Abstract
Objective:
To elaborate the role of quantitative magnetic resonance imaging (MRI) parameters in the evaluation of treatment response in malignant tumors.
Data Sources:
Data cited in this review were obtained mainly from PubMed in English from 1999 to 2014, with keywords “dynamic contrast-enhanced (DCE)-MRI,” “diffusion-weighted imaging (DWI),” “microcirculation,” “apparent diffusion coefficient (ADC),” “treatment response” and “oncology.”
Study Selection:
Articles regarding principles of DCE-MRI, principles of DWI, clinical applications as well as opportunity and aspiration were identified, retrieved and reviewed.
Results:
A significant correlation between ADC values and treatment response was reported in most DWI studies. Most quantitative DCE-MRI studies showed a significant correlation between Ktrans values and treatment response. However, in different tumors and studies, both high and low pretreatment ADC or Ktrans values were found to be associated with response rate. Both DCE-MRI and DWI demonstrated changes in their parameters hours to days after treatment, showing a decrease in Ktrans or an increase in ADC associated with response in most cases.
Conclusions:
Combinations of quantitative MRI play an important role in the evaluation of treatment response of malignant tumors and hold promise for use as a cancer treatment response biomarker. However, validation is hampered by the lack of reproducibility and standardization. MRI acquisition protocols and quantitative image analysis approaches should be properly addressed prior to further testing the clinical use of quantitative MRI parameters in the assessment of treatments.
Keywords: Apparent Diffusion Coefficient, Diffusion-weighted Imaging, Dynamic Contrast-enhanced Magnetic Resonance Imaging, Microcirculation, Oncology, Treatment Response
INTRODUCTION
Incidences of malignant tumors continue to increase due to a combination of factors, including smoking, aging population and carcinogenic substances in the environment. Attempts with therapeutic options include radiotherapy, various chemotherapeutic regimens and individualized molecularly targeted drugs have highlighted the limitations of the evaluation of treatment response based on tumor size reduction only, which may not always typically occur until days or weeks after fractionated regimens. Another considerable problem is that the functional changes acted by any effective therapy in the tumor microenvironment are not directly evaluated on anatomic imaging.
The identification of factors that enable evaluation of treatment response is important with regard to the selection of the optimal following therapy. Tumors identified to be less responsive can be subjected to alternative treatment strategies or investigational therapies. Unfortunately, the histologic treatment response information acquired by morphologic imaging is limited. Therefore, there is a need for clinically significant noninvasive evaluation of treatment response.
In view of the diversity of magnetic resonance imaging (MRI) sequences available, an ever-increasing body of research indicates that quantitative MRI parameters may play an important role on this respect of research.[1,2,3,4,5,6,7,8,9] In this review, we will focus on the recent development in this field, especially dynamic contrast-enhanced MRI (DCE-MRI) and diffusion-weighted imaging (DWI).
Principles of dynamic contrast-enhanced magnetic resonance imaging
Dynamic contrast-enhanced-MRI is a promising imaging technique that can enable noninvasive quantitative assessment of tissue microvasculature, such as tissue perfusion, capillary permeability, and integrity.[10,11] The information acquired from data can be applied to a study of angiogenesis, hypoxia, and evaluation of various biomarkers. Regions of necrosis, muscle, vessel, and viable information inside the tumor display distinct signal enhancement in dynamic images. DCE-MRI in combination with tumor morphologic imaging could provide a possible prognostic response to antiangiogenic treatment.[7] Quantitative measures generated from DCE-MRI data involve evaluation of some combination of principal kinetic parameters: The influx volume transfer constant (Ktrans), the efflux rate constant (kep), the relative extravascular extracellular space (EES) fractional volume (ve), and the relative vascular plasma space (vp) from a two-compartment pharmacokinetic model proposed by Tofts et al.,[10] and the corresponding differences (ΔKtrans, Δkep, Δve, and Δvp) between outer and inner tumor.[6,8,12,13,14] The Ktrans (min−1) is transendothelial transport of contrast medium from the vascular compartment to the tumor interstitium. The kep (min−1) reflects the reverse transport of contrast medium back into the vascular space. Ktrans and ve relate to the tissue's basic physiology, whereas the rate constant (kep) is the ratio of the transfer constant to the EES:[10]
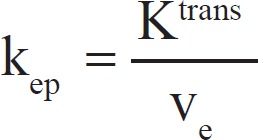
kep can be derived from the shape of the gadolinium concentration versus time results, whereas Ktrans and ve require knowledge of the absolute values of gadolinium concentration. Ktrans has several physiologic interpretations, depending on the tissue's vascular permeability and perfusion.
Compared with the complex quantitative parameters, a qualitative assessment of perfusion based on the shape of the signal intensity versus time curve may also be useful clinically for assessing response to treatment. Tissues are described using a number of descriptors including onset of enhancement, maximum signal intensity, and the washout gradient and so on.[15] These semi-quantitative parameters have the advantage of being relatively straightforward to calculate and can be used when quantitative techniques fail.
Principles of diffusion-weighted imaging
Diffusion-weighted-MRI is a technique that is acquired to make the MR signal sensitive to the molecular motion of water by applying strong magnetic field gradients. Movement including directed perfusion and passive thermally-induced diffusion can be measured, which is constrained by the cell size, structure, membrane, and organelles form physical boundaries. The rate of diffusion of water molecules in cellular tissues is usually described by means of an apparent diffusion coefficient (ADC), which is always lower than that of free water. Many treatments, such as radiation and/or chemotherapy, can result in the loss of the cell membrane integrity that may affect water diffusion and perfusion. Thus, DW-MRI can provide microstructural information on the cellular level in a detectable way long before tumor size reductions are seen. This ability aids in determining prognosis as well as planning treatment strategies.
Diffusion-weighted images acquired at a minimum of two sensitivity levels defined by the sequence b-value provide an estimate of ADC, which represents the magnitude of random motion of water molecules. ADC value is impeded by cellular packing, cell membrane and macromolecules presented in the various tissues, and displays lower especially in tumors. On the other hand, ADC values are often increased in the necrotic regions and tissues with damaged or permeable cell membranes. Therefore, variation in ADC values reflects the alteration and redistribution of water molecules between intracellular and extracellular compartments of tissue.[16]
The b-value determines the extent of DW in a scan. The proper selection of b-value is an important technical consideration, and dependent on the given body DWI application and objective. Observed signal attenuation differs in different tissues, which may follow the two types of decay models: Bi-exponential behavior and a single exponential behavior. In most clinical DWI studies, the bi-exponential behavior of signal attenuation is not observed due to the low b-values used. Therefore, it is common practice to fit the DWI data to a single exponential decay model.
CLINICAL APPLICATIONS
Differentiating malignant tumors from benign ones
Quantitative DCE-MRI parameters can be used to differentiate malignant tumors from benign ones. This application of research is mainly documented in breast and prostate cancer, where malignant lesions usually have higher Ktrans than benign tumors and tumor-like growths.[6,7,13] Malignant lesions have rapid and high amplitude contrast medium wash-in followed by a relatively rapid wash-out, whereas benign lesions are characterized by contrast medium wash-in followed by persistent enhancement, due to a lower degree of angiogenesis.[17,18] These results are consistent with recently reported documents of malignant tumors in the head and neck including the sinonasal area.[19,20] Xian et al. demonstrated a significant difference in ve between epithelial and nonepithelial malignant tumors and a significant difference in kep and ve between malignant epithelial tumors and lymphomas in the sinonasal region.[20]
Tumors differ in their cellularity, and this difference may reflect their histologic composition and biologic aggressiveness. In a study that included carcinomas, lymphomas, benign salivary gland adenomas and benign cysts,[21] the mean ADC value of benign solid lesions significantly higher than that of malignant tumors was found. In the same way, investigators have found that benign liver lesions, such as cysts and hemangiomas, have higher mean ADC values than malignant lesions, such as metastases and hepatocellular carcinoma (HCC).[22,23]
Monitoring treatment response in different sites
Many DCE-MRI studies have suggested that quantitative parameters can be used as potential markers for prediction of treatment response and long-term survival.[8,24] Ktrans reflects a combination of tumor blood flow and microvascular permeability. On the other hand, Ktrans values are also used to monitor response to cancer therapies.[25] In addition to Ktrans, the other kinetic analysis of DCE-MRI data, ve, vp and kep, are also used to describe the uptake of low molecular weight gadolinium-medium contrast agents. The parameter ve reflects the EES, which is composed of interstitial fluid and connective tissue arranged in a supportive frame structure and restricted by blood vessel walls and cell plasma membranes. Interestingly, controversy exists in the previous and current research, as other recently published papers exhibited the importance of ve, vp and kep as prognostic biomarkers of clinical outcome in patients with cancers of other regions.[26,27,28] Given the inherently different physiologic information that these parameters offer, we believe these parameters may play a complementary role in the evaluation of all oncological treatment response when used in combination with other quantitative and qualitative parameters. If confirmed in a larger trial, the prognostic significance of the DCE-MRI parameters would be useful to stratify patients in future clinical trials and to identify therapeutic regimen individually.
Diffusion-weighted imaging-MRI can be used to detect microstructural changes that precede changes in tumor size as an indication of tumor response to therapy. For many organs and tumor types, a tumor initially demonstrates decreased ADCs relative to the surrounding tissue due to diffusion restriction caused by high cell density in the tumor. Effective treatment on oncology results in tumor lysis, loss of cell membrane integrity, increased extracellular space. So, a positive response to treatment may tend to result in an increase in ADC.
These quantitative MRI parameters have been investigated extensively in the evaluation of treatment response in different malignant tumors. Recent research focusing on these aspects includes data on breast cancer, prostate cancer, colonic and rectal cancer, malignant liver lesions, and head and neck cancer.
Breast cancer
Diffusion-weighted imaging and DCE-MRI are under development for monitoring response to cancer therapies.[25] Incremental ADC increase over chemotherapeutic treatment cycles is found in a number of breast cancer studies[29,30,31,32] while the morphological change did not detect until second cycle of chemotherapy.[33] The increase in ADC values was due to both activation of apoptosis and presentation of cell death within the tumor,[34] which can be detected as early as 3 days after treatment.[35] Decrease in the transfer of gadolinium contrast from blood vessels to tumor tissue, measured by Ktrans, is observed with cytotoxic therapy[29] and antiangiogenic therapies in breast cancer.[36] This result supports the idea that successful treatment where killing of tumor cells causes vascular shutdown due to loss of the proangiogenic cytokine may result in apoptosis of proliferating endothelial cells.[37]
Prostate cancer
Apparent diffusion coefficient increase was found in response to both radiotherapy and antihormonal therapy,[38,39] and patients with high Ktrans value before treatment were demonstrated response to chemoradiation therapy and prolonged survival.[7] In the evaluation of local tumor progression of prostate cancer after high-intensity focused ultrasonic ablation, DCE-MRI was more sensitive than T2-weighted MRI with DWI, but T2-weighted MRI with DWI was more specific than DCE-MRI[40] or T2-weighted MRI alone.[41] In another study, multiparametric MRI, including DW-MRI, can achieve accuracy levels of 80–90% in the detection of recurrent prostate cancer after radiation therapy, which may have implications for determining presence or absence of local recurrence and subsequent local salvage therapy.[42]
Colonic and rectal cancer
Change of the ADC value in rectal cancer was unlike others. Pretreatment ADC values in rectal cancer patients were found to be negatively correlated with percentage size change of tumors after chemotherapy and chemoradiation: The presence of higher pretreatment ADC values reflected necrotic tumors that were resistant to therapy. Persistence of low ADC in responders after chemotherapy could represent a loss of a nonviable fraction of the treated tumor.[43] Another explanation is that this manifestation was attributed to the possible increase in fibrosis and proctitis in response to treatment.[44]
In the study by DeVries et al.,[45] a significant difference in the intratumoral frequencies of perfusion index values before therapy was found between patients responding to chemoradiation and those not responding. This finding allows the formulation of the hypotheses for advanced primary rectal carcinoma: The response to chemoradiation is determined by tumor microcirculation before therapy.
Malignant liver lesions
Several studies demonstrated that ADC increases correlated with response to systemic chemotherapy of liver metastases of breast and colorectal cancer.[46,47,48] In a study of 24 patients with unresectable HCC undergoing transcatheter arterial chemoembolization (TACE), mean tumor size was unchanged up to 4 weeks after TACE whereas reduction in tumor ADC was significant 1–2 weeks after therapy.[49] In this way, the use of DW-MRI seems to be an advantage over tumor morphology.
Magnetic resonance perfusion derived HCC parameters, Ktrans and Kep, were sensitive imaging biomarkers. Higher baseline Ktrans value and more substantial drop in Ktrans and Kep at 2 weeks after therapy correlated with better clinical outcome.[50] This manifestation reflected the underlying tumor permeability changes induced by antiangiogenic therapy.
Head and neck cancer
Apparent diffusion coefficient value can be used as a biomarker for the prediction and early detection of response to concurrent chemoradiation therapy in head and neck cancer. Primary tumors and metastatic lymph nodes with lower ADC values are more likely to have a better response to the treatment.[51,52] Follow-up and early response to treatment have shown ADC increase in both primary tumor and nodal metastasis.[9,52,53,54] In complete responders significant increase in ADC was observed within 1-week of treatment that continued until the end of treatment.[51]
Malignant tumors responsive to chemoradiation therapy have significantly higher Ktrans values before treatment than those partial response or no response.[8,20,24] The responders show prolonged survival in the follow-up study. These results support the idea that relatively higher blood flow in tumors associated with increased oxygenation levels may result in better access to chemotherapeutic drugs and radiosensitivity.[55] However, one study of squamous cell carcinoma in head and neck failed to show a significant difference in ve, vp and keP values between responders and nonresponders.[19]
Osteosarcoma
Diffusion-weighted-MRI may be useful in assessing response to cytotoxic chemotherapy. Tumors with no increase in ADC showed a poor response to chemotherapy on their histology results.[56,57] At mid-course of treatment, the ADC differential (ADCpost − ADCpre) had 100% sensitivity and 57% specificity for predicting poor responders.[56] In another study, the patients with a good response had a significantly higher minimum ADC ratio ([ADCpost − ADCpre]/ADCpre) than those with a poor response.[57] DCE-MRI was also a prognostic factor for indicative of histologic response to neoadjuvant therapy. In the analysis by Guo et al.,[26] response was found as a time-dependent covariate and Ktrans, vp, and Δkep at week 9 were significantly different between responders and nonresponders.
CONCLUSION
As more individualized, biomarker-driven therapies are developed, and with cost incentives for comparative effectiveness research to assess these individualized therapies, the multidisciplinary nature of oncology research must be accommodated. The multiparametric MRI approach including anatomical and functional imaging techniques proves to be the optimal approach in the diagnosis of tumors and prediction and evaluation of treatment response.[30,58,59] However, prospective and multi-center studies must be conducted to assess quantitative MRI parameters as the most appropriate imaging biomarkers.
Footnotes
Edited by: Li-Min Chen
Source of Support: This work was supported by the grants from National Natural Science Foundation of China (No. 81271557 and No. 81201075).
Conflict of Interest: None declared.
REFERENECES
- 1.Sasaki M, Sumi M, Eida S, Ichikawa Y, Sumi T, Yamada T, et al. Multiparametric MR imaging of sinonasal diseases: Time-signal intensity curve- and apparent diffusion coefficient-based differentiation between benign and malignant lesions. AJNR Am J Neuroradiol. 2011;32:2154–9. doi: 10.3174/ajnr.A2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medved M, Karczmar G, Yang C, Dignam J, Gajewski TF, Kindler H, et al. Semiquantitative analysis of dynamic contrast enhanced MRI in cancer patients: Variability and changes in tumor tissue over time. J Magn Reson Imaging. 2004;20:122–8. doi: 10.1002/jmri.20061. [DOI] [PubMed] [Google Scholar]
- 3.Heijmen L, Verstappen MC, Ter Voert EE, Punt CJ, Oyen WJ, de Geus-Oei LF, et al. Tumour response prediction by diffusion-weighted MR imaging: Ready for clinical use? Crit Rev Oncol Hematol. 2012;83:194–207. doi: 10.1016/j.critrevonc.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Malayeri AA, El Khouli RH, Zaheer A, Jacobs MA, Corona-Villalobos CP, Kamel IR, et al. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics. 2011;31:1773–91. doi: 10.1148/rg.316115515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thoeny HC, Ross BD. Predicting and monitoring cancer treatment response with diffusion-weighted MRI. J Magn Reson Imaging. 2010;32:2–16. doi: 10.1002/jmri.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harry VN, Semple SI, Parkin DE, Gilbert FJ. Use of new imaging techniques to predict tumour response to therapy. Lancet Oncol. 2010;11:92–102. doi: 10.1016/S1470-2045(09)70190-1. [DOI] [PubMed] [Google Scholar]
- 7.Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol. 2006;24:3293–8. doi: 10.1200/JCO.2006.06.8080. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Loevner LA, Quon H, Kilger A, Sherman E, Weinstein G, et al. Prediction of response to chemoradiation therapy in squamous cell carcinomas of the head and neck using dynamic contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 2010;31:262–8. doi: 10.3174/ajnr.A1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandecaveye V, Dirix P, De Keyzer F, de Beeck KO, Vander Poorten V, Roebben I, et al. Predictive value of diffusion-weighted magnetic resonance imaging during chemoradiotherapy for head and neck squamous cell carcinoma. Eur Radiol. 2010;20:1703–14. doi: 10.1007/s00330-010-1734-6. [DOI] [PubMed] [Google Scholar]
- 10.Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T (1)-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–32. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 11.Verstraete KL, Lang P. Bone and soft tissue tumors: The role of contrast agents for MR imaging. Eur J Radiol. 2000;34:229–46. doi: 10.1016/s0720-048x(00)00202-3. [DOI] [PubMed] [Google Scholar]
- 12.Miller JC, Pien HH, Sahani D, Sorensen AG, Thrall JH. Imaging angiogenesis: Applications and potential for drug development. J Natl Cancer Inst. 2005;97:172–87. doi: 10.1093/jnci/dji023. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Yu Y, Zhang Y, Bao S, Wu C, Wang X, et al. A clinically feasible method to estimate pharmacokinetic parameters in breast cancer. Med Phys. 2009;36:3786–94. doi: 10.1118/1.3152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yankeelov TE, Luci JJ, Lepage M, Li R, Debusk L, Lin PC, et al. Quantitative pharmacokinetic analysis of DCE-MRI data without an arterial input function: A reference region model. Magn Reson Imaging. 2005;23:519–29. doi: 10.1016/j.mri.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Yabuuchi H, Fukuya T, Tajima T, Hachitanda Y, Tomita K, Koga M. Salivary gland tumors: Diagnostic value of gadolinium-enhanced dynamic MR imaging with histopathologic correlation. Radiology. 2003;226:345–54. doi: 10.1148/radiol.2262011486. [DOI] [PubMed] [Google Scholar]
- 16.Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia. 2009;11:102–25. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choyke PL, Dwyer AJ, Knopp MV. Functional tumor imaging with dynamic contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging. 2003;17:509–20. doi: 10.1002/jmri.10304. [DOI] [PubMed] [Google Scholar]
- 18.Lipnick S, Liu X, Sayre J, Bassett LW, Debruhl N, Thomas MA. Combined DCE-MRI and single-voxel 2D MRS for differentiation between benign and malignant breast lesions. NMR Biomed. 2010;23:922–30. doi: 10.1002/nbm.1511. [DOI] [PubMed] [Google Scholar]
- 19.Chawla S, Kim S, Dougherty L, Wang S, Loevner LA, Quon H, et al. Pretreatment diffusion-weighted and dynamic contrast-enhanced MRI for prediction of local treatment response in squamous cell carcinomas of the head and neck. AJR Am J Roentgenol. 2013;200:35–43. doi: 10.2214/AJR.12.9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xian J, Du H, Wang X, Yan F, Zhang Z, Hao H, et al. Feasibility and value of quantitative dynamic contrast enhancement MR imaging in the evaluation of sinonasal tumors. Chin Med J (Engl) 2014;127:2259–64. [PubMed] [Google Scholar]
- 21.Wang J, Takashima S, Takayama F, Kawakami S, Saito A, Matsushita T, et al. Head and neck lesions: Characterization with diffusion-weighted echo-planar MR imaging. Radiology. 2001;220:621–30. doi: 10.1148/radiol.2202010063. [DOI] [PubMed] [Google Scholar]
- 22.Taouli B, Vilgrain V, Dumont E, Daire JL, Fan B, Menu Y. Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: Prospective study in 66 patients. Radiology. 2003;226:71–8. doi: 10.1148/radiol.2261011904. [DOI] [PubMed] [Google Scholar]
- 23.Kim T, Murakami T, Takahashi S, Hori M, Tsuda K, Nakamura H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol. 1999;173:393–8. doi: 10.2214/ajr.173.2.10430143. [DOI] [PubMed] [Google Scholar]
- 24.Chawla S, Kim S, Loevner LA, Hwang WT, Weinstein G, Chalian A, et al. Prediction of disease-free survival in patients with squamous cell carcinomas of the head and neck using dynamic contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 2011;32:778–84. doi: 10.3174/ajnr.A2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLaughlin R, Hylton N. MRI in breast cancer therapy monitoring. NMR Biomed. 2011;24:712–20. doi: 10.1002/nbm.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo J, Reddick WE, Glass JO, Ji Q, Billups CA, Wu J, et al. Dynamic contrast-enhanced magnetic resonance imaging as a prognostic factor in predicting event-free and overall survival in pediatric patients with osteosarcoma. Cancer. 2012;118:3776–85. doi: 10.1002/cncr.26701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Connor JP, Jackson A, Parker GJ, Roberts C, Jayson GC. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol. 2012;9:167–77. doi: 10.1038/nrclinonc.2012.2. [DOI] [PubMed] [Google Scholar]
- 28.Türkbey B, Thomasson D, Pang Y, Bernardo M, Choyke PL. The role of dynamic contrast-enhanced MRI in cancer diagnosis and treatment. Diagn Interv Radiol. 2010;16:186–92. doi: 10.4261/1305-3825.DIR.2537-08.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yankeelov TE, Lepage M, Chakravarthy A, Broome EE, Niermann KJ, Kelley MC, et al. Integration of quantitative DCE-MRI and ADC mapping to monitor treatment response in human breast cancer: Initial results. Magn Reson Imaging. 2007;25:1–13. doi: 10.1016/j.mri.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li XR, Cheng LQ, Liu M, Zhang YJ, Wang JD, Zhang AL, et al. DW-MRI ADC values can predict treatment response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. Med Oncol. 2012;29:425–31. doi: 10.1007/s12032-011-9842-y. [DOI] [PubMed] [Google Scholar]
- 31.Shin HJ, Baek HM, Ahn JH, Baek S, Kim H, Cha JH, et al. Prediction of pathologic response to neoadjuvant chemotherapy in patients with breast cancer using diffusion-weighted imaging and MRS. NMR Biomed. 2012;25:1349–59. doi: 10.1002/nbm.2807. [DOI] [PubMed] [Google Scholar]
- 32.Sharma U, Danishad KK, Seenu V, Jagannathan NR. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed. 2009;22:104–13. doi: 10.1002/nbm.1245. [DOI] [PubMed] [Google Scholar]
- 33.Pickles MD, Gibbs P, Lowry M, Turnbull LW. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging. 2006;24:843–7. doi: 10.1016/j.mri.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Lee KC, Moffat BA, Schott AF, Layman R, Ellingworth S, Juliar R, et al. Prospective early response imaging biomarker for neoadjuvant breast cancer chemotherapy. Clin Cancer Res. 2007;13:443–50. doi: 10.1158/1078-0432.CCR-06-1888. [DOI] [PubMed] [Google Scholar]
- 35.Kim H, Morgan DE, Zeng H, Grizzle WE, Warram JM, Stockard CR, et al. Breast tumor xenografts: Diffusion-weighted MR imaging to assess early therapy with novel apoptosis-inducing anti-DR5 antibody. Radiology. 2008;248:844–51. doi: 10.1148/radiol.2483071740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Connor JP, Jackson A, Parker GJ, Jayson GC. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer. 2007;96:189–95. doi: 10.1038/sj.bjc.6603515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Padhani AR, Khan AA. Diffusion-weighted (DW) and dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) for monitoring anticancer therapy. Target Oncol. 2010;5:39–52. doi: 10.1007/s11523-010-0135-8. [DOI] [PubMed] [Google Scholar]
- 38.Song I, Kim CK, Park BK, Park W. Assessment of response to radiotherapy for prostate cancer: Value of diffusion-weighted MRI at 3 T. AJR Am J Roentgenol. 2010;194:W477–82. doi: 10.2214/AJR.09.3557. [DOI] [PubMed] [Google Scholar]
- 39.Nemoto K, Tateishi T, Ishidate T. Changes in diffusion-weighted images for visualizing prostate cancer during antiandrogen therapy: Preliminary results. Urol Int. 2010;85:421–6. doi: 10.1159/000321233. [DOI] [PubMed] [Google Scholar]
- 40.Kim CK, Park BK, Lee HM, Kim SS, Kim E. MRI techniques for prediction of local tumor progression after high-intensity focused ultrasonic ablation of prostate cancer. AJR Am J Roentgenol. 2008;190:1180–6. doi: 10.2214/AJR.07.2924. [DOI] [PubMed] [Google Scholar]
- 41.Kim CK, Park BK, Lee HM. Prediction of locally recurrent prostate cancer after radiation therapy: Incremental value of 3T diffusion-weighted MRI. J Magn Reson Imaging. 2009;29:391–7. doi: 10.1002/jmri.21645. [DOI] [PubMed] [Google Scholar]
- 42.Arumainayagam N, Kumaar S, Ahmed HU, Moore CM, Payne H, Freeman A, et al. Accuracy of multiparametric magnetic resonance imaging in detecting recurrent prostate cancer after radiotherapy. BJU Int. 2010;106:991–7. doi: 10.1111/j.1464-410X.2010.09291.x. [DOI] [PubMed] [Google Scholar]
- 43.Dzik-Jurasz A, Domenig C, George M, Wolber J, Padhani A, Brown G, et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360:307–8. doi: 10.1016/S0140-6736(02)09520-X. [DOI] [PubMed] [Google Scholar]
- 44.Jang KM, Kim SH, Choi D, Lee SJ, Park MJ, Min K. Pathological correlation with diffusion restriction on diffusion-weighted imaging in patients with pathological complete response after neoadjuvant chemoradiation therapy for locally advanced rectal cancer: Preliminary results. Br J Radiol. 2012;85:e566–72. doi: 10.1259/bjr/24557556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Devries AF, Griebel J, Kremser C, Judmaier W, Gneiting T, Kreczy A, et al. Tumor microcirculation evaluated by dynamic magnetic resonance imaging predicts therapy outcome for primary rectal carcinoma. Cancer Res. 2001;61:2513–6. [PubMed] [Google Scholar]
- 46.Theilmann RJ, Borders R, Trouard TP, Xia G, Outwater E, Ranger-Moore J, et al. Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia. 2004;6:831–7. doi: 10.1593/neo.03343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh DM, Scurr E, Collins D, Kanber B, Norman A, Leach MO, et al. Predicting response of colorectal hepatic metastasis: Value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol. 2007;188:1001–8. doi: 10.2214/AJR.06.0601. [DOI] [PubMed] [Google Scholar]
- 48.Cui Y, Zhang XP, Sun YS, Tang L, Shen L. Apparent diffusion coefficient: Potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology. 2008;248:894–900. doi: 10.1148/radiol.2483071407. [DOI] [PubMed] [Google Scholar]
- 49.Kamel IR, Liapi E, Reyes DK, Zahurak M, Bluemke DA, Geschwind JF. Unresectable hepatocellular carcinoma: Serial early vascular and cellular changes after transarterial chemoembolization as detected with MR imaging. Radiology. 2009;250:466–73. doi: 10.1148/radiol.2502072222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahani DV, Jiang T, Hayano K, Duda DG, Catalano OA, Ancukiewicz M, et al. Magnetic resonance imaging biomarkers in hepatocellular carcinoma: Association with response and circulating biomarkers after sunitinib therapy. J Hematol Oncol. 2013;6:51. doi: 10.1186/1756-8722-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S, Loevner L, Quon H, Sherman E, Weinstein G, Kilger A, et al. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res. 2009;15:986–94. doi: 10.1158/1078-0432.CCR-08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martínez Barbero JP, Rodríquez Jiménez I, Martin Noguerol T, Luna Alcalá A. Utility of MRI diffusion techniques in the evaluation of tumors of the head and neck. Cancers (Basel) 2013;5:875–89. doi: 10.3390/cancers5030875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galbán CJ, Mukherji SK, Chenevert TL, Meyer CR, Hamstra DA, Bland PH, et al. A feasibility study of parametric response map analysis of diffusion-weighted magnetic resonance imaging scans of head and neck cancer patients for providing early detection of therapeutic efficacy. Transl Oncol. 2009;2:184–90. doi: 10.1593/tlo.09175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.King AD, Mo FK, Yu KH, Yeung DK, Zhou H, Bhatia KS, et al. Squamous cell carcinoma of the head and neck: Diffusion-weighted MR imaging for prediction and monitoring of treatment response. Eur Radiol. 2010;20:2213–20. doi: 10.1007/s00330-010-1769-8. [DOI] [PubMed] [Google Scholar]
- 55.Cooper RA, Carrington BM, Loncaster JA, Todd SM, Davidson SE, Logue JP, et al. Tumour oxygenation levels correlate with dynamic contrast-enhanced magnetic resonance imaging parameters in carcinoma of the cervix. Radiother Oncol. 2000;57:53–9. doi: 10.1016/s0167-8140(00)00259-0. [DOI] [PubMed] [Google Scholar]
- 56.Baunin C, Schmidt G, Baumstarck K, Bouvier C, Gentet JC, Aschero A, et al. Value of diffusion-weighted images in differentiating mid-course responders to chemotherapy for osteosarcoma compared to the histological response: Preliminary results. Skeletal Radiol. 2012;41:1141–9. doi: 10.1007/s00256-012-1360-2. [DOI] [PubMed] [Google Scholar]
- 57.Oka K, Yakushiji T, Sato H, Hirai T, Yamashita Y, Mizuta H. The value of diffusion-weighted imaging for monitoring the chemotherapeutic response of osteosarcoma: A comparison between average apparent diffusion coefficient and minimum apparent diffusion coefficient. Skeletal Radiol. 2010;39:141–6. doi: 10.1007/s00256-009-0830-7. [DOI] [PubMed] [Google Scholar]
- 58.Padhani AR. Integrating multiparametric prostate MRI into clinical practice. Cancer Imaging. 2011;11:S27–37. doi: 10.1102/1470-7330.2011.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kitajima K, Kaji Y, Fukabori Y, Yoshida K, Suganuma N, Sugimura K. Prostate cancer detection with 3 T MRI: Comparison of diffusion-weighted imaging and dynamic contrast-enhanced MRI in combination with T2-weighted imaging. J Magn Reson Imaging. 2010;31:625–31. doi: 10.1002/jmri.22075. [DOI] [PubMed] [Google Scholar]


