Abstract
Cardiovascular disease is the leading cause of death worldwide. Achieving the next phase of potential treatment strategies and better prognostic tools will require a concerted effort from interdisciplinary fields. Biomaterials-based cardiac tissue models are revolutionizing the area of preclinical research and translational applications. The goal of in vitro cardiac tissue modeling is to create physiological functional models of the human myocardium, which is a difficult task due to the complex structure and function of the human heart. This review describes the advances made in area of in vitro cardiac models using biomaterials and bioinspired platforms. The field has progressed extensively in the past decade, and we envision its applications in the areas of drug screening, disease modeling, and precision medicine.
Graphical abstract
1. Introduction
Drug discovery and development is a challenging road, and current methods to evaluate drug safety and efficacy are costly and inefficient. The average time between drug discovery and commercialization is 10 - 15 years, with median costs over $5 billion [1]. During preclinical and clinical development, cardiotoxicity remains a major cause of failure, with high rates of post-approval withdrawal of medicines [2]. Furthermore, effective pre-clinical evaluation of drugs is essential for treating cardiovascular diseases affecting 17.5 million people worldwide and accounting for 31% of all global deaths in 2012 [3]. However, major barriers inhibit current research in human drug screening: experimental in vivo interventions have unacceptably high risks for humans enrolled in clinical trials, and non-human animal models fail to fully recapitulate human physiology. For example, the resting heart rate in mice is tenfold higher than in humans, while the mouse QT interval is one-fourth of a typical human [4]. Due to inter-species differences in ion channels, biological pathways, and pharmacokinetic properties, animal models do not faithfully predict human cardiotoxicity. Thus, human in vitro models of cardiac tissue that are predictive of human drug response would be a significant advancement for understanding, studying, and developing new drugs and strategies for treating cardiac diseases.
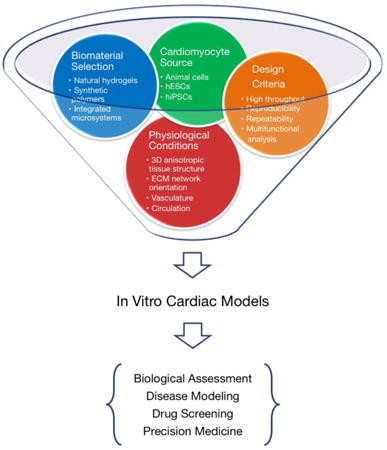
An ideal in vitro cardiac model should accurately recapitulate the physiological or pathological conditions of the human heart, including three-dimensional (3D) anisotropic tissue structure, orientation of the extracellular matrix (ECM) network, vascularization, and circulation (Figure 1). Traditional 2D in vitro systems, although informative [5, 6], cannot accurately mimic the complex 3D conditions due to their inability to recapitulate the dynamics of the biological and mechanical properties of the in vivo microenvironment [7]. The 3D models are characterized by establishment of adhesion complexes and tissue polarity, and by changes in cytoskeletal structure and cell volume that are significantly different from those found in cells cultured as monolayers. As a result, the translational results in 2D conditions are fundamentally different from those in 3D [8].
Figure 1.
Overview of in vitro cardiac tissue model. New in vitro biomaterial-based cardiac tissue models have the potential to be used for fundamental research and translational applications. In particular, the areas of drug discovery, disease modeling, and precision medicine could benefit immensely from these emerging technologies.
Human cardiovascular conditions in vitro can be achieved by developing engineered physiologically relevant 3D models, for instance by embedding cells in biomaterial matrices or microfabricated devices. For the purpose of in vitro modeling, biomaterials and microsystems not only serve as scaffolds for tissue formation, but also provide a highly-controllable microenvironment that incorporates key niche elements to enable precise regulation of cell fate and function [9-11]. Specifically, the complex tissue and organ architecture of the heart is maintained by extensive 3D ECM networks, including fibrous proteins (e.g. collagen, elastin), adhesive glycoproteins (e.g. laminin, fibronectin) and proteoglycans [12]. This ECM network, primarily in the form of perimysial collagen fibers, guides the anisotropic alignment of cardiomyocytes (CMs), mechanically confines the cells to connect each other, and contributes to stress-strain relationships for the heart [13]. Perimysial collagen fibers are comprised of bundles of twisted constituent fibrils (∼ 40 - 50 nm in diameter), forming fibers that range from ∼ 0.5 - 10 μm in diameter and ∼100-200 μm in spacing, allowing several CMs to fit in-between[14]. Furthermore, the perimysial collagen fibers are arranged parallel with the long axis of cardiac muscle and therefore are one of the most significant components of the myocardium that contributes to its non-linear passive stiffness in the direction of the cardiac muscle fibers [15]. The perimysial fibers interact with the CMs via various mechanotransduction pathways, and ultimately affect normal cardiac function. For example, the fibrillar collagen networks register sarcomere Z-line across the CM membrane, and thereby ensure equal stretching of contiguous cells and maintenance of the mechanical continuity between CMs [16]. Given the key role of ECM in heart development and mechanical functions, development of an in vitro cardiac model requires biomaterials, methods, and systems to host the cells, control the cell-cell and cell-ECM interactions, and regulate the cell fate and functions.
In this review, we focus on the important role of biomaterials and microsystems used for in vitro cardiac models. First, we briefly discuss the cell source used for cardiac tissue models, and emphasize human induced pluripotent stem cells (hiPSCs) as the most promising cell type for generation of human CMs. Then, we highlight key properties of different in vitro models, along with their advantages and limitations for applications such as drug cardiotoxicity screening and human heart disease modeling.
2. Cell Sources for Cardiac Tissue Models
In the adult human heart, CMs account for roughly 75% of the heart volume, although they represent only about 33% of the total cell number [17, 18]. Therefore, identifying the optimal source of beating CMs is the first step in the development of a functioning in vitro cardiac model. Early cardiac tissue models depended on either immortalized human cell lines or primary cells isolated from multiple species. The immortalized human ventricular AC cell line was developed using fusion of primary ventricular CMs with a SC-40 transformed fibroblast cell line [19]. Primary CMs isolated from embryonic chicken and neonatal mice and rats were the next most common cell sources for cardiac models [20-22], but increased awareness that animal cell-based models cannot truly recapitulate human physiology has led to the development of more sophisticated cells to build human-like tissue models.
The advancement of stem cell biology has spearheaded the development of in vitro cardiac models that employ differentiated pluripotent stem and progenitor cells [23]. Originally, mesenchymal stem cells (MSCs) were widely used for cardiac tissue models to investigate their beneficial effects on damaged cardiac tissues, either through transdifferentiation or paracrine signaling [24-26]. However, MSCs suboptimal capability for cardiac differentiation has limited the use of these cells in cardiac tissue models.
For better recapitulation of human physiology and pathology, in vitro cardiac models now focus on human pluripotent stem cells, including human embryonic stem cells (hESCs) and hiPSCs [27, 28] (Table 1). Contracting CMs were first generated from hiPSCs through co-culture with END2 mouse endoderm-like cells, a methodology restricted by its reliance on animal cells [29]. hiPSCs suspended in fetal bovine serum to make 3D aggregates of embryoid bodies was later used to generate CMs. Initial protocols produced contracting embryoid bodies with only 5%-15% efficiency [30], and subsequent optimization with timely addition of growth factors (such as Activin A, BMP4 and FGF) improved this efficiency to over 70% [31]. Nowadays, monolayer differentiation, which involves simple, serum-free, and scalable protocols, has largely replaced embryoid body formation [32, 33]. Meanwhile, Activin A and BMP4 have been replaced by small molecules CHIR99021 and IWP4, which leads to greater reliability and higher efficiency [34]. Recently, chemically defined method to replace Matrigel-coating with synthesized vitronectin peptide, and “B27” with L-ascorbic acid 2-phosphate and recombinant human albumin has been used to generate CMs at 85% purity, that can be enriched to 95% with sodium lactate [35]. Based on these advances, City of Hope scientists funded by California Institute of Regenerative Medicine (CIRM) are currently developing a bag-based bioreactor system for scalable and controllable production of Good Manufacturing Practices (GMP)-level hESC-CMs, which will remove a key barrier to developing regenerative medicine products, especially for cardiac repair requiring for high doses of human CMs [36].
Table 1.
Generation of cardiomyocytes from human pluripotent stem cells.
| Methods | Media | Yield | Disadvantage |
|---|---|---|---|
| Feeder Layer | Serum-based media Mouse END-2 cells [29] | 35% | Low yield Serum media Requirement of mouse feeder cells |
| Embryoid Bodies | Serum-based media [30] | 5-15% | Low yield Serum media |
| RPMI+B27 supplement ActivinA + BMP4 [31] | 60% | Medium yield Requirement of EB formation Batch variability of growth factors Chemical undefined “B27” |
|
| Bioreactor suspension culture [36] RPMI+B27 supplement Small molecules | 90% | Chemical undefined “B27” | |
| Monolayer | RPMI+B27 supplement ActivinA + BMP4 [33] | 35% | Low yield Batch variability of growth factors Chemical undefined “B27” |
| RPMI+B27 supplement Matrigel Sandwich ActivinA + BMP4 [32] | 90% | Batch variability of Matrigel and growth factors Chemical undefined “B27” |
|
| RPMI+B27 supplement Small molecules [34] | 90% | Chemical undefined “B27” | |
| RPMI + human albumin L-ascorbic acid 2-phosphate (AA 2-P) Small molecules [35] | 85% |
Exciting advances in genome-editing methods by endonuclease (ZFN or TALEN) or palindromic repeat (CRISPR) are being introduced to engineer cardiac disease-associated gene mutations into hiPSC lines with the same genetic background, which will be instrumental for generating libraries of disease-specific CMs for drug testing and disease modeling [37, 38]. To work effectively in the area of patient-specific cells and disease models, a high degree of collaboration and coordination amongst academic laboratories and industry is required. To this end various institutions like CIRM, Cellular Dynamics International (CDI), Coriell Institute for Medical Research, Axiogenesis, and Stanford University are working cohesively to establish a bank of hiPSCs, which will ensure the development of standard operating procedures and practices in order to achieve efficiency, consistency, and high throughput [39, 40]. Making this hiPSC bank available to a broader base of researchers would strongly support a more thorough understanding of the nature of cardiovascular diseases, and the development of cures and stem cell therapies for said diseases.
One key area of research that needs to be addressed prior to full-scale use of iPSCs for cardiac drug screening and development is the maturity of the CMs. During heart development, cardiac muscle cells undergo a complex series of structural changes that ultimately result in their adult phenotype [41]. CM maturation in vivo is also regulated by diverse factors, including topographical, electrical, mechanical, biochemical, and cellular interaction cues. However, hiPSC-CMs in vitro retain a relatively immature phenotype and exhibit relatively small size, reduced electrical excitability, impaired excitation-contraction coupling, and incomplete adrenergic sensitivity [42]. This is one of the critical obstacles to the successful development of predictive drug and toxicology screens, as well as safe and efficient cardiac therapies. Currently, efforts focus on dissecting the external cues (e.g., chemical, physical, electrical), deciphering signaling pathways, and harnessing this information to accelerate the maturation process [43]. Hereby, engineering methods will play a crucial role to stimulate the in vitro processing of hiPSC-CMs maturation by providing relevant environmental motifs, such as anisotropic morphology, external electrical stimulation, mechanical loading, and extracellular matrices.
3. Cell Micropatterning for 2D CM Alignment
An optimal in vitro model would incorporate the aforementioned hiPSCs into an in vivo-like tissue structure while providing researchers with precise control over cell types, ECM composition, cell-cell interactions, and microenvironment geometry. In early studies on the effect of CM anisotropic morphology, cardiac cells were aligned on a thin collagen surface coating that was spread using a cell scraper and polymerized while slowly being poured within a slightly tilted dish [44]. Later, microabrasion was employed to create aligned CMs with anisotropic sarcomeric structure, by unidirectional abrading polyvinyl chloride (PVC) coverslips using lapping papers with different grit sizes [45].
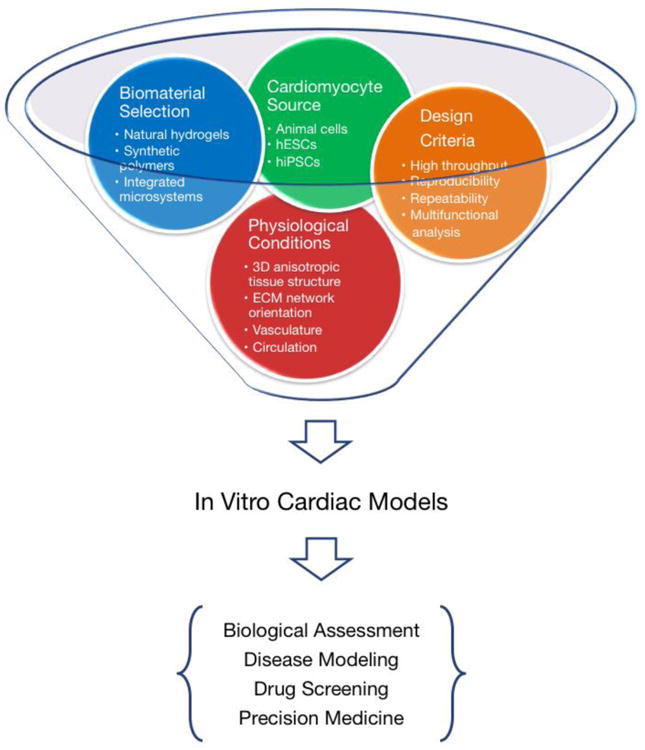
More recently, microfabrication-based patterning techniques (Figure 2) have been used to establish in vitro culture models and investigate the fundamental physiological and pathological characteristics of CMs. Cell alignment can be controlled by surface topography [46-50] or by micromolding with microchannels fabricated from PDMS [51-53]. Microcontact printing ECM proteins created cell-adhesive areas of various shapes on cell-repelling surfaces, using, for instance, laminin onto polyacrylamide thin films [54, 55], fibronectin onto alginate [56], laminin onto PDMS [57], or repelling areas on adhesive surfaces using chitosan and hyaluronic acid onto PDMS and glass [58, 59]. Significant observations in calcium handling, action potentials, and conductional velocities were more similar to adult mouse myocardium in aligned CMs as compared to those grown in randomly oriented cultures [60, 61]. Monolayer of aligned neonatal rat CMs created by microcontact-printing method was found to undergo fibrosis after activation of TGF-β signaling pathway and reduce electrical conduction due to the mechanical interactions between myofibroblasts and CMs [62].
Figure 2.
Micropatterned 2D cardiac models. Topographical alignment of CMs with (A) microfabricated nanostructured surface [50]; (B) prestressed thermoplastic shrink film with tunable multi-scaled wrinkles [49]; and (C) microcontact-printed patterns of pattern CMs into (D) aligned stripes to mimic adult cardiac tissue structure [55] and (E) circular colonies for high-throughput screening [64]. (F) Using oxygen plasma to etch PEG surfaces under a PDMS stencil protection allows micropatterning hiPSCs and determining stem cell fate during cardiac differentiation [72].
Micropatterning hiPSC-derived CMs (hiPSC-CMs) by microcontact printing collagen onto polyacrylamide has been used to increase the maturity level of hiPSC-CMs with optimized culture media [63]. A similar microcontact printing approach with laminin was used to generate hESC-CM microarrays for functional analysis and drug screening, assessing the effects of treatment with H2O2 on CM viability and contractility [64].
Micropatterning techniques also enable precise control over the shape and size of cell colonies and are regularly used to generate uniform embryoid bodies (EBs) for studies of embryogenesis and cardiomyogenesis. Contained in poly(ethylene glycol) (PEG) hydrogel microwells, mouse ESCs formed homogeneous EBs of different sizes. The size of the EBs modulated differential expression of WNT5a and WNT11, leading to higher CM differentiation in large EBs, compared to higher endothelial differentiation in small EBs [65]. The size of 3D polyurethane microwells was also found to modulate cell-cell contact and canonical Wnt/β-catenin signaling in human ESCs, resulting in higher CM differentiation in larger wells [66, 67]. A more extensive study of microwells in silicone rubber sheets fabricated via laser cutting revealed that cell patterning resulted in homogeneous expression of pluripotent markers in hiPSCs and improved yield and reproducibility of cardiac differentiation [68]. Studies on the effects of patterning sizes on embryogenesis and cardiogenesis were also conducted by microcontact printing Matrigel to generate uniform EBs. Patterned EBs revealed that the ratio of Gata6 (endoderm-associated marker) to Pax6 (neural-associated marker) expression increased with decreasing colony size. Larger EBs with endoderm-biased (high Gata6/Pax6) gene expression at early stages exhibited higher mesoderm and cardiac induction[69]. This approach was further used for high-throughput analysis of cell fate determination and endogenous signaling pathway activation and differentiation bias [70].
Recently, researchers found that the geometric confinement from the micropatterned substrate was able to trigger self-organization of hESCs, which recapitulated spatial cell fate patterning during early embryonic development. In response to BMP4, colonies reproducibly differentiated to an outer trophectoderm-like ring, an inner ectodermal circle, and a ring of mesendoderm expressing primitive-streak markers in between [71]. Synergism of biochemical cues and geometric confinement on micropatterned hiPSCs can induce self-organizing lineage specification and creation of a 3D beating human cardiac microchamber, which resembles the developing primitive human heart. These in vitro cardiac microchambers were used to screen drugs likely to generate cardiac malformations during development. For example, applying thalidomide during the cardiac differentiation not only reduced differentiation efficiency, but also significantly damaged the formation of cardiac microchambers with smaller size, lower contractility, and decreased beat rates compared to the control [72].
Although micropatterning methods can confine colony geometry, regulate cell morphology and functions, and support high-throughput analysis, these 2D culture platforms lack the full architecture and functional properties of 3D human tissues and organs, and thus are of limited use for cardiac research. These 2D results have been seen as the first step towards engineering cardiac models, which can be used as templates for 3D tissue structure. Ongoing 2D research would focus on single-cell micropatterning and analysis, which can provide insight on cellular machinery, characterize the heterogeneity of cell population, and enable high-throughput screening for single-cell response to different environmental factors. Compared to CM alignment for mimicking heart muscle tissue, single CM micropatterning is extensively involved in exploring myofibrillogenesis and its relationship with extracellular factors. By microcontact printing ECM protein on the coverslip to shape single CM into the predesigned patterns, researchers found that not only cell shape was defined but also the cytoskeleton was under reorganization into the predicted architecture [73]. It was noticed that the spatial configuration of ECM played a key role in regulating the other three factors: cell shape [20], sarcomere orientation [74], and nuclear morphology [75].
4. Biomaterials Used to Generate 3D Cardiac Models
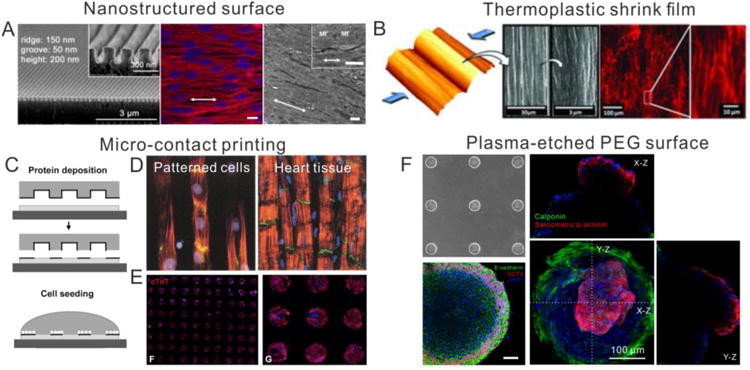
Engineering a 3D cardiac tissue with physiologically relevant microenvironment and cell morphology presents a significant challenge for in vitro cardiac modeling. Biomaterials have played a major role in creating 3D tissue models, since they not only support cell attachment and alignment, but also transmit load, provide physiologically relevant stiffness, and ideally can be degraded and replaced over time by cell-secreted ECM proteins. Several representative natural and synthetic biomaterials-based engineered heart tissue (EHT) systems are shown in Figure 3. We have classified theses as either hydrogel based or fibrous cardiac models, each is discussed in greater detail below.
Figure 3.
Biomaterial-based 3D cardiac models. (A) Engineered heart mini-tissues (millimeter scale) were made from fibrin and hiPSCs for implantation [78] and drug-screening purpose [79]. (B) Engineered heart micro-tissue (micron scale) made from collagen were used to model the dilated cardiomyopathy caused by titin mutation [82]. (C) Fibrin-based cardiac tissue patch was generated by soft lithography with controllable size and architecture and its drug response to isoproterenol [85]. (D) A biowires platform combining architectural and electrical cues generated a microenvironment conducive to the maturation of hiPSC-derived cardiac tissues [95]. (E) Electrospun nanofiber scaffolds were made for creating the continuous anisotropic cardiac tissue [89]. (F) Aligned nanofiber scaffolds made by rotary jet spinning promoted better sarcomere formation in CMs [91]. (G) High-defined filamentous scaffolds made by two-photon initiated polymerization were used to create an aligned hiPSC-CMs-based cardiac model for drug screening [92].
4.1 Natural hydrogel-based cardiac models
Hydrogels consisting of two naturally occurring proteins, collagen and fibrin, have been widely used to generate EHT. Matrigel was often used as a supplemental material to increase cell viability and attachment due to its various growth factors and matrix components. The first EHT consisted of a 3D scaffold of collagen I with embryonic chick CMs [76]. Later, they were further developed into ring structures with neonatal rat CMs [77], which could be stacked and implanted for successful improvement of the function of infarcted rat heart [78]. Currently, EHTs are primarily designed in a two-post configuration, allowing for characterization of contraction forces. Parallel EHT arrays consisting of a mixture of fibrinogen, Matrigel, thrombin, and neonatal rat CMs [79] or hESC-CMs [80], on a silicone post rack casted from Teflon molds, were used for preliminary drug screening. Proarrhythmic compounds chromanol and erythromycin was shown to affect EHT repolarization inhibition, and the cardiotoxic drug doxorubicin affected EHT force generation in a time- and dose-dependent manner [79]. Isoprenaline and carbachol were found to affect the spontaneous contractile rate. Repolarization was inhibited by E-4031 (3 nM IC50), procainamide (100 μM IC50), sertindole (10 nM IC50), quinidine (1000 nM IC50), and cisapride (30 nM IC50). [80].
EHTs of collagen I and fibrinogen were also generated on microfabricated devices with micron-scale standing posts, and researchers found that the matrix composition affected the dynamic and static contractility of the cardiac tissues [81]. Using these micro-EHTs, researchers were able to model dilated cardiomyopathy caused by titin mutation, and demonstrated that 3D titin-mutant EHTs exhibited lower contraction forces compared to WT EHTs, such difference in contractile function was not possible to be detected by single-cell assays [82]. This configuration was further employed to generate EHTs based on a mixture of collagen I, Matrigel, and hESC-CMs for preclinical drug screening and gene transfer. The 610 nM IC50 value generated for verapamil in these EHTs surpassed the 160 nM IC50 for traditional iPSC cells in 2D culture, indicating better recapitulation of human physiology compared to a 2D culture system. However, an insufficient response to isoproterenol suggested cardiac tissue immaturity [83]. A multi-post configuration with collagen I and Matrigel was used to design and formulate cardiac microtissues using hiPSC-CMs, and researchers found that tissue structure and non-CM population played important roles in tissue integrity and maturation [84]. This multi-post platform was further applied to establish a tachycardiac model of arrhythmogenesis for in vitro patient-specific disease modeling.
The 3D cardiac tissue structure was also created using fibrin-based hydrogel matrix generated by soft lithography technique with controllable size and architecture; these EHTs demonstrated increased spontaneous beat rate and twitch amplitude upon exposure to isoproterenol, with an EC50 of 95 nM falling within the reported 30-160 nM range for adult human ventricular tissue. CMs differentiated from ESCs and from cardiac progenitor cells (CPCs) were seeded into this engineered hydrogel to yield highly aligned CMs and robust intercellular coupling with rapid action potential conduction (22–25 cm/s) and significant contractile forces (up to 2 mN) [85, 86].
In all post-based in vitro cardiac microtissues, various natural biomaterials (e.g. collagen I, fibrin, Matrigel) served as an initial scaffold and ECM to support cell attachment, whereas the posts stabilized the developing tissue as the cells condensed and remodeled the scaffold, which had the effect of aligning in 3D the encapsulated CMs. The flexible PDMS posts additionally served as the sensor enabling the measurement of contraction force generated by the beating CMs. These contractile forces are a key output of EHTs and are coupled with CM electrophysiology and hypertrophy within EHTs; however, these forces are highly dependent on the biomaterial composition, making it difficult to compare drug responses among different EHTs developed by different research groups. A high degree of natural material variability is of major concern in efforts to establish standardized assays for drug screening with the requirements of consistency, reproducibility, and high-throughput capability. Such material variability will affect tissue formation and cellular responses, which will eventually lead to the variation of functional readout, such as contractile force measured by the posts.
4.2 Synthetic fibrous cardiac models
Synthetic biomaterials provide an attractive alternative to natural materials, as researchers can control the entire synthesis process as well as the materials' mechanical properties, topography, and structure. A number of synthetic polymers have also been used to create 3D cardiac scaffolds for either in vitro models or implantable patches to repair and regenerate the infarcted tissue. Key requirements for synthetic scaffolds are that they recapitulate the native 3D hierarchical fibrillar structure, possess biomimetic surface properties, and demonstrate mechanical integrity. The most frequently used synthetic polymers for cardiac tissue engineering are polyurethane, poly e-caprolactone (PCL), polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers. One example of synthetic material-based cardiac constructs were generated with neonatal rat CMs and poly(glycerol sebacate) (PGS) and maintained in a bioreactor with simultaneous culture medium perfusion and electrical conditioning, which led to enhanced organization and functionality of engineered cardiac tissue [87].
Cell alignment can be obtained with electrospun nanofiber-based scaffolds, which provide flexible matrices and topographic properties offering support and guidance for the CMs. CMs organized into anisotropic cardiac tissue on aligned PCL/gelatin composite electrospun nanofibrous scaffolds to structurally mimic the oriented ECM in myocardium [88]. The orientation and density of electrospun polymethylglutarimide (PMGI) nanofibers defined the overall architecture of the cardiac tissue, which was optimized for best alignment with 30-50 fibers/mm and an average distance between fibers of under 30 μm [89]. An aligned fibrous mesh of electrospun polyester blend, poly(3–hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), P(L-D,L)LA, and poly(glycerol sebacate) (PGS) was shown to enhance cardiomyogenic differentiation of human umbilical cord mesenchymal stem cells [90]. Similarly, rotary jet spinning was used to fabricate highly aligned nanofiber constructs from a blend of collagen, gelatin, and PCL polymer, which promotes better sarcomere formation in CMs [91].
Electrospun 3D scaffolds with aligned nanofibers using synthetic polymers successfully mimic the structure and orientation of native ECM in the myocardium and help CMs self-organize with anisotropic structure. However, the micron scale porosity of these scaffolds limits cell infiltration into the matrix and thereby the creation of a 3D tissue. As such, the scaffolds are 3D in nature, but the tissue is really a 2D structure similar to those created on micropatterned surfaces. To address this limitation, a highly defined scaffold structure was fabricated by two-photon initiated polymerization (TPIP) with unprecedented control over a wide range of matrix features. A human cardiac disease model was created by seeding hiPSC-CMs, with long QT syndrome type 3 (LQT3), on the TPIP-fabricated synthetic filamentous scaffolds. Tailoring the mechanical properties of the scaffolds modulated the contractility of residing hiPSC-CMs and, more importantly, recapitulated the abnormal contractility of long QT syndrome. Treatment with caffeine increased the spontaneous contractile rate and maximum contractile velocity and high doses of caffeine and nifedipine both caused cessation of beating. In contrast, treatment with E-4031 indicated irregular beating patterns, and propranolol induced significant uncoordinated beating, suggestive of cardiac arrhythmias, in a dose-dependent manner [92].
A collagen-based cardiac tissue model, termed ‘biowire’, combined architectural and electrical cues to generate a microenvironment conducive to maturation of hiPSC-derived cardiac tissues [93]. The hiPSC-CMs were seeded with collagen type I into a microfabricated well and subjected to electrical stimulation with a progressive increase in frequency. Biowires submitted to electrical stimulation had markedly increased myofibril ultrastructural organization, elevated conduction velocity, and improved both electrophysiological and Ca2+ handling properties compared to non-stimulated controls. These changes suggested enhanced CM maturation that depended on the stimulation rate. The biowire maturation represented an intermediate phenotype as CMs undergo maturation from the embryonic state, as evidenced by low membrane conductance. The use of electrical stimulation in conjunction with stretch as a mimic of cardiac load, concurrently or sequentially, might be required to induce terminal differentiation and maturation in hiPSC-CMs [94, 95].
These findings collectively suggest that 3D tissue engineered models with defined cellular microenvironments hold great promise for high-content drug screening and cardiotoxicity testing. The integration of biomaterials with existing iPSC-based disease models could better recapitulate disease pathology and may represent superior scalability and flexibility for creating large numbers of personalized models to meet diverse and urgent patient needs.
5. Microdevices for 3D Cardiac Models
Moving away from scaffold-based cardiac models, highly miniaturized and integrated microphysiological systems are currently being developed as “heart-on-a-chip” technology to provide more controlled 3D microenvironments, with enhanced multiple functionalities and increased throughput. Such microphysiological systems (Figure 4) combined with hiPSC technology are expected to not only better predict on toxicity and efficacy of potential drugs in human physiologically relevant conditions, but also provide a more in-depth understanding of human cardiac disease in complex and heterogeneous microenvironments.
Figure 4.
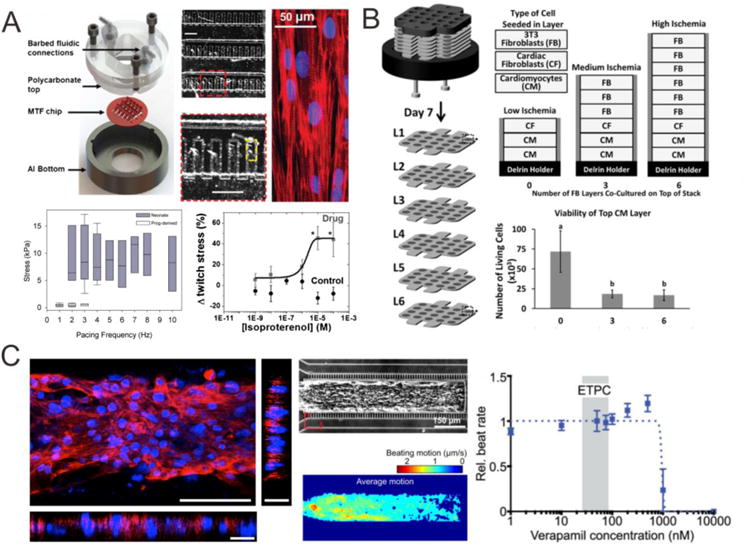
Microdevice-based 3D cardiac models. (A) PIPAAm-based ‘heart-on-chip’ microsystem [97] can measure the deformation of the elastomeric thin film to characterize the contractility of cardiac tissue derived from various cell types and assess the drug response to isoproterenol [98]. (B) A stacked-paper culture system containing CMs was used to mimic the pathological microenvironment occurring during cardiac ischemia [101]. (C) A microfluidic-based microphysiological system was designed to recapitulate a minimal organoid of the human myocardium with highly aligned tissue architecture and anisotropic beating behavior, allowing for accurate prediction of drug cardiotoxicity [102].
An engineered anisotropic ventricular myocardium was first developed by micropatterning neonatal rat CMs on poly(N-isopropylacrylamide) (PIPAAm)/PDMS-based thin elastomeric film, which can simultaneously measure the contractile function, quantify the electrical propagation, and evaluate cytoskeletal architecture in cardiac tissues during pharmacological interventions. A dose-dependent increase in spontaneous beat rate and stress was reported in response to epinephrine. [96]. This microsystem was further incorporated with fluidic control for drug washout, a heating element for temperature control, and embedded electrodes for electrical field stimulation [97]. This system was not only used to characterize the cardiac tissue derived from various cell types (primary neonatal mouse CMs, mouse iPSC-CMs, and human iPSC-CMs) [98], but also to model maladaptive cardiac hypertrophy [99] and patient-specific mitochondrial cardiomyopathy, specifically, the Barth Syndrome (BTHS) - a mitochondrial disorder caused by mutation of the gene encoding tafazzin (TAZ) [100]. To study the pathophysiology underlying BTHS the group generated hiPSC-CMs from two patients with BTHS and discovered metabolic, structural and functional abnormalities associated with TAZ mutation. This elegant study provided new insights into the pathogenesis of Barth syndrome, and pointed to a new treatment strategy for BTHS.
For improved modeling specific types of cardiac disease, unique platforms should be designed to mimic the pathological microenvironment occurring during the disease progression. A paper-based culture system was developed with multiple layers of paper-containing cells, suspended in hydrogels, stacked to form a layered 3D model of a cardiac tissue. Mass transport of oxygen and glucose into this 3D system was modulated to induce an ischemic environment in the bottom layers of the stack. This in vitro cardiac model mechanistically studied cellular motility and viability, and recapitulated the cellular interactions and gradients of molecules in the heart under ischemia. However, the cardiospheres in the stacked papers lacked the aligned structure to mimic the in vivo tissue structure. Moreover, this system currently makes it difficult to determine the concentration of small molecules (e.g., oxygen, glucose, or cytokines) in situ and to measure the contractility of CMs without complex optics [101].
To allow accurate prediction of drug cardiotoxicity, a microfluidic-based microphysiological system was designed to recapitulate a minimal organoid of the human myocardium [102]. Pharmacological studies on this system with verapamil (950 nM IC50), isoproterenol (315 nM EC50), metoprolol (244 μM IC50), and E-4031 (392 nM IC50) predicted a higher safety margin and had better concordance with tissue-scale values and clinical observations, compared to those in cellular-scale studies and large-scale animals. The human cardiac microphysiological system was proposed to complement animal models, and in the future may have the potential to replace animal studies, which often are expensive, unethical, and unable to accurately predict the drug's actual effect.
Since the discovery of Moore's law semiconductor industry has come a long way and the development of new microfabrication techniques has equipped the bioengineering community with tools, which can be employed for basic and translational applications. Microengineered in vitro models with multiple readouts have a great potential to better mimic the in vivo physiology and provide a deeper understanding of the physiological events that characterize cardiac development and function. These systems provide fine control over fluid flow creating microcirculation mimicking the in vivo transport; massive parallelization for high content readouts; miniaturization of large systems for convenient operation and reduction of reagent use leading to lower operational costs; and unprecedented control of system architecture and dimensions at the biological scale (nm to μm). We envision the use of microtechnologies coupled with hiPSC biology to revolutionize the areas of drug screening, disease modeling, and personalized medicine.
6. Perspective and Conclusions
The heart is a powerful, complex organ that has intrigued both artists and scientists for millennia. In vitro cardiac tissue models present great opportunities for regenerative medicine, drug screening, and disease modeling. The opportunities, however, coincide with enormous challenges due to the complexity of cardiac structure and function. A standardized, reproducible, and scalable process for differentiating hiPSCs to CMs is required for consistent cell quality. Recent developments in the cardiogenic differentiation open the possibility of obtaining such human CMs in the laboratory [34, 35].
The immaturity of hiPSC-CMs complicates the cells' adoption as a reliable readout for translational applications. Such immature embryonic or neonatal-like CMs cannot compare morphologically with large and stiff ventricular CMs in the adult human heart [103]. Thus, cardiac tissues constructed from hiPSC-CMs have significantly lower field potentials and contraction forces than adult ventricular tissue, so at this point cannot be considered an exact in vitro model of mature myocardium. It has been suggested that tissue-engineering methods would necessitate the maturation of hiPSC-CMs in a physiologically mimicked microenvironment [63, 86, 93]. This suggests that genetic and environmental factors interact and lead to CM maturation, though the mechanism and process is not fully understood.
We summarize the current in vitro cardiac tissue models, along with their advantages and limitations for applications, such as drug cardiotoxicity screening and human heart disease modeling (Table 2). An ideal in vitro cardiac tissue model should be physiologically relevant with multiple biological, mechanical, and electrical readouts, ensuring different functional endpoints for a particular application. Appropriate biomaterials used for the cardiac tissue models need to be chosen carefully according to the specific applications. For example, microsystems with conventional PDMS as a substrate result in drug stability problems and unpredictable device performance, due to its absorption and retention of highly hydrophobic compounds [104, 105]. Acceptance of these models will require automation, robustness, and easy integration into the workflow at pharmaceutical companies. Specifically for drug development and testing, the microfluidic-based system with standardized fabrication and process holds great promise on high-content screening with electrical and mechanical measurement and integration with multiple organs to achieve “human on a chip.”
Table 2.
Analysis of in vitro cardiac tissue models and the corresponding mechanical, electrophysiological, and biological outcomes.
| Characteristics | Outcomes | ||||||
|---|---|---|---|---|---|---|---|
| Platform | Cell Type | Materials | Coating | Mechanical | Beat Rate (bpm) | Biological | |
| Micropatterning | Surface topography | nrCM | PVC cover slips | Fibronectin [45] | * | + | |
| nrCM | PDMS | Laminin [46] | * | ++ | |||
| nrCM | Polyurethane, polystyrene | [47] | 15-50 | + | |||
| nmCM | PDMS | Fibronectin, laminin, collagen I [48] | * | ++ | |||
| Microcontact printing | nrCM | Polyacrylamide | Laminin, Matrigel [54] | Young's modulus 5-35 kPa | * | ++ | |
| nrCM | Alginate | Fibronectin [56] | Young's modulus 57 kPa | 60-240 (pacing) | ++ | ||
| nrCM | PDMS, Stretch device | Collagen [57] | * | + | |||
| nrCM | Polystyrene | Chitosan [58] | * | ++ | |||
| nrCM | PDMS | Hyaluronic acid, fibronectin [59] | 60-100 | + | |||
| nmCM | Glass, photoresist | [60] | * | ++ | |||
| hESC-CM, hiPSC-CM | Polyacrylamide | Gelatin [63] | Contractile stress 0.2-0.5 mN/mm2 | 60-180 (pacing) | ++ | ||
| hESC-CM | Polyacrylamide | Laminin [64] | Elastic modulus 15-35 kPa | 50 | ++ | ||
| Microfabrication | Microposts | hESC-CM, nrCM | Silicone post racks | Fibrin, Matrigel [80] | Contractile force 100-300 μN | 300 | ++ |
| nrCM hiPSC-CM | PDMS | Collagen, fibrinogen [81, 82] | Contractile force 2-6 μN | 33-60 | ++ | ||
| hESC-CM | PDMS posts | Collagen, Matrigel [83] | Contractile force 0.3 mN | 70 | ++ | ||
| hESC-CM, nrCM | PDMS posts | Collagen, Matrigel [84] | * | ++ | |||
| hESC-CM | PDMS posts | Fibrin [85] | Contractile force 2 mN | * | ++ | ||
| hESC-CM | PDMS posts, patch | Fibrin, Matrigel [86] | Contractile force 3 mN | 60-180 (pacing) | ++ | ||
| Perfusion Bioreactor | nrCM | Poly(glycerol sebacate), channels | Laminin | Elastic modulus 34.55±1.26 kPa, Pore size 75-150 μm | 180 (pacing) | ++ | |
| Biowires | hESC-CM | PDMS | Collagen [93, 94] | Conduction velocity 11-16 cm/s, Young's modulus 1-6 kPa | 60-360 (pacing) | ++ | |
| Two-photon polymerization | hiPSC-CM, Long QT3 syndrome | Filamentous matrix | Fibronectin [92] | Maximal contraction velocity 15-25 μm/s | 90 | ++ | |
| S syst | nrCM, hESC-CM, hiPSC-CM, Barth syndrome | PDMS, PIPAAm | Fibronectin Gelatin [96, 97, 99,100] | Young's modulus 1.52 MPa, Systolic stress 15-20 kPa, Diastolic stress 8.0 kPa | 120 (pacing) | ++ | |
| hiPSC-CM | PDMS | Fibronectin [102] | Average contraction velocity 3 μm/s | 55 - 80 | ++ | ||
nrCM Neonatal rat CMs
nmCM Neonatal mouse CMs
hESC-CM Human embryonic stem cell-derived CMs
hiPSC-CM Human induced pluripotent stem cell-derived CMs
Beat rate Beat per minute (BPM);
Spontaneous contractions reported without beat rate
Indications of CMs are limited to: cell alignment and elongation, morphological assessment, genetic assessment
Indications of CMs include: sarcomeres, functional gap junctions, appropriate responses to drug treatments, as well as indications from ‘+’
A more futuristic application is envisioned in the area of precision medicine, an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person [106, 107]. The promise of precision medicine for cancer therapeutics is already being realized with the recent introduction of several targeted therapies, some with companion diagnostic tests that identify patients most likely to benefit from treatment [108]. Moving forward, we hope to see physiologically functional in vitro cardiac models of individual- and disease-specific hiPSCs on chips, which can be termed as “patient on a chip”. This approach will help to diagnose and design better treatment strategy for individual patients. Success, however, will depend on how effectively and how efficiently engineering and biology can be integrated to create such systems.
Acknowledgments
This work was supported in part from the NIH R01HL096525, UH2TR000487, UH3TR000487, and R21 EB21003. A.M. is a postdoctoral fellow of CIRM training program TG2-01164. P.L. is supported by a postdoctoral fellowship from the German Research Foundation, LO 2081/1-1. S.J. is supported by the National Science Foundation Graduate Research Fellowship DGE 1106400.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Herper M. The cost of creating a new drug now $5 billion, pushing big pharma to change. Forbes. 2013 [Google Scholar]
- 2.Ferri N, Siegl P, Corsini A, Herrmann J, Lerman A, Benghozi R. Drug attrition during pre-clinical and clinical development: understanding and managing drug-induced cardiotoxicity. Pharmacol Ther. 2013;138(3):470–84. doi: 10.1016/j.pharmthera.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, Mc Guire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB C. American Heart Association Statistics, and S. Stroke Statistics. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453(7193):322–9. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 5.Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, Ingber DE. Engineering cell shape and function. Science. 1994;264(5159):696–8. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- 6.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–8. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 7.Shao Y, Sang J, Fu J. On human pluripotent stem cell control: The rise of 3D bioengineering and mechanobiology. Biomaterials. 2015;52:26–43. doi: 10.1016/j.biomaterials.2015.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver VM, Fischer AH, Peterson OW, Bissell MJ. The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochem Cell Biol. 1996;74(6):833–51. doi: 10.1139/o96-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouten CV, Dankers PY, Driessen-Mol A, Pedron S, Brizard AM, Baaijens FP. Substrates for cardiovascular tissue engineering. Adv Drug Deliv Rev. 2011;63(4-5):221–41. doi: 10.1016/j.addr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Hirt MN, Hansen A, Eschenhagen T. Cardiac tissue engineering: state of the art. Circ Res. 2014;114(2):354–67. doi: 10.1161/CIRCRESAHA.114.300522. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, Feric NT, Thavandiran N, Nunes SS, Radisic M. The role of tissue engineering and biomaterials in cardiac regenerative medicine. Can J Cardiol. 2014;30(11):1307–22. doi: 10.1016/j.cjca.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker KK, Ingber DE. Extracellular matrix, mechanotransduction and structural hierarchies in heart tissue engineering. Philos Trans R Soc Lond B Biol Sci. 2007;362(1484):1267–79. doi: 10.1098/rstb.2007.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson TF, Geraci MA, Sonnenblick EH, Factor SM. Coiled perimysial fibers of papillary muscle in rat heart: morphology, distribution, and changes in configuration. Circ Res. 1988;63(3):577–92. doi: 10.1161/01.res.63.3.577. [DOI] [PubMed] [Google Scholar]
- 14.Kanzaki Y, Terasaki F, Okabe M, Fujita S, Katashima T, Otsuka K, Ishizaka N. Three-dimensional architecture of cardiomyocytes and connective tissue in human heart revealed by scanning electron microscopy. Circulation. 2010;122(19):1973–4. doi: 10.1161/CIRCULATIONAHA.110.979815. [DOI] [PubMed] [Google Scholar]
- 15.MacKenna DA, Vaplon SM, McCulloch AD. Microstructural model of perimysial collagen fibers for resting myocardial mechanics during ventricular filling. Am J Physiol. 1997;273(3 Pt 2):H1576–86. doi: 10.1152/ajpheart.1997.273.3.H1576. [DOI] [PubMed] [Google Scholar]
- 16.Baicu CF, Stroud JD, Livesay VA, Hapke E, Holder J, Spinale FG, Zile MR. Changes in extracellular collagen matrix alter myocardial systolic performance. Am J Physiol Heart Circ Physiol. 2003;284(1):H122–32. doi: 10.1152/ajpheart.00233.2002. [DOI] [PubMed] [Google Scholar]
- 17.Borg TK, Ranson WF, Moslehy FA, Caulfield JB. Structural basis of ventricular stiffness. Lab Invest. 1981;44(1):49–54. [PubMed] [Google Scholar]
- 18.Caulfield JB, Borg TK. The collagen network of the heart. Lab Invest. 1979;40(3):364–72. [PubMed] [Google Scholar]
- 19.Davidson MM, Nesti C, Palenzuela L, Walker WF, Hernandez E, Protas L, Hirano M, Isaac ND. Novel cell lines derived from adult human ventricular cardiomyocytes. J Mol Cell Cardiol. 2005;39(1):133–47. doi: 10.1016/j.yjmcc.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Parker KK, Tan J, Chen CS, Tung L. Myofibrillar architecture in engineered cardiac myocytes. Circ Res. 2008;103(4):340–2. doi: 10.1161/CIRCRESAHA.108.182469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badie N, Bursac N. Novel micropatterned cardiac cell cultures with realistic ventricular microstructure. Biophys J. 2009;96(9):3873–85. doi: 10.1016/j.bpj.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneko T, Kojima K, Yasuda K. An on-chip cardiomyocyte cell network assay for stable drug screening regarding community effect of cell network size. Analyst. 2007;132(9):892–8. doi: 10.1039/b704961g. [DOI] [PubMed] [Google Scholar]
- 23.Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10(1):16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figeac F, Lesault PF, Le Coz O, Damy T, Souktani R, Trebeau C, Schmitt A, Ribot J, Mounier R, Guguin A, Manier C, Surenaud M, Hittinger L, Dubois-Rande JL, Rodriguez AM. Nanotubular crosstalk with distressed cardiomyocytes stimulates the paracrine repair function of mesenchymal stem cells. Stem Cells. 2014;32(1):216–30. doi: 10.1002/stem.1560. [DOI] [PubMed] [Google Scholar]
- 25.Haneef K, Naeem N, Khan I, Iqbal H, Kabir N, Jamall S, Zahid M, Salim A. Conditioned medium enhances the fusion capability of rat bone marrow mesenchymal stem cells and cardiomyocytes. Mol Biol Rep. 2014;41(5):3099–112. doi: 10.1007/s11033-014-3170-1. [DOI] [PubMed] [Google Scholar]
- 26.Citro L, Naidu S, Hassan F, Kuppusamy ML, Kuppusamy P, Angelos MG, Khan M. Comparison of human induced pluripotent stem-cell derived cardiomyocytes with human mesenchymal stem cells following acute myocardial infarction. PLoS One. 2014;9(12):e116281. doi: 10.1371/journal.pone.0116281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dell'Era P, Benzoni P, Crescini E, Valle M, Xia E, Consiglio A, Memo M. Cardiac disease modeling using induced pluripotent stem cell-derived human cardiomyocytes. World J Stem Cells. 2015;7(2):329–42. doi: 10.4252/wjsc.v7.i2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartman ME, Dai DF, Laflamme MA. Human pluripotent stem cells: Prospects and challenges as a source of cardiomyocytes for in vitro modeling and cell-based cardiac repair. Adv Drug Deliv Rev. 2015 doi: 10.1016/j.addr.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R, Tertoolen L. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107(21):2733–40. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 30.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108(3):407–14. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8(2):228–40. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, Barron MR, Hou L, Soerens AG, Yu J, Palecek SP, Lyons GE, Thomson JA, Herron TJ, Jalife J, Kamp TJ. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res. 2012;111(9):1125–36. doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25(9):1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 34.Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat Protoc. 2013;8(1):162–75. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11(8):855–60. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen VC, Couture SM, Ye J, Lin Z, Hua G, Huang HI, Wu J, Hsu D, Carpenter MK, Couture LA. Scalable GMP compliant suspension culture system for human ES cells. Stem Cell Res. 2012;8(3):388–402. doi: 10.1016/j.scr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Miyaoka Y, Chan AH, Judge LM, Yoo J, Huang M, Nguyen TD, Lizarraga PP, So PL, Conklin BR. Isolation of single-base genome-edited human iPS cells without antibiotic selection. Nat Methods. 2014;11(3):291–3. doi: 10.1038/nmeth.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Zhang WY, Hu S, Lan F, Lee AS, Huber B, Lisowski L, Liang P, Huang M, de Almeida PE, Won JH, Sun N, Robbins RC, Kay MA, Urnov FD, Wu JC. Genome editing of human embryonic stem cells and induced pluripotent stem cells with zinc finger nucleases for cellular imaging. Circ Res. 2012;111(12):1494–503. doi: 10.1161/CIRCRESAHA.112.274969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor CJ, Bolton EM, Pocock S, Sharples LD, Pedersen RA, Bradley JA. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet. 2005;366(9502):2019–25. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]
- 40.Stacey GN, Crook JM, Hei D, Ludwig T. Banking human induced pluripotent stem cells: lessons learned from embryonic stem cells? Cell Stem Cell. 2013;13(4):385–8. doi: 10.1016/j.stem.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473(7347):326–35. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robertson C, Tran DD, George SC. Concise review: maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells. 2013;31(5):829–37. doi: 10.1002/stem.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res. 2014;114(3):511–23. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simpson DG, Terracio L, Terracio M, Price RL, Turner DC, Borg TK. Modulation of cardiac myocyte phenotype in vitro by the composition and orientation of the extracellular matrix. J Cell Physiol. 1994;161(1):89–105. doi: 10.1002/jcp.1041610112. [DOI] [PubMed] [Google Scholar]
- 45.Bursac N, Parker KK, Iravanian S, Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy: a model for functional electrophysiological studies of cardiac muscle. Circ Res. 2002;91(12):e45–54. doi: 10.1161/01.res.0000047530.88338.eb. [DOI] [PubMed] [Google Scholar]
- 46.Motlagh D, Hartman TJ, Desai TA, Russell B. Microfabricated grooves recapitulate neonatal myocyte connexin43 and N-cadherin expression and localization. J Biomed Mater Res A. 2003;67(1):148–57. doi: 10.1002/jbm.a.10083. [DOI] [PubMed] [Google Scholar]
- 47.Wang PY, Yu J, Lin JH, Tsai WB. Modulation of alignment, elongation and contraction of cardiomyocytes through a combination of nanotopography and rigidity of substrates. Acta Biomater. 2011;7(9):3285–93. doi: 10.1016/j.actbio.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 48.Luna JI, Ciriza J, Garcia-Ojeda ME, Kong M, Herren A, Lieu DK, Li RA, Fowlkes CC, Khine M, McCloskey KE. Multiscale biomimetic topography for the alignment of neonatal and embryonic stem cell-derived heart cells. Tissue Eng Part C Methods. 2011;17(5):579–88. doi: 10.1089/ten.TEC.2010.0410. [DOI] [PubMed] [Google Scholar]
- 49.Chen A, Lieu DK, Freschauf L, Lew V, Sharma H, Wang J, Nguyen D, Karakikes I, Hajjar RJ, Gopinathan A, Botvinick E, Fowlkes CC, Li RA, Khine M. Shrink-film configurable multiscale wrinkles for functional alignment of human embryonic stem cells and their cardiac derivatives. Adv Mater. 2011;23(48):5785–91. doi: 10.1002/adma.201103463. [DOI] [PubMed] [Google Scholar]
- 50.Kim DH, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, Suh KY, Tung L, Levchenko A. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci U S A. 2010;107(2):565–70. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma Z, Liu Q, Liu H, Yang H, Yun JX, Eisenberg C, Borg TK, Xu M, Gao BZ. Laser-patterned stem-cell bridges in a cardiac muscle model for on-chip electrical conductivity analyses. Lab Chip. 2012;12(3):566–73. doi: 10.1039/c2lc20699d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma Z, Liu Q, Yang H, Runyan RB, Eisenberg CA, Xu M, Borg TK, Markwald R, Wang Y, Gao BZ. Laser patterning for the study of MSC cardiogenic differentiation at the single-cell level. Light Sci Appl. 2013;2 doi: 10.1038/lsa.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Annabi N, Selimovic S, Acevedo Cox JP, Ribas J, Afshar Bakooshli M, Heintze D, Weiss AS, Cropek D, Khademhosseini A. Hydrogel-coated microfluidic channels for cardiomyocyte culture. Lab Chip. 2013;13(18):3569–77. doi: 10.1039/c3lc50252j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cimetta E, Pizzato S, Bollini S, Serena E, De Coppi P, Elvassore N. Production of arrays of cardiac and skeletal muscle myofibers by micropatterning techniques on a soft substrate. Biomed Microdevices. 2009;11(2):389–400. doi: 10.1007/s10544-008-9245-9. [DOI] [PubMed] [Google Scholar]
- 55.McDevitt TC, Angello JC, Whitney ML, Reinecke H, Hauschka SD, Murry CE, Stayton PS. In vitro generation of differentiated cardiac myofibers on micropatterned laminin surfaces. J Biomed Mater Res. 2002;60(3):472–9. doi: 10.1002/jbm.1292. [DOI] [PubMed] [Google Scholar]
- 56.Agarwal A, Farouz Y, Nesmith AP, Deravi LF, McCain ML, Parker KK. Micropatterning Alginate Substrates for In Vitro Cardiovascular Muscle on a Chip. Advanced Functional Materials. 2013;23(30):3738–3746. doi: 10.1002/adfm.201203319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Camelliti P, Gallagher JO, Kohl P, McCulloch AD. Micropatterned cell cultures on elastic membranes as an in vitro model of myocardium. Nat Protoc. 2006;1(3):1379–91. doi: 10.1038/nprot.2006.203. [DOI] [PubMed] [Google Scholar]
- 58.Karp JM, Yeo Y, Geng W, Cannizarro C, Yan K, Kohane DS, Vunjak-Novakovic G, Langer RS, Radisic M. A photolithographic method to create cellular micropatterns. Biomaterials. 2006;27(27):4755–64. doi: 10.1016/j.biomaterials.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 59.Khademhosseini A, Eng G, Yeh J, Kucharczyk PA, Langer R, Vunjak-Novakovic G, Radisic M. Microfluidic patterning for fabrication of contractile cardiac organoids. Biomed Microdevices. 2007;9(2):149–57. doi: 10.1007/s10544-006-9013-7. [DOI] [PubMed] [Google Scholar]
- 60.Thomas SP, Bircher-Lehmann L, Thomas SA, Zhuang J, Saffitz JE, Kleber AG. Synthetic strands of neonatal mouse cardiac myocytes: structural and electrophysiological properties. Circ Res. 2000;87(6):467–73. doi: 10.1161/01.res.87.6.467. [DOI] [PubMed] [Google Scholar]
- 61.Pong T, Adams WJ, Bray MA, Feinberg AW, Sheehy SP, Werdich AA, Parker KK. Hierarchical architecture influences calcium dynamics in engineered cardiac muscle. Exp Biol Med (Maywood) 2011;236(3):366–73. doi: 10.1258/ebm.2010.010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson SA, Copeland CR, Reich DH, Tung L. Mechanical coupling between myofibroblasts and cardiomyocytes slows electric conduction in fibrotic cell monolayers. Circulation. 2011;123(19):2083–93. doi: 10.1161/CIRCULATIONAHA.110.015057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ribeiro MC, Tertoolen LG, Guadix JA, Bellin M, Kosmidis G, D'Aniello C, Monshouwer-Kloots J, Goumans MJ, Wang YL, Feinberg AW, Mummery CL, Passier R. Functional maturation of human pluripotent stem cell derived cardiomyocytes in vitro--correlation between contraction force and electrophysiology. Biomaterials. 2015;51:138–50. doi: 10.1016/j.biomaterials.2015.01.067. [DOI] [PubMed] [Google Scholar]
- 64.Serena E, Cimetta E, Zatti S, Zaglia T, Zagallo M, Keller G, Elvassore N. Micro-arrayed human embryonic stem cells-derived cardiomyocytes for in vitro functional assay. PLoS One. 2012;7(11):e48483. doi: 10.1371/journal.pone.0048483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang YS, Chung BG, Ortmann D, Hattori N, Moeller HC, Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc Natl Acad Sci U S A. 2009;106(40):16978–83. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohr JC, Zhang J, Azarin SM, Soerens AG, de Pablo JJ, Thomson JA, Lyons GE, Palecek SP, Kamp TJ. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials. 2010;31(7):1885–93. doi: 10.1016/j.biomaterials.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Azarin SM, Lian X, Larson EA, Popelka HM, de Pablo JJ, Palecek SP. Modulation of Wnt/beta-catenin signaling in human embryonic stem cells using a 3-D microwell array. Biomaterials. 2012;33(7):2041–9. doi: 10.1016/j.biomaterials.2011.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Myers FB, Silver JS, Zhuge Y, Beygui RE, Zarins CK, Lee LP, Abilez OJ. Robust pluripotent stem cell expansion and cardiomyocyte differentiation via geometric patterning. Integr Biol (Camb) 2013;5(12):1495–506. doi: 10.1039/c2ib20191g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bauwens CL, Peerani R, Niebruegge S, Woodhouse KA, Kumacheva E, Husain M, Zandstra PW. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26(9):2300–10. doi: 10.1634/stemcells.2008-0183. [DOI] [PubMed] [Google Scholar]
- 70.Nazareth EJ, Ostblom JE, Lucker PB, Shukla S, Alvarez MM, Oh SK, Yin T, Zandstra PW. High-throughput fingerprinting of human pluripotent stem cell fate responses and lineage bias. Nat Methods. 2013;10(12):1225–31. doi: 10.1038/nmeth.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Warmflash A, Sorre B, Etoc F, Siggia ED, Brivanlou AH. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods. 2014;11(8):847–54. doi: 10.1038/nmeth.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma Z, Wang J, Loskill P, Huebsch N, Koo S, Svedlund FL, Marks N, Hua EW, Grigoropoulos CP, Conklin BR, Healy KE. Self-organizing human cardiac microchambers mediated by geometric confinement. Nat Comm. 2015 doi: 10.1038/ncomms8413. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geisse NA, Sheehy SP, Parker KK. Control of myocyte remodeling in vitro with engineered substrates. In Vitro Cell Dev Biol Anim. 2009;45(7):343–50. doi: 10.1007/s11626-009-9182-9. [DOI] [PubMed] [Google Scholar]
- 74.Bray MA, Sheehy SP, Parker KK. Sarcomere alignment is regulated by myocyte shape. Cell Motil Cytoskeleton. 2008;65(8):641–51. doi: 10.1002/cm.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bray MA, Adams WJ, Geisse NA, Feinberg AW, Sheehy SP, Parker KK. Nuclear morphology and deformation in engineered cardiac myocytes and tissues. Biomaterials. 2010;31(19):5143–50. doi: 10.1016/j.biomaterials.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, Zimmermann W, Dohmen HH, Schafer H, Bishopric N, Wakatsuki T, Elson EL. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J. 1997;11(8):683–94. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 77.Zimmermann WH, Fink C, Kralisch D, Remmers U, Weil J, Eschenhagen T. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol Bioeng. 2000;68(1):106–14. [PubMed] [Google Scholar]
- 78.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12(4):452–8. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 79.Hansen A, Eder A, Bonstrup M, Flato M, Mewe M, Schaaf S, Aksehirlioglu B, Schwoerer AP, Uebeler J, Eschenhagen T. Development of a drug screening platform based on engineered heart tissue. Circ Res. 2010;107(1):35–44. doi: 10.1161/CIRCRESAHA.109.211458. [DOI] [PubMed] [Google Scholar]
- 80.Schaaf S, Shibamiya A, Mewe M, Eder A, Stohr A, Hirt MN, Rau T, Zimmermann WH, Conradi L, Eschenhagen T, Hansen A. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. Plos One. 2011;6(10) doi: 10.1371/journal.pone.0026397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB, Chen CS. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng Part A. 2012;18(9-10):910–9. doi: 10.1089/ten.tea.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S, Gorham J, Yang L, Schafer S, Sheng CC, Haghighi A, Homsy J, Hubner N, Church G, Cook SA, Linke WA, Chen CS, Seidman JG, Seidman CE. HEART DISEASE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science. 2015;349(6251):982–6. doi: 10.1126/science.aaa5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turnbull IC, Karakikes I, Serrao GW, Backeris P, Lee JJ, Xie C, Senyei G, Gordon RE, Li RA, Akar FG, Hajjar RJ, Hulot JS, Costa KD. Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. FASEB J. 2014;28(2):644–54. doi: 10.1096/fj.13-228007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thavandiran N, Dubois N, Mikryukov A, Masse S, Beca B, Simmons CA, Deshpande VS, McGarry JP, Chen CS, Nanthakumar K, Keller GM, Radisic M, Zandstra PW. Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc Natl Acad Sci U S A. 2013;110(49):E4698–707. doi: 10.1073/pnas.1311120110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liau B, Christoforou N, Leong KW, Bursac N. Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function. Biomaterials. 2011;32(35):9180–7. doi: 10.1016/j.biomaterials.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34(23):5813–20. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maidhof R, Tandon N, Lee EJ, Luo J, Duan Y, Yeager K, Konofagou E, Vunjak-Novakovic G. Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. J Tissue Eng Regen Med. 2012;6(10):e12–23. doi: 10.1002/term.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kai D, Prabhakaran MP, Jin G, Ramakrishna S. Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering. J Biomed Mater Res B Appl Biomater. 2011;98(2):379–86. doi: 10.1002/jbm.b.31862. [DOI] [PubMed] [Google Scholar]
- 89.Orlova Y, Magome N, Liu L, Chen Y, Agladze K. Electrospun nanofibers as a tool for architecture control in engineered cardiac tissue. Biomaterials. 2011;32(24):5615–24. doi: 10.1016/j.biomaterials.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 90.Kenar H, Kose GT, Toner M, Kaplan DL, Hasirci V. A 3D aligned microfibrous myocardial tissue construct cultured under transient perfusion. Biomaterials. 2011;32(23):5320–9. doi: 10.1016/j.biomaterials.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Badrossamay MR, Balachandran K, Capulli AK, Golecki HM, Agarwal A, Goss JA, Kim H, Shin K, Parker KK. Engineering hybrid polymer-protein super-aligned nanofibers via rotary jet spinning. Biomaterials. 2014;35(10):3188–97. doi: 10.1016/j.biomaterials.2013.12.072. [DOI] [PubMed] [Google Scholar]
- 92.Ma Z, Koo S, Finnegan MA, Loskill P, Huebsch N, Marks NC, Conklin BR, Grigoropoulos CP, Healy KE. Three-dimensional filamentous human diseased cardiac tissue model. Biomaterials. 2014;35(5):1367–77. doi: 10.1016/j.biomaterials.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Masse S, Gagliardi M, Hsieh A, Thavandiran N, Laflamme MA, Nanthakumar K, Gross GJ, Backx PH, Keller G, Radisic M. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013;10(8):781–7. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miklas JW, Nunes SS, Sofla A, Reis LA, Pahnke A, Xiao Y, Laschinger C, Radisic M. Bioreactor for modulation of cardiac microtissue phenotype by combined static stretch and electrical stimulation. Biofabrication. 2014;6(2):024113. doi: 10.1088/1758-5082/6/2/024113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xiao Y, Zhang B, Liu H, Miklas JW, Gagliardi M, Pahnke A, Thavandiran N, Sun Y, Simmons C, Keller G, Radisic M. Microfabricated perfusable cardiac biowire: aplatform that mimics native cardiac bundle. Lab Chip. 2014;14(5):869–82. doi: 10.1039/c3lc51123e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grosberg A, Alford PW, McCain ML, Parker KK. Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. Lab Chip. 2011;11(24):4165–73. doi: 10.1039/c1lc20557a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Agarwal A, Goss JA, Cho A, McCain ML, Parker KK. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip. 2013;13(18):3599–608. doi: 10.1039/c3lc50350j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feinberg AW, Ripplinger CM, van der Meer P, Sheehy SP, Domian I, Chien KR, Parker KK. Functional differences in engineered myocardium from embryonic stem cell-derived versus neonatal cardiomyocytes. Stem Cell Reports. 2013;1(5):387–96. doi: 10.1016/j.stemcr.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCain ML, Sheehy SP, Grosberg A, Goss JA, Parker KK. Recapitulating maladaptive, multiscale remodeling of failing myocardium on a chip. Proc Natl Acad Sci U S A. 2013;110(24):9770–5. doi: 10.1073/pnas.1304913110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang G, Mc Cain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang DZ, Li K, Wang J, Wanders RJ, Kulik W, Vaz FM, Laflamme MA, Murry CE, Chien KR, Kelley RI, Church GM, Parker KK, Pu WT. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014;20(6):616–23. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mosadegh B, Dabiri BE, Lockett MR, Derda R, Campbell P, Parker KK, Whitesides GM. Three-dimensional paper-based model for cardiac ischemia. Adv Healthc Mater. 2014;3(7):1036–43. doi: 10.1002/adhm.201300575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mathur A, Loskill P, Shao K, Huebsch N, Hong S, Marcus SG, Marks N, Mandegar M, Conklin BR, Lee LP, Healy KE. Human iPSC-based cardiac microphysiological system for drug screening applications. Sci Rep. 2015;5:8883. doi: 10.1038/srep08883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22(14):1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abate AR, Lee D, Do T, Holtze C, Weitz DA. Glass coating for PDMS microfluidic channels by sol-gel methods. Lab Chip. 2008;8(4):516–8. doi: 10.1039/b800001h. [DOI] [PubMed] [Google Scholar]
- 105.Sun L, Luo Y, Gao Z, Zhao W, Lin B. Easy-to-fabricate thin-film coating on PDMS substrate with super hydrophilicity and stability. Electrophoresis. 2015;36(6):889–92. doi: 10.1002/elps.201400366. [DOI] [PubMed] [Google Scholar]
- 106.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–5. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ashley EA. The Precision Medicine Initiative: A New National Effort. JAMA. 2015 doi: 10.1001/jama.2015.3595. [DOI] [PubMed] [Google Scholar]
- 108.Tsimberidou AM, Eggermont AM, Schilsky RL. Precision cancer medicine: the future is now, only better. Am Soc Clin Oncol Educ Book. 2014:61–9. doi: 10.14694/EdBook_AM.2014.34.61. [DOI] [PubMed] [Google Scholar]


