Abstract
A bidirectional relationship has been observed for kidney disease and cancer. On the one hand, cancer is an important complication noted in kidney disease as well as a major cause of morbidity and mortality in this group. On the other hand, improved cancer treatment has prolonged survival, but also increased the development of acute and chronic kidney disease. The combination of cancer and kidney disease makes it challenging for clinicians to provide comprehensive and safe therapies for this group of patients. As such, clinicians caring for this group must develop expertise and become competent in the practice of a newly evolving subspecialty of nephrology known as ‘onco-nephrology’. This brief narrative review will focus on the cancer risk in patients with underlying kidney disease, the therapies such as erythropoiesis-stimulating agents on cancer progression and other outcomes, and the appropriate dosing of anti-cancer agents in patients with underlying kidney disease.
Keywords: albuminuria, cancer, dosage adjustment, erythropoiesis-stimulating agents, kidney disease
INTRODUCTION
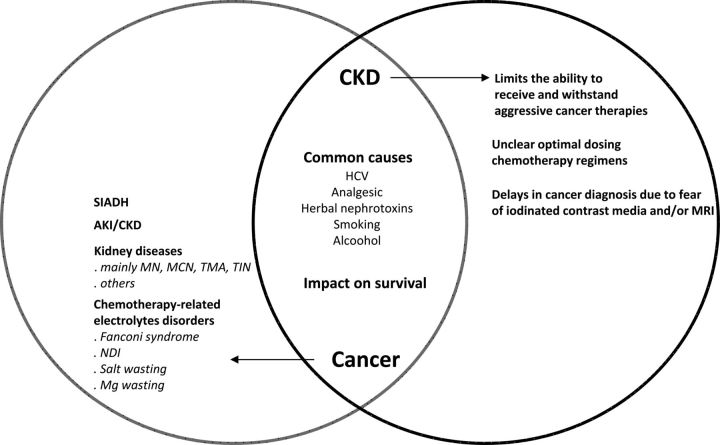
Cancer is becoming increasingly recognized as a complication and a major cause of morbidity and mortality in the chronic kidney disease (CKD) population. In addition, dramatic progress in therapy of cancer with an associated improvement in survival has made kidney disease a growing concern in this population. The combination of cancer and kidney disease also influences therapies used for both disease processes. Thus, there is a bidirectional relationship between kidney disease and cancer (Figure 1). As such, clinicians caring for patients with this combination of disease must develop expertise and become competent ‘onco-nephrologists’. This brief commentary will focus on the cancer risk in patients with underlying kidney disease, the influence of underlying cancer on therapies such as erythropoiesis-stimulating agents (ESAs) and the appropriate dosing of commonly employed anti-neoplastic agents in patients with underlying kidney disease.
FIGURE 1:
Bidirectional relationship between kidney disease and cancer. SIADH, syndrome of inappropriate antidiuretic hormone secretion; AKI/CKD, acute kidney injury/chronic kidney disease; MN, membranous nephropathy; MCN, minimal change nephropathy; TMA, thrombotic microangiopathy; TIN, tubulointerstitial nephritis; NDI, nephrogenic diabetes insipidus; MRI, magnetic resonance imaging with gadolinium.
First, we must evaluate the actual significance of the cancer risk in patients with underlying kidney disease. Large observational studies have consistently shown a 2- to 3-fold increased risk of cancer among kidney transplant recipients [1–6] and an excess risk of 20–50% for all cancers among people both with early stage CKD and on dialysis [1]. Piselli et al. [7] confirmed the increased risk for cancer following kidney transplant, and also suggested a possible protective effect of mammalian target of rapamycin inhibitors in reducing the frequency of post-transplant cancers. In addition, the relative cancer-specific mortality rate is approximately five to six times greater than the age- and gender-matched general population [8]. Among 19 103 kidney transplant procedures performed in England between April 2001 and March 2012 (median follow-up 4.4 years), 2085 deaths occurred, of which 376 (18.0%) were due to malignancy (crude mortality rate 361 malignancy-related deaths per 100 000 person-years) [9]. Thus, malignancy as a cause of post-kidney transplantation death is common and requires heightened surveillance [9]. In Table 1, we have compiled a list of specific issues of cancer in various degrees of kidney dysfunction (i.e. CKD stages 3–5, dialysis and transplantation) [9–18]. On the other hand, CKD is common in patients with cancer [19] and is suggested to be an independent risk factor for death from cancer by recent studies [12, 20, 21]. The relationship between kidney disease and cancer is multiple.
Table 1.
Specific issues of cancer in various degrees of kidney dysfunction
| Authors | Study source | n | Follow-up (median, years) | Death |
Common site of malignancy and comments | |
|---|---|---|---|---|---|---|
| All | Malignancy-related (%) | |||||
| CKD 3–5 stages | ||||||
| Lai et al. [10] | Chinese prospective cohort study | 739 | 4.5 | 18 | 35.7 | NA |
| Dubose et al. [11] | Case-matched, retrospective review of a prospectively maintained database of Louisiana | 1223 | 5 | Five-year OS, 75% (CKD) versus 85% (non-CKD), P = 0.47; 5-year DFS, 64% CKD versus 81% (non-CKD), P = 0.45 |
Study on breast cancer. CKD does not appear to have a significant impact on outcomes in patients with breast cancer | |
| Weng et al. [12] | Four health screening centres in Taiwan | 123 717 | 7.06 | 2710 | 41.2 | Lung, kidney, and urinary tract increased mortality due to cancer incidence/worse response to cancer treatment |
| Dialysis | ||||||
| Chantrel et al. [13] | Registre REIN | 63 311 | 9 | 21 818 | 10.5 | NA |
| Rosa-Diez et al. [14] | RLADTR | 543 669 000 | 20 | NA | 10 | NA |
| Renal transplant | ||||||
| Webster et al. [15] | Australia and New Zealand Dialysis and Transplant Registry | 15 185 | 7.2 | NA | 1642 | Cancer rates in kidney recipients are similar to non-transplanted people 20–30 years older, but absolute risk differs across patient groups |
| Kiberd et al. [16] | URSDS | 164 078 | 5.05 | 1937 | 3 | Up to 25% of cancer-related deaths occurred after allograft failure. Cancer is a major cause of premature death in all kidney transplant recipients |
| Koukourgianni et al. [17] | French single-centre experience | 240 | 20 | NA | 16 cancers, mortality rate of 25% | PTLPD, lymphoma, renal papillary carcinoma, thyroid carcinoma, ovarian seminoma, renal cancer |
| Ma et al. [18] | Australian and New Zealand Dialysis and Transplant Registry | 7040 | 4.4 | NA | 468 due to cancer (6.6%) | Genitourinary, PTPLD. Recipients of expanded criteria deceased-donor kidneys are at substantially increased risk of cancer, especially cancers with a viral aetiology |
| Farrugia et al. [9] | English retrospective observational cohort study | 19 103 | 4.4 | 2085 | 18.0 | Increased age, pretransplant history of malignancy and deceased-donor kidney transplantation are independent risks for post-transplant death from malignancy |
CKD, chronic kidney disease; NA, not available; OS, overall survival; DFS, disease-free survival; PTLPD, post-transplantation lymphoproliferative disease; RLADTR, Latin American Dialysis and Renal Transplant Registry; USRDS, United States Renal Data System (between January 1990 and December2004).
Magee [22] has elegantly emphasized the various links between the kidney and cancer. First, cancer and CKD may have the same cause, that is, analgesics and herbal nephrotoxins induce interstitial nephritis and urothelial cancers; HCV infection causes liver cancer and membranoproliferative disease (MPGN), and smoking exposes to bladder/kidney cancer and renovascular disease. Secondly, some kidney diseases may be related to cancer, mainly membranous nephropathy, minimal change disease, crescentic GN and thrombotic microangiopathy. Thirdly, underlying CKD often limits treatment options or diagnosis of cancer [22]. Examples include the inability of patients to receive and withstand aggressive cancer therapies and/or undergo unclear optimal dosing chemotherapy regimens. In addition, there is concern over multiple and, at times serious drug–drug interactions, as well as exposure to potentially nephrotoxic-iodinated contrast media and multiorgan toxic gadolinium as manifested by nephrogenic systemic fibrosis [22]. This multifaceted link between cancer and kidney disease can ultimately impact on the survival of both CKD and cancer.
As seen with the patients who fall into the CKD definition, albuminuria, also, appears to be an independent risk marker for cancer incidence [21] and cancer deaths [23]. It is uncertain whether the poor survival in people with CKD and cancer is due to a more aggressive form of cancer, ineffective treatment of the underlying malignancy for the reasons stated above, a greater competing risk of death from CKD-related comorbidities or some combination of these factors [24].
CKD AS AN INDEPENDENT RISK FACTOR FOR DEATH FROM CANCER
Albuminuria and cancer
Albuminuria may reflect a paraneoplastic renal disease and/or may be a marker of cancer incidence and associated mortality (Table 2) [25–32]. Increased urinary albumin/creatinine ratio (UACR) and cancer risk appear to mirror the results of studies of individuals on dialysis, who have higher cancer rates than the general population [4, 33]. The Tromsø study showed that elevated UACR at baseline was correlated with subsequent cancer incidence. A total 590 of 5425 participants without diabetes mellitus or previous cancer had the first diagnosis of cancer at 10.3 years of follow-up. Each standard deviation higher rise in the logarithmically transformed UACR was associated with a relative risk (RR) of 1.17 for cancer (P < 0.001). Independently from smoking, participants with UACR in the highest quintile were 8.3- and 2.4-fold more likely to receive a diagnosis of bladder and lung cancer, respectively [21].
Table 2.
Association of albuminuria, CKD and cancer incidence/mortality
| Authors | Study design (n) | n (age, years) | Follow-up (years) | Definition | Comments |
|---|---|---|---|---|---|
| Yuyun et al. [25] | Prospective population-based cohort study EPIC-Norfolk | 20 911 (40–79) | Average, 6.3 | UACR | Increased mortality rate across categories of albuminuria and CVD and non-CVD only significant in men. |
| Fried et al. [20] | Prospective community-based cohort study from the ACHS | 4637 (74 ± 5) | 9.5 | Cystatin C and eGFR | Higher (19.9 versus 13.9/1000 patient-years) unadjusted rates of death secondary to cancer in older patients with CKD than those without CKD. |
| Jørgensen et al. [21] | Population-based longitudinal study, Tromsø study | 5425 | 10.3 | UACR | Correlation between baseline ACR and incidence of cancer Compared with lowest UACR quintile, participants with ACR in the highest quintile were 8.3- and 2.4-fold more likely to receive a diagnosis of bladder cancer and lung cancer, respectively. |
| Wong et al. [24] | Prospective-based cohort study from the BMES and NSWCR, Australia | 3654 (49–74) | 10.1 | eGFR | Moderate CKD (eGFR of 55 mL/min) increases the risk for all cancers in men by 40%. For every 10-mL/min decrement in eGFR, the risk for cancer increased by 29%. |
| Lin et al. [23] | Prospective-based NHANES III (1988–94) | 6112 (≥50) | 12.4 | UACR | Increased mortality risk associated with UACR for all cancer [RR, 1.20; 95% CI 1.06–1.36], lung cancer [RR, 1.22; 95% CI 1.05–1.43) and prostate cancer mortality [RR, 1.40; 95% CI 1.01–1.95] in men only. |
| Weng et al. [12] | Population-based longitudinal study from Taiwan | 123 717 (61 ± 11.9) | 7.06 | eGFR/MDRD | Significant graded relationship between the severity of non- dialysis-dependent CKD and cancer mortality. |
| Whaley-Connell et al. [26] | Prospective study. Database from NKF KEEP | 109 285 (63 ± 14.5) | Albuminuria and eGFR | 26 and 36.4% had CKD defined by albuminuria or eGFR <60 mL/min/1.73 m2 and cancer, respectively. | |
| Christensson et al. [27] | Prospective population-based cohort study | 24 552 (26–61) | 28 | CKD EPI | Significant association between moderate CKD and kidney cancer risk in younger men (HR, 3.38; 95% CI 1.48–7.71; P < 0.004). |
| Lin et al. [28] | Population-based retrospective cohort study Taiwan | 1 million | ESRD patients with secondary HPT have a 10.2-fold higher risk of developing thyroid cancer than ESRD patients without secondary HPT. | ||
| Zaidan et al. [29] | Retrospective French cohort study from 1996 to 2011 | 170 | Mean lithium exposure, 21.4 years | Incidence ratio of renal cancer was higher in lithium-treated patients compared with the general population: 7.51 (95% CI 1.51–21.95) and 13.69 (95% CI 3.68–35.06) in men and women, respectively. | |
| Iff et al. [30] | Prospective population-based cohort study from the BMES | 4077 (49–97) | Median, 12.8 (8.6–15.8) | eGFR | For every 10-mL/min/1.73 m2 reduction in eGFR, an increase in cancer-specific mortality of 18% (P < 0.001). Excess all cancer mortality [HR,1.27 (95% CI 1.00–1.60; P = 0.05)], breast [adjusted HRs, 1.99 (95% CI 1.05–3.85; P = 0.01)] and urinary tract [adjusted HRs, 2.54 (95% CI 1.02–6.44; P = 0.04)] cancer deaths. |
| Nakamura et al. [31] | Retrospective Japanese cohort study | 231 (63.6 ± 12.7) | 1 | MDRD | Increased mortality in patients with CKD (HR 14-fold higher) than in those without CKD. |
| Na et al. [32] | Retrospective Korean cohort study | 8223 | 1 | eGFR | CKD was associated with an increased risk of death in cancer patients: eGFR stage III, adjusted HR 1.12 (95% CI 1.01–1.26, P = 0.04); eGFR stage IV–V, adjusted HR 1.75 (95% CI 1.32–2.32, P < 0.001). |
EPIC: Norfolk, European Prospective Investigation into Cancer: Norfolk Cohort; CVD, cardiovascular disease; non-CVD, non-cardiovascular disease; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; UACR, urinary albumin/creatinine ratio; NHANES III; RR, relative risk; MDRD, Modification of Diet in Renal Disease; NKF KEEP, National Kidney Foundation's Kidney Early Evaluation Program; ACHS, American Cardiovascular Health Study; BMES, Blue Mountain Eye Study; NSWCR, New South Wales Cancer Registry; HPT, hyperparathyroidism.
Albuminuria was also associated with an increased risk of cancer deaths from all-cause, lung and prostate cancers in men aged 50 and older in the USA [23]. Report from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–94) found an increased mortality risk associated with logarithmically transformed UACR for all-cause cancer (RR, 1.20), lung cancer (RR, 1.22) and prostate cancer mortality (RR, 1.40) in men. No mortality association between UACR and cancer was apparent in women. In the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study, a 2-fold increase of albuminuria was associated with a 29% increased risk of CVD mortality and a 12% increased risk of non-CVD mortality in the general population, which was attributed primarily to malignant neoplasms [34]. In contrast, a study from Norfolk [35] did not find an association between albuminuria and cancer. Despite the growing recognition for albuminuria as a paraneoplastic and inflammatory phenomenon, the underlying mechanisms for the association between albuminuria and cancer incidence and mortality are largely unknown [35–38]. One may speculate that the renin–angiotensin system may participate in generating the increased cancer risk associated with albuminuria. Angiotensin II has been implicated in the development or invasion of several kinds of cancer [39]. Angiotensin could exert mitogenic activity through the angiotensin II type I (ATII-I) receptors, whose receptor expression is higher in cancerous prostate [40].
Kidney disease and cancer
As with albuminuria, end-stage renal disease (ESRD) is also a predictor of non-cardiovascular mortality [20], which is related to increased cancer risk attributed to kidney and urinary tract cancer [41–44]. An increased rate of incidence of thyroid cancer, other endocrine cancers, virus infection-related cancers, skin cancer and liver cancer has also been reported in ESRD patients [4, 45, 46]. However, this excess cancer risk in men with CKD does not appear to be limited to those with ESRD or with a transplant, but is also described in those with moderate CKD (Table 2). Wong et al. [24] reported an increased incidence rate of of lung cancer and urinary tract cancer among at least Stage 3 CKD patients in a prospective, population-based cohort study and the excess risk began at an estimated GFR (eGFR) of 55 mL/min/1.73 m2 (adjusted hazard ratio [HR]) and increased linearly as GFR declined. For every 10-mL/min/1.73 m2 decrement in eGFR, the risk for cancer increased by 29% (adjusted HR 1.29), with the greatest risk at an eGFR of <40 mL/min/1.73 m2 (adjusted HR 3.01).
Recently, Iff et al. [30] also reported that, in older people, eGFR <60 mL/min/1.73 m2 is associated with an increased risk of cancer death with a linear relationship between reduction in eGFR and incidence of cancer-specific mortality, independent of age, smoking status, sex, blood pressure as well as serum fibrinogen and fasting blood glucose levels. The association between reduced kidney function and cancer death also appears to be site-specific for breast cancers (2-fold higher) and cancers of the urinary tract system (2.5-fold higher) [30].
In their large and highly representative population-based cohort from a cross-sectional screening programme with a median follow-up of 28 years, Christensson et al. [27] found that the long-term risk of kidney cancer was significantly higher only among younger men with moderately impaired kidney function when compared with those with normal or mild baseline kidney function impairment. However, Wong et al. [24] were unable to find any increased risk among those with stage 2 CKD.
In other studies, an increased incidence and aggressiveness of upper urinary tract urothelial carcinoma, found among CKD patients [47, 48], was observed with analgesic nephropathy and Chinese herb nephropathy (aristolochic acid nephropathy) [49, 50]. In a retrospective French study, the standardized incidence ratio of renal cancer was significantly higher in long-term lithium-treated patients when compared with the general population: 7.51 and 13.69 in men and women, respectively [29]. In their population-based retrospective cohort consisting of original claim data of 1 million beneficiaries randomly sampled from the Taiwan National Health Insurance Research Database (NHIRD), Lin et al. [28] observed that ESRD patients with secondary hyperparathyroidism exhibited a 10.1-fold increased risk of thyroid cancer than did ESRD patients without this parathyroid complication, after adjusting for comorbidities.
In a longitudinal population-based prospective cohort focusing on cancer-related deaths in Taiwan, a significant graded relationship between the severity of CKD and cancer mortality was found when compared with non-CKD patients. Deaths from cancer of the liver, kidney and urinary tract increased incrementally with the severity of renal impairment with an adjusted HR of 1.74, 3.3 and 7.3, respectively [12].
Continued research on this subject is required to tease out the relative higher incidence and mortality of cancers in CKD patients [22]. Pending this information, we must apply common sense strategies to reduce cancer death risk in CKD patients such as smoking cessation, exclude or limit the use of potential carcinogenic drugs (e.g. cyclophosphamide) and judicious screening for cancers [22]. In light of this sentiment, the use of ESAs in this population will be examined next.
ESAs AND CANCER
Anaemia and ESAs in oncology
Approximately 32% of cancer patients present with anaemia at diagnosis and ∼54% of initially non-anaemic cancer patients develop anaemia during treatment [51, 52]. In those patients, anaemia can be a result of underlying disease (cancer-related anaemia) or chemotherapy-induced anaemia (CIA) [53]. Clinicians should carefully evaluate the impact of anaemia on quality of life [54] as well as its association with shorter overall survival [55].
ESAs are approved in the USA and Europe for treating CIA based on randomized, placebo-controlled trials, showing that these drugs reduce red blood cell transfusions. Three different ESAs are available to date: epoetin α (Procrit®, Johnson & Johnson; Epogen®, Amgen), epoetin β (NeoRecormon®, Roche) and darbepoetin α (Aranesp®, Amgen). Other novel molecules such as the continuous erythropoietin receptor activator [56, 57] and biosimilars that have been developed [58, 59] are available. More than 80 randomized controlled trials (RCTs) and 20 meta-analyses and systematic reviews on the effects of ESAs in cancer patients have been published [60]. Although ESAs continue to be indicated for the management of CIA, their use in clinical practice has become controversial. ESA safety issues include thromboembolic events [61, 62] and concerns regarding whether ESAs increase disease progression and/or mortality in cancer patients [61–66].
Disease progression and/or mortality in oncology
The ESA-product labelling describes eight clinical trials of concern that suggest ESA use increases disease progression and/or mortality in cancer patients [67–74]. Two studies were performed in the non-indicated setting of radiotherapy treatment only [69, 71], two in the non-indicated anaemia-of-cancer setting (patients received neither chemotherapy nor radiotherapy) [68, 73] and four in the indicated chemotherapy setting [69, 70, 72, 74]. Although the ENHANCE and DAHANCA-10 trials suggested that ESA use increases disease progression, this finding was not replicated in two RCTs in the radiotherapy setting for the treatment of patients with head-and-neck cancer; the Radiation Therapy Oncology Group (RTOG 99-03) trial [75] and the controlled EPO-GBR-7 trial [76]. Nonetheless, based on the ENHANCE and DAHANCA-10 studies, the ESA-product labelling does not recommend ESA use in the radiotherapy-only setting. Hence, several meta-analyses of RCTs exploring the risk of worsening the life prognosis for cancer patients on ESA were reported [61–66, 77–81]. The majority of the clinical trials reviewed in these meta-analyses were conducted with haemoglobin (Hb) concentrations higher than the accepted standard stated in the current package inserts of ESA in the USA and the EU (Hb concentrations at the beginning of ESA therapy to be 10 g/dL or lower, and target Hb concentration of 10–12 g/dL) and/or clinical trials were conducted in patients not receiving chemotherapy. However, large RCTs reported more deaths in ESA patients compared with controls in various clinical settings, that is head-and-neck cancers undergoing radiotherapy [69, 71], metastatic breast cancer undergoing chemotherapy [69] and advanced stage cancers not receiving chemotherapy [73]. As a result, an increased risk to death cannot be excluded in ESA-treated cancer patients. Two large RCTs to determine the effects of ESAs on overall and progression-free survival in patients with Hb levels of <110 g/L are currently underway [82, 83] and will provide a definitive answer to this important question. Pending the outcome of these two studies in progress, the clinician should refer to the International recommendations for the treatment of chemotherapy-induced anaemia [84–88].
ESA use and cancer risk in CKD
Data on CKD patients with cancer and ESA use are scant. In the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) [89], more than 4000 patients with Type 2 diabetes mellitus and CKD stage 3/4 were randomly assigned to darbepoetin α to achieve an Hb level of 13 g/dL or to placebo. Although there was no significant difference in patients reporting a cancer-related adverse event [darbepoetin α group 6.9% and placebo group 6.4% (P = 0.53)], a trend towards an increased risk of death attributed to cancer [darbepoetin α group, 39 deaths; placebo group, 25 deaths (P = 0.08)] was noted. However, the pre-specified secondary analyses showed a higher risk of cancer-related death in patients with a malignancy present at least 5 years prior to randomization (14 of 188 in the darbepoetin group versus 1 of 160 in the placebo group, P = 0.002) [90]. An observational study also showed an increased incidence of stroke or thromboembolic events in CKD patients with cancer, who, however, received two to four times higher doses of ESA compared with those without cancer [91]. In contrast, a Japanese study in CKD stage 4/5 patients failed to show an increased incidence of cancer with ESA therapy [92]. Cancers were reported in 2.7% (101/3762) and 2.4% (88/3653), and annualized event rates were 5.3 and 4.8%, respectively. However, lower Hb average (10.1 g/dL) and short surveillance times limited the study results [91].
Venous thromboembolic events
Owing to the hypercoagulable state of malignancy, cancer patients are at an increased risk to develop thrombovascular events. Nevertheless, it appears to be a rare event. Data for venous thromboembolic events (VTEs) are available from about 50 RCTs comparing ESA with no ESA treatment in cancer patients, and evaluated in nine meta-analyses [61, 62, 66, 77, 78, 93–96]. Although limitations and potential biases exist, meta-analyses on epoetin β (seven trials including 2112 patients) [95] and darbepoetin α (12 trials including 2297 patients) [96] suggest an increased risk for VTEs in cancer patients receiving ESAs. In these trials, RR for VTEs was 1.62 [95] and 1.57 [96], respectively. ESAs have the potential to increase thombogenic activity either by augmented haemoglobin levels through changes in rheology or other mechanisms such as increased or enhanced platelet function. Healthy volunteers receiving recombinant ESAs demonstrate increased platelet reactivity and endothelial activation [97]. Unproven hypotheses are that ESAs could increase the incidence of VTEs by stimulating platelet production [98–103] or by an association between VTEs and JAK2 kinase activation [104]. Physicians treating cancer patients with ESAs might be more aware of the complication of VTEs in patients receiving ESAs.
CHEMOTHERAPY DOSAGE ADJUSTMENT IN CKD
Anti-cancer drugs can be eliminated primarily by renal and/or non-renal pathways, which are defined as the fraction eliminated via a renal route <15%. One of the important drug-related problems in patients with renal impairment is inappropriate medication use and dosing errors [105, 106]. Along this line, many cytotoxic drugs and their active/toxic metabolites are eliminated through the kidney depending on how much of the substance undergoes renal filtration, tubular secretion and/or tubular reabsorption. Hence, patients with both acute kidney injury (AKI) and CKD receiving chemotherapeutic agents often possess alterations in their pharmacokinetic parameters such as drug absorption, distribution, protein binding, biotransformation and renal excretion, which may result in the accumulation of potentially toxic components and over-dosage [107]. Therefore, clinicians must be wary to appropriately adjust doses of drugs that are excreted primarily by the kidneys. This requires dosing according to the calculated or measured creatinine clearance or eGFR formulas, which will allow the safe use of chemotherapy in patients with underlying kidney disease. Table 3 summarizes the dosage adjustment of the most commonly used chemotherapeutic agents for which dose modification may be required in the setting of underlying kidney disease [108–110].
Table 3.
| Agent | Creatinine clearance (mL/min) |
Haemodialysis | ||
|---|---|---|---|---|
| 90–60 | 60–30 | 30–15 | ||
| Bleomycin | 100% | 75% | 75% | 50%a |
| Capecitabine | 100% | 75% | Avoid | Avoid |
| Carboplatin | Dosing based on AUC. Calvert formula: total carboplatin dose, mg = target AUC × (eGFR + 25). AUC varies between 5 (treated patients) and 7 mg/mL min (untreated patients) | In dialysis patients, consider GFR = 0 and the target dose = 125–175 mg. E: 84 ± 3%b | ||
| Carmustine | 100% | 80% if CrCl ≥45; 75% if CrCl <45 | Consider alternative | Consider alternative |
| Cisplatin | 100% | 50% | 50% | 25–50%b |
| Crizotinib | 100% | 100% | 50% | NA |
| Cyclophosphamide | 100% | 75–100% | 75% | 75%a—E:40–90% |
| Cytarabine (high dose) | 100% | 60% if CrCl ≥45; 50% if CrCl <45 | 30% or consider alternative | 30% consider alternativea |
| Dacarbazine | 100% | 80–70% | NA | 100 mg daily for 5 days per cycle |
| Eribulin | 100% | ≥40 mL/min: 100%; <40 mL/min: NA | NA | NA |
| Etoposide | 100% | 75% | 75% | 50%c |
| Fludarabine | 100% | 80% (USA); 50% (UK) | Avoid | Avoid |
| Hydroxyurea | 100% | 50% | 50% | 20%b |
| Ifosfamide | 100% | 75–100% | 75–100% | 75%,b E: 87% |
| Irinotecan | 100% | NA | NA | 30–40% |
| Lenalidomide | 25 mg daily | 10 mg daily | 15 mg every 2 days | 15 mg every 2 days—E:31%b |
| Lomustine | 100% | 75% | 75% | 25–50% |
| Melphalan | 100% | 75% | 75% | 50%a |
| Methotrexate | 100% | 80% | 50% | Avoid—HD Cl: from 82 to 102 mL/minb |
| Mitomycin | 100% | 100% | 100% | 75%a |
| Oxaliplatin | 100% | 100% | Avoid if CrCl <30 (Canadian) or <20 (USA) | Avoid |
| Pentazocine | 100% | 66% | 66% | 50% |
| Pentostatin | 100% | 50–75% | 50% | 50% (1–2 h before the dialysis session) |
| Pemetrexed | 100% | 100% | Avoid | Avoid |
| Regorafenib | 100% | 100% | NA | NA |
| Sorafenib | 100% | 100% | 100% | 25% of the dose and increase to 100% according to clinical safety and efficacyc |
| Sunitinib | 100% | 100% | 100% | 25% then increase to 100% according to clinical safety and efficacy. E: 0c |
| Topotecan | 100% | 50% | 50% | 25%b |
| Vandetanib | 100% | 25% | NA | NA |
AUC, target area under the concentration versus time curve in mg/mL•min; E, extraction coefficient (%); NA, not available.
aIn the absence of data on its removal during dialysis, the drug will be administered after the session, the haemodialysis days.
bThe drug is dialyzable. Therefore, it will be administered after the session, the days with HD.
cThe drug may be administered indifferently before or after the haemodialysis session.
Importantly, CKD alters also the pharmacokinetics of drugs that are cleared by non-renal mechanisms since it has been shown that uraemia (both AKI and CKD) affects hepatic drug metabolism and coupled transport. Therefore, it will be a challenge for clinicians to prescribe some drugs and develop a rational strategy for drug dosing in the CKD population [111]. Indeed, uraemic toxins interfere with transcriptional activation, cause down-regulation of gene expression mediated by proinflammatory cytokines and directly inhibit the activity of the cytochrome P450s and drug transporters [112]. As of now, the clinician will need to watch for signs of over-dosage and toxicity when the patient appears to have had appropriate ‘renal’ dosing of their chemotherapeutic regimen. Thus, special considerations should be taken when these drugs are prescribed to CKD patients to optimize exposure to cytotoxic drugs and to reduce the risk of adverse effects [110]. Such precautions include the use of less nephrotoxic agents and documented preventive measures, which are well integrated into oncology practice as well as vigilant surveillance [111–113].
CONCLUSION
Kidney disease and cancer are intertwined in many ways. Underlying kidney disease appears to increase cancer risk and its associated morbidity and mortality. Improved treatment of many malignancies has also extended life with kidney disease as an unfortunate but not necessarily unexpected consequence. This bidirectional relationship has created a clinical challenge for nephrologists as they strive to provide comprehensive and safe management of these patients. This requires that nephrology fellowship training programmes and nephrology societies provide education and updates on the management of this group of patients. This will allow clinicians to develop expertise and competence as ‘onco-nephrologists’ practicing in this new and exciting territory. We are hopeful that this paper starts this educational mission by briefly reviewing cancer risk in patients with underlying CKD, the therapies such as the ESAs on cancer progression and other outcomes, and the appropriate dosing of anti-cancer agents in patients with kidney disease.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA 2006; 296: 2823–2831 [DOI] [PubMed] [Google Scholar]
- 2.Brunner FP, Landais P, Selwood NH. Malignancies after renal transplantation: the EDTA-ERA registry experience. European Dialysis and Transplantation Association-European Renal Association. Nephrol Dial Transplant 1995; 10(Suppl 1): 74–80 [DOI] [PubMed] [Google Scholar]
- 3.Adami J, Gabel H, Lindelof B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer 2003; 89: 1221–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birkeland SA, Lokkegaard H, Storm HH. Cancer risk in patients on dialysis and after renal transplantation. Lancet 2000; 355: 1886–1887 [DOI] [PubMed] [Google Scholar]
- 5.Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007; 370: 59–67 [DOI] [PubMed] [Google Scholar]
- 6.Kyllonen L, Salmela K, Pukkala E. Cancer incidence in a kidney transplanted population. Transpl Int 2000; 13(Suppl 8): 394–398 [DOI] [PubMed] [Google Scholar]
- 7.Piselli P, Serraino D, Segoloni GP, et al. Risk of de novo cancers after transplantation: results from a cohort of 7217 kidney transplant recipients, Italy 1997-2009. Eur J Cancer 2013; 49: 336–344 [DOI] [PubMed] [Google Scholar]
- 8.Australia and New Zealand dialysis and Transplant Registry. The Twenty Eighth Report. Chapter 10 cancer Report 2005; 132–139, http://ghdx.healthdata.org/organizations/australia-and-new-zealand-dialysis-and-transplant-registry-anzdata
- 9.Farrugia D, Mahboob S, Cheshire J, et al. Malignancy-related mortality following kidney transplantation is common. Kidney Int 2014; 85: 1395–1403 [DOI] [PubMed] [Google Scholar]
- 10.Lai X, Zhang AH, Chen SY, et al. Outcomes of stage 1-5 chronic kidney disease in Mainland China. Ren Fail 2014; 36: 520–525 [DOI] [PubMed] [Google Scholar]
- 11.Dubose AC, Chu QD, Li BD, et al. Is chronic kidney disease an independent risk factor for mortality in breast cancer? J Surg Res 2013; 184: 260–264 [DOI] [PubMed] [Google Scholar]
- 12.Weng PH, Hung KY, Huang HL, et al. Cancer-specific mortality in chronic kidney disease: longitudinal follow-up of a large cohort. Clin J Am Soc Nephrol 2011; 6: 1121–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chantrel F, de Cornelissen F, Deloumeaux J, et al. [Survival and mortality in ESRD patients]. Nephrol Ther 2013; 9(Suppl 1): S127–S137 [DOI] [PubMed] [Google Scholar]
- 14.Rosa-Diez G, Gonzalez-Bedat M, Pecoits-Filho R, et al. Renal replacement therapy in Latin American end-stage renal disease. Clin Kidney J 2014; 7: 431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webster AC, Craig JC, Simpson JM, et al. Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: a cohort study of 15183 recipients. Am J Transplant 2007; 7: 2140–2151 [DOI] [PubMed] [Google Scholar]
- 16.Kiberd BA, Rose C, Gill JS. Cancer mortality in kidney transplantation. Am J Transplant 2009; 9: 1868–1875 [DOI] [PubMed] [Google Scholar]
- 17.Koukourgianni F, Harambat J, Ranchin B, et al. Malignancy incidence after renal transplantation in children: a 20-year single-centre experience. Nephrol Dial Transplant 2010; 25: 611–616 [DOI] [PubMed] [Google Scholar]
- 18.Ma MK, Lim WH, Turner RM, et al. The risk of cancer in recipients of living-donor, standard and expanded criteria deceased donor kidney transplants: a registry analysis. Transplantation 2014; 98: 1286–1293 [DOI] [PubMed] [Google Scholar]
- 19.Janus N, Launay-Vacher JN, Byloos E, et al. Cancer and renal insufficiency results of the IRMA study. Br J Cancer 2010; 103: 1815–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol 2005; 16: 3728–3735 [DOI] [PubMed] [Google Scholar]
- 21.Jørgensen L, Heuch I, Jenssen T, et al. Association of albuminuria and cancer incidence. J Am Soc Nephrol 2008; 19: 992–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magee C. Kidney disease and death from cancer. Am J Kidney Dis 2014; 63: 7–9 [DOI] [PubMed] [Google Scholar]
- 23.Lin YS, Chiu FC, Lin JW, et al. Association of albuminuria and cancer mortality. Cancer Epidemiol Biomarkers Prev 2010; 19: 2950–2957 [DOI] [PubMed] [Google Scholar]
- 24.Wong G, Webster AC, Chapman JR, et al. Reported cancer screening practices of nephrologists: results from a national survey. Nephrol Dial Transplant 2009; 24: 2136–2143 [DOI] [PubMed] [Google Scholar]
- 25.Yuyun M, Khaw K, Luben R, et al. Microalbuminuria independently predicts all cause and cardiovascular mortality in a British population: the European prospective investigation into cancer in Norfolk (EPIC Norfolk) population study. Int J Epidemiol 2004; 33: 189–198 [DOI] [PubMed] [Google Scholar]
- 26.Whaley-Connell A, Shlipak MG, Inker LA, et al. Awareness of kidney disease and relationship to end-stage renal disease and mortality. Am J Med 2012; 125: 661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensson A, Savage C, Sjoberg DD, et al. Association of cancer with moderately impaired renal function at baseline in a large, representative, population-based cohort followed for up to 30 years. Int J Cancer 2013; 133: 1452–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin SY, Lin WM, Lin CL, et al. The relationship between secondary hyperparathyroidism and thyroid cancer in end stage renal disease: a population based cohort study. Eur J Intern Med 2014; 25: 276–280 [DOI] [PubMed] [Google Scholar]
- 29.Zaidan M, Stucker F, Stengel B, et al. Increased risk of solid renal tumors in lithium-treated patients. Kidney Int 2014; 86: 184–190 [DOI] [PubMed] [Google Scholar]
- 30.Iff S, Craig JC, Turner R, et al. Reduced estimated GFR and cancer mortality. Am J Kidney Dis 2014; 63: 23–30 [DOI] [PubMed] [Google Scholar]
- 31.Nakamura Y, Tsuchiya K, Nitta K, et al. [Prevalence of anemia and chronic kidney disease in cancer patients: clinical significance for 1-year mortality]. Nihon Jinzo Gakkai Shi 2011; 53: 38–45 [PubMed] [Google Scholar]
- 32.Na SY, Sung JY, Chang JH, et al. Chronic kidney disease in cancer patients: an independent predictor of cancer-specific mortality. Am J Nephrol 2011; 33: 121–130 [DOI] [PubMed] [Google Scholar]
- 33.Iseki K, Osawa A, Fukiyama K. Evidence for increased cancer deaths in chronic dialysis patients. Am J Kidney Dis 1993; 22: 308–313 [DOI] [PubMed] [Google Scholar]
- 34.Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106: 1777–1782 [DOI] [PubMed] [Google Scholar]
- 35.McCarthy ET, Sharma R, Sharma M, et al. TNF-alpha increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J Am Soc Nephrol 1998; 9: 433–438 [DOI] [PubMed] [Google Scholar]
- 36.Mahmud N, O'Connell MA, Stinson J, et al. Tumour necrosis factor-α and microalbuminuria in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 1995; 7: 215–219 [PubMed] [Google Scholar]
- 37.Vaglio A, Buzio L, Cravedi P, et al. Prognostic significance of albuminuria in patients with renal cell cancer. J Urol 2003; 170: 1135–1137 [DOI] [PubMed] [Google Scholar]
- 38.Gekle M, Knaus P, Nielsen R, et al. Transforming growth factor-β1 reduces megalin- and cubilin-mediated endocytosis of albumin in proximal-tubule-derived opossum kidney cells. J Physiol 2003; 552: 471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heffelfinger SC. The renin angiotensin system in the regulation of angiogenesis. Curr Pharm Des 2007; 13: 1215–1229 [DOI] [PubMed] [Google Scholar]
- 40.Uemura H, Ishiguro H, Kubota Y. Pharmacology and new perspectives of angiotensin II receptor blocker in prostate cancer treatment. Int J Urol 2008; 15: 19–26 [DOI] [PubMed] [Google Scholar]
- 41.Stewart JH, Buccianti G, Agodoa L, et al. Cancers of the kidney and urinary tract in patients on dialysis for end-stage renal disease: analysis of data from the United States, Europe, and Australia and New Zealand. J Am Soc Nephrol 2003; 14: 197–207 [DOI] [PubMed] [Google Scholar]
- 42.Curtis J. Cancer and patients with end-stage renal failure. Br Med J 1982; 284: 69–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishikawa I, Kovacs G. High incidence of papillary renal cell tumours in patients on chronic haemodialysis. Histopathology 1993; 22: 135–139 [DOI] [PubMed] [Google Scholar]
- 44.Matas AJ, Simmons RL, Kjellstrand CM, et al. Increased incidence of malignancy during chronic renal failure. Lancet 1975; 1: 883–886 [DOI] [PubMed] [Google Scholar]
- 45.Maisonneuve P, Agodoa L, Gellert R, et al. Cancer in patients on dialysis for endstage renal disease: an international collaborative study. Lancet 1999; 354: 93–99 [DOI] [PubMed] [Google Scholar]
- 46.Buccianti G, Maisonneuve P, Ravasi B, et al. Cancer among patients on renal replacement therapy: a population-based survey in Lombardy, Italy. Int J Cancer 1996; 66: 591–593 [DOI] [PubMed] [Google Scholar]
- 47.Chen CY, Liao YM, Tsai WM, et al. Upper urinary tract urothelial carcinoma in eastern Taiwan: high proportion among all urothelial carcinomas and correlation with chronic kidney disease. J Formos Med Assoc 2007; 106: 992–998 [DOI] [PubMed] [Google Scholar]
- 48.Hung PH, Shen CH, Chiu YL, et al. The aggressiveness of urinary tract urothelial carcinoma increases with the severity of chronic kidney disease. BJU Int 2009; 104: 1471–1474 [DOI] [PubMed] [Google Scholar]
- 49.Bengtsson U, Johansson S, Angervall L. Malignancies of the urinary tract and their relation to analgesic abuse. Kidney Int 1978; 13: 107–113 [DOI] [PubMed] [Google Scholar]
- 50.Nortier JL, Martinez MC, Schmeiser HH, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med 2000; 342: 1686–1692 [DOI] [PubMed] [Google Scholar]
- 51.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med 2004; 116: 11S–26S [DOI] [PubMed] [Google Scholar]
- 52.Ludwig H, Van Belle S, Barrett-Lee P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 2004; 40: 2293–2306 [DOI] [PubMed] [Google Scholar]
- 53.Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst 1999; 91: 1616–1634 [DOI] [PubMed] [Google Scholar]
- 54.Ludwig H, Strasser K. Symptomatology of anemia. Semin Oncol 2001; 28: 7–14 [DOI] [PubMed] [Google Scholar]
- 55.Caro JJ, Salas M, Ward A, et al. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer 2001; 91: 2214–2221 [PubMed] [Google Scholar]
- 56.Dmoszynska A, Kloczko J, Rokicka M, et al. A dose exploration, phase I/II study of administration of continuous erythropoietin receptor activator once every 3 weeks in anemic patients with multiple myeloma receiving chemotherapy. Haematologica 2007; 92: 493–501 [DOI] [PubMed] [Google Scholar]
- 57.Osterborg A, Steegmann JL, Hellmann A, et al. Phase II study of three dose levels of continuous erythropoietin receptor activator (C.E.R.A.) in anaemic patients with aggressive non-Hodgkin's lymphoma receiving combination chemotherapy. Br J Haematol 2007; 136: 736–744 [DOI] [PubMed] [Google Scholar]
- 58.Krzakowski M. Epoetin delta: efficacy in the treatment of anaemia in cancer patients receiving chemotherapy. Clin Oncol (R Coll Radiol) 2008; 20: 705–713 [DOI] [PubMed] [Google Scholar]
- 59.Jelkmann W, Bohlius J, Hallek M, et al. The erythropoietin receptor in normal and cancer tissues. Crit Rev Oncol Hematol 2008; 67: 39–61 [DOI] [PubMed] [Google Scholar]
- 60.Quirt I, Micucci S, Moran LA, et al. Erythropoietin in the management of patients with nonhematologic cancer receiving chemotherapy. Systemic Treatment Program Committee. Cancer Prev Control 1997; 1: 241–248 [PubMed] [Google Scholar]
- 61.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 2008; 299: 914–924 [DOI] [PubMed] [Google Scholar]
- 62.Glaspy J, Crawford J, Vansteenkiste J, et al. Erythropoiesis-stimulating agents in oncology: a study-level metaanalysis of survival and other safety outcomes. Br J Cancer 2010; 102: 301–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lambin P, Ramaekers BL, van Mastrigt GA, et al. Erythropoietin as an adjuvant treatment with (chemo) radiation therapy for head and neck cancer. Cochrane Database Syst Rev 2009; 3: CD006158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohashi Y, Uemura Y, Fujisaka Y, et al. Meta-analysis of epoetin beta and darbepoetin alfa treatment for chemotherapy-induced anemia and mortality: individual patient data from Japanese randomized, placebo-controlled trials. Cancer Sci 2013; 104: 481–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bohlius J, Schmidlin K, Brillant C, et al. Recombinant human erythropoiesis- stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet 2009; 373: 1532–1542 [DOI] [PubMed] [Google Scholar]
- 66.Tonelli M, Hemmelgarn B, Reiman T, et al. Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis. CMAJ 2009; 180: E62–E71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal haemoglobin levels with epoetin alfa in mainly non anemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol 2005; 23: 5865–5868 [DOI] [PubMed] [Google Scholar]
- 68.Wright JR, Ung YC, Julian JA, et al. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol 2007; 25: 1027–1032 [DOI] [PubMed] [Google Scholar]
- 69.Henke M, Laszig R, Rübe C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet 2003; 362: 1255–1260 [DOI] [PubMed] [Google Scholar]
- 70.Hedenus M, Adriansson M, San Miguel J, et al. Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol 2003; 122: 394–403 [DOI] [PubMed] [Google Scholar]
- 71.Overgaard J, Sr, Hoff CM, Hansen HS, et al. Danish Head and Neck Cancer Group (DAHANCA): Randomized study of darbepoetin alfa as modifier of radiotherapy in patients with primary squamous cell carcinoma of the head and neck (HNSCC): final outcome of the DAHANCA 10 trial (abstract 6007) 2009 ASCO Annual Meeting. http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview= abst_detail_view&conf ID=65&abstract ID=35348 [Google Scholar]
- 72.Thomas G, Ali S, Hoebers FJ, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dl with erythropoietin vs above 10.0 g/dl without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol 2008; 108: 317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith RE, Jr, Aapro MS, Ludwig H, et al. Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol 2008; 26: 1040–1050 [DOI] [PubMed] [Google Scholar]
- 74.Untch M, Fasching PA, Konecny GE, et al. PREPARE trial: a randomized phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel and CMF versus a standard-dosed epirubicin/cyclophosphamide followed by paclitaxel ± darbepoetin alfa in primary breast cancer—results at the time of surgery. Ann Oncol 2011; 22: 1988–1998 [DOI] [PubMed] [Google Scholar]
- 75.Machtay M, Pajak TF, Suntharalingam M, et al. Radiotherapy with or without erythropoietin for anemic patients with head and neck cancer: a randomized trial of the Radiation Therapy Oncology Group (RTOG 99-03). Int J Radiat Oncol Biol Phys 2007; 69: 1008–1017 [DOI] [PubMed] [Google Scholar]
- 76.Hoskin PJ, Robinson M, Slevin N, et al. Effect of epoetin alfa on survival and cancer treatment-related anemia and fatigue in patients receiving radical radiotherapy with curative intent for head and neck cancer. J Clin Oncol 2009; 27: 5751–5756 [DOI] [PubMed] [Google Scholar]
- 77.Ross SD, Allen IE, Henry DH, et al. Clinical benefits and risks associated with epoetin and darbepoetin in patients with chemotherapy-induced anemia: a systematic review of the literature. Clin Ther 2006; 28: 801–831 [DOI] [PubMed] [Google Scholar]
- 78.Seidenfeld J, Piper M, Bohlius J, et al. Comparative Effectiveness of Epoetin and Darbepoetin for Managing Anemia in Patients Undergoing Cancer Treatment. Rockville: Agency for Healthcare Research and Quality, 2006, www.effectivehealthcare.ahrq.gov/reports/final.cfm [PubMed] [Google Scholar]
- 79.Hedenus M, Vansteenkiste J, Kotasek D, et al. Darbepoetin alfa for the treatment of chemotherapy-induced anemia: disease progression and survival analysis from four randomized, double blind, placebo-controlled trials. J Clin Oncol 2005; 23: 6941–6948 [DOI] [PubMed] [Google Scholar]
- 80.Boogaerts M, Coiffier B, Kainz C. Epoetin Beta QOL Working Group. Impact of epoetin beta on quality of life in patients with malignant disease. Br J Cancer 2003; 88: 988–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leyland-Jones B. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol 2003; 4: 459–460 [DOI] [PubMed] [Google Scholar]
- 82.Anemia treatment for advanced non-small cell lung cancer (NSCLC) patients receiving chemotherapy. National Institutes of Health, 2012. http://clinicaltrials.gov/show/NCT00858364 (28 November 2012, date last accessed) [Google Scholar]
- 83.A study of epoetin alfa plus standard supportive care versus standard supportive care only in anemic patients with metastatic breast cancer receiving standard chemotherapy. National Institutes of Health, 2012. http://clinicaltrials.gov/ct2/show/NCT00338286 (28 November 2012, date last accessed) [Google Scholar]
- 84.Rizzo JD, Brouwers M, Hurley P, et al. American Society of ClinicalOncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients withcancer. J Clin Oncol 2010; 28: 4996–5010 [DOI] [PubMed] [Google Scholar]
- 85.European Medicines Agency (EMA). Press release: EMEA recom-mends a new warning for epoetins for their use in cancer patients, 2008. http://www.ema.europa.eu/docs/en GB/document library/Press release/2009/11/WC500015069.pdf (28 November 2012, date last accessed) [Google Scholar]
- 86.European Medicines Agency (EMA). Public statement: epoetins and the risk of tumour growth progression and thromboembolic events in cancer patients and cardiovascular risks in patients with chronic kidney disease, 2007. http://www.ema.europa.eu/docs/enGB/documentlibrary/Publicstatement/2009/11/WC500015604.pdf (28 November 2012, date last accessed) [Google Scholar]
- 87.Aapro MS, Link H. September 2007 update on EORTC guidelines and anemia management with erythropoiesis-stimulating agents. Oncologist 2008; 13: 33–36 [DOI] [PubMed] [Google Scholar]
- 88.Rodgers GM, Blinder M, Cella D, et al. Cancer- and chemotherapy-induced anemia. Clinical practice guidelines in oncology. v.1.2013, 2012. http://www.nccn.org/professionals/physician gls/pdf/anemia.pdf (28 November 2012, date last accessed) [Google Scholar]
- 89.Locatelli F, Aljama P, Canaud B, et al. Anaemia Working Group of European Renal Best Practice (ERBP). Target haemoglobin to aim for with erythropoiesis-stimulating agents: a position statement by ERBP following publication of the Trial to reduce cardiovascular events with Aranesp therapy (TREAT) study. Nephrol Dial Transplant 2010; 25: 2846–2850 [DOI] [PubMed] [Google Scholar]
- 90.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]
- 91.Seliger SL, Zhang AD, Weir MR, et al. Erythropoiesis-stimulating agents increase the risk of acute stroke in patients with chronic kidney disease. Kidney Int 2011; 80: 288–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Imai E, Yamamoto R, Suzuki H, et al. Incidence of symptomatic stroke and cancer in chronic kidney disease patients treated with epoetins. Clin Exp Nephrol 2010; 14: 445–452 [DOI] [PubMed] [Google Scholar]
- 93.Bohlius J, Langensiepen S, Schwarzer G, et al. Recombinant human erythropoietin and overall survival in cancer patients: results of a comprehensive meta-analysis. J Natl Cancer Inst 2005; 97: 489–498 [DOI] [PubMed] [Google Scholar]
- 94.Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 2006; 98: 708–714 [DOI] [PubMed] [Google Scholar]
- 95.Ludwig H, Crawford J, Osterborg A, et al. Pooled analysis of individual patient-level data from all randomized, double-blind, placebo-controlled trials of darbepoetin alfa in the treatment of patients with chemotherapy-induced anemia. J Clin Oncol 2009; 27: 2838–2847 [DOI] [PubMed] [Google Scholar]
- 96.Aapro M, Scherhag A, Burger HU. Effect of treatment with epoetin-beta on survival, tumour progression and thromboembolic events in patients with cancer: an updated meta-analysis of 12 randomised controlled studies including 2,301 patients. Br J Cancer 2008; 99: 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lackner E, Mensik C, Eichler HG, et al. Effects of erythropoietin on platelet reactivity and thrombopoiesis in humans. Blood 2000; 95: 2983–2989 [PubMed] [Google Scholar]
- 98.Fraser JK, Lin FK, Berridge MV. Expression of high affinity receptors for erythropoietin on human bone marrow cells and on the human erythroleukemic cell line, HEL. Exp Hematol 1998; 16: 836–842 [PubMed] [Google Scholar]
- 99.Grossi A, Vannucchi AM, Rafanelli D, et al. Recombinant human erythropoietin has little influence on megakaryocytopoiesis in mice. Br J Haematol 1989; 71: 463–468 [DOI] [PubMed] [Google Scholar]
- 100.Yonemura Y, Kawakita M, Fujimoto K, et al. Effects of short-term administration of recombinant human erythropoietin on rat megakaryopoiesis. Int J Cell Cloning 1992; 10: 18–27 [DOI] [PubMed] [Google Scholar]
- 101.Ait-Oudhia S, Scherrmann JM, Krzyzanski W. Simultaneous pharmacokinetics/pharmacodynamics modeling of recombinant human erythropoietin upon multiple intravenous dosing in rats. J Pharmacol Exp Ther 2010; 334: 897–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buss DH, Cashell AW, O'Connor ML, et al. Occurrence, etiology, and clinical significance of extreme thrombocytosis: a study of 280 cases. Am J Med 1994; 96: 247–253 [DOI] [PubMed] [Google Scholar]
- 103.Basser RL, Rasko JE, Clarke K, et al. Randomized, blinded, placebo-controlled phase I trial of pegylated recombinant human megakaryocyte growth and development factor wit filgrastim after dose-intensive chemotherapy in patients with advanced cancer. Blood 1997; 89: 3118–3128 [PubMed] [Google Scholar]
- 104.Seidel HM, Lamb P, Rosen J. Pharmaceutical intervention in the JAK/STAT signaling pathway. Oncogene 2000; 19: 2645–2656 [DOI] [PubMed] [Google Scholar]
- 105.Yeung CK, Shen DD, Thummel KE, et al. Effects of chronic kidney disease and uremia on hepatic drug metabolism and transport. Kidney Int 2014; 85: 522–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perazella MA, Parikh C. Pharmacology. Am J Kidney Dis 2005; 46: 1129–1139 [DOI] [PubMed] [Google Scholar]
- 107.Perazella M. Onco-nephrology: renal toxicities of chemotherapeutic agents. Clin J Am Soc Nephrol 2012; 7: 1713–1721 [DOI] [PubMed] [Google Scholar]
- 108.Janus N, Thariat J, Boulanger H, et al. Proposal for dosage adjustment and timing of chemotherapy in hemodialyzed patients. Ann Oncol 2010; 21: 1395–1403 [DOI] [PubMed] [Google Scholar]
- 109.Dooley MJ, Poole SG, Rischin D. Dosing of cytotoxic chemotherapy: impact of renal function estimates on dose. Ann Oncol 2013; 24: 2746–2752 [DOI] [PubMed] [Google Scholar]
- 110.Kintzel PE, Dorr RT. Anticancer drug renal toxicity and elimination: dosing guidelines for altered renal function. Cancer Treat Rev 1995; 21: 33–64 [DOI] [PubMed] [Google Scholar]
- 111.Perazella MA, Markowitz GS. Drug-induced acute interstitial nephritis. Nat Rev Nephrol 2010; 6: 461–470 [DOI] [PubMed] [Google Scholar]
- 112.Salahudeen AK, Bonventre JV. Onconephrology: the latest frontier in the war against kidney disease. J Am Soc Nephrol 2013; 24: 26–30 [DOI] [PubMed] [Google Scholar]
- 113.Salahudeen AK, Doshi SM, Pawar T, et al. Incidence, rate, clinical correlates, and outcomes of AKI in patients admitted to a comprehensive cancer center. Clin J Am Soc Nephrol 2013; 8: 347–354 [DOI] [PMC free article] [PubMed] [Google Scholar]