Abstract
Background
We practice the timely placement of an arteriovenous fistula (AVF) in patients facing chronic hemodialysis. We have anecdotally observed after AVF creation that there appears to be a slowing of the decline in kidney function as measured by the estimated glomerular filtration rate (eGFR). There are physiologically plausible explanations as to how an AVF might alter kidney function, but this clinical observation has been attributed to improved compliance and/or other practices. The present retrospective observational analysis was performed to assess the possibility that a successfully created AVF could be associated with the slowing of the eGFR trajectory.
Methods
We identified 123 patients between 2005 and 2010 with at least two eGFR determinations for 2 years before and up to 2 years after AVF creation. Inclusion eligibility was that the fistula was maturing by the nephrologists' initial post-creation examination. Termination events were death, starting dialysis or transplantation. Each subject served as their own control for the pre- and post-AVF-creation eGFR measurements.
Results
Subjects' median age was 68 years and 56% were diabetic. The rate of change of the eGFR for the 2 years prior to AVF creation was −5.9 mL/min/year (95% CI: −5.3, −6.5) and after AVF creation −0.5 mL/min/year (95% CI: −1.1, 0.1) (interaction (P < 0.001).
Conclusions
A functioning AVF may be associated with a slowing of the eGFR decline. Agreeing to timely AVF creation selects patients in an otherwise typical population and other confounders have not yet been eliminated. To do so a thorough prospective observational study is indicated.
Keywords: arteriovenous fistula, chronic kidney disease, eGFR trajectory, HD access, hemodialysis
INTRODUCTION
Nearly 50 years ago, Cimino and Brescia described the creation of an arteriovenous fistula (AVF) for repeated venipuncture for chronic hemodialysis (HD) treatments [1]. While always thought to be the best form of HD vascular access, this was formalized in the 1997 National Kidney Foundation's Dialysis Outcome Quality Initiative (NKF-DOQI) Clinical Practice Guidelines on Hemodialysis Vascular Access [2]. The preference for the AVF access has been based on its potentially smaller surgery, durability, longevity, less need for interventions, maintenance of the skin as a protective barrier against infection, absence of a foreign body and ease of accessibility. Systemic physiologic effects follow the creation of an arteriovenous fistula. However, those effects on kidney function have not been described in detail. For example, an AVF has not been considered as playing any role in slowing down the process of kidney functional decline in progressive chronic kidney disease (CKD).
As advocates for the early creation of AVFs in progressive CKD, we have often noted that there appeared to be the decline in the estimated glomerular filtration rate (eGFR) after AVF creation. In conversations, other nephrologists have observed this phenomenon. We and they have attributed this to coincidence or an improved patient behavioral compliance with therapy. However, many patients agreeing to a timely fistula creation are very compliant. This raises at least two questions. Is slowing of the eGFR decline real, and if so, what is the physiology? The local and systemic macrovascular effects of AVFs have been appreciated for a long time [3]. Recent investigations into the microcirculatory effects of AVFs suggest biological plausibility for AVFs to affect the eGFR [4]. Delaying the time to initiate dialysis has many advantages including beneficial psycho-social considerations, reduced costs and possibly even lower mortality. Furthermore, the presence of a functioning AVF at the initiation of dialysis means a lower frequency of using catheters. Thus, the significance of a functioning AVF is that it takes on a major role in determining a positive outcome. To test the hypothesis that the creation of an AVF might alter (slow) the decline of the GFR trajectory in progressive CKD, we examined the eGFR decline of patients before and after successfully created AVFs.
MATERIALS AND METHODS
Vanderbilt University Medical Center's electronic medical records (EMRs) were searched using Current Procedural Terminology (CPT) billing codes to identify any patient who underwent an AVF creation between 2005 and 2010. We assumed that AVF creation was performed for CKD, but did not cross-reference the CPT codes to CKD diagnoses. We obtained eGFR values calculated within the EMR by the Modification of Diet in Renal Disease (MDRD) equation [GFR (mL/min/1.73 m2) = 186 × (Scr)−1.154 × (Age)−0.203 × (0.742 if female) × (1.212 if African American)] [5] and adjusted for temporal modifications within the Vanderbilt EMR. Age, race, sex, medications and comorbid conditions were determined from the patient's EMR at the time of the AVF creation, but were not utilized in the analysis because statistically this would have required a sample size that was beyond our capacity. Weight was assumed to be stable, i.e. weight changes were not taken into consideration. Furthermore, medication changes and seminal events that might precipitate initiation of dialysis were not ascertained and, again, the reason for AVF creation was assumed to be CKD.
All patients >18 years of age at the time of the surgery for AVF creation, receiving an AVF during 2005–2010 were analyzed if followed in a Vanderbilt nephrology clinic, so that the AVF could be evaluated by the primary nephrologist and determined to be ‘functional’. Patients had to have at least 2 data points before AVF creation and 2 data points after AVF creation for slope analysis. Data points were gathered for 2 years prior to AVF creation and for 2 years after creation, or until death, started on dialysis or transplanted. Patients not meeting these criteria, including those with an AVF created but not deemed functional or with any previous organ transplants, were excluded.
Using the PS software, it was determined that in order to detect a difference in the rate of decline in the eGFR by 0.5 mL/min per year, a sample size of 170 patients is needed. An interim analysis for an abstract deadline was performed and led to the present results. The effect of AVF creation on kidney function was assessed using generalized linear regression with compound symmetry correlation structure to account for the repeat measurements within each patient. Statistical analysis was performed with RStudio 3.0.1 (http://www.rstudio.com/), and 2-sided P < 0.05 was considered statistically significant.
RESULTS
One hundred twenty-three patients met our inclusion criteria. The median age was 68 years (interquartile range 59.0–76.0), 41% were female, 77% were Caucasian, 20% were African-American, 97% were hypertensive, 56% were diabetic and 41% smoked. These patients represented our practice's typical population and represented every practicing clinician.
There was a median observation time of 638 days before AVF creation, with the mean eGFR at this onset of observation at 28.3 mL/min [standard deviation (SD) = 11.93]. The median number of observations between the onset of the study and the creation of the AVF was 9 (range 6–11). The mean eGFR at the time of AVF creation was 16.9 mL/min (SD = 6.84). After AVF creation the median follow-up time was 549 days, median of eGFR observations was 10 (range 6–14) per patient and the mean eGFR was 15.8 mL/min (SD = 8.67) at the end of the observation period. By this time 72 patients had begun hemodialysis, 37 were alive not yet dialyzed or transplanted, 6 were transplanted, 4 died and another 4 were lost to follow-up.
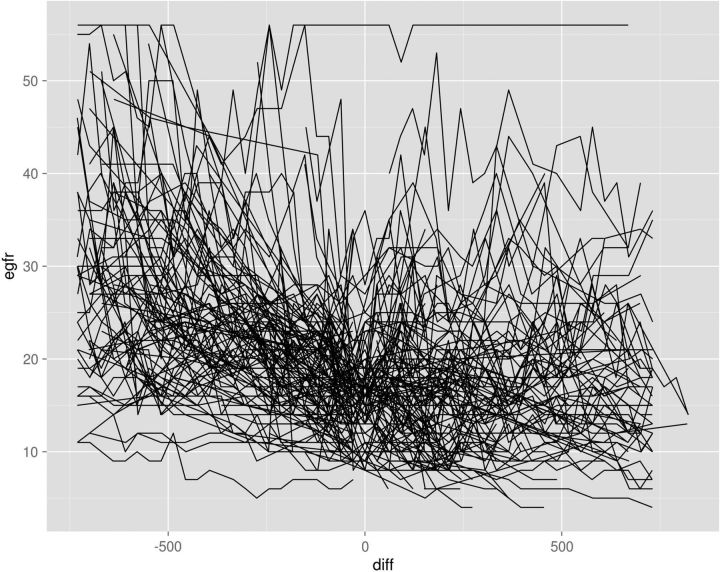
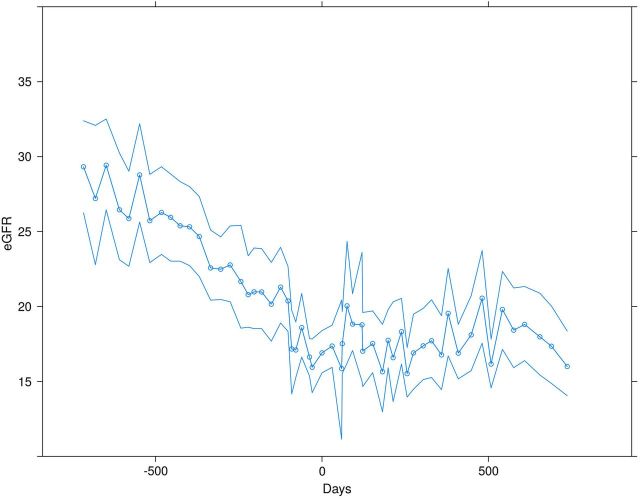
The eGFR curves of all 123 patients are shown in Figure 1. For some patients the durations of data observation before AVF creation or after AVF creation are full 2-year observation periods. For others, the durations of observations are less, as one would expect in a typical practice of late-stage CKD patients. The mean and 95% confidence intervals for the 123 patients analyzed collectively are shown in Figure 2, with a visibly clear change in slope of the eGFR post AVF creation. Prior to AVF formation, the rate of change was −5.90 mL/min per year (95% CI: −5.28, −6.51), while after AVF formation the rate of change was −0.46 mL/min per year (95% CI: −1.05, 0.14). The slopes of the eGFR decline (i.e. negative rate of change) before and after AVF creation are statistically different, indicating a slower decline after AVF creation (interaction P < 0.001). Thus, the interim analysis led to termination of the data collection as the effect was greater than projected for power calculations.
FIGURE 1:
The eGFR curves of all 123 successfully created AV fistula patients. The eGFR is plotted on the vertical axis, time in days on the horizontal axis. Zero on the horizontal axis is the time point for AVF creation, with eGFR values to the left of zero representing the days before the access creation and to the right of zero the days after creation. Patient 17 had an AVF created for recurrent plasma exchanges with thrombotic thrombocytopenic purpura and the eGFR was unchanged (>50 mL/min) over the course of the observation period.
FIGURE 2:
The eGFR trajectory prior to AVF creation, where zero is the time of fistula creation, and after AVF creation (mean and bootstrapped 95% confidence intervals). Prior to AVF formation, the rate of change was −5.90 mL/min/year (95% CI −5.28, −6.51), while after AVF formation the rate of change was −0.46 mL/min/year (95% CI −1.05, 0.14) (interaction P < 0.001).
DISCUSSION
Because circulatory changes temporally accompany the successful creation of an AVF, our simple goal of this retrospective observational study was to ascertain if there is a signal that the functioning fistula might slow the deterioration of the eGFR. If no such signal was present, there would be no rationale to pursue this further. Thus, only successful fistula patients were selected. Recent work by Korsheed and his associates has suggested that there may be physiological benefits of a functioning AVF, in contradistinction to those patients whose fistulas were not functional. In only those patients with successful fistulas, they noted systemic consequences [4, 6], namely local and remote changes in the microcirculation. AVFs reduce non-endothelial-dependent vasodilation in the contralateral forearms of late-stage (not on dialysis) CKD patients [4]. This was attributed to changes in function and structure of the endothelium, induced by the local sheer wall stress and wider systemic hemodynamic effects of the functioning fistula, such as decreased total peripheral resistance and systolic and diastolic blood pressure, and increased stroke volume, left ventricular ejection fraction and cardiac output [6]. Arterial stiffness appears to accompany progressive CKD [7, 8]. This stiffening occurring in renal vascular beds may be contributing to dysregulation and further loss of renal function, creating a cycle of each contributing to the worsening of the other. Thus, in later stages when an AVF is created, endothelial cells responding to the sheer wall stress downstream from the fistula react in a manner which appears to mitigate the arterial stiffening both locally and systemically. This was shown by Korsheed in contralateral subcutaneous dermal capillaries [4]. Interestingly, dermal capillaries have been shown to behave similarly to kidney capillaries [9]. Therefore, it is plausible that the ‘downstream’ effects of the fistula may reach renal vascular beds at both the macro- and microvascular levels. Thus, by this extrapolation, a systemic effect of reducing endothelial and non-endothelial-derived vasodilation could lead to perfusion of previously under-perfused tissue. This could occur in renal vascular beds and may represent a recruitment of untapped renal functional reserve [10]. It could also occur in skeletal muscle vascular beds. A similar phenomenon on the slowing of renal functional decline has been observed in a mouse model from products of the skeletal muscle [11]. Perhaps the effect of the functioning AVF is mediated indirectly through what happens to skeletal muscle post fistula creation, as there is a very metabolically active muscle ‘secretome’ as recently discussed by Rondon-Berrios et al. [12]. Thus, there is biological plausibility of stabilization of renal function or a slowing of its loss after the creation of a functional AV fistula. On the other hand, it is possible that a successful fistula merely selects patients in whom the eGFR stabilizes. Even if so, the same question about the physiological mechanism(s) apply.
Could our observations be an artifact or attributed in some unexplained way to ‘better medical compliance?’ The former must be addressed in a more comprehensive study as discussed below and the latter would still require some physiological explanation. Our present hypothesis that the fistula induces beneficial vascular changes that affect eGFR seems at least equally or more likely to explain the slowing of the eGFR trajectory. Previous to this report, the concept that the functioning fistula might slow the loss of kidney function had not been considered, despite our anecdotal observations that it might be occurring. The ramifications of delaying dialysis with a functioning fistula are profound. Not only would the patient have the preferred access for hemodialysis and thus avoid a catheter, but also the actual dialysis may be delayed with its psycho-social, economic and possible mortality reduction benefits. To clinicians these benefits are obvious, but to the patient they are more theoretical and less clearly understood. Even when patients are followed by a nephrologist in a nationalized health-care system, the timeliness of AVF creation is less than ideal, suggesting that unexplained barriers still exist [13]. One must consider the possibility that creation of a fistula in a timely manner that could delay the onset of the initiation of dialysis may be an incentive for patients to accept and even advocate to undergo the procedure.
Using the trajectory of the decline of the eGFR has its limitations. These may include end-points that are often only clear in hindsight, a survivor bias, and that in a given patient the trajectory may not be linear, even if no untoward events are detected [14–20]. This retrospective analysis seems the most reasonable approach to begin to test our hypothesis that a successfully created AVF might slow the trajectory of eGFR decline. It is for this reason that we are presenting all of the 123 patients' eGFR plots in Figure 1. We also recognize that normalization to body surface area is standard for eGFR estimations. However, volume expansion from the AVF creation has not been shown to alter serum creatinine concentration or body composition [6, 21] and so we assume that our observed eGFR effect is not a simple artifact of dilution. Medication changes and renal function-altering events supervene and can affect renal function, but were not analyzed in this project for lack of adequate statistical power.
A very much larger sample size would have been required to assess the role of predetermined confounders in this complex patient population. For example, one would like to adjust for the presence or absence of renoprotection such as the numerous medications to suppress the immune system, or to alter glomerular hemodynamics such as renin–angiotensin–aldosterone system antagonists. How to account for the intermittent nature of these therapies is complicated. Furthermore, other medications such as diuretics, other anti-hypertensive agents, non-steroidal anti-inflammatory agents, antibiotics, proton pump inhibitors, H2 blockers and contrast agents are confounders. Intercurrent illnesses which commonly affect the eGFR in later stages of CKD include myocardial infarction, stroke, pneumonia, urinary tract infection, diarrhea or other volume-depleting states and for which all would need accounted. The underlying renal disease and control of blood pressure and glycemia affect GFR decline. Lastly, a change in body composition (e.g. diminished muscle mass and thus creatinine generation), which occurs as the GFR declines, may explain the slowing of the eGFR trajectory. As body mass shrinks with progressive uremia, the eGFR declines less rapidly simply because of the way the eGFR is calculated. However, our practice uses edema-free weight loss as an indication to initiate dialysis, and many months to years of such a loss seems unlikely. However, this would be tracked carefully in a prospective study. A single center is unlikely to have enough fistulas created in a reasonable time frame to statistically account for all these variables. It is clear that to explore the role of the many variables that affect the initiation of dialysis and the possibility that a functioning AVF may influence it will require a thorough and properly designed study powered appropriately to address the predetermined variables. Such an undertaking will require multiple centers contributing these data.
CONCLUSION
In conclusion, there may be a temporal association between successful AVF creation and a slowing of the eGFR trajectory. This phenomenon is biologically plausible. A properly designed prospective observational trial will detect the role of confounding variables. The results of this initial investigation suggest that such a trial may show a previously unexpected and highly beneficial effect of timely creation of a preparatory AV fistula, namely a slowing of the decline of renal function, perhaps delaying the initiation of dialysis. This has obvious cost saving and decreased morbidity and mortality implications involving a very large number of patients.
CONFLICT OF INTEREST STATEMENT
None declared.
ACKNOWLEDGEMENTS
Dialysis Clinics, Incorporated supported the fellowship of P.M.H. We are grateful to Drs Alp Ikizler, Ray Hakim, Fred Luft, Ray Harris and the journal reviewers who provided advice along the way. These data were presented in poster format at the American Society of Nephrology Annual Meeting in Philadelphia, PN, on 16 November 2014.
REFERENCES
- 1.Brescia MJ, Cimino JE, Appel K, et al. Chronic hemodialysis using venipuncture and a surgically created fistula. N Engl J Med 1966; 275: 1089–1092 [DOI] [PubMed] [Google Scholar]
- 2.NKF-DOQI Clinical Practice Guidelines for Vascular Access. New York, National Kidney Foundation, 1997, pp. 22–23 [PubMed] [Google Scholar]
- 3.Warren JV, Nickerson JL, Elkin DC. The cardiac output in patients with arteriovenous fistula. J Clin Invest 1951; 30: 210–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korsheed S. The cardiovascular and functional consequences or arteriovenous fistula formation in chronic kidney disease. Thesis submitted to University of Nottingham Nottingham England, United Kingdom, 2011 [Google Scholar]
- 5.Levey AS, Stevens LA, Schmid CH, et al. for CKD-EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korsheed S, Eldehni MT, John SG, et al. Effects of arteriovenous fistula formation on arterial stiffness and cardiovascular performance and function. Nephrol Dial Transplant 2011; 26: 3296–3302 [DOI] [PubMed] [Google Scholar]
- 7.Gosse P, Safar ME. Arterial stiffness and plasma creatinine in untreated hypertensive patients. Am J Hypertens 2005; 18: 1140–1145 [DOI] [PubMed] [Google Scholar]
- 8.Wang MC, Tsai WC, Chen JY, et al. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis 2005; 45: 494–501 [DOI] [PubMed] [Google Scholar]
- 9.Economides PA, Caselli A, Zuo CS, et al. Kidney oxygenation during water diuresis and endothelial function in patients with type 2 diabetes and subjects at risk to develop diabetes. Metabolism 2004; 53: 222–227 [DOI] [PubMed] [Google Scholar]
- 10.Bosch JP, Saccaggi A, Lauer A, et al. Renal functional reserve in humans: effect of protein intake on glomerular filtration rate. Am J Med 1983; 75: 943–950 [DOI] [PubMed] [Google Scholar]
- 11.Hanatani S, Izumiya Y, Araki S, et al. Akt-1-mediated fast/glycolytic skeletal muscle growth attenuates renal damage in experimental kidney disease. J Am Soc Nephrol 2014; 25: 2800–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rondon-Berrios H, Wang Y, Mitch WE. Can muscle-kidney cross talk slow progression of CKD? J Am Soc Nephrol 2014; 25: 2681–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Jaisihi AA, Lok C, Garg AX, et al. Vascular access creation before hemodialysis initiation and use: a population-based cohort study. Clin J Am Soc Nephrol 2015; online doi 10.2215/CJN.06220614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene T. A model for a proportional treatment effect on disease progression. Biometrics 2001; 57: 354–360 [DOI] [PubMed] [Google Scholar]
- 15.Greene T, Lai J, Levey AS. Interpretation of clinical studies of renal disease. Chapter 40 In Neilson EG, Couser WG. (eds). Immunologic Renal Disease, 1st edn Philadelphia, Pennsylvania: Lippincott-Raven Publishers, 1997, pp. 887–914 [Google Scholar]
- 16.Al-Aly Z, Zeringue A, Fu J, et al. Rate of kidney function decline associates with mortality. J Am Soc Nephrol 2010; 21: 1961–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Astor BC, Lewis J, et al. Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis 2012; 59: 504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Hare AM, Batten A, Burrows NR, et al. Trajectories of kidney function decline in the 2 years before initiation of long-term dialysis. Am J Kidney Dis 2012; 59: 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong Y, Munoz A, Schwartz GJ, et al. Nonlinear trajectory of GFR in children before RRT. J Am Soc Nephrol 2014; 25: 913–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coresh J, Turin TC, Matsushita K, et al. for the CKD Consortium. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014; 311: 2518–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandhu JS, Wander GS, Gupta ML, et al. Hemodynamic effects of arteriovenous fistula in end-stage renal failure. Ren Fail 2004; 26: 695–701 [DOI] [PubMed] [Google Scholar]