Abstract
Plasmodium vivax is the most prevalent malaria parasite on the American continent. It generates a global burden of 80–100 million cases annually and represents a tremendous public health problem, particularly in the American and Asian continents. A malaria vaccine would be considered the most cost-effective measure against this vector-borne disease and it would contribute to a reduction in malaria cases and to eventual eradication. Although significant progress has been achieved in the search for Plasmodium falciparum antigens that could be used in a vaccine, limited progress has been made in the search for P. vivax components that might be eligible for vaccine development. This is primarily due to the lack of in vitro cultures to serve as an antigen source and to inadequate funding. While the most advanced P. falciparum vaccine candidate is currently being tested in Phase III trials in Africa, the most advanced P. vivax candidates have only advanced to Phase I trials. Herein, we describe the overall strategy and progress in P. vivax vaccine research, from antigen discovery to preclinical and clinical development and we discuss the regional potential of Latin America to develop a comprehensive platform for vaccine development.
Keywords: malaria, Plasmodium vivax, vaccine platform, discovery, vaccine development, neglected disease, Latin America
On the American continent, an estimated 170 million people living in a total of 21 countries of the Latin America (LA) and Caribbean regions are continuously exposed to Plasmodium vivax and Plasmodium falcipa-rum infections (Guerra et al. 2008, 2010). Approximately 60% of the malaria cases are reported in Brazil, whereas 40% of the cases are from Colombia (14.2%), Peru (8.8%), Venezuela (5.4%), Bolivia (1.9%) and Ecuador (1.1%). In addition, Haiti (2.8%), of the Caribbean region, and Central American countries, including Guatemala (3.8%), Panama (0.4%) and Honduras (1.5%), contribute to the overall malaria burden. In terms of Plasmodium species, approximately 74% of infections are caused by P. vivax, 25% by P. falciparum and the remaining 1% by Plasmodium malariae. The infections result in an estimated mortality rate of 1% (Guerra et al. 2010).
Significant regional efforts have been aimed at controlling malaria and the Global Malaria Eradication Program (GMEP) led to the elimination of, or significant reduction in, malaria outbreaks throughout the American continent by 1960 (Gabaldon et al. 1961, Gabaldon 1983). However, the decades that followed were characterized by a trend of increasing malaria transmission, with periodic epidemic outbreaks in some areas (Gusmão 1999, WHO 2010). At the beginning of the GMEP, countries such as Venezuela, Colombia, Peru and Panama had a significantly diminished number of malaria cases. However, for multiple reasons, control activities were not sustainable. Parasites and mosquitoes became refractory to antimalarial drugs and insecticides, respectively, leading to a malaria resurgence (Alonso et al. 2011, Najera et al. 2011). Despite the failure of the control measures to eradicate malaria in South America and the persistence of the disease in the whole American continent, countries such as El Salvador, Costa Rica, Guatemala and Nicaragua have witnessed a decrease of > 90% in malaria transmission over the last decade. A similar pattern has been observed in other regions worldwide, which has generated growing enthusiasm about the possibility of malaria elimination in multiple countries, including the Mesoamerican region (Feachem et al. 2009, ISM2015 2010).
While P. falciparum dominates malaria transmission on the African continent (90%), in the rest of the world, including LA, P. vivax is predominant (70%), with coexistence of both parasite species in numerous malaria-endemic regions (Mendis et al. 2009).
Although species co-existence simplifies some control activities, it makes others more difficult: vectors for both parasite species in a given region may be controlled in a similar fashion, but clinical manifestations, susceptibility to antimalarials, virulence, mortality and life cycle forms (development of hypnozoites in P. vivax) are different (Das et al. 2009). Moreover, the immune responses induced by both parasite species, the mechanisms of protection and factors involved with pathogenesis may be different. An additional complication is that in regions where more than one species is present, the control or elimination of one species could lead to an increase in the number of cases caused by the remaining species (Oliveira-Ferreira et al. 2010). Therefore, efforts to develop an effective malaria vaccine must consider the inclusion of components from both parasite species. The most advanced P. falciparum vaccine is undergoing Phase III testing and several others candidates are currently being assessed in Phases I and II. The most advanced P. vivax vaccine candidates, in contrast, have only been tested in Phase I trials and a few others are only now being tested in preliminary studies (Arévalo-Herrera et al. 2010, Crompton et al. 2010, Goodman & Draper 2010). It is likely that the current malaria epidemiological scenario would not benefit from a P. falciparum monovalent vaccine due to the high P. vivax prevalence in most endemic areas.
Because of the high prevalence and epidemiological importance of P. vivax in LA region, as well as the potential for researchers from LA to contribute to the development of an effective malaria vaccine, attempts to establish proper conditions for accelerating the identification and characterisation of new P. vivax vaccine candidates are being made in Colombia and other countries of the region. The available facilities and strategies, as well as the current perspectives, are described here.
Acquired immunity and evidence of malaria immune protection - Naturally-acquired immunity
Despite the fact that mechanisms of malaria immune protection are only partially understood, it has been observed that natural immunity is progressively acquired during the first two decades of life in subjects residing in malaria-endemic regions (Collins & Jeffery 1999). If individuals are persistently exposed to malaria, they progressively develop milder clinical manifestations, lower parasitaemia levels and eventually asymptomatic infections. Sterile immunity, however, is never achieved (Bottius et al. 1996). The immunity that is acquired is species and stage specific (Cohen 1979), depends on continuous antigenic stimulation and will decrease rapidly in the absence of exposure to the parasite (Yount & Coggeshall 1949, Doolan et al. 2009). Clinical immunity significantly contributes to the decrease in the economic burden of the disease, as individuals exposed to malaria for long periods of time eventually develop an almost normal life activity. This provides indirect evidence for the feasibility of a malaria vaccine that would decrease morbidity and mortality.
Naturally-acquired immunity to malaria has been extensively documented in P. falciparum (Day & Marsh 1991, Doolan et al. 2009). Reports available on P. vivax infection in Papua New Guinea (PNG) indicate that there is a high transmission intensity. Therefore clinical immunity to P. vivax is acquired faster in young children than is immunity to P. falciparum. This is demonstrated by an increased ability of children to control P. vivax parasitaemia (Lin et al. 2010). In contrast, most malaria-endemic regions of LA have low, unstable malaria transmission, and therefore the acquisition of clinical immunity is likely different from that in PNG. It has been assumed that immunity is rarely acquired in areas with relatively low malaria transmission (Macdonald 1951). However, in studies conducted in the Amazon Basin of Brazil, a high frequency of subclinical infections with sub-patent parasitaemia occurred in all age groups with disease significantly decreasing with age (Alves et al. 2005, Coura et al. 2006, Suarez-Mutis et al. 2007, Ladeia-Andrade et al. 2009). In the Peruvian Amazon, despite low transmission and supervised administration of the recommended radical malaria cure regimen, patients with P. vivax infections remained sub-patent and asymptomatic for several consecutive months, until spontaneous clearance without treatment (Van den Eede et al. 2011). Similar observations were made in studies conducted in Colombia. The prevalence of sub-patent parasitaemias ranged from 0–33% regardless of age, based on thick smear assays in areas of high malaria transmission on the Pacific coast (Terrientes et al. 1994, González et al. 1997, Mendez et al. 2000, Osorio et al. 2004). In the northern area of Colombia (Tierralta, Cordoba), the prevalence of Plasmodium infection was approximately 18%, whereas the prevalence of asymptomatic infection as measured with polymerase chain reaction was 15% (Cucunubá et al. 2008). Although there is a lack of systematic studies in LA to determine the grade of clinical immunity and its correlation with age, the high prevalence of subclinical infection observed in LA suggests that individuals who are permanently exposed to malaria acquire a level of immunity that allows them to live a normal life. This may also explain the maintenance of low to moderate malaria transmission in LA.
Experimental studies have shown that sterile immunity can be achieved by different means. Retrospective analysis of data collected from patients who had received malaria therapy for the treatment of neurosyphilis during the first decades of the XX century showed that some strain-specific immunity can be developed, even after only the first parasite exposure. The development of species-specific immunity, in contrast, required several infectious exposures with heterologous strains (Collins & Jeffery 1999, 2004). Second, immunization of human malaria-naïve volunteers with irradiated malaria sporozoites (attenuated) has consistently been shown to confer sterile protection against experimental challenge with live sporozoite mosquito bites (Clyde 1990). Third, passive transfer of antibodies from hyper-immune malaria adults to malaria-naïve individuals has shown consistent parasite clearance of asexual blood stage parasites (Cohen et al. 1961, McGregor 1964, Sabchareon et al. 1991). Fourth, malaria-naïve subjects, as well as individuals from malaria-endemic communities, may be partially protected by vaccination with the RTS,S vaccine, a recombinant P. falciparum vaccine candidate based on the circumsporozoite (CS) protein (Aide et al. 2011, Olotu et al. 2011).
Evidence of immunization-induced immunity
Although it has been proposed that the intracellular development of the parasite is a mechanism to evade the immune response, immune factors are capable of eliminating intracellular parasites within both hepatocytes and red blood cells (Hoffman et al. 1996, Riley et al. 2006). The accepted theory of the last two decades has been that parasites can be targeted during the short extracellular time periods prior to hepatocyte invasion, erythrocyte reinvasion or gamete fertilization (Hoffman & Miller 1996, Herrera 2005, Girard et al. 2007). According to this concept, vaccines targeting the pre-erythrocytic stage would induce antibody-mediated inhibition of hepatocyte invasion by sporozoites (Hollingdale 1990). Nevertheless, some antigens have been shown to delay the development of liver stage schizonts by parasites through cytolytic immune responses mediated by CD8+ lymphocytes or cytokines, such as gamma interferon (IFN-γ) (Rodrigues et al. 1991, Scheller & Azad 1995, BenMohamed et al. 2004, Hafalla et al. 2011). Given that tissue schizogony is asymptomatic and is followed by the clinical manifestations of the infection, inhibiting this phase may provide sterile immunity (Nussenzweig & Nussenzweig 1989, Richie & Saul 2002). During asexual parasite development, vaccines could induce specific antibodies that block merozoite invasion of erythrocytes (Holder 1996), facilitate erytrophagocytosis (Khusmith et al. 1982, La Raja 2002), induce complement-mediated erythrocyte lysis (Healer et al. 1997) or inhibit intraerythrocytic parasite development through soluble immune mediators, such as oxygen radicals that are released in response to antibody dependent cell inhibition (Bouharoun-Tayoun et al. 1995). It has been proposed that erythrocytic stage vaccines would not prevent blood infection and that they are intended simply to diminish the severity of the disease. However, there is the possibility that a highly effective vaccine targeting the asexual blood stage would block the invasion of the first-generation merozoite upon the burst of a liver schizont, and that this could occur prior to the appearance of clinical manifestations (Holder 1996, Lusingu et al. 2009). Furthermore, the parasite could be targeted during the fertilization process by antibodies that recognise gametocytes (Tsuboi et al. 2003, Dinglasan et al. 2007), thus blocking the sporogonic parasite development in the mosquito midgut.
Strategies for malaria vaccine discovery - Classic strategies for parasite antigen discovery and production
Although malaria vaccine research has been conducted for several decades, the introduction of recombinant DNA technology and peptide synthesis in the early 1980s (Nussenzweig 1984, Miller et al. 1986), together with the development of long-term P. falciparum culture methods (Trager & Jensen 1976), greatly stimulated vaccine candidate discovery. Using these technologies, malaria antigen selection, expression and purification became more efficient and the first vaccine candidates were proposed (Nussenzweig & Nussenzweig 1989). Thereafter, parasite DNA obtained from in vitro cultures, infected animals, patients or mosquitoes allowed easy gene cloning and the generation of DNA expression libraries. Such libraries were further screened with semi-immune sera as well as with monoclonal antibodies in the search for antigenic proteins. Recombinant technology allowed the mass production of proteins that are suitable for use in large scale immunization (Mahanty et al. 2003).
The production of synthetic peptides has also contributed to malaria vaccine development, allowing the characterization of T helper cell (Herrera et al. 1992), CD8+ T cell (Arévalo-Herrera et al. 2002) and B cell epitopes (Arévalo-Herrera et al. 1998, Good & Doolan 2010).
In addition, mass production of asexual blood parasites from P. falciparum cultures contributed to the isolation of parasite strains that were able to develop mature, infective gametocytes in vitro (Ponnudurai et al. 1982). The latter have not only facilitated the identification of relevant antigens on sexually differentiated parasites (gametocytes), but have also permitted the establishment of effective methods of infecting colonies of Anopheles mosquitoes (Gamage-Mendis et al. 1993, Toure et al. 1998, Solarte et al. 2011). This has made it possible to produce mature, viable and functionally active sporozoites (Ponnudurai et al. 1989) that can be used to assess the capacity of antibodies to block parasite transmission from human to mosquito, ex vivo (Hurtado et al. 1997, Sattabongkot et al. 2003, Arévalo-Herrera et al. 2005c, 2011a, Diallo et al. 2008, Solarte et al. 2011).
Unfortunately, the lack of a method to continuously cultivate P. vivax has made this process significantly more complex, for several reasons. First, parasites have to be obtained directly from patients or from infected non-human primates, which makes the process expensive and cumbersome (Collins et al. 1986, Arévalo-Herrera et al. 2005b). Second, P. vivax has a high propensity for reticulocyte invasion and parasitaemias in both humans and monkeys, which seldom exceeds 1% (Herrera et al. 2002, Jordán-Villegas et al. 2005). Consequently, only a limited number of sites that are located close to endemic areas have access to both human P. viva x-infected blood samples and laboratories with conditions to effectively produce different P. viva x parasite forms (i.e., asexual, gametocytes or sporozoites) (Mendis et al. 1987, Sattabongkot et al. 1991, Salas et al. 1994, Ramsey et al. 1996, Carter et al. 2000, McClean et al. 2010, Solarte et al. 2011).
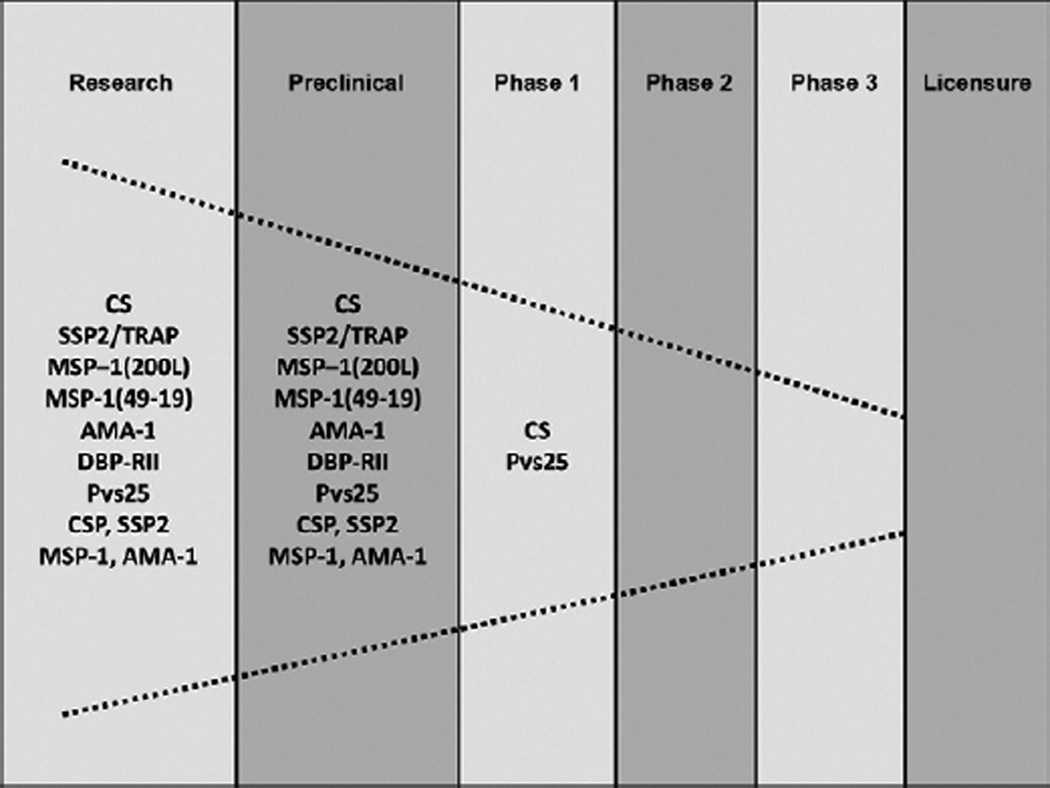
Because of these constraints, only a few P. vivax antigens with vaccine potential have been identified (Herrera et al. 2007, Arévalo-Herrera et al. 2010). Stage-specific antigens described to date are as follows: the CS protein (McCarthy & Clyde 1977) and the sporozoite surface protein/trombospondin related adhesive protein (SSP2/TRAP) (Templeton & Kaslow 1997), which are present at the pre-erythrocytic stage of the parasite, the Duffy binding protein (DBP) (Fang et al. 1991, Chitnis & Miller 1994), the merozoite surface protein (MSP)-1 (Del Portillo et al. 1991) and the apical membrane antigen (AMA)-1 (Kocken et al. 1999), which are present at the asexual erythrocytic stages, and the Pvs25 and Pvs28, which are present in stages developed in the mosquito midgut, i.e., ookinetes/oocysts (Tsuboi et al. 1998) (Figure).
Figure.
Plasmodium vivax vaccines pipeline.
Although there have been no sexually differentiated erythrocytic stage (gametocyte) antigens reported to date, several genes from both gametocytes and the sporogonic cycle have been identified. These have yet to be expressed as proteins that can reach preclinical studies (Moreira et al. 2004).
Pre-erythrocytic vaccines
The CSP has been the most widely studied malaria protein. It is abundantly expressed on the sporozoite surface and it is involved in parasite invasion of hepatic cells. It was cloned in 1985 (Arnot et al. 1985) and several studies examining its characterization, its immunogenicity in animals (see P. vivax preclinical vaccine studies) and its safety, tolerability and immunogenicity in clinical studies have been conducted (see Clinical P. vivax vaccine studies).
SSP2/PvTRAP is a protein present on the surface and in the micronemes of sporozoites and the liver-stages of the parasite and it is involved in motility and cell adhesion (Sultan et al. 1997). It was first cloned in 1997 (Templeton & Kaslow 1997), and its immunogenicity and protective efficacy in rodents and Aotus monkeys has been tested (Castellanos et al. 2007). It has also been shown to be effective in mice as a DNA vaccine (Rogers et al. 1999) (see Preclinical studies using P. vivax subunit vaccines: immunogenicity and protective efficacy).
The antigenicity of the CSP protein has been widely studied in malaria-endemic regions of LA, particularly in Colombia (Herrera et al. 1997, 2004), Brazil (Kremsner et al. 1992, Curado et al. 1997, Oliveira-Ferreira et al. 2004, Arruda et al. 2007) and Mexico (Mota et al. 1996).
In these studies, several CS-derived synthetic peptide fragments corresponding to the N-terminal, C-terminal and central repeat domain peptide (R) have been tested using sera of subjects from LA-endemic areas who showed significant seropositivity rates for this protein, specifically for the R fragment (Herrera et al. 2004, Oliveira-Ferreira et al. 2004, Arruda et al. 2007). In addition, when sera from San Luis Potosi, Mexico (Mota et al. 1996) and Colombia was tested against synthetic peptides corresponding to the VK210 and VK247 variants of CS, recognition of the VK210 variant was higher than recognition of the VK247 variant (Arévalo-Herrera et al. 1998, González et al. 2001).
Concerning cellular responses, CD8+ T-lymphocyte responses to long synthetic peptides (LSP) that corresponded to the C -terminal region of CSP induced the production of considerable levels of IFN-γ. Thus, this region has the potential to induce CSP-specific cellular immune responses (Arévalo-Herrera et al. 2002).
Together, these studies confirm the antigenicity of the protein and the prevalent recognition of the repetitive region, and they demonstrate the induction of cellular immune responses by the C-terminal peptide. These findings have important implications for development of P. vivax vaccine.
Vaccines for the asexual blood stages
DBP, MSP-1 and AMA-1 have received considerable attention as vaccine candidates. DBP is considered an antigen with great vaccine potential due to the fact that P. vivax invasion of erythrocytes depends on the interaction of this protein with the Duffy blood group (Fy) antigen. As a result, individuals who lack Fy antigen (Fy-) are naturally protected from P. vivax blood infection (Herrera et al. 2005b). This antigen has been tested with sera from PNG, the Brazilian Amazon and Colombia (Herrera et al. 2005b, King et al. 2008, Cole-Tobian et al. 2009, Maestre et al. 2010). In different populations of the Brazilian Amazon, anti-DBP antibodies were detected and shown to correlate with long-term exposure to malaria (Ceravolo et al. 2009, Souza-Silva et al. 2010). In Colombia, despite the low transmission of P. vivax infection, a high prevalence of anti-DBP antibodies (40%) was observed in sera from Fy+ individuals from the Pacific coast, particularly in those > 40 years old (Michon et al. 1998). This is in contrast to recent studies in the northern endemic region where only 9% of the Fy+ sera showed reactivity against a DBP recombinant protein (Maestre et al. 2010).
MSP-1 is a protein expressed on the merozoite surface of all malaria species. Two highly conserved MSP-1 fragments, the PvMSP-119 (located at the C-terminal) and the PvMSP-1–200L (Pv200L located at the N-terminal), have been extensively studied and proven to be highly immunogenic (Soares & Rodrigues 2002, Valderrama-Aguirre et al. 2005, Bargieri et al. 2010, Storti-Melo et al. 2011). Studies using sera from individuals naturally exposed to P. vivax in the Brazilian Amazon showed that 50–90% of sera from naturally exposed individuals contain antibodies against these proteins (Soares et al. 1997, Bastos et al. 2007, Storti-Melo et al. 2011). Similarly, the majority of individuals from Buenaventura, a malaria-endemic area of Colombia, presented antibodies to Pv200L (Valderrama-Aguirre et al. 2005), supporting the potential of this fragment as a vaccine target.
The AMA-1 protein is expressed initially in the merozoite micronemes, and further translocates to the parasite surface during erythrocyte invasion where it is involved in the apical re-orientation of merozoites after initial attachment. This protein is highly immunogenic during natural infection in humans and it displays limited polymorphism in Brazil (Rodrigues et al. 2003). The extracellular domain, DII, is the most frequently recognized domain (Mufalo et al. 2008).
Transmission blocking antigens
Pvs25 and Pvs28 are highly conserved ookinete surface antigens composed of four tandem epidermal growth factor-like domains. They are putatively anchored to the parasite surface by a glycosyl-phosphatidyl-inositol moiety (Toure et al. 1998). Cloning and expression of these genes as recombinant proteins has allowed their characterization and use as transmission-blocking vaccines. They have been tested in animal models, allowing the completion of Phase I clinical studies (see Preclinical studies using P. vivax subunit vaccines: immunogenicity and protective efficacy and Phase I clinical trials - CS).
Novel approaches for parasite antigen discovery
Since the P. falciparum genome sequence was completed and reported in 2002 (Gardner et al. 2002), and that of P. vivax more recently (Carlton et al. 2008, Dharia et al. 2010), a substantial amount of information about these two parasites has become available. Comparative studies have shown that these Plasmodium species have the potential to express approximately 5,000 putative proteins of which 77% are orthologous with proteins in other malaria species. Despite the enormous efforts for malaria vaccine development during the past decades, vaccine candidates represent no more than 0.1% of the complete P. vivax predicted proteome. However, during the last few years, studies describing the P. falciparum and P. vivax transcriptomes (Bozdech et al. 2008, Westenberger et al. 2010) and proteomes have revealed promising new directions. Novel, potential antigenic targets (Acharya et al. 2009), including a few variant surface antigens from the Plasmodium interspersed repeat multigene family (Cunningham et al. 2010), have been identified and progress has been made in understanding the parasite transcription process, as well as in elucidating the P. falciparum intra-erythrocytic transcriptomic/proteomic process (Foth et al. 2011). All of the currently available information has been integrated into an accessible data base, PlasmoDB (Aurrecoechea et al. 2009), which provides substantial information for the selection of antigens with vaccine potential. Among them, several novel P. vivax theoretical adhesins and adhesion-like molecules have been recently identified (Ansari et al. 2008), which would expand the list of potential P. vivax vaccine candidates.
Pilot studies aimed at providing a more efficient system for P. vivax antigen discovery have been conducted. In a cooperative effort between the Malaria Vaccine and Drug Development Centre (MVDC) in Cali, Colombia, and The Institute for Genomic Research in Rockville, Maryland, a first approach was performed to identify novel antigens with vaccine potential. A wide P. vivax genome screening was conducted. A total of 18 genes were selected based on criteria indicating their possible expression on the surface of sporozoites. Genes were cloned, expressed in eukaryotic cells and tested to determine their potential to stimulate B and T cell responses in vitro. Based on the fact that Fy- individuals who are permanently exposed to P. vivax malaria in endemic regions develop immune responses mainly to pre-erythrocytic stage antigens (Herrera et al. 2005b, Wang et al. 2005), sera and peripheral blood mononuclear cells (PBMC) were obtained from individuals residing in malaria-endemic areas of Colombia to test the antigenicity of these proteins (Herrera et al. 1992, Arévalo-Herrera et al. 1998).
To assess B cell responses, transient transfection of human embryonic kidney cells (HEK-293) with known sporozoite (CSP, SSP2), blood stage (AMA-1 and MSP-1) and novel genes was carried out. Transfected cells expressing these antigens were tested with semi-immune sera. Likewise, to determine the efficacy of T cell responses elicited by these and other selected antigens, autologous PBMC were transfected and used as antigen-presenting cells (APCs) to autologous mononuclear cells (T cells). The T cell response was measured by IFN-γ production. Novel antigens that elicited cellular and humoral responses were considered to be eligible for further evaluation of their vaccine potential (Wang et al. 2005, Herrera et al. 2007).
Recently, a group of 11 novel genes and two other known antigens (CS, MSP-1) were assessed using the methodology described above. In this study, HEK-293 cells that were transfected with each gene were effectively recognized by sera of individuals from endemic areas of Colombia. Again, autologous PBMCs were transfected with the genes and used as APCs for T cells from individuals of endemic areas. As determined by measuring IFN-γ production through the enzyme-linked immunosorbent spot assay, all genes elicited specific, cellular immune responses. In order to further determine the immunogenicity of these antigens in rodents, a proof-of-principle study was performed using six of these genes cloned into an expression vector containing a cytomegalovirus promoter (pcDNA3.1 D/V5-His-TOPO, Invitrogen). These vectors were used as DNA vaccines. BALB/c mice were immunized with four novel and two known (CS, MSP-1) genes. Sera of vaccinated mice were tested for a response by immunoflorescence (IFAT) and enzyme linked immunosorbent assay (ELISA), and the results indicated that most vaccinated animals produced specific humoral responses after the second or third immunization (unpublished data). In addition, all genes induced T cell responses. A screening approach like this one would facilitate the identification of genes/antigens capable of inducing predominantly T cell responses. This would be particularly useful in terms of identifying IFN-γ responses, which are known to be crucial for protection during the pre-erythrocytic phase of infection (Hafalla et al. 2011).
By means of collaboration between MVDC and the Institute of Biochemistry at the University of Lausanne (Switzerland), a bioinformatic search was combined with chemical peptide synthesis and serological screening for rapid identification of novel vaccine candidates. Based on the observation that α-helical protein domains are present in several P. falciparum malaria antigens and are targets of antibodies (B cell epitopes), a comprehensive bioinformatic search was performed on the P. falciparum genome. A total of 100 segments present on pre-erythrocytic parasite stages were identified and 93 of these were chemically synthesized, purified and analyzed by mass spectrometry (MALDI-TOF). All peptides appeared to be antigenic, as each was recognized based on ELISA conducted with sera from individuals from malaria-endemic areas of Colombia, Burkina Faso and Tanzania. Peptide-specific antibodies were purified from these immune sera using affinity chromatography and they were tested for recognition of native proteins in IFAT. P. falciparum parasitized red blood cells were recognized by antibodies purified from 18 human sera (Villard et al. 2007).
A similar approach is currently being used for P. vivax antigen discovery. A total of 50 P. vivax genes that are orthologous to P. falciparum have been identified and processed as described. Antigenicity of these peptides is being analyzed with sera of individuals from endemic areas of Colombia, PNG and Burkina Faso. Preliminary ELISA results have shown that all 50 peptides are antigenic, but significant variability in recognition has been observed. Specific anti-peptide antibodies have been purified and their protective efficacy is being assessed through passive transfer experiments in P. vivax-infected primates. Additionally, the selected peptides are being evaluated for their immunogenicity in BALB/c mice and preclinical studies will be conducted in Aotus monkeys to evaluate the peptides protective efficacy.
Recently, the construction of LSP (up to 185 residues) for vaccine development has contributed to the rapid production of clean, functional, synthetic proteins containing fragments that have induced specific immune responses in preclinical and clinical studies (Perlaza et al. 2001, Herrera et al. 2005a, 2011, Corradin et al. 2010, Olugbile et al. 2010, Arévalo-Herrera et al. 2011b).
Despite limited financial support, significant advances have been made in the discovery and development of potential P. vivax vaccines. Unfortunately, in contrast to P. falciparum, genome wide studies on P. vivax are still limited. This is due to the current lack of continuous cultures and to the limited amount of DNA that can be derived from human blood draws.
Mosquito components as candidates for malaria vaccine development
Another proposed vaccine strategy does not target parasite components, but instead targets mosquito components. Targeted components are essential for mosquito survival or for the vector-parasite interaction. These components include glycans (carbohydrates), glycoproteins and enzymes, including the Anophelesgam -biae aminopeptidase N (AgAPN1). The inhibition of this enzyme by anti-AgAPN1 IgG has been shown to strongly inhibit both Plasmodium berghei and P. falciparum development in different mosquito species, suggesting an important role for this protein in the ookinete invasion of the mosquito midgut (Dinglasan & Jacobs-Lorena 2008). Targeting vital mosquito molecules or parasite host-cell interactions could lead to the blockage of human to human parasite transmission (Lavazec et al. 2007, Dinglasan & Jacobs-Lorena 2008, Takeo et al. 2009).
P. vivax preclinical vaccine studies - Animal models
Rodents and non-human primates are the preferred animal models for malaria vaccine preclinical testing (Stowers & Miller 2001, Herrera et al. 2002). Four rodent malaria species [P. berghei, Plasmodium chabaudi (subspecies chabaudi and adami), Plasmodium vinckei, and Plasmodium yoelii] have been isolated from wild African rodents and are currently being used for preclinical research. While each model will not provide enough information individually to reliably extrapolate the results to humans, together these models will provide relevant information about the antigens and immune mechanisms and about the immuno-pathogenesis of malaria (Taylor-Robinson 2010).
New World non-human primates have proven to be a very valuable model for malaria vaccine and drug testing (WHO 1988, Herrera et al. 2002). Among them, primates from the Aotus and Saimiri genera are the most extensively used for malaria research and they are still abundant in several countries of LA (Collins 1992). These primates are currently considered to be endangered and some countries have banned their exportation to other countries or restricted their capture even for scientific purposes. Nonetheless, a number of primate centres located in LA provide a valuable resource for vaccine studies (Table). Currently, there are multiple primate centres in Panama, Colombia, Peru and Brazil that harbour these genera and would allow significant vaccine progress if funds were available.
TABLE.
Latin American primate centres
| Primary species | |||||
|---|---|---|---|---|---|
| Primate centre | Country |
Saimiri sciureus |
Aotus | Aotus species | References |
| Gorgas Memorial Laboratory/ Gorgas Memorial Institute of Health Studies, Panama |
Panama | + | + |
Aotus lemurinus lemurinus, Aotus trivirgatus |
Rossan et al. (1975), Inselburg et al. (1991), Galinski et al. (1992) |
| Centre of Reproduction and Conservation of Non Human Primates/Universidad Nacional Mayor de San Marcos, Iquitos |
Peru | + |
Aotus nancymai, Aotus vociferans |
Gozalo and Montoya (1990), Montoya et al. (1994) |
|
| Fundación Centro de Primates/ Caucaseco Scientific Research Centre, Cali |
Colombia | + | + | Aotus lemurinus griseimembra |
Herrera et al. (1997, 2002), Arévalo-Herrera et al. (1998, 2005a), Valderrama-Aguirre et al. (2005), Jordán-Villegas et al. (2011) |
| Fiocruz Primate Centre, Rio de Janeiro |
Brazil | + |
Andrade et al. (2004), Alves et al. (2010) |
||
| National Primate Centre/ Fundação Nacional de Saúde, Belém |
Brazil | + | + | Aotus infulatus | Carvalho et al. (2000, 2004, 2005) |
Both Aotus and Saimiri monkeys are susceptible to experimental infection with previously-adapted, human Plasmodium strains, but there are some susceptibility differences (Young et al. 1966). Aotus lemurinus griseimembra appears to be the New World monkey species most susceptible to infection with human malaria parasites. They have been shown to be susceptible to a series of isolates including the P. falciparum Santa Lucia and the P. vivax Salvador I (Sal I) strains. Both parasite species are able to produce mature gametocytes that are infective to Anopheles mosquitoes and able to produce sporozoites (Salas et al. 1994, Hurtado et al. 1997, Jordán-Villegas et al. 2005), thus allowing studies with all P. falciparum and P. vivax parasite stages.
Preclinical studies using P. vivax subunit vaccines: immunogenicity and protective efficacy - Pre-erythrocytic stages
Several preclinical studies have been carried out to assess the safety and immunogenicity of PvCSP formulations (Collins 1990, Stowers & Miller 2001, Arévalo-Herrera et al. 2011b). After detailed immunological characterization of PvCS that included mapping of relevant B and T cell epitopes, mapping of three LSP-containing fragments of the N-terminal and C-terminal regions and mapping of R of the PvCS, various vaccine formulations were tested for immunogenicity in mice and Aotus monkeys (Herrera et al. 2004, Arévalo-Herrera et al. 2011b). In Aotus, the three LSP fragments that were formulated with Montanide ISA 720 (Seppic, France) induced strong antibody responses. Antibodies recognized the native protein on the sporozoite and Aotus PBMCs responded with in vitro production of IFN-γ (Herrera et al. 2004, Arévalo-Herrera et al. 2011b). Monkeys responded preferentially to the N peptide with both adjuvant formulations (Herrera et al. 1997, Lopez et al. 1997, Arévalo-Herrera et al. 1998, 2011b). These studies provided the impetus for the use of these vaccine formulations in Phase I clinical trials (see Clinical P. vivax vaccine studies).
A recombinant version of the P. vivax CSP, which contained a R composed of both VK210 (South Korean Type I) and VK247, was produced in Escherichia coli and used for immunogenicity studies in mice. BALB/c mice were inoculated with recombinant CSP formulated with Montanide ISA 720, Montanide ISA 51, and incomplete Freund’s adjuvants, and these formulations were highly immunogenic and elicited antibodies that agglutinated live sporozites (Bell et al. 2009).
P. vivax SSP2/TRAP has been tested in mice and monkeys and was shown to induce high levels of specific antibodies in both animal models (Rogers et al. 1999). More recently, LSP formulated with Montanide ISA 720 was tested in BALB/c mice and Aotus monkeys and it resulted in specific antibody responses in both animal species. Although Aotus monkeys showed partial protection against challenge with P. vivax sporozoites, no further studies have been reported with this or other SSP2/TRAP constructs (Castellanos et al. 2007).
Asexual blood stages
P. vivax merozoites have been shown to require interaction with Fy antigen for reticulocyte invasion through DBP and the antibody-mediated immune blockage of this interaction represents a promising mechanism for vaccine development. Several pre-clinical studies have been conducted with PvRII-DBP, a polymorphic and functional domain located in the N-terminal region of the DBP. Immunogenicity studies in mice showed that recombinant PvRII formulated with any of five adjuvants elicited high titres of antibodies. Two of the formulations [Montanide ISA 720 and AS02A (GlaxoSmithKline)] were highly effective at inducing antibodies capable of blocking PvRII binding to erythrocytes in a functional assay (Yazdani et al. 2004). Studies in rhesus monkeys provided evidence that these proteins were also highly immunogenic when formulated with Montanide ISA 720 and AS02A, and antibody titres correlated with in vitro binding inhibition assays (Moreno et al. 2008).
Immunization of Aotus monkeys formulated with Freund’s adjuvant as well as with Montanide ISA 720 showed high immunogenicity. Monkeys immunized with PvRII formulated with Freund’s adjuvant showed partial protection against intravenous challenge of animals with P. vivax asexual blood forms and had higher antibody titres than monkeys immunized with the Montanide ISA 720 formulation. Despite being immunogenic, the Montanide ISA 720 formulation did not induce protection (Arévalo-Herrera et al. 2005a, Chitnis & Sharma 2008). Notwithstanding the discouraging results, this protein is currently being proposed as a vaccine candidate for clinical development due to its functional role (Moorthy & Hill 2002, Alonso et al. 2011).
The antigenicity of the MSP-1 fragments has been demonstrated in several studies using different regions of this protein (PvMSP1 N and C-terminal regions and Pv200L) (Valderrama-Aguirre et al. 2005, Yeom et al. 2008). Additionally, P. vivax MSP-119 has elicited partial protection in Saimiri boliviensis (Stowers et al. 2001).
BALB/c mice and Aotus monkeys immunized with a recombinant Pv200L protein that was produced in E. coli showed high IgG antibody titres that cross-reacted with P. vivax parasites. Pv200L formulated with Freund’s adjuvant displayed unexpectedly high antibody responses and recognition of parasite antigens in vitro. Immunized primates were partially protected against an experimental heterologous challenge with P. vivax, as measured by lower and self-limiting parasitaemias and protection against malaria (Valderrama-Aguirre et al. 2005).
A nucleic acid vaccine has been developed using the P. vivax AMA-1 gene, which induces high antibody titres in mice (Rogers et al. 1999). Additionally, a recombinant P. vivax AMA-1 protein produced in Pichia pastoris and adjuvanted with SBAS2 proved to be highly immunogenic in rhesus monkeys, inducing high IgG antibody titres (Kocken et al. 1999).
Transmission blocking vaccines
Both Pvs25 and Pvs28 have been expressed as recombinant proteins in Saccharomyces cerevisae and have been shown to be highly immunogenic and to induce blocking antibodies in animal models (Arévalo-Herrera et al. 2005b). Additionally, a recombinant Pvs25 formulated with Montanide ISA 720 has been tested in Aotus mon keys and it el icits antibodies wit h transmission-blocking activity and complete inhibition of parasite development in Anopheles albimanus mosquitoes fed with P. viva x-infected human blood by artificial membrane feeding assays (Arévalo-Herrera et al. 2005b). No vaccine studies using antigens expressed on the P. vivax gametocytes have been reported.
Preclinical studies using P. vivax irradiated sporozoites
One of the most valuable malaria vaccine models is the vaccination of human and various animal species with radiation-attenuated sporozoites (Clyde 1990). Numerous trials have been conducted with P. falciparum and with rodent malaria species, but only two volunteers have been vaccinated with P. vivax irradiated sporozoites. Although this vaccination system has repeatedly demonstrated the possibility of inducing sterile immunity in malaria and has become the malaria vaccine gold standard, until recently this vaccine strategy was not considered an option for mass production.
Recently, Sanaria Inc has embarked in the production of a viable, metabolically active, cryopreserved, radiation-attenuated P. falciparum sporozoite vaccine for human use (Hoffman et al. 2010). Although, it is too early to assess the feasibility of this strategy for industrial mass production or to evaluate the protective efficacy of this vaccine candidate in humans, it is clear that such a system would not be applicable for P. vivax because a method for continuous in vitro culture of this species is not available. In preparation to establish this system in humans, a preclinical study was recently conducted in Colombia in which Aotus monkeys were used in a dose-escalating study: groups of animals were subjected to two, five and 10 intravenous injections of P. vivax irradiated sporozoites (106/dose). After vaccination, animals were challenged with 104 viable P. vivax sporozoites. Aotus vaccinated 10 times were partially protected from infection, whereas those receiving fewer attenuated parasite doses were not protected (Jordán-Villegas et al. 2011). Although this model may require improvement, it represents a valuable experimental system to study mechanisms of protection against the P. vivax pre-erythrocytic phase and it may be complementary to studies in human (see Prospects for vaccination of human volunteers with P. vivax irradiated sporozoites).
Clinical P. vivax vaccine studies
In contrast to the number of P. falciparum vaccine candidates that have undergone clinical testing, only two P. vivax vaccine candidates have been tested in Phase I trials and none have progressed to clinical Phases II and III (Figure).
Phase I clinical trials - CS
Two clinical trials to assess the safety, immunogenicity and tolerability of P. vivax CS vaccine candidates based on LSP have been conducted at MVDC. The first trial tested three synthetic peptides, N, R or C of the CS protein, formulated with Montanide ISA 720. The second trial assessed varying doses of a mixture of three LSP (N, R and C peptides) formulated with Montanide ISA 720 or Montanide ISA 51. In both trials, antibodies to the three peptides recognized P. vivax sporozoites in an immunofluorescent antibody test and PBMC from most immunized volunteers (> 90%) also produced IFN-γ upon in vitro peptide stimulation (Herrera et al. 2005a). Vaccines adjuvanted with Montanide ISA 51 showed higher sporozoite protein recognition and IFN-γ production (Herrera et al. 2011). Both vaccines proved to be immunogenic, safe and well tolerated, with no serious adverse events (AE) reported.
Pvs25
Thus far, two Phase I clinical trials have been conducted using Pvs25. One was a dose-escalating trial conducted using a recombinant Pvs25 expressed in S. cerevisiae, formulated with Alhydrogel®. This was called Pvs25H. AE related to vaccination were mild or moderate in severity and no serious AE were reported. The formulation induced antibody titres with transmission blocking activity in an antibody concentration-dependent manner and antibody remained detectable in most volunteers for a year after the first vaccination (Malkin et al. 2005). The second study assessed the safety and immunogenicity of recombinant Pfs25 and Pvs25 vaccine candidates formulated with Montanide ISA 51. Despite the elicitation of serum anti-Pfs25 antibody responses with transmission blocking activity, this trial had to be stopped when half of the volunteers developed local AE and the presence of a few systemic serious AE (Wu et al. 2008).
Preparation for Phase II clinical trials - Prospects for Phase II trials with P. vivax CS protein
Based on the success of the previous Phase I trials conducted with the PvCS peptide-based vaccine, efforts are being made to advance to Phase II studies in which the protective efficacy of these vaccine formulations would be assessed. Recently, two Phase II P. vivax sporozoite challenge trials have been performed to assess safety and to determine the minimal sporozoite effective dose. A total of 34 malaria-naïve volunteers have been exposed to two-10 infective mosquito bites. All volunteers developed pre-patent periods ranging from nine-16 days, with a mean of 10.2 days. Treatment was administered at detection of patent parasitaemia by thick blood smear. All volunteers successfully recovered after the treatment and no parasite relapses were documented in a two year follow-up period. No serious AE were reported (Herrera et al. 2009, 2011). A Phase II sporozoite challenge study to compare the responses of naïve and pre-immune individuals to sporozoites after two-four bites by infected A. albimanus is currently being planned at MVDC. The success of this study would allow comparative analysis of the protective efficacy of vaccination of naïve and preimmune individuals with the PvCS.
Prospects for vaccination of human volunteers with P. vivax irradiated sporozoites
Based on the successful preclinical studies conducted in Aotus monkeys with P. vivax irradiated sporozoites that were described in section Preclinical studies using P. vivax irradiated spo-rozoites (Jordán-Villegas et al. 2011), and in view of the scarcity of data for this malaria species, a Phase I/II clinical trial is being prepared in Colombia to vaccinate naïve, human volunteers with P. vivax irradiated sporozoites. The aim of this study, sponsored by the National Heart, Lung and Blood Institute, is to determine the re-producibility of this model in P. vivax and to investigate the mechanisms of protection and the antigens targeted by protective immune responses. Reagents (sera and cells) derived from the vaccinated volunteers would be stored and used for P. vivax antigen discovery.
Establishment of field sites for further Phase III clinical trials
There are current efforts aimed at establishing field sites for Phase II and III vaccine studies in the LA region. For this purpose, the Centro Latino Americano de Investigación en Malaria (CLAIM), a research centre being established with support from the National Institute of Allergy and Infectious Diseases, will significantly contribute to the goal of establishing field sites for malaria vaccine testing. CLAIM initially consisted of research groups and field sites in Colombia, Guatemala, Panama and Peru, but will extend its activities to other countries of South and Central America. This will increase our understanding of malaria epidemiology, vector biology, vector control and the clinical features of malaria, with the aim of contributing to malaria control and elimination in the region. Given that vaccines would represent a useful tool for malaria elimination, CLAIM will concentrate its efforts on studying the immune responses to novel and classical P. vivax antigens and will prepare sites for future P. vivax vaccine testing.
Conclusions and perspectives
Thus far, only a limited number of P. vivax antigens, i.e. PvCS and Pv25, have been adequately characterized to enter the process of preclinical and Phase I studies. Part of the effort to obtain novel vaccine candidates includes the combination of bioinformatics tools with chemical peptide synthesis and serological characterization.
In spite of the progress in genomic and proteomic technology, a lack of funding remains. Sustained efforts are needed for identification of novel antigens that could serve as vaccines candidates for testing in preclinical and clinical studies.
In order to be able to move forward in the development of the currently available vaccine candidates, and in preparation for the testing of novel compounds, it is critical to increase the efforts to develop long-term P. vivax culture methods. This resource would advance the standardization of a challenge model system and this is a necessary step to advance to Phase II clinical P. vivax vaccines trials.
Greater efforts and resources are urgently needed not only in the improvement of the immunologic and safety profiles of the currently available P. vivax vaccine candidates, but also in research and testing for new compounds that can help to advance development of an effective P. vivax vaccine.
Acknowledgments
To Dr Mark James, for critical reading of the manuscript.
Financial support: COLCIENCIAS (2304-04-14476, 2304-12-16787, 2304-04-19524, 2304-452-21222, 2304-459-21564, 2304-493-26202, 2304-493-26209), NHLBI/NIH (RHL086488A), NIAID/NIH/TMRC (AI49486-01), ICEMR, CLAIM (U19AI089702)
REFERENCES
- Acharya P, Pallavi R, Chandran S, Chakravarti H, Middha S, Acharya J, Kochar S, Kochar D, Subudhi A, Boopathi AP, Garg S, Das A, Tatu U. A glimpse into the clinical proteome of human malaria parasites Plasmodium falciparum and Plasmodium vivax. Proteomics Clin Appl. 2009;3:1314–1325. doi: 10.1002/prca.200900090. [DOI] [PubMed] [Google Scholar]
- Aide P, Dobaño C, Sacarlal J, Aponte JJ, Mandomando I, Guinovart C, Bassat Q, Renom M, Puyol L, Macete E, Herreros E, Leach A, Dubois MC, Demoitie MA, Lievens M, Vekemans J, Loucq C, Ballou WR, Cohen J, Alonso PL. Four year im-munogenicity of the RTS,S/AS02(A) malaria vaccine in Mozambican children during a phase IIb trial. Vaccine. 2011 doi: 10.1016/j.vaccine.2011.03.041. [DOI] [PubMed] [Google Scholar]
- Alonso L, Brown G, Arévalo-Herrera M, Binka F, Chitnis C, Collins F, Doumbo OK, Greenwood B, Hall BF, Levine M, Mendis K, Newman R, Plowe C, Rodríguez M, Sinden R, Slutsker L, Tanner M. A research agenda to underpin malaria eradication. PLoS Medicine. 2011;8:e1000406. doi: 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves FA, Souza MT, Goncalves EC, Schneider MP, Marinho AM, Muniz JA, Fragoso SP, Krieger MA, Goldenberg S, Daniel-Ribeiro CT, Carvalho LJ. DNA sequencing of 13 cytokine gene fragments of Aotus infulatus and Saimiri sciureus, two non-human primate models for malaria. Cytokine. 2010;52:151–155. doi: 10.1016/j.cyto.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Alves FP, Gil LH, Marrelli MT, Ribolla PE, Camargo EP, Da Silva LH. Asymptomatic carriers of Plasmodium spp as infection source for malaria vector mosquitoes in the Brazilian Amazon. J Med Entomol. 2005;42:777–779. doi: 10.1093/jmedent/42.5.777. [DOI] [PubMed] [Google Scholar]
- Andrade MC, Ribeiro CT, Silva VF, Molinaro EM, Gonçalves MA, Marques MA, Cabello PH, Leite JP. Biologic data of Macaca mulatta, Macaca fascicularis and Saimiri sciureus used for research at the Fiocruz Primate Center. Mem Inst Oswaldo Cruz. 2004;99:581–589. [PubMed] [Google Scholar]
- Ansari FA, Kumar N, Bala Subramanyam M, Gnanamani M, Ramachandran S. MAAP: malarial adhesins and adhesin-like proteins predictor. Proteins. 2008;70:659–666. doi: 10.1002/prot.21568. [DOI] [PubMed] [Google Scholar]
- Arévalo-Herrera M, Castellanos A, Yazdani SS, Shakri AR, Chitnis CE, Dominik R, Herrera S. Immunogenicity and protective efficacy of recombinant vaccine based on the receptor-binding domain of the Plasmodium vivax Duffy binding protein in Aotus monkeys. Am J Trop Med Hyg. 2005a;73:25–31. doi: 10.4269/ajtmh.2005.73.5_suppl.0730025. [DOI] [PubMed] [Google Scholar]
- Arévalo-Herrera M, Chitnis C, Herrera S. Current status of Plasmodium vivax vaccine. Hum Vaccin. 2010;6:124–132. doi: 10.4161/hv.6.1.9931. [DOI] [PubMed] [Google Scholar]
- Arévalo-Herrera M, Roggero MA, González JM, Vergara J, Corradin G, Lopez JA, Herrera S. Mapping and comparison of the B-cell epitopes recognized on the Plasmodium vivax circum-sporozoite protein by immune Colombians and immunized Aotus monkeys. Ann Trop Med Parasitol. 1998;92:539–551. [PubMed] [Google Scholar]
- Arévalo-Herrera M, Solarte Y, Rocha L, Alvarez D, Beier JC, Herrera S. Characterization of Plasmodium vivax transmission-blocking activity in low to moderate malaria transmission settings of the Colombian Pacific coast. Am J Trop Med Hyg. 2011a;84:71–77. doi: 10.4269/ajtmh.2011.10-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arévalo-Herrera M, Solarte Y, Yasnot MF, Castellanos A, Rincón A, Saul A, Mu J, Long C, Miller L, Herrera S. Induction of transmission-blocking immunity in Aotus monkeys by vaccination with a Plasmodium vivax clinical grade PVS25 recombinant protein. Am J Trop Med Hyg. 2005b;73(Suppl. 5):32–37. doi: 10.4269/ajtmh.2005.73.32. [DOI] [PubMed] [Google Scholar]
- Arévalo-Herrera M, Solarte Y, Zamora F, Mendez F, Yasnot M, Rocha L, Long C, Miller L, Herrera S. Plasmodium vivax: transmission-blocking immunity in a malaria-endemic area of Colombia. Am J Trop Med Hyg. 2005c;73:38–43. doi: 10.4269/ajtmh.2005.73.5_suppl.0730038. [DOI] [PubMed] [Google Scholar]
- Arévalo-Herrera M, Valencia AZ, Vergara J, Bonelo A, Fleischhauer K, González JM, Restrepo JC, Lopez JA, Valmori D, Corradin G, Herrera S. Identification of HLA-A2 restricted CD8(+) T-lymphocyte responses to Plasmodium vivax circumsporozoite protein in individuals naturally exposed to malaria. Parasite Immunol. 2002;24:161–169. doi: 10.1046/j.1365-3024.2002.00449.x. [DOI] [PubMed] [Google Scholar]
- Arévalo-Herrera M, Vera O, Castellanos A, Cespedes N, Soto L, Cor-radin G, Herrera S. Preclinical vaccine study of Plasmodium vivax circumsporozoite protein derived-synthetic polypeptides formulated in montanide ISA 720 and Montanide ISA 51 adjuvants. Am J Trop Med Hyg. 2011b;84:21–27. doi: 10.4269/ajtmh.2011.10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnot DE, Barnwell JW, Tam JP, Nussenzweig V, Nussenzweig RS, Enea V. Circumsporozoite protein of Plasmodium vivax: gene cloning and characterization of the immunodominant epitope. Science. 1985;230:815–818. doi: 10.1126/science.2414847. [DOI] [PubMed] [Google Scholar]
- Arruda ME, Zimmerman RH, Souza RM, Oliveira-Ferreira J. Prevalence and level of antibodies to the circumsporozoite protein of human malaria parasites in five states of the Amazon Region of Brazil. Mem Inst Oswaldo Cruz. 2007;102:367–371. doi: 10.1590/s0074-02762007005000041. [DOI] [PubMed] [Google Scholar]
- Aurrecoechea C, Brestelli J, Brunk BP, Dommer J, Fischer S, Gajria B, Gao X, Gingle A, Grant G, Harb OS, Heiges M, Innamorato F, Iodice J, Kissinger JC, Kraemer E, Li W, Miller JA, Nayak V, Pennington C, Pinney DF, Roos DS, Ross C, Stoeckert CJ, Jr, Treatman C, Wang H. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 2009;37:D539–D543. doi: 10.1093/nar/gkn814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargieri DY, Leite JA, Lopes SC, Sbrogio-Almeida ME, Braga CJ, Ferreira LC, Soares IS, Costa FT, Rodrigues MM. Immuno-genic properties of a recombinant fusion protein containing the C-terminal 19 kDa of Plasmodium falciparum merozoite surface protein-1 and the innate immunity agonist FliC flagellin of Salmonella typhimurium. Vaccine. 2010;28:2818–2826. doi: 10.1016/j.vaccine.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Bastos MS, da Silva-Nunes M, Malafronte RS, Hoffmann EH, Wun-derlich G, Moraes SL, Ferreira MU. Antigenic polymorphism and naturally acquired antibodies to Plasmodium vivax merozoite surface protein 1 in rural Amazonians. Clin Vaccine Immunol. 2007;14:1249–1259. doi: 10.1128/CVI.00243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell BA, Wood JF, Bansal R, Ragab H, Cargo, Washington MA, Wood CL, Ware LA, Ockenhouse CF, Yadava A. Process development for the production of an E. coli produced clinical grade recombinant malaria vaccine for Plasmodium vivax. Vaccine. 2009;27:1448–1453. doi: 10.1016/j.vaccine.2008.12.027. [DOI] [PubMed] [Google Scholar]
- BenMohamed L, Thomas A, Druilhe P. Long-term multiepitopic cytotoxic-T-lymphocyte responses induced in chimpanzees by combinations of Plasmodium falciparum liver-stage peptides and lipopeptides. Infect Immun. 2004;72:4376–4384. doi: 10.1128/IAI.72.8.4376-4384.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottius E, Guanzirolli A, Trape JF, Rogier C, Konate L, Druilhe P. Malaria: even more chronic in nature than previously thought; evidence for subpatent parasitaemia detectable by the polymerase chain reaction. Trans R Soc Trop Med Hyg. 1996;90:15–19. doi: 10.1016/s0035-9203(96)90463-0. [DOI] [PubMed] [Google Scholar]
- Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druilhe P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med. 1995;182:409–418. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdech Z, Mok S, Hu G, Imwong M, Jaidee A, Russell B, Ginsburg H, Nosten F, Day NP, White NJ, Carlton JM, Preiser PR. The transcriptome of Plasmodium vivax reveals divergence and diversity of transcriptional regulation in malaria parasites. Proc Natl Acad Sci USA. 2008;105:16290–16295. doi: 10.1073/pnas.0807404105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton JM, Adams JH, Silva JC, Bidwell SL, Lorenzi H, Caler E, Crabtree J, Angiuoli SV, Merino EF, Amedeo P, Cheng Q, Coul-son RM, Crabb BS, Del Portillo HA, Essien K, Feldblyum TV, Fernandez-Becerra C, Gilson PR, Gueye AH, Guo X, Kang’a S, Kooij TW, Korsinczky M, Meyer EV, Nene V, Paulsen I, White O, Ralph SA, Ren Q, Sargeant TJ, Salzberg SL, Stoeckert CJ, Sullivan SA, Yamamoto MM, Hoffman SL, Wortman JR, Gardner MJ, Galinski MR, Barnwell JW, Fraser-Liggett CM. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature. 2008;455:757–763. doi: 10.1038/nature07327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R, Mendis KN, Miller LH, Molineaux L, Saul A. Malaria transmission-blocking vaccines-how can their development be supported? Nat Med. 2000;6:241–244. doi: 10.1038/73062. [DOI] [PubMed] [Google Scholar]
- Carvalho LJ, Alves FA, Bianco C, Jr, Oliveira SG, Zanini GM, Soe S, Druilhe P, Theisen M, Muniz JA, Daniel-Ribeiro CT. Immunization of Saimiri sciureus monkeys with a recombinant hybrid protein derived from the Plasmodium falciparum antigen glutamate-rich protein and merozoite surface protein 3 can induce partial protection with Freund and Montanide ISA 720 adjuvants. Clin Diagn Lab Immunol. 2005;12:242–248. doi: 10.1128/CDLI.12.2.242-248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LJ, Oliveira SG, Alves FA, Brígido MC, Muniz JA, Daniel-Ribeiro CT. Aotus infulatus monkey is susceptible to Plasmodium falciparum infection and may constitute an alternative experimental model for malaria. Mem Inst Oswaldo Cruz. 2000;95:363–365. doi: 10.1590/s0074-02762000000300011. [DOI] [PubMed] [Google Scholar]
- Carvalho LJ, Oliveira SG, Theisen M, Alves FA, Andrade MC, Zanini GM, Brigido MC, Oeuvray C, Povoa MM, Muniz JA, Druilhe P, Daniel-Ribeiro CT. Immunization of Saimiri sciureus monkeys with Plasmodium falciparum merozoite surface protein-3 and glutamate-rich protein suggests that protection is related to antibody levels. Scand J Immunol. 2004;59:363–372. doi: 10.1111/j.0300-9475.2004.01409.x. [DOI] [PubMed] [Google Scholar]
- Castellanos A, Arévalo-Herrera M, Restrepo N, Gulloso L, Corradin G, Herrera S. Plasmodium vivax thrombospondin related adhesion protein: immunogenicity and protective efficacy in rodents and Aotus monkeys. Mem Inst Oswaldo Cruz. 2007;102:411–416. doi: 10.1590/s0074-02762007005000047. [DOI] [PubMed] [Google Scholar]
- Ceravolo IP, Sanchez BA, Sousa TN, Guerra BM, Soares IS, Braga EM, McHenry AM, Adams JH, Brito CF, Carvalho LH. Naturally acquired inhibitory antibodies to Plasmodium vivax Duffy binding protein are short-lived and allele-specific following a single malaria infection. Clin Exp Immunol. 2009;156:502–510. doi: 10.1111/j.1365-2249.2009.03931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis CE, Miller LH. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J Exp Med. 1994;180:497–506. doi: 10.1084/jem.180.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis CE, Sharma A. Targeting the Plasmodium vivax Duffy-binding protein. Trends Parasitol. 2008;24:29–34. doi: 10.1016/j.pt.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Clyde DF. Immunity to falciparum and vivax malaria induced by irradiated sporozoites: a review of the University of Maryland studies, 1971-75. Bull World Health Organ. 1990;68:9–12. [PMC free article] [PubMed] [Google Scholar]
- Cohen S. Immunity to malaria. Proc R Soc Lond B Biol Sci. 1979;203:323–345. doi: 10.1098/rspb.1979.0001. [DOI] [PubMed] [Google Scholar]
- Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Cole-Tobian JL, Michon P, Biasor M, Richards JS, Beeson JG, Mueller I, King CL. Strain-specific Duffy binding protein antibodies correlate with protection against infection with homologous compared to heterologous Plasmodium vivax strains in Papua New Guinean children. Infect Immun. 2009;77:4009–4017. doi: 10.1128/IAI.00158-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WE. Testing of Plasmodium vivax CS proteins in Saimiri monkeys. Bull World Health Organ. 1990;68:42–46. [PMC free article] [PubMed] [Google Scholar]
- Collins WE. South American monkeys in the development and testing of malarial vaccines - A review. Mem Inst Oswaldo Cruz. 1992;87(Suppl. III):401–406. doi: 10.1590/s0074-02761992000700068. [DOI] [PubMed] [Google Scholar]
- Collins WE, Jeffery GM. A retrospective examination of secondary sporozoite- and trophozoite-induced infections with Plasmodium falciparum: development of parasitologic and clinical immunity following secondary infection. Am J Trop Med Hyg. 1999;61:20–35. doi: 10.4269/tropmed.1999.61-020. [DOI] [PubMed] [Google Scholar]
- Collins WE, Jeffery GM, Roberts JM. A retrospective examination of reinfection of humans with Plasmodium vivax. Am J Trop Med Hyg. 2004;70:642–644. [PubMed] [Google Scholar]
- Collins WE, McClure HM, Swenson RB, Mehaffey PC, Skinner JC. Infection of mosquitoes with Plasmodium vivax from chimpanzees using membrane feeding. Am J Trop Med Hyg. 1986;35:56–60. doi: 10.4269/ajtmh.1986.35.56. [DOI] [PubMed] [Google Scholar]
- Corradin G, Kajava AV, Verdini A. Long synthetic peptides for the production of vaccines and drugs: a technological platform coming of age. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3001387. 50rv3. [DOI] [PubMed] [Google Scholar]
- Coura JR, Suárez-Mutis M, Ladeia-Andrade S. A new challenge for malaria control in Brazil: asymptomatic Plasmodium infection - A review. Mem Inst Oswaldo Cruz. 2006;101:229–237. doi: 10.1590/s0074-02762006000300001. [DOI] [PubMed] [Google Scholar]
- Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. J Clin Invest. 2010;120:4168–4178. doi: 10.1172/JCI44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucunubá ZM, Guerra AP, Rahirant SJ, Rivera JA, Cortés LJ, Ni-cholls RS. Asymptomatic Plasmodium spp infection in Tierralta, Colombia. Mem Inst Oswaldo Cruz. 2008;103:668–673. doi: 10.1590/s0074-02762008000700007. [DOI] [PubMed] [Google Scholar]
- Cunningham D, Lawton J, Jarra W, Preiser P, Langhorne J. The pir multigene family of Plasmodium: antigenic variation and beyond. Mol Biochem Parasitol. 2010;170:65–73. doi: 10.1016/j.molbiopara.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Curado I, Duarte AM, Lal AA, Oliveira SG, Kloetzel JK. Antibodies anti bloodstream and circumsporozoite antigens (Plasmodium vivax and Plasmodium malariae/P. brasilianum) in areas of very low malaria endemicity in Brazil. Mem Inst Oswaldo Cruz. 1997;92:235–243. doi: 10.1590/s0074-02761997000200017. [DOI] [PubMed] [Google Scholar]
- Das A, Sharma M, Gupta B, Dash AP. Plasmodium falciparum and Plasmodium vivax: so similar, yet very different. Parasitol Res. 2009;105:1169–1171. doi: 10.1007/s00436-009-1521-y. [DOI] [PubMed] [Google Scholar]
- Day KP, Marsh K. Naturally acquired immunity to Plasmodium falciparum. Immunol Today. 1991;12:A68–A71. doi: 10.1016/s0167-5699(05)80020-9. [DOI] [PubMed] [Google Scholar]
- Del Portillo HA, Longacre S, Khouri E, David PH. Primary structure of the merozoite surface antigen 1 of Plasmodium vivax reveals sequences conserved between different Plasmodium species. Proc Natl Acad Sci USA. 1991;88:4030–4034. doi: 10.1073/pnas.88.9.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharia NV, Bright AT, Westenberger SJ, Barnes SW, Batalov S, Kuhen K, Borboa R, Federe GC, McClean CM, Vinetz JM, Neyra V, Llanos-Cuentas A, Barnwell JW, Walker JR, Winzeler EA. Whole-genome sequencing and microarray analysis of ex vivo Plasmodium vivax reveal selective pressure on putative drug resistance genes. Proc Natl Acad Sci USA. 2010;107:20045–20050. doi: 10.1073/pnas.1003776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo M, Toure AM, Traore SF, Niare O, Kassambara L, Konare A, Coulibaly M, Bagayogo M, Beier JC, Sakai RK, Toure YT, Doumbo OK. Evaluation and optimization of membrane feeding compared to direct feeding as an assay for infectivity. Malar J. 2008;7:248. doi: 10.1186/1475-2875-7-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinglasan RR, Jacobs-Lorena M. Flipping the paradig m on malaria transmission-blocking vaccines. Trends Parasitol. 2008;24:364–370. doi: 10.1016/j.pt.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinglasan RR, Kalume DE, Kanzok SM, Ghosh AK, Muratova O, Pandey A, Jacobs-Lorena M. Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. Proc Natl Acad Sci USA. 2007;104:13461–13466. doi: 10.1073/pnas.0702239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clinical Microbiology Reviews. 2009;22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang XD, Kaslow DC, Adams JH, Miller LH. Cloning of the Plasmodium vivax Duffy receptor. Mol Biochem Parasitol. 1991;44:125–132. doi: 10.1016/0166-6851(91)90228-x. [DOI] [PubMed] [Google Scholar]
- Feachem RGA, Phillips AA, Targett GA. Shrinking the malaria map: a prospectus on malaria elimination. San Francisco: The Global Health Group; 2009. Available from: map.ox.ac.uk/PDF/MEG_prospectus.pdf. [Google Scholar]
- Foth BJ, Zhang N, Chaal BK, Sze SK, Preiser PR, Bozdech Z. Quantitative time-course profiling of parasite and host cell proteins in the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics. 2011 doi: 10.1074/mcp.M110.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaldon A. Malaria eradication in Venezuela: doctrine, practice and achievement after twenty years. Am J Trop Med Hyg. 1983;32:203–211. doi: 10.4269/ajtmh.1983.32.203. [DOI] [PubMed] [Google Scholar]
- Gabaldon A, Berti LA, Guerrero L, Garcia Martin G. Eradication of malaria in Venezuela. Program, progress and present status. Rev Sanid Asist Soc. 1961;(Suppl. 3):290–336. [PubMed] [Google Scholar]
- Galinski MR, Medina CC, Ingravallo P, Barnwell JW. A reticulocyte-binding protein complex of Plasmodium vivax merozoites. Cell. 1992;69:1213–1226. doi: 10.1016/0092-8674(92)90642-p. [DOI] [PubMed] [Google Scholar]
- Gamage-Mendis AC, Rajakaruna J, Weerasinghe S, Mendis C, Carter R, Mendis KN. Infectivity of Plasmodium vivax and P. falciparum to Anopheles tessellatus; relationship between oocyst and sporozoite development. Trans R Soc Trop Med Hyg. 1993;87:3–6. doi: 10.1016/0035-9203(93)90396-8. [DOI] [PubMed] [Google Scholar]
- Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard MP, Reedb ZH, Friede M, Kieny MP. A review of human vaccine research and development: malaria. Vaccine. 2007;25:1567–1580. doi: 10.1016/j.vaccine.2006.09.074. [DOI] [PubMed] [Google Scholar]
- González JM, Hurtado S, Arévalo-Herrera M, Herrera S. Variants of the Plasmodium vivax circumsporozoite protein (VK210 and VK247) in Colombian isolates. Mem Inst Oswaldo Cruz. 2001;96:709–712. doi: 10.1590/s0074-02762001000500023. [DOI] [PubMed] [Google Scholar]
- González JM, Olano V, Vergara J, Arévalo-Herrera M, Carrasquilla G, Herrera S, Lopez JA. Unstable, low-level transmission of malaria on the Colombian Pacific coast. Ann Trop Med Para-sitol. 1997;91:349–358. [PubMed] [Google Scholar]
- Good MF, Doolan DL. Malaria vaccine design: immunological considerations. Immunity. 2010;33:555–566. doi: 10.1016/j.immuni.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Goodman AL, Draper SJ. Blood-stage malaria vaccines - recent progress and future challenges. Ann Trop Med Parasitol. 2010;104:189–211. doi: 10.1179/136485910X12647085215534. [DOI] [PubMed] [Google Scholar]
- Gozalo A, Montoya E. Reproduction of the owl monkey (Aotusnancymai) (primates: Cebidae) in captivity. Am J Primatol. 1990;21:61–68. doi: 10.1002/ajp.1350210107. [DOI] [PubMed] [Google Scholar]
- Guerra CA, Gikandi PW, Tatem AJ, Noor AM, Smith DL, Hay SI, Snow RW. The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS Medicine. 2008;5:e38. doi: 10.1371/journal.pmed.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IRF, Baird JK, Snow RW, Hay SI. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusmão R. Overview of malaria control in the Americas. Parassitologia. 1999;41:355–360. [PubMed] [Google Scholar]
- Hafalla JC, Silvie O, Matuschewski K. Cell biology and immunology of malaria. Immunol Rev. 2011;240:297–316. doi: 10.1111/j.1600-065X.2010.00988.x. [DOI] [PubMed] [Google Scholar]
- Healer J, Mcguinness D, Hopcroft P, Haley S, Carter R, Riley E. Complement-mediated lysis of Plasmodium falciparum gametes by malaria-immune human sera is associated with antibodies to the gamete surface antigen Pfs230. Infect Immun. 1997;65:3017–3023. doi: 10.1128/iai.65.8.3017-3023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera S. La malaria: estrategias actuales para el desarrollo de una vacuna efectiva. Rev Acad Colomb Cienc. 2005;29:535–546. [Google Scholar]
- Herrera S, Bonelo A, Perlaza BL, Fernández OL, Victoria L, Lenis AM, Soto L, Hurtado H, Acuña LM, Vélez JD, Palacios R, Chen-Mok M, Corradin G, Arévalo-Herrera M. Safety and elicitation of humoral and cellular responses in Colombian ma-laria-naïve volunteers by a Plasmodium vivax circumsporozoite protein-derived synthetic vaccine. Am J Trop Med Hyg. 2005a;73:3–9. doi: 10.4269/ajtmh.2005.73.3. [DOI] [PubMed] [Google Scholar]
- Herrera S, Bonelo A, Perlaza BL, Valencia AZ, Cifuentes C, Hurtado S, Quintero G, López JA, Corradin G, Arévalo-Herrera M. Use of long synthetic peptides to study the antigenicity and immunogenicity of the Plasmodium vivax circumsporozoite protein. Int J Parasitol. 2004;34:1535–1546. doi: 10.1016/j.ijpara.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Herrera S, Corradin G, Arévalo-Herrera M. An update on the search for a Plasmodium vivax vaccine. Trends Parasitol. 2007;23:122–128. doi: 10.1016/j.pt.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Herrera S, De Plata C, Gonzalez M, Perlaza BL, Bettens F, Corradin G, Arévalo-Herrera M. Antigenicity and immunogenicity of multiple antigen peptides (MAP) containing P. vivax CS epitopes in Aotus monkeys. Parasite Immunol. 1997;19:161–170. doi: 10.1046/j.1365-3024.1997.d01-193.x. [DOI] [PubMed] [Google Scholar]
- Herrera S, Escobar P, de Plata C, Avila GI, Corradin G, Herrera MA. Human recognition of T cell epitopes on the Plasmodium vivax circumsporozoite protein. J Immunol. 1992;148:3986–3990. [PubMed] [Google Scholar]
- Herrera S, Fernández O, Manzano MR, Murrain B, Vergara J, Blanco P, Palacios R, Vélez JD, Epstein JE, Chen-Mok M, Reed ZH, Arévalo-Herrera M. Successful sporozoite challenge model in human volunteers with Plasmodium vivax strain derived from human donors. Am J Trop Med Hyg. 2009;81:740–746. doi: 10.4269/ajtmh.2009.09-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera S, Fernández OL, Vera O, Cardenas W, Ramirez O, Palacios R, Chen-Mok M, Corradin G, Arévalo-Herrera M. Phase I safety and immunogenicity trial of Plasmodium vivax CS derived long synthetic peptides adjuvanted with Montanide ISA 720 or Montanide ISA 51. Am J Trop Med Hyg. 2011;84:12–20. doi: 10.4269/ajtmh.2011.09-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera S, Gómez A, Vera O, Vergara J, Valderrama-Aguirre A, Maestre A, Méndez F, Wang R, Chitnis CE, Yazdani SS, Arévalo-Herrera M. Antibody response to Plasmodium vivax antigens in Fy-negative individuals from the Colombian Pacific coast. Am J Trop Med Hyg. 2005b;73:44–49. doi: 10.4269/ajtmh.2005.73.44. [DOI] [PubMed] [Google Scholar]
- Herrera S, Perlaza BL, Bonelo A, Arévalo-Herrera M. Aotus monkeys: their great value for anti-malaria vaccines and drug testing. Int J Parasitol. 2002;32:1625–1635. doi: 10.1016/s0020-7519(02)00191-1. [DOI] [PubMed] [Google Scholar]
- Hoffman SL, Billingsley PF, James E, Richman A, Loyevsky M, Li T, Chakravarty S, Gunasekera A, Chattopadhyay R, Li M, Stafford R, Ahumada A, Epstein JE, Sedegah M, Reyes S, Richie TL, Lyke KE, Edelman R, Laurens MB, Plowe CV, Sim BK. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin. 2010;6:97–106. doi: 10.4161/hv.6.1.10396. [DOI] [PubMed] [Google Scholar]
- Hoffman SL, Fracke DE, Hollingdale MR, Druilhe P. Attacking the infected hepatocyte. In: Hoffman S, editor. Malaria vaccine development: a multi-immune response approach. Washington: ASM Press; 1996. p. 35. [Google Scholar]
- Hoffman SL, Miller L. Perspectives on malaria vaccine development. In: Hoffman S, editor. Malaria vaccine development. Washington: ASM Press; 1996. pp. 1–13. [Google Scholar]
- Holder A. Preventing the merozoite invasion of erythrocyte. In: Hoffman S, editor. Malaria vaccine development: a multi-immune response approach. Washington: ASM Press; 1996. pp. 77–104. [Google Scholar]
- Hollingdale MR. Anti-sporozoite antibodies. Bull World Health Organ. 1990;68(Suppl.):47–51. [PMC free article] [PubMed] [Google Scholar]
- Hurtado S, Salas ML, Romero JF, Zapata JC, Ortiz H, Arévalo-Her-rera M, Herrera S. Regular production of infective sporozoites of Plasmodium falciparum and P. vivax in laboratory-bred Anopheles albimanus. Ann Trop Med Parasitol. 1997;91:49–60. doi: 10.1080/00034983.1997.11813111. [DOI] [PubMed] [Google Scholar]
- Inselburg J, Bzik DJ, Li WB, Green KM, Kansopon J, Hahm BK, Bathurst IC, Barr PJ, Rossan RN. Protective immunity induced in Aotus monkeys by recombinant SERA proteins of Plasmodium falciparum. Infect Immun. 1991;59:1247–1250. doi: 10.1128/iai.59.4.1247-1250.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordán-Villegas A, Perdomo AB, Epstein JE, Lopez J, Castellanos A, Manzano MR, Hernandez MA, Soto L, Mendez F, Richie TL, Hoffman SL, Arévalo-Herrera M, Herrera S. Immune responses and protection of Aotus monkeys immunized with irradiated Plasmodium vivax sporozoites. Am J Trop Med Hyg. 2011;84:43–50. doi: 10.4269/ajtmh.2011.09-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordán-Villegas A, Zapata JC, Perdomo AB, Quintero GE, Solarte Y, Arévalo-Herrera M, Herrera S. Aotus lemurinus griseimembra monkeys: a suitable model for Plasmodium vivax sporozoite infection. Am J Trop Med Hyg. 2005;73:10–15. doi: 10.4269/ajtmh.2005.73.10. [DOI] [PubMed] [Google Scholar]
- Khusmith S, Druilhe P, Gentilini M. Enhanced Plasmodium falciparum merozoite phagocytosis by monocytes from immune individuals. Infect Immun. 1982;35:874–879. doi: 10.1128/iai.35.3.874-879.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CL, Michon P, Shakri AR, Marcotty A, Stanisic D, Zimmerman PA, Cole-Tobian JL, Mueller I, Chitnis CE. Naturally acquired Duffy-binding protein-specific binding inhibitory antibodies confer protection from blood-stage Plasmodium vivax infection. Proc Natl Acad Sci USA. 2008;105:8363–8368. doi: 10.1073/pnas.0800371105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocken CH, Dubbeld MA, Van Der Wel A, Pronk JT, Waters AP, Langermans JA, Thomas AW. High-level expression of Plasmodium vivax apical membrane antigen 1 (AMA-1) in Pichia pastoris: strong immunogenicity in Macaca mulatta immunized with P. vivax AMA-1 and adjuvant SBAS2. Infect Immun. 1999;67:43–49. doi: 10.1128/iai.67.1.43-49.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremsner PG, Neifer S, Zotter GM, Bienzle U, Rocha RM, Maracic M, Clavijo P, Nussenzweig RS, Cochrane AH. Prevalence and level of antibodies to the circumsporozoite proteins of human malaria parasites, including a variant of Plasmodium vivax, in the population of two epidemiologically distinct areas in the state of Acre, Brazil. Trans R Soc Trop Med Hyg. 1992;86:23–27. doi: 10.1016/0035-9203(92)90423-a. [DOI] [PubMed] [Google Scholar]
- La Raja M. Erythrophagocytosis by peripheral monocytes in Plasmodium falciparum malaria. Haematologica. 2002;87:EIM14. [PubMed] [Google Scholar]
- Ladeia-Andrade S, Ferreira MU, de Carvalho ME, Curado I, Coura JR. Age-dependent acquisition of protective immunity to malaria in riverine populations of the Amazon Basin of Brazil. Am J Trop Med Hyg. 2009;80:452–459. [PubMed] [Google Scholar]
- Lavazec C, Boudin C, Lacroix R, Bonnet S, Diop A, Thiberge S, Boisson B, Tahar R, Bourgouin C. Carboxypeptidases B of Anopheles gambiae as targets for a Plasmodium falciparum transmission-blocking vaccine. Infect Immun. 2007;75:1635–1642. doi: 10.1128/IAI.00864-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E, Kiniboro B, Gray L, Dobbie S, Robinson L, Laumaea A, Schopflin S, Stanisic D, Betuela I, Blood-Zikursh M, Siba P, Felger I, Schofield L, Zimmerman P, Mueller I. Differential patterns of infection and disease with P. falciparum and P. vivax in young Papua New Guinean children. PLoS ONE. 2010;5:e9047. doi: 10.1371/journal.pone.0009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JA, Gonzalez JM, Kettner A, Arevalo-Herrera M, Herrera S, Corradin G, Roggero MA. Synthetic polypeptides corresponding to the non-repeat regions from the circumsporozoite protein of Plasmodium falciparum: recognition by human T-cells and immunogenicity in owl monkeys. Ann Trop Med Parasitol. 1997;91:253–265. [PubMed] [Google Scholar]
- Lusingu JP, Gesase S, Msham S, Francis F, Lemnge M, Seth M, Sembuche S, Rutta A, Minja D, Segeja MD, Bosomprah S, Cousens S, Noor R, Chilengi R, Druilhe P. Satisfactory safety and immunogenicity of MSP3 malaria vaccine candidate in Tanzanian children aged 12–24 months. Malar J. 2009;8:163. doi: 10.1186/1475-2875-8-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald G. Community aspects of immunity to malaria. Br Med Bull. 1951;8:33–36. doi: 10.1093/oxfordjournals.bmb.a074051. [DOI] [PubMed] [Google Scholar]
- Maestre A, Muskus C, Duque V, Agudelo O, Liu P, Takagi A, Ntumngia FB, Adams JH, Sim KL, Hoffman SL, Corradin G, Velez ID, Wang R. Acquired antibody responses against Plasmodium vivax infection vary with host genotype for Duffy antigen receptor for chemokines (DARC) PLoS ONE. 2010;5:e11437. doi: 10.1371/journal.pone.0011437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty S, Saul A, Miller LH. Progress in the development of recombinant and synthetic blood-stage malaria vaccines. J Exp Biol. 2003;206:3781–3788. doi: 10.1242/jeb.00646. [DOI] [PubMed] [Google Scholar]
- Malkin EM, Durbin AP, Diemert DJ, Sattabongkot J, Wu Y, Miura K, Long CA, Lambert L, Miles AP, Wang J, Stowers A, Miller LH, Saul A. Phase I vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine. 2005;23:3131–3138. doi: 10.1016/j.vaccine.2004.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy VC, Clyde DF. Plasmodium vivax: correlation of circumsporozoite precipitation (CSP) reaction with sporozoite-induced protective immunity in man. Exp Parasitol. 1977;41:167–171. doi: 10.1016/0014-4894(77)90142-4. [DOI] [PubMed] [Google Scholar]
- McClean CM, Alvarado HG, Neyra V, Llanos-Cuentas A, Vinetz JM. Optimized in vitro production of Plasmodium vivax ookinetes. Am J Trop Med Hyg. 2010;83:1183–1186. doi: 10.4269/ajtmh.2010.10-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IA. The passive transfer of human malarial immunity. Am J Trop Med Hyg. 1964;13:9. doi: 10.4269/ajtmh.1964.13.237. [DOI] [PubMed] [Google Scholar]
- Mendez F, Carrasquilla G, Munoz A. Risk factors associated with malaria infection in an urban setting. Trans R Soc Trop Med Hyg. 2000;94:367–371. doi: 10.1016/s0035-9203(00)90106-8. [DOI] [PubMed] [Google Scholar]
- Mendis K, Rietveld A, Warsame M, Bosman A, Greenwood B, Wer-nsdorfer WH. From malaria control to eradication: the WHO perspective. Trop Med Int Health. 2009;14:1–7. doi: 10.1111/j.1365-3156.2009.02287.x. [DOI] [PubMed] [Google Scholar]
- Mendis KN, Munesinghe YD, de Silva YN, Keragalla I, Carter R. Malaria transmission-blocking immunity induced by natural infections of Plasmodium vivax in humans. Infect Immun. 1987;55:369–372. doi: 10.1128/iai.55.2.369-372.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michon PA, Arevalo-Herrera M, Fraser T, Herrera S, Adams JH. Serologic responses to recombinant Plasmodium vivax Duffy binding protein in a Colombian village. Am J Trop Med Hyg. 1998;59:597–599. doi: 10.4269/ajtmh.1998.59.597. [DOI] [PubMed] [Google Scholar]
- Miller LH, Howard RJ, Carter R, Good MF, Nussenzweig V, Nussenzweig RS. Research toward malaria vaccines. Science. 1986;234:1349–1356. doi: 10.1126/science.2431481. [DOI] [PubMed] [Google Scholar]
- Montoya E, Moro J, Gózalo A, Samaméb H. Reproduccion de Aotus vociferans (primates cebidae) en cautiverio. Investigaciones Pecuarias. 1994;7:122–126. [Google Scholar]
- Moorthy V, Hill AV. Malaria vaccines. Br Med Bull. 2002;62:59–72. doi: 10.1093/bmb/62.1.59. [DOI] [PubMed] [Google Scholar]
- Moreira CK, Marrelli MT, Jacobs-Lorena M. Gene expression in Plasmodium: from gametocytes to sporozoites. Int J Parasitol. 2004;34:1431–1440. doi: 10.1016/j.ijpara.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Moreno A, Caro-Aguilar I, Yazdani SS, Shakri AR, Lapp S, Strobert E, McClure H, Chitnis CE, Galinski MR. Preclinical assessment of the receptor-binding domain of Plasmodium vivax Duffy-binding protein as a vaccine candidate in Rhesus macaques. Vaccine. 2008;26:4338–4344. doi: 10.1016/j.vaccine.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota J, Coreno O, Cochrane AH, Ramos C. Prevalence of antibodies to the repeat epitope of the circumsporozoite protein of Plasmodium vivax in San Luis Potosi, Mexico. Arch Med Res. 1996;27:233–236. [PubMed] [Google Scholar]
- Mufalo BC, Gentil F, Bargieri DY, Costa FT, Rodrigues MM, Soares IS. Plasmodium vivax apical membrane antigen-1: comparative recognition of different domains by antibodies induced during natural human infection. Microbes Infect. 2008;10:1266–1273. doi: 10.1016/j.micinf.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Najera J, Gonzalez-Silva M, Alonso P. Some lessons for the future from the global malaria eradication programme (1955–1969) PLoS Medicine. 2011;8:e1000412. doi: 10.1371/journal.pmed.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig V. Synthetic peptides and malaria surface antigens. Ann Sclavo Collana Monogr. 1984;1:187–189. [PubMed] [Google Scholar]
- Nussenzweig V, Nussenzweig RS. Rationale for the development of an engineered sporozoite malaria vaccine. Adv Immunol. 1989;45:283–334. doi: 10.1016/s0065-2776(08)60695-1. [DOI] [PubMed] [Google Scholar]
- Oliveira-Ferreira J, Lacerda MV, Brasil P, Ladislau JL, Tauil PL, Daniel-Ribeiro CT. Malaria in Brazil: an overview. Malar J. 2010;9:115. doi: 10.1186/1475-2875-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Ferreira J, Pratt-Riccio LR, Arruda M, Santos F, Ribeiro CT, Goldberg AC, Banic DM. HLA class II and antibody responses to circumsporozoite protein repeats of P. vivax (VK210, VK247 and P. vivax-like) in individuals naturally exposed to malaria. Acta Trop. 2004;92:63–69. doi: 10.1016/j.actatropica.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Olotu A, Lusingu J, Leach A, Lievens M, Vekemans J, Msham S, Lang T, Gould J, Dubois MC, Jongert E, Vansadia P, Carter T, Njuguna P, Awuondo KO, Malabeja A, Abdul O, Gesase S, Mturi N, Drakeley CJ, Savarese B, Villafana T, Lapierre D, Ballou WR, Cohen J, Lemnge MM, Peshu N, Marsh K, Riley EM, von Seidlein L, Bejon P. Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5–17 months in Kenya and Tanzania: a randomised controlled trial. Lancet Infect Dis. 2011;11:102–109. doi: 10.1016/S1473-3099(10)70262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olugbile S, Habel C, Servis C, Spertini F, Verdini A, Corradin G. Malaria vaccines - The long synthetic peptide approach: technical and conceptual advancements. Curr Opin Mol Ther. 2010;12:64–76. [PubMed] [Google Scholar]
- Osorio L, Todd J, Bradley D. Absence of asymptomatic malaria in school children of Quibdo, Choco. Biomedica. 2004;24:13–19. [PubMed] [Google Scholar]
- Perlaza BL, Sauzet JP, Balde AT, Brahimi K, Tall A, Corradin G, Druilhe P. Long synthetic peptides encompassing the Plasmodium falciparum LSA3 are the target of human B and T cells and are potent inducers of B helper, T helper and cytolytic T cell responses in mice. Eur J Immunol. 2001;31:2200–2209. doi: 10.1002/1521-4141(200107)31:7<2200::aid-immu2200>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Ponnudurai T, Lensen AHW, GemertGJA V, Bensink MPE, Bolmer M, Meuwissen JHET. Sporozoite load of mosquitoes infected with Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1989;83:67–70. doi: 10.1016/0035-9203(89)90708-6. [DOI] [PubMed] [Google Scholar]
- Ponnudurai T, Meuwissen JH, Leeuwenberg AD, Verhave JP, Lensen AH. The production of mature gametocytes of Plasmodium falciparum in continuous cultures of different isolates infective to mosquitoes. Trans R Soc Trop Med Hyg. 1982;76:242–250. doi: 10.1016/0035-9203(82)90289-9. [DOI] [PubMed] [Google Scholar]
- Ramsey J, Salinas E, Rodriguez M. Acquired transmission-blocking immunity to Plasmodium vivax in a population of southern coastal Mexico. Am J Trop Med Hyg. 1996;54:458–463. doi: 10.4269/ajtmh.1996.54.458. [DOI] [PubMed] [Google Scholar]
- Richie TL, Saul A. Progress and challenges for malaria vaccines. Nature. 2002;415:694–701. doi: 10.1038/415694a. [DOI] [PubMed] [Google Scholar]
- Riley EM, Wahl S, Perkins DJ, Schofield L. Regulating immunity to malaria. Parasite Immunol. 2006;28:35–49. doi: 10.1111/j.1365-3024.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues MH, Cunha MG, Machado RL, Ferreira OC, Jr, Rodrigues MM, Soares IS. Serological detection of Plasmodium vivax malar ia using recombina nt proteins cor responding to t he 19-kDa C-terminal region of the merozoite surface protein-1. Malar J. 2003;2:39. doi: 10.1186/1475-2875-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MM, Cordey AS, Arreaza G, Corradin G, Romero P, Mary-anski JL, Nussenzweig RS, Zavala F. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int Immunol. 1991;3:579–585. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- Rogers WO, Gowda K, Hoffman SL. Construction and immunogenicity of DNA vaccine plasmids encoding four Plasmodium vivax candidate vaccine antigens. Vaccine. 1999;17:3136–3144. doi: 10.1016/s0264-410x(99)00146-2. [DOI] [PubMed] [Google Scholar]
- Rossan RN, Young MD, Baerg DC. Chemotherapy of Plasmodium vivax in Saimiri and Aotus models. Am J Trop Med Hyg. 1975;24:168–173. doi: 10.4269/ajtmh.1975.24.168. [DOI] [PubMed] [Google Scholar]
- Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, Foucault C, Chongsuphajaisiddhi T, Druilhe P. Parasitologic and clinical human response to immuno-globulin administration in falciparum malaria. Am J Trop Med Hyg. 1991;45:297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- Salas ML, Romero JF, Solarte Y, Olano V, Herrera MA, Herrera S. Development of sporogonic cycle of Plasmodium vivax in experimentally infected Anopheles albimanus mosquitoes. Mem Inst Oswaldo Cruz. 1994;89(Suppl. II):115–119. doi: 10.1590/s0074-02761994000600024. [DOI] [PubMed] [Google Scholar]
- Sattabongkot J, Maneechai N, Phunkitchar V, Eikarat N, Khuntirat B, Sirichaisinthop J, Burge R, Coleman RE. Comparison of artificial membrane feeding with direct skin feeding to estimate the infectiousness of Plasmodium vivax gametocyte carriers to mosquitoes. Am J Trop Med Hyg. 2003;69:529–535. [PubMed] [Google Scholar]
- Sattabongkot J, Maneechai N, Rosenberg R. Plasmodium vivax: gametocyte infectivity of naturally infected Thai adults. Parasitology. 1991;102:27–31. doi: 10.1017/s0031182000060303. [DOI] [PubMed] [Google Scholar]
- Scheller LF, Azad AF. Maintenance of protective immunity against malaria by persistent hepatic parasites derived from irradiated sporozoites. Proc Natl Acad Sci USA. 1995;92:4066–4068. doi: 10.1073/pnas.92.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISM2015. [homepage on the Internet. Panamá: Iniciativa Salud Mesoamérica; 2010. 2015. Available from: sm2015.org/es/salud-mesoamerica-2015/salud-mesoamerica-2015-inicio,1904.html. [Google Scholar]
- Soares IS, Levitus G, Souza JM, Del Portillo HA, Rodrigues MM. Acquired immune responses to the N- and C-terminal regions of Plasmodium vivax merozoite surface protein 1 in individuals exposed to malaria. Infect Immun. 1997;65:1606–1614. doi: 10.1128/iai.65.5.1606-1614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares IS, Rodrigues MM. Immunogenic properties of the Plasmodium vivax vaccine candidate MSP1(19) expressed as a secreted non-glycosylated polypeptide from Pichia pastoris. Parasitology. 2002;124:237–246. doi: 10.1017/s003118200100110x. [DOI] [PubMed] [Google Scholar]
- Solarte Y, Manzano MR, Rocha L, Hurtado H, James MA, Arevalo-Herrera M, Herrera S. Plasmodium vivax sporozoite production in Anopheles albimanus mosquitoes for vaccine clinical trials. Am J Trop Med Hyg. 2011;84:28–34. doi: 10.4269/ajtmh.2011.09-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Silva FA, da Silva-Nunes M, Sanchez BA, Ceravolo IP, Malafronte RS, Brito CF, Ferreira MU, Carvalho LH. Naturally acquired antibodies to Plasmodium vivax Duffy-binding protein (DBP) in Brazilian Amazon. Am J Trop Med Hyg. 2010;82:185–193. doi: 10.4269/ajtmh.2010.08-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storti-Melo LM, Souza-Neiras WC, Cassiano GC, Taveira LC, Cordeiro AJ, Couto VS, Povoa MM, Cunha MG, Echeverry DM, Rossit AR, Arévalo-Herrera M, Herrera S, Machado RL. Evaluation of the naturally acquired antibody immune response to the Pv200L N-terminal fragment of Plasmodium vivax merozoite surface protein-1 in four areas of the Amazon Region of Brazil. Am J Trop Med Hyg. 2011;84:58–63. doi: 10.4269/ajtmh.2011.10-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers AW, Cioce V, Shimp RL, Lawson M, Hui G, Muratova O, Kaslow DC, Robinson R, Long CA, Miller LH. Efficacy of two alternate vaccines based on Plasmodium falciparum mero-zoite surface protein 1 in an Aotus challenge trial. Infect Immun. 2001;69:1536–1546. doi: 10.1128/IAI.69.3.1536-1546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers AW, Miller LH. Are trials in New World monkeys on the critical path for blood-stage malaria vaccine development? Trends Parasitol. 2001;17:415–419. doi: 10.1016/s1471-4922(01)02011-6. [DOI] [PubMed] [Google Scholar]
- Suarez-Mutis MC, Cuervo P, Leoratti FM, Moraes-Avila SL, Ferreira AW, Fernandes O, Coura JR. Cross sectional study reveals a high percentage of asymptomatic Plasmodium vivax infection in the Amazon Rio Negro area, Brazil. Rev Inst Med Trop Sao Paulo. 2007;49:159–164. doi: 10.1590/s0036-46652007000300005. [DOI] [PubMed] [Google Scholar]
- Sultan AA, Thathy V, Frevert U, Robson KJ, Crisanti A, Nussenzweig V, Nussenzweig RS, Menard R. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- Takeo S, Hisamori D, Matsuda S, Vinetz J, Sattabongkot J, Tsuboi T. Enzymatic characterization of the Plasmodium vivax chitinase, a potential malaria transmission-blocking target. Parasitol Int. 2009;58:243–248. doi: 10.1016/j.parint.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson AW. Regulation of immunity to Plasmodium: implications from mouse models for blood stage malaria vaccine design. Exp Parasitol. 2010;126:406–414. doi: 10.1016/j.exppara.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Templeton TJ, Kaslow DC. Cloning and cross-species comparison of the thrombospondin-related anonymous protein (TRAP) gene from Plasmodium knowlesi Plasmodium vivax and Plasmodium gallinaceum. Mol Biochem Parasitol. 1997;84:13–24. doi: 10.1016/s0166-6851(96)02775-2. [DOI] [PubMed] [Google Scholar]
- Terrientes ZI, Kramer K, Herrera MA, Chang SP. Naturally acquired antibodies against the major merozoite surface coat protein (MSP-1) of Plasmodium falciparum acquired by residents in an endemic area of Colombia. Mem Inst Oswaldo Cruz. 1994;89(Suppl. II):55–61. doi: 10.1590/s0074-02761994000600014. [DOI] [PubMed] [Google Scholar]
- Toure YT, Doumbo O, Toure A, Bagayoko M, Diallo M, Dolo A, Vernick KD, Keister DB, Muratova O, Kaslow DC. Gametocyte infectivity by direct mosquito feeds in an area of seasonal malaria transmission: implications for Bancoumana, Mali, as a transmission-blocking vaccine site. Am J Trop Med Hyg. 1998;59:481–486. doi: 10.4269/ajtmh.1998.59.481. [DOI] [PubMed] [Google Scholar]
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, Kaslow DC, Gozar MM, Tachibana M, Cao YM, Torii M. Sequence polymorphism in two novel Plasmodium vivax ookinete surface proteins, Pvs25 and Pvs28, that are malaria transmission-blocking vaccine candidates. Mol Med. 1998;4:772–782. [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, Tachibana M, Kaneko O, Torii M. Transmission-blocking vaccine of vivax malaria. Parasitol Int. 2003;52:1–11. doi: 10.1016/s1383-5769(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Valderrama-Aguirre A, Quintero G, Gómez A, Castellanos A, Pérez Y, Méndez F, Arévalo-Herrera M, Herrera S. Antigenicity, immunogenicity and protective efficacy of Plasmodium vivax MSP1 Pv200L: a potential malaria vaccine subunit. Am J Trop Med Hyg. 2005;73:16–24. doi: 10.4269/ajtmh.2005.73.16. [DOI] [PubMed] [Google Scholar]
- Van den Eede P, Soto-Calle VE, Delgado C, Gamboa D, Grande T, Rodriguez H, Llanos-Cuentas A, Anne J, D’Alessandro U, Erhart A. Plasmodium vivax sub-patent infections after radical treatment are common in Peruvian patients: results of a 1-year prospective cohort study. PLoS ONE. 2011;6:e16257. doi: 10.1371/journal.pone.0016257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard V, Agak GW, Frank G, Jafarshad A, Servis C, Nebie I, Sirima SB, Felger I, Arévalo-Herrera M, Herrera S, Heitz F, Backer V, Druilhe P, Kajava AV, Corradin G. Rapid identification of malaria vaccine candidates based on alpha-helical coiled coil protein motif. PloS ONE. 2007;2:e645. doi: 10.1371/journal.pone.0000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Arévalo-Herrera M, Gardner MJ, Bonelo A, Carlton JM, Gomez A, Vera O, Soto L, Vergara J, Bidwell SL, Domingo A, Fraser CM, Herrera S. Immune responses to Plasmodium vivax pre-erythrocytic stage antigens in naturally exposed Duffy-negative humans: a potential model for identification of liver-stage antigens. Eur J Immunol. 2005;35:1859–1868. doi: 10.1002/eji.200425807. [DOI] [PubMed] [Google Scholar]
- Westenberger SJ, McClean CM, Chattopadhyay R, Dharia NV, Carlton JM, Barnwell JW, Collins WE, Hoffman SL, Zhou Y, Vinetz JM, Winzeler EA. A systems-based analysis of Plasmodium vivax lifecycle transcription from human to mosquito. PLoS Negl Trop Dis. 2010;4:e653. doi: 10.1371/journal.pntd.0000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO - World Health Organization. Role of non-human primates in malaria vaccine development: memorandum from a WHO meeting. Bull World Health Organ. 1988;66:719–728. [PMC free article] [PubMed] [Google Scholar]
- WHO - World Health Organization. World malaria report: 2010. 2010 Available from: who.int/malaria/world_malaria_report_2010/en/index.html.
- Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, Fay MP, Narum D, Rausch K, Miles AP, Aebig J, Orcutt A, Muratova O, Song G, Lambert L, Zhu D, Miura K, Long C, Saul A, Miller LH, Durbin AP. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with mon-tanide ISA 51. PloS ONE. 2008;3:e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani SS, Shakri AR, Mukherjee P, Baniwal SK, Chitnis CE. Evaluation of immune responses elicited in mice against a recombinant malaria vaccine based on Plasmodium vivax Duffy-binding protein. Vaccine. 2004;22:3727–3737. doi: 10.1016/j.vaccine.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Yeom JS, Kim ES, Lim KJ, Oh JH, Sohn MJ, Yoo SB, Kim E, Bae I, Jung YJ, Park JW. Naturally acquired IgM antibody response to the C-terminal region of the merozoite surface protein 1 of Plasmodium vivax in Korea: use for serodiagnosis of vivax malaria. J Parasitol. 2008;94:1410–1414. doi: 10.1645/GE-1484.1. [DOI] [PubMed] [Google Scholar]
- Young MD, Porter JA, Jr, Johnson CM. Plasmodium vivax transmitted from man to monkey to man. Science. 1966;153:1006–1007. doi: 10.1126/science.153.3739.1006. [DOI] [PubMed] [Google Scholar]
- Yount EH, Jr, Coggeshall LT. Status of immunity following cure of recurrent vivax malaria. Am J Trop Med Hyg. 1949;29:701–705. doi: 10.4269/ajtmh.1949.s1-29.701. [DOI] [PubMed] [Google Scholar]