Abstract
Residual proteinuria, the amount of proteinuria that remains during optimally dosed renin-angiotensin-aldosterone system (RAAS) blockade, is an independent risk factor for progressive renal function loss and cardiovascular complications in chronic kidney disease (CKD) patients. Dual RAAS blockade may reduce residual proteinuria but without translating into improved cardiorenal outcomes at least in diabetic nephropathy; rather, dual RAAS blockade may increase the risk of adverse events. These findings have challenged the concept of residual proteinuria as an absolute treatment target. Therefore, new strategies must be explored to address whether by further reduction of residual proteinuria using interventions not primarily targeting the RAAS benefit in terms of cardiorenal risk reduction would accrue. Both clinical and experimental intervention studies have demonstrated that vitamin D can reduce residual proteinuria through both RAAS-dependent and RAAS-independent pathways. Future research should prospectively explore vitamin D treatment as an adjunct to RAAS blockade in an interventional trial exploring clinically relevant cardiorenal end points.
Keywords: cardiovascular disease, chronic kidney disease, proteinuria, vitamin D
PROTEINURIA: A TARGET FOR THERAPY IN CHRONIC KIDNEY DISEASE
The presence of proteinuria is a risk factor for adverse cardiorenal outcome across the spectrum of chronic kidney disease (CKD) stages. In the general population, proteinuria is associated with renal function loss independently of baseline renal function [1], while in patients with established CKD, the presence of proteinuria is a risk factor for the development of end-stage renal disease (ESRD) [2]. Recent large-scale analyses from the CKD prognosis consortium revealed that the relative risk for ESRD by estimated glomerular filtration rate (eGFR) and albuminuria was independent of the presence of diabetes [3] or hypertension [4], highlighting the importance of proteinuria per se as a predictor of clinical outcomes. Proteinuria is also strongly associated with cardiovascular mortality, independent of other cardiovascular risk factors [5].
Successful proteinuria reduction has been shown to lower the risk of reaching both renal and cardiovascular end points in concert with, but also independent of, blood pressure reduction [6, 7], while further blood pressure reduction over and above adequate proteinuria reduction in non-diabetic CKD may have no additional benefit in terms of ESRD prevention [8]. Proteinuria is thus proposed as an independent treatment target that should be resolutely addressed to reduce the risk of progressive renal function loss and cardiovascular complications.
RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM BLOCKADE FOR PROTEINURIA: ASSETS AND BOUNDARIES
Renin-angiotensin-aldosterone system (RAAS) blockade is the mainstay of treatment for proteinuric CKD, both in diabetic and in non-diabetic patients. RAAS blockade reduces, but rarely abrogates, proteinuria, resulting in ‘residual proteinuria’ (or ‘treatment-resistant proteinuria’). Bolstering the importance of addressing residual proteinuria, the extent of residual proteinuria is associated with the rate of renal function loss across populations [9] while it also determines the remaining cardiovascular risk [6]. Under optimal conditions, each RAAS-blocking agent is able to reduce proteinuria by around 40% (ratio of means angiotensin receptor blocker [ARB] versus placebo 0.66, ARB and ACEi equally effective) [10]. In adherence to ‘the rule of halves’—one drug halves the amount of proteinuria, whereas the additive antiproteinuric effect of the second drug is only a modest 25% reduction, i.e. ‘halved’ effect—addition of another RAAS-blocking agent resulted in only 25% further reduction of proteinuria (ratio of means 0.76–0.78 of combination therapy versus ARB or ACEi, respectively). Exposure to high doses of two agents targeting the same pathway may provide further reductions in blood pressure and proteinuria; however, this no longer translates into further incremental outcome benefits but rather clear safety signals emerging in the form of hyperkalemia and acute kidney injury (Table 1). Combination of a RAAS-blocking compound (ACEi or ARB) with the direct renin inhibitor aliskiren even increased the cardiorenal risk, along with a higher incidence of hyperkalemia and hypotension [12].
Table 1.
Three major randomized controlled trials aimed at reducing residual proteinuria and cardiovascular outcome by combined RAAS blockade
| ONTARGET [11] | ALTITUDE [12] | VA NEPHRON-D [13] | |
|---|---|---|---|
| Population | 25 620 patients ≥55 years with diabetes and end organ damage or with atherosclerotic vascular disease | 8561 patients ≥35 years with diabetes and microalbuminuria, macroalbuminuria or cardiovascular disease | 1448 patients with type 2 diabetes and macroalbuminuria |
| Renal function | Mean eGFR 74 mL/min/1.73 m2 eGFR < 60 mL/min/1.73 m2: n = 8034 eGFFR < 30 mL/min/1.73 m2: n = 263 |
Mean eGFR 57 mL/min/1.73 m2 eGFR < 60 mL/min/1.73 m2: n = 5778 eGFR < 30 mL/min/1.73 m2: n = 210 |
Mean eGFR 54 mL/min/1.73 m2 eGFR < 60 mL/min/1.73 m2: n = 894 eGFR < 30 mL/min/1.73 m2: none |
| Intervention |
|
ACEi/ARB therapy combined with:
|
Losartan 100 mg/day combined with:
|
| Median follow-up | 56 months | 32.9 months (study halted prematurely) | 26.4 months (study halted prematurely) |
| Proteinuria outcome | Combination therapy reduced the increase in albuminuria compared with ramipril monotherapy (21 versus 31%, P = 0.0009) | Combination therapy decreased albumin-to-creatinine ratio more than monotherapy [between group difference: 14% (95% CI 11–17%)] | Combination therapy decreased albumin-to-creatinine ratio more (786–517 mg/g) than losartan monotherapy (829–701 mg/g), P < 0.001 |
| Primary outcome | Combination therapy had an increased occurrence of the composite renal end point [dialysis, doubling serum creatinine, death; HR 1.09 (1.01–1.18), P = 0.037] | Combination therapy showed no beneficial effect on the primary composite end point (cardiovascular events; renal events, i.e. ESRD, RRT needed but not given, death by renal cause, doubling of creatinine) | There was no benefit of combination therapy on primary end point (eGFR decline of ≥30 mL/min/1.73 m2 or 50% reduction, ESRD or death), secondary end point (eGFR decline or ESRD) or tertiary end points (cardiovascular events, eGFR slope) |
| Safety concern | Increased occurrence of the primary composite renal end point with combination therapy | Combination therapy was associated with higher incidence of hyperkalemia (11.6 versus 7.2%) and hypotension (12.1 versus 8.3%), both P < 0.001 | Combination therapy increased the risk of hyperkalemia (6.3 versus 2.6 events per 100 person-years) and acute kidney injury (12.2 versus 6.7 events per 100 person-years), both P < 0.001) |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate, ESRD, end-stage renal disease; HR, hazard ratio.
The dissociation of proteinuria reduction from improved cardiorenal outcomes during and beyond optimally dosed RAAS blockade raises the question of whether we have reached the maximum beneficial effect of RAAS blockade or whether we are reaching the toxicity threshold of the pharmacological instruments we choose to deploy. Agents with a different pharmacological and side effect profile, i.e. with limited or no effect on blood pressure and serum potassium concentrations, are urgently needed to address whether the relationship between proteinuria and cardiorenal outcomes may persist when using a different interventional treatment modality to further reduce proteinuria. One important type of drug currently under investigation to this extent is the endothelin antagonist. Atrasentan, a selective endothelin A receptor antagonist, was recently demonstrated to reduce albuminuria and improve blood pressure and lipid spectrum with manageable fluid overload-related adverse events in patients with type 2 diabetic nephropathy receiving RAAS inhibitors [14]. The effect of atrasentan on hard end points in this population is investigated by the currently ongoing SONAR trial in over 4000 patients (NCT01858532).
VITAMIN D IN CKD
CKD is characterized by a progressive inability to generate active vitamin D (1,25(OH)2-vitamin D, calcitriol) from its precursor 25(OH)-vitamin D (calcidiol). CKD patients are also more commonly deficient in 25(OH)-vitamin D in comparison with subjects with normal renal function [15]. The urinary loss of vitamin D bound to albumin and its carrier protein vitamin D-binding protein (VDBP) might predispose patients to vitamin D deficiency. In line with this concept, proteinuria reduction by ACE inhibition also reduced urinary VDBP loss [16]. The impact of urinary VDBP loss on vitamin D status and the potential of antiproteinuric therapy to improve vitamin D status in patients with non-nephrotic range proteinuria, however, remain to be established. Genetic variation in VDBP may also influence 25(OH)-vitamin D concentrations, although this may not translate into different concentrations of bioavailable 25(OH)-vitamin D [17]. Moreover, in CKD the vitamin D-activating enzyme 1-alpha hydroxylase (Cyp27b1) is suppressed by increased fibroblast growth factor 23 (FGF23) concentrations [18]. The progressive deregulation of vitamin D metabolism with deteriorating renal function is mirrored by the fact that vitamin D deficiency itself may contribute to progressive renal function loss [19]. If untreated, this vicious circle may well be one of the forces driving progressive kidney disease, bone disease and possibly also cardiovascular disease in CKD patients.
VITAMIN D, RAAS BLOCKADE AND CKD PROGRESSION
Over the past decade, several animal studies demonstrated the capacity of supplementation with either endogenous vitamin D (cholecalciferol, calcidiol or calcitriol) or its analogues (e.g. paricalcitol) to reduce proteinuria along with renal inflammation, glomerulosclerosis and interstitial fibrosis in models of fibrotic and inflammatory CKD, as reviewed elsewhere [20]. In humans, observational studies have documented associations between the use of vitamin D supplements (calcitriol and calcitriol or alfacalcidol, respectively) and survival in CKD [21, 22]. Together, these findings set the stage for a number of prospective randomized controlled trials (RCTs) addressing the effect of vitamin D analogues on proteinuria as a surrogate end point. We recently performed a systematic review of all RCTs with either endogenous-active vitamin D (calcitriol) or its synthetic analogue paricalcitol as an antiproteinuric intervention. During follow-up, active vitamin D analogues reduced proteinuria on average by 16%, whereas proteinuria increased by 6% in patients receiving control treatment (P < 0.001) [23].
Of interest, these results were obtained in the majority of cases (84% overall) against the background of pre-existing chronic RAAS blockade, underlining the capacity of vitamin D analogues to reduce residual proteinuria. The additive effect of RAAS blockade and vitamin D on proteinuria could point towards interactions between the RAAS and vitamin D [24] as well as towards RAAS-independent renoprotective effects of vitamin D. Of note, a recent study found that ramipril reduces FGF23 levels in Stage 1–2 CKD patients with diabetic nephropathy, without clear effects on parathyroid hormone, 25(OH)-vitamin D or calcium levels [25]. In a very recent study, it was found that proteinuria in itself affects renal phosphate handling, increasing serum phosphate and FGF23 levels [26]. This may very well explain the observed relationship between proteinuria (reduction) and FGF23. In turn, reduced FGF23 levels as a consequence of ACE inhibitor therapy may very well increase the bioavailability of active 1,25(OH)2-vitamin D, contributing to proteinuria control. Thus, the capacity of ACE inhibitors to lower proteinuria may in fact be in part through an effect of FGF23 and vitamin D. Departing from a long known association between calcitriol and renin levels, elegant experimental studies have elucidated interference of the vitamin D receptor (VDR) with a transcription factor binding site in the (pro)renin promoter region, inhibiting renin expression [27]. Consequently, VDR−/− mice display strongly increased renal and circulating renin concentrations as well as increased angiotensin II generation [28]. In man, vitamin D deficiency is accompanied by increased circulating angiotensin II concentrations and blunted renal plasma flow responses to infused angiotensin II, indicating both systemic and intrarenal RAAS activation [29]. The capacity of vitamin D analogues to reduce the compensatory induction of renin during RAAS blockade may at least in part explain their renoprotective effect in addition to RAAS blockade.
Despite this relatively well-defined negative regulatory effect on renin production, VDR agonists should not be considered equal to conventional RAAS blockers (Figure 1). From a clinical perspective, vitamin D analogues such as paricalcitol may display limited to no effects on blood pressure [30], nor have hyperkalemia as a side effect, making them more suitable for combination with RAAS blockers further to downtitrate proteinuria in more advanced CKD, where hyperkalemia can be more problematic (Table 1).
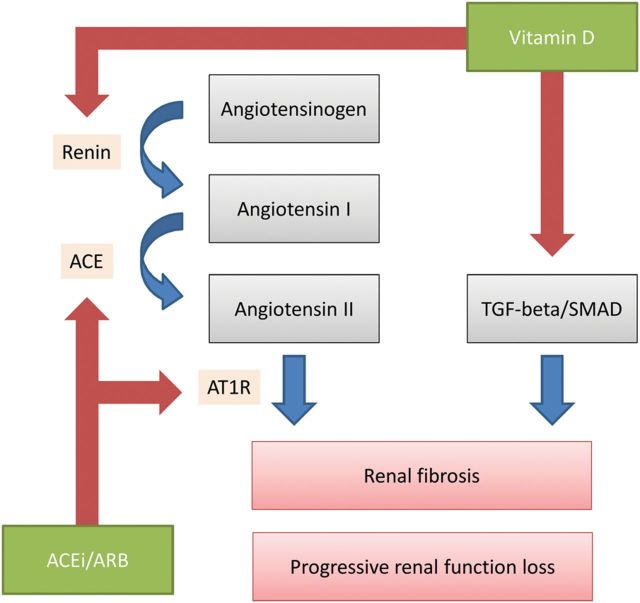
FIGURE 1:
Schematic representation of complementary renoprotective actions of ACEi/ARB and vitamin D in chronic kidney disease. Vitamin D may provide renoprotection through RAAS-mediated effects, i.e. by suppression of renin gene expression. This effect is through VDR signaling. The second renoprotective pathway of vitamin D is through reduction of TGF-beta/SMAD signaling. Negative regulation indicated by blue arrows, positive regulation by red arrows. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AT1R, angiotensin II type 1 receptor; TGF-beta, transforming growth factor-beta. See text for references.
Vitamin D supplementation yields renoprotective effects beyond the RAAS. Recently published elegant studies revealed that novel vitamin D analogues may also exert antifibrotic effects by influencing the transforming growth factor (TGF)-beta/SMAD pathway [31]. Using synthetic ligands based on the structure of the VDR–ligand complex that were specific to reduce TGF-beta/SMAD signaling, Ito et al. reduced renal fibrosis in an animal model, while hypercalcemia was avoided. The use of 1,25(OH)2-vitamin D-derived synthetic ligands may allow more potent blockade of pro-fibrotic pathways in CKD, with a more limited tendency to hypercalcemia, which commonly limits vitamin D uptitration in clinical practice. A question that remains to be addressed is whether chemical compounds specifically targeting TGF-beta/SMAD but without activating classical VDR-mediated pathways will take away not only hypercalcemia but also beneficial effects such as those resulting from renin suppression. Vitamin D supplementation also exerts beneficial effects on endothelial function. Vitamin D directly regulates endothelial nitric oxide synthase (eNOS) in mice, and mice lacking VDR demonstrated endothelial dysfunction and arterial stiffness [32]. In line, VDR activation by paricalcitol improves endothelium-dependent vasodilatation in patients with Stage 3–4 CKD [33].
Interestingly, also the precursor vitamin D compound cholecalciferol may lower proteinuria. In a recently published clinical trial, patients with Stage 3–4 CKD and albuminuria were randomized to receiving 666 IU/day of cholecalciferol or no treatment. After 6 months of follow-up, the albumin-to-creatinin ratio (ACR) had decreased by 53.2% (95% CI 66.0–27.0%), whereas the ACR increased by 7.1% (−25.3 to +53.3%) in the control group (P = 0.005 between groups) [34].
POTENTIAL SIDE EFFECTS OF VITAMIN D TREATMENT
A finding that should be interpreted as a warning sign is the increased calcium phosphate product observed in patients treated with vitamin D when given either as cholecalciferol [34] or as active vitamin D (analogues), as documented in some but not all trials [23]. On one hand, an increased calcium load elicited by vitamin D may predispose to the development of adynamic bone disease, accompanied by a higher fracture risk but possibly also vascular calcification [35]. On the other hand, a higher level of serum phosphate may not only promote vascular calcification but also (partially) blunt the renoprotective effect of RAAS blockade [36]. The long-term effects of a higher serum phosphate level induced by vitamin D treatment may warrant close monitoring. Nevertheless, it is reassuring that even in the high-risk population of hemodialysis patients, serum calcium is only associated with increased mortality when its levels are higher than 2.75 mmol/L [37]. Treatment decisions should be guided by individual patient characteristics, e.g. favoring paricalcitol over calcitriol in the case of a high–normal serum calcium in the CKD-Mineral Bone Disease (MBD) patient at increased risk for vascular calcification [38]. Thus, the benefit–risk ratio of vitamin D added to RAAS blockade, compared with dual RAAS blockade, is driven by different efficacy and safety profiles; whether this translates into clinically relevant benefits overwhelming its side effects remains to be established.
VITAMIN D AND CARDIOVASCULAR OUTCOME IN CKD
The cardiovascular effects of vitamin D deficiency and its supplementation in CKD are not well understood. Epidemiological studies have suggested associations between vitamin D deficiency and cardiovascular morbidity and mortality in CKD patients [39], and the use of vitamin D supplements (predominantly the native form cholecalciferol) has been associated with a survival advantage [40]. However, prospective intervention studies designed to establish the cardioprotective effects of vitamin D supplementation have yielded mixed results. In the Women's Health Initiative study conducted in postmenopausal women, combined calcium and vitamin D3 supplementation failed to materially reduce the risk for myocardial infarction or death by coronary heart disease [41]. In the PRIMO trial, paricalcitol treatment had no effect on left ventricular mass index [30], but did lower left atrial volume index and brain natriuretic peptide in CKD patients [42]. Therefore, at this moment, it is premature to conclude that vitamin D supplementation can influence the strongly increased cardiovascular risk to which CKD patients are exposed. On the other hand, since a considerable number of studies have now documented reduction of (residual) proteinuria—a well-established cardiorenal marker in CKD—it is very worthwhile to further clarify the position of vitamin D treatment as an adjunct to RAAS blockade. Of note, a large randomized trial addressing, among others, whether cholecalciferol can provide primary prevention against cardiovascular disease in 20 000 men in the USA (VITAL) is currently ongoing (ClinicalTrials.gov identifier: NCT01169259).
A secondary analysis of the other VITAL study, which was performed in patients with diabetic nephropathy [43], suggested that the capacity of paricalcitol to reduce albuminuria depends on the patient's dietary sodium status. This is relevant given the fact that dietary sodium intake, through impacting volume status, is another modifiable determinant of the antiproteinuric response to RAAS blockade.
RAAS BLOCKADE AND DIETARY SODIUM
High dietary sodium intake is associated with attenuated antiproteinuric and long-term renoprotective effects of RAAS blockade in both diabetic and non-diabetic proteinuric CKD. Furthermore, numerous trials have now demonstrated that sodium restriction further reduces residual proteinuria during RAAS blockade, with a possible role for volume markers to identify patients who might benefit most from sodium restriction, as reviewed in Ref. [44]. In hypertensive patients, every 1 g of sodium intake per day was associated with a 14% increased risk of coronary heart disease [45]. These reports underscore the necessity to reduce sodium intake below 5 g of sodium chloride per day in CKD patients, as indicated in current guidelines. A recent double-blinded, placebo-controlled trial demonstrated that proteinuria can be halved in just 2 weeks by reducing sodium excretion from 168 to 75 mmol/day in hypertensive Stage 3–4 CKD [46]. Notwithstanding recent data reporting absence of an association of high sodium excretion and renal failure per se [47], high sodium intake remained associated with cardiovascular morbidity and mortality in a worldwide study [48]. Taking into account that a reduction of dietary sodium by 33–44 mmol/day lowered cardiovascular risk by 25% in 744 prehypertensive patients after 10–15 years follow-up [49], efforts to reduce and optimize sodium intake in patients at risk according to current guidelines should be increased. The persistent effect across different populations—particularly in CKD—favors modulation of sodium intake as a strategy to intensify RAAS blockade efficacy. Alternative strategies to increase the cardiorenal protective effect of RAAS blockade include combination therapy with diuretics, targeting obesity, and moderate protein restriction, as reviewed elsewhere [50].
As both vitamin D and dietary sodium restriction have a strong potential to lower residual proteinuria during RAAS blockade, the combination of these strategies seems prudent. A multicenter, randomized controlled crossover trial addressing the combined impact of dietary sodium restriction and the vitamin D analogue paricalcitol on residual proteinuria in 50 non-diabetic patients is currently ongoing; results are expected in 2015 (ViRTUE trial, Dutch trial register NTR2898). The interaction between sodium intake and paricalcitol on albuminuria is currently under investigation in 112 diabetic patients (PROCEED trial, ClinicalTrials.gov identifier: NCT01393808).
SUMMARY AND FUTURE DIRECTIONS
Although proteinuria has been considered the key target for renoprotective therapy, recent studies using double RAAS blockade have dissociated proteinuria reduction per se from progressive renal function loss. Since the boundaries of RAAS blockade-based treatment are now better defined, this paves the way to study adjunctive antiproteinuric therapies with different pharmacological and side effect profiles. Given the emerging independent observations that vitamin D analogues are able to reduce proteinuria, even when added to RAAS blockade, future studies are warranted to investigate whether further reduction of proteinuria beyond the maximally tolerated dose of RAAS blockade can further improve cardiorenal prognosis of CKD patients. Disturbed calcium phosphate metabolism is probably the most prominent safety signal that should be accounted for in such studies. How cardiorenal protective and adverse effects translate into hard patient-specific outcomes remains therefore to be established in future large-scale prospective RCTs, designed to address the effects of vitamin D supplementation added to background RAAS blockade on hard cardiorenal end points.
FUNDING
This work is supported by a consortium grant from the Dutch Kidney Foundation (NIGRAM consortium, grant no CP10.11). M.H.D.B. is supported by grants from the Dutch Kidney Foundation (grant no KJPB.08.07) and the Netherlands Organization for Scientific Research (Veni grant). The funding sources had no role in the preparation, review, or approval of the manuscript.
CONFLICT OF INTEREST STATEMENT
D.J.A.G. has received speaking and consulting honoraria from Abbott, Amgen, Genzyme, Keryx Pharmaceuticals and Shire. R.T. has received a coordinating center grant from Abbott to the Massachusetts General Hospital and speaker's fees and travel support from Abbott. R.T. is also a consultant to Fresenius Medical Care. M.H.D.B. has received non-financial support from Abbott to the University Medical Center Groningen for an ongoing clinical trial, and lecture fees from Amgen. J.K.H. has none to declare.
REFERENCES
- 1.Turin TC, James M, Ravani P, et al. Proteinuria and rate of change in kidney function in a community-based population. J Am Soc Nephrol 2013; 24: 1661–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McQuarrie EP, Traynor JP, Taylor AH, et al. Association between urinary sodium, creatinine, albumin, and long-term survival in chronic kidney disease. Hypertension 2014; 64: 111–117 [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012; 380: 1662–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahmoodi BK, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet 2012; 380: 1649–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106: 1777–1782 [DOI] [PubMed] [Google Scholar]
- 6.Holtkamp FA, de Zeeuw D, de Graeff PA, et al. Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: a post hoc analysis of the combined RENAAL and IDNT trials. Eur Heart J 2011; 32: 1493–1499 [DOI] [PubMed] [Google Scholar]
- 7.Schmieder RE, Mann JF, Schumacher H, et al. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 2011; 22: 1353–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet 2005; 365: 939–946 [DOI] [PubMed] [Google Scholar]
- 9.Ruggenenti P, Perna A, Remuzzi G, et al. Retarding progression of chronic renal disease: the neglected issue of residual proteinuria. Kidney Int 2003; 63: 2254–2261 [DOI] [PubMed] [Google Scholar]
- 10.Kunz R, Friedrich C, Wolbers M, et al. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med 2008; 148: 30–48 [DOI] [PubMed] [Google Scholar]
- 11.Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372: 547–553 [DOI] [PubMed] [Google Scholar]
- 12.Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367: 2204–2213 [DOI] [PubMed] [Google Scholar]
- 13.Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369: 1892–1903 [DOI] [PubMed] [Google Scholar]
- 14.de Zeeuw D, Coll B, Andress D, et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol 2014; 25: 1083–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaClair RE, Hellman RN, Karp SL, et al. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis 2005; 45: 1026–1033 [DOI] [PubMed] [Google Scholar]
- 16.Doorenbos CR, de Cuba MM, Vogt L, et al. Antiproteinuric treatment reduces urinary loss of vitamin D-binding protein but does not affect vitamin D status in patients with chronic kidney disease. J Steroid Biochem Mol Biol 2012; 128: 56–61 [DOI] [PubMed] [Google Scholar]
- 17.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 2013; 369: 1991–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 2004; 19: 429–435 [DOI] [PubMed] [Google Scholar]
- 19.Goncalves JG, de Braganca AC, Canale D, et al. Vitamin D deficiency aggravates chronic kidney disease progression after ischemic acute kidney injury. PLoS ONE 2014; 9: e107228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirkovic K, van den Born J, Navis G, et al. Vitamin D in chronic kidney disease: new potential for intervention. Curr Drug Targets 2011; 12: 42–53 [DOI] [PubMed] [Google Scholar]
- 21.Naves-Diaz M, Alvarez-Hernandez D, Passlick-Deetjen J, et al. Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney Int 2008; 74: 1070–1078 [DOI] [PubMed] [Google Scholar]
- 22.Shoben AB, Rudser KD, de Boer IH, et al. Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol 2008; 19: 1613–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Borst MH, Hajhosseiny R, Tamez H, et al. Active vitamin D treatment for reduction of residual proteinuria: a systematic review. J Am Soc Nephrol 2013; 24: 1863–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Borst MH, Vervloet MG, ter Wee PM, et al. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol 2011; 22: 1603–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yilmaz MI, Sonmez A, Saglam M, et al. Ramipril lowers plasma FGF-23 in patients with diabetic nephropathy. Am J Nephrol 2014; 40: 208–214 [DOI] [PubMed] [Google Scholar]
- 26.de Seigneux S, Courbebaisse M, Rutkowski JM, et al. Proteinuria increases plasma phosphate by altering its tubular handling. J Am Soc Nephrol 2015; 26: 1608–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan W, Pan W, Kong J, et al. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem 2007; 282: 29821–29830 [DOI] [PubMed] [Google Scholar]
- 28.Li YC, Kong J, Wei M, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002; 110: 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forman JP, Williams JS, Fisher ND. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension 2010; 55: 1283–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 2012; 307: 674–684 [DOI] [PubMed] [Google Scholar]
- 31.Ito I, Waku T, Aoki M, et al. A nonclassical vitamin D receptor pathway suppresses renal fibrosis. J Clin Invest 2013; 123: 4579–4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrukhova O, Slavic S, Zeitz U, et al. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol Endocrinol 2014; 28: 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zoccali C, Curatola G, Panuccio V, et al. Paricalcitol and endothelial function in chronic kidney disease trial. Hypertension 2014; 64: 1005–1011 [DOI] [PubMed] [Google Scholar]
- 34.Molina P, Gorriz JL, Molina MD, et al. The effect of cholecalciferol for lowering albuminuria in chronic kidney disease: a prospective controlled study. Nephrol Dial Transplant 2014; 29: 97–109 [DOI] [PubMed] [Google Scholar]
- 35.Brandenburg VM, Floege J. Adynamic bone disease—bone and beyond. NDT Plus 2008; 1: 135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zoccali C, Ruggenenti P, Perna A, et al. Phosphate may promote CKD progression and attenuate renoprotective effect of ACE inhibition. J Am Soc Nephrol 2011; 22: 1923–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Floege J, Kim J, Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 2011; 26: 1948–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazzaferro S, Goldsmith D, Larsson TE, et al. Vitamin D metabolites and/or analogs: which D for which patient? Curr Vasc Pharmacol 2014; 12: 339–349 [DOI] [PubMed] [Google Scholar]
- 39.Drechsler C, Pilz S, Obermayer-Pietsch B, et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J 2010; 31: 2253–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 2007; 167: 1730–1737 [DOI] [PubMed] [Google Scholar]
- 41.Hsia J, Heiss G, Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007; 115: 846–854 [DOI] [PubMed] [Google Scholar]
- 42.Tamez H, Zoccali C, Packham D, et al. Vitamin D reduces left atrial volume in patients with left ventricular hypertrophy and chronic kidney disease. Am Heart J 2012; 164: 902–9.e2 [DOI] [PubMed] [Google Scholar]
- 43.de Zeeuw D, Agarwal R, Amdahl M, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 2010; 376: 1543–1551 [DOI] [PubMed] [Google Scholar]
- 44.Humalda JK, Navis G. Dietary sodium restriction: a neglected therapeutic opportunity in chronic kidney disease. Curr Opin Nephrol Hypertens 2014; 23: 533–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joosten MM, Gansevoort RT, Mukamal KJ, et al. Sodium excretion and risk of developing coronary heart disease. Circulation 2014; 129: 1121–1128 [DOI] [PubMed] [Google Scholar]
- 46.McMahon EJ, Bauer JD, Hawley CM, et al. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol 2013; 24: 2096–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan L, Tighiouart H, Levey AS, et al. Urinary sodium excretion and kidney failure in nondiabetic chronic kidney disease. Kidney Int 2014; 86: 582–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371: 612–623 [DOI] [PubMed] [Google Scholar]
- 49.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 2007; 334: 885–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laverman GD, de Zeeuw D, Navis G. Between-patient differences in the renal response to renin-angiotensin system intervention: clue to optimising renoprotective therapy? J Renin Angiotensin Aldosterone Syst 2002; 3: 205–213 [DOI] [PubMed] [Google Scholar]