, iNOS, eNOS, HIF-1α, HIF-2α, and VEGF were detected by Western blotting [Figure 3a]. The Hx-induced COX-2 and iNOS could be reduced by TXL in a concentration-dependent manner, but PGIS merely increased significantly in high dose TXL group and difference of eNOS was hardly seen among groups [Figure 3b and c]. The Hx-induced HIF-2α decreased by TXL in a concentration-dependent manner, however, HIF-1α and VEGF were reduced significantly only in high-dose TXL group [Figure 3d]. The protein content changes were in accordance with that of their mRNA expressions, confirming the effects of TXL on COX-2, iNOS, and HIF-2α/VEGF in HCMEC.
Figure 3.
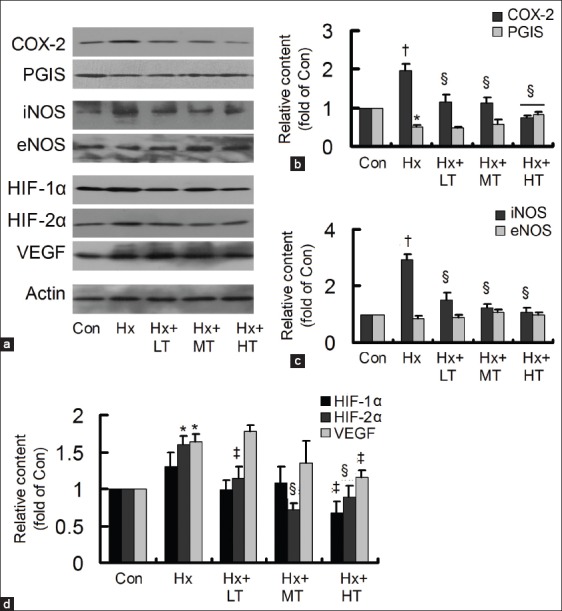
Effects of tongxinluo (TXL) on the hypoxia-induced protein content changes. (a) The protein contents of cyclooxygenase-2 (COX-2), prostacyclin synthase (PGIS), inducible nitric oxide synthase (iNOS), endothelial NOS, hypoxia-inducible factor (HIF)-1α, HIF-2α, and vascular endothelial growth factor (VEGF) were detected by Western blotting; (b) TXL decreased the hypoxia-induced COX-2, with increase of PGIS; (c) TXL decreased the hypoxia-induced iNOS; (d) TXL decreased the hypoxia-induced HIF-1α, HIF-2α, and VEGF. *P < 0.05 or †P < 0.01, compared with control (Con) group; ‡P < 0.05 or §P < 0.01, compared with hypoxia group. The data were repeated twice and represented as mean ± standard division. LT: Low dose TXL; MT: Middle dose TXL; HT: High-dose TXL.
Tongxinluo attenuated the hypoxia-induced human cardiac microvascular endothelial cell injury
After the cells were treated with low-, middle-, and high-dose TXL for 24 h, their morphological alterations were observed under light microscope [Figure 4a]. As illustrated, in contrast to control group, the cells shrank, turned round and seem to die in Hx group. However, for TXL treatments, the morphological injury was attenuated gradually with its concentration ascending. Moreover, PGE2 increased significantly in Hx group, and high dose TXL could inhibit the Hx-induced increase [Figure 4b]. Meanwhile, the Hx-induced accumulation of NT content could be attenuated obviously by high dose TXL treatment [Figure 4c]. The results demonstrated that TXL could attenuate the Hx-induced HCMEC injury.
Figure 4.
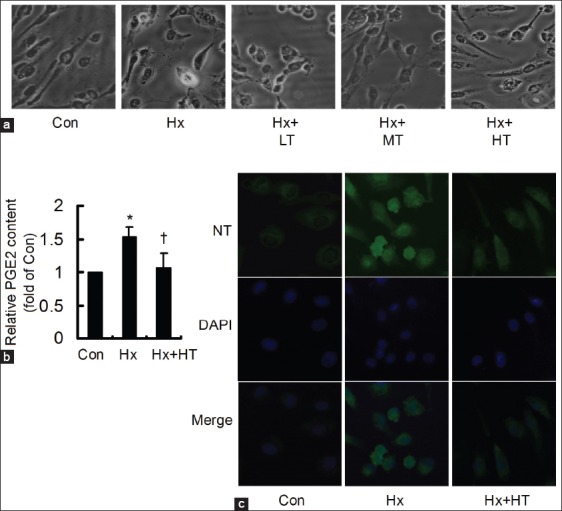
Tongxinluo (TXL) attenuated the hypoxia (Hx)-induced injury (×400). (a) Compared to control (Con) group, the cells shrank, turned round and seem to die in Hx group, which could be obviously attenuated by TXL treatments; (b) Prostaglandin E2 content increased in Hx group, and significantly decreased in Hx plus high dose TXL (HT) group, *P < 0.05, compared with control (Con) group; †P < 0.05, compared with hypoxia group; (c) Nitrotyrosine content dramatically increased in Hx group, and was obviously inhibited in Hx plus HT group. LT: Low dose TXL; MT: Middle dose TXL.
DISCUSSION
Endothelial dysfunction is considered as the initiating process and pathological basis of cardiovascular disease. TXL could effectively improve the cardiac microcirculation to antagonize Hx, however, the underlying mechanism is still not fully clarified. Inflammation, oxidative stress, and HIFs make a vicious cycle to drive the pathological process of endothelial dysfunction. Whether and how TXL affects COX-2, PGIS, iNOS, eNOS, HIF-1α, HIF-2α, and VEGF in Hx-stimulated HCMEC is unknown. In this study, it was found that TXL could mainly inhibit the Hx-induced COX-2, iNOS, HIF-2α/VEGF in a concentration-dependent manner, with attenuation of cell morphological injury, PGE2, and NT contents. The results manifested that TXL modulated inflammation-related COX-2, oxidative stress-related iNOS, and HIF-2α/VEGF to antagonize the Hx-induced HCMEC injury.
The immense endothelium surface area in microcirculation makes this region more vulnerable to injury. Since the endothelial cell layer constitutes the primary barrier and co-ordinates the overlying smooth muscle cell layer, microvascular endothelial dysfunction is believed to be the initiating process and pathological basis of cardiovascular disease.[3,4,5,29] In this study, HCMEC was adopted to investigate the Hx-induced heart microvascular endothelial cell injury.
Inflammation and oxidative stress have been widely believed to be the key pathological process of cardiac microvascular endothelial cells. COX-2 and PGIS are enzymes with opposing actions in inflammation, and in contrary to iNOS, eNOS plays constitutive roles in physiological vasodilatation.[8,9,10,11,12] The mutual promotion COX-2 and iNOS may contribute to vascular disease, which has also been proved by our previous study.[30] Meanwhile, HIF-1α and HIF-2α are induced by Hx, which can drive VEGF expression, resulting in abnormal proliferation.[13,14] Besides, VEGF, COX-2, and iNOS are other target genes of HIFs in Hx. Whether these factors are involved in the Hx-induced HCMEC pathological process is unknown.
At first, the mRNA expressions of COX-2, PGIS, iNOS, eNOS, HIF-1α, HIF-2α, and VEGF in Hx-stimulated HCMEC were detected. The results found that Hx could dramatically induce COX-2, iNOS, HIF-2α and VEGF in a time-dependent manner, validating the involvement of inflammation, oxidative stress and HIFs/VEGF in the Hx-induced HCMEC dysfunction.
Tongxinluo has satisfactory beneficial effects on cardiovascular disease, particularly in the modulation of microcirculation.[19,20,21,25,26] About the mechanism of TXL, several researchers focus on the regulation of eNOS, and still others explore its effect on micro RNAs.[22,31] Although inflammation, oxidative stress and HIFs/VEGF have been considered as the key pathological process in Hx stimulation, whether and how TXL affects the inflammation-related COX-2, oxidative stress-related iNOS, and HIF-2α/VEGF have not been explored. Therefore, based on the Hx-induced expression of COX-2, iNOS, HIF-2α, and VEGF, the effects of TXL were investigated in Hx-stimulated HCMEC.
In this study, it first revealed that TXL could inhibit the mRNA expressions of COX-2, iNOS, HIF-2α and VEGF in a concentration-dependent manner, with up-regulation of PGIS and eNOS. Then their protein contents showed that TXL could concentration-dependently suppress COX-2, iNOS, HIF-2α, and VEGF, with slight up-regulation of PGIS and down-regulation of HIF-1α. These data manifested that TXL mainly inhibited the Hx-induced COX-2, iNOS, HIF-2α/VEGF in HCMEC.
Furthermore, the morphological alterations illustrated that Hx brought the conspicuous injury to HCMEC, and TXL could obviously attenuate the pathological alterations with its concentration ascending. As known, COX-2 synthesizes PGE2 to mediate inflammation and iNOS causes extensive oxidative stress injury, which can be reflected by NT content. And besides VEGF, HIFs also exhibit Hx-induced effects via iNOS. In this study, it was found that PGE2 and NT contents increased in Hx group, reflecting the Hx-induced HCMEC injury, and high-dose TXL could obviously reduce the induced PGE2 and NT contents. The results demonstrated that TXL could antagonize Hx-induced HCMEC morphological, inflammatory, and oxidative stress injury.
In conclusion, hypoxia triggered inflammation-related COX-2, oxidative stress-related iNOS, and HIF-2α/VEGF to cause HCMEC injury, and TXL treatment could inhibit COX-2, iNOS, HIF-2α/VEGF to antagonize the Hx-induced injury.
Footnotes
Edited by: Li-Shao Guo
Source of Support: This work was supported by grants from the Major State Basic Research Development Program of China (973 Program) (No. 2012CB518601), the National Natural Science Foundation of China (No. 81470595), the Hebei Natural Science Foundation (No. H2012206005).
Conflict of Interest: None declared.
REFERENECES
- 1.Tano JY, Gollasch M. Hypoxia and ischemia-reperfusion: A BiK contribution? Am J Physiol Heart Circ Physiol. 2014;307:H811–7. doi: 10.1152/ajpheart.00319.2014. [DOI] [PubMed] [Google Scholar]
- 2.Schiano C, Casamassimi A, Vietri MT, Rienzo M, Napoli C. The roles of mediator complex in cardiovascular diseases. Biochim Biophys Acta. 2014;1839:444–51. doi: 10.1016/j.bbagrm.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Ding Y, Zhang B, Zhou K, Chen M, Wang M, Jia Y, et al. Dietary ellagic acid improves oxidant-induced endothelial dysfunction and atherosclerosis: Role of Nrf2 activation. Int J Cardiol. 2014;175:508–14. doi: 10.1016/j.ijcard.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 4.Steyers CM, 3rd, Miller FJ., Jr Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. 2014;15:11324–49. doi: 10.3390/ijms150711324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tousoulis D, Simopoulou C, Papageorgiou N, Oikonomou E, Hatzis G, Siasos G, et al. Endothelial dysfunction in conduit arteries and in microcirculation. Novel therapeutic approaches. Pharmacol Ther. 2014;144:253–67. doi: 10.1016/j.pharmthera.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Mangge H, Becker K, Fuchs D, Gostner JM. Antioxidants, inflammation and cardiovascular disease. World J Cardiol. 2014;6:462–77. doi: 10.4330/wjc.v6.i6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Lian K, Zhang L, Wang R, Yi F, Gao C, et al. TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2014;109:415. doi: 10.1007/s00395-014-0415-z. [DOI] [PubMed] [Google Scholar]
- 8.Li YN, Wu YL, Jia ZH, Qi JS. Interaction between COX-2 and iNOS aggravates vascular lesion and antagonistic effect of ginsenoside. J Ethnopharmacol. 2008;119:305–11. doi: 10.1016/j.jep.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Tunctan B, Korkmaz B, Sari AN, Kacan M, Unsal D, Serin MS, et al. Contribution of iNOS/sGC/PKG pathway, COX-2, CYP4A1, and gp91(phox) to the protective effect of 5,14-HEDGE, a 20-HETE mimetic, against vasodilation, hypotension, tachycardia, and inflammation in a rat model of septic shock. Nitric Oxide. 2013;33:18–41. doi: 10.1016/j.niox.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He C, Choi HC, Xie Z. Enhanced tyrosine nitration of prostacyclin synthase is associated with increased inflammation in atherosclerotic carotid arteries from type 2 diabetic patients. Am J Pathol. 2010;176:2542–9. doi: 10.2353/ajpath.2010.090783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camacho M, Rodríguez C, Guadall A, Alcolea S, Orriols M, Escudero JR, et al. Hypoxia upregulates PGI-synthase and increases PGI2 release in human vascular cells exposed to inflammatory stimuli. J Lipid Res. 2011;52:720–31. doi: 10.1194/jlr.M011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu B, Kuang L, Liu J. Bariatric surgery relieves type 2 diabetes and modulates inflammatory factors and coronary endothelium eNOS/iNOS expression in db/db mice. Can J Physiol Pharmacol. 2014;92:70–7. doi: 10.1139/cjpp-2013-0034. [DOI] [PubMed] [Google Scholar]
- 13.Skuli N, Majmundar AJ, Krock BL, Mesquita RC, Mathew LK, Quinn ZL, et al. Endothelial HIF-2a regulates murine pathological angiogenesis and revascularization processes. J Clin Invest. 2012;122:1427–43. doi: 10.1172/JCI57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapitsinou PP, Sano H, Michael M, Kobayashi H, Davidoff O, Bian A, et al. Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J Clin Invest. 2014;124:2396–409. doi: 10.1172/JCI69073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramakrishnan S, Anand V, Roy S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J Neuroimmune Pharmacol. 2014;9:142–60. doi: 10.1007/s11481-014-9531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan J, Lata C, Santilli A, Green D, Roy S, Santilli S. Supplemental oxygen reverses hypoxia-induced smooth muscle cell proliferation by modulating HIF-alpha and VEGF levels in a rabbit arteriovenous fistula model. Ann Vasc Surg. 2014;28:725–36. doi: 10.1016/j.avsg.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao CX, Luo CL, Wu XH. Hypoxia promotes 786-O cells invasiveness and resistance to sorafenib via HIF-2a/COX-2. Med Oncol. 2015;32:419. doi: 10.1007/s12032-014-0419-4. [DOI] [PubMed] [Google Scholar]
- 18.Branco-Price C, Zhang N, Schnelle M, Evans C, Katschinski DM, Liao D, et al. Endothelial cell HIF-1a and HIF-2a differentially regulate metastatic success. Cancer Cell. 2012;21:52–65. doi: 10.1016/j.ccr.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li XD, Yang YJ, Geng YJ, Jin C, Hu FH, Zhao JL, et al. Tongxinluo reduces myocardial no-reflow and ischemia-reperfusion injury by stimulating the phosphorylation of eNOS via the PKA pathway. Am J Physiol Heart Circ Physiol. 2010;299:H1255–61. doi: 10.1152/ajpheart.00459.2010. [DOI] [PubMed] [Google Scholar]
- 20.Li XD, Yang YJ, Cheng YT, Dou KF, Tian Y, Meng XM. Protein kinase A-mediated cardioprotection of tongxinluo relates to the inhibition of myocardial inflammation, apoptosis, and edema in reperfused swine hearts. Chin Med J (Engl) 2013;126:1469–79. [PubMed] [Google Scholar]
- 21.Jia Y, Bao F, Huang F, Leung SW. Is tongxinluo more effective than isosorbide dinitrate in treating angina pectoris? A systematic review and meta-analysis of randomized controlled trials. J Altern Complement Med. 2011;17:1109–17. doi: 10.1089/acm.2010.0788. [DOI] [PubMed] [Google Scholar]
- 22.Zhang RN, Zheng B, Li LM, Zhang J, Zhang XH, Wen JK. Tongxinluo inhibits vascular inflammation and neointimal hyperplasia through blockade of the positive feedback loop between miR-155 and TNF-a. Am J Physiol Heart Circ Physiol. 2014;307:H552–62. doi: 10.1152/ajpheart.00936.2013. [DOI] [PubMed] [Google Scholar]
- 23.Liang JQ, Wu K, Jia ZH, Liu C, Ding J, Huang SN, et al. Chinese medicine Tongxinluo modulates vascular endothelial function by inducing eNOS expression via the PI-3K/Akt/HIF-dependent signaling pathway. J Ethnopharmacol. 2011;133:517–23. doi: 10.1016/j.jep.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 24.Bai WW, Xing YF, Wang B, Lu XT, Wang YB, Sun YY, et al. Tongxinluo improves cardiac function and ameliorates ventricular remodeling in mice model of myocardial infarction through enhancing angiogenesis. Evid Based Complement Alternat Med 2013. 2013 doi: 10.1155/2013/813247. 813247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai W, Wei C, Kong H, Jia Z, Han J, Zhang F, et al. Effect of the traditional Chinese medicine tongxinluo on endothelial dysfunction rats studied by using urinary metabonomics based on liquid chromatography-mass spectrometry. J Pharm Biomed Anal. 2011;56:86–92. doi: 10.1016/j.jpba.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Cui H, Li X, Li N, Qi K, Li Q, Jin C, et al. Induction of autophagy by Tongxinluo through the MEK/ERK pathway protects human cardiac microvascular endothelial cells from hypoxia/reoxygenation injury. J Cardiovasc Pharmacol. 2014;64:180–90. doi: 10.1097/FJC.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Zhao M, Li B, Qi J. Dynamic localization and functional implications of C-peptide might for suppression of iNOS in high glucose-stimulated rat mesangial cells. Mol Cell Endocrinol. 2013;381:255–60. doi: 10.1016/j.mce.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Hald BO, Jacobsen JC, Sandow SL, Holstein-Rathlou NH, Welsh DG. Less is more: Minimal expression of myoendothelial gap junctions optimizes cell-cell communication in virtual arterioles. J Physiol. 2014;592:3243–55. doi: 10.1113/jphysiol.2014.272815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Qi J, Liu K, Li B, Wang H, Jia J. Peroxynitrite-induced nitration of cyclooxygenase-2 and inducible nitric oxide synthase promotes their binding in diabetic angiopathy. Mol Med. 2010;16:335–42. doi: 10.2119/molmed.2010.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B, Yang Q, Bai WW, Xing YF, Lu XT, Sun YY, et al. Tongxinluo protects against pressure overload-induced heart failure in mice involving VEGF/Akt/eNOS pathway activation. PLoS One. 2014;9:e98047. doi: 10.1371/journal.pone.0098047. [DOI] [PMC free article] [PubMed] [Google Scholar]


