Abstract
Vitamin C is an important antioxidant and cofactor which is involved in the regulation of development, function and maintenance of several cell types in the body. Deficiencies in vitamin C can lead to conditions such as scurvy, which, among other ailments, causes gingivia, bone pain and impaired wound healing. This review examines the functional importance of vitamin C as it relates to the development and maintenance of bone tissues. Analysis of several epidemiological studies and genetic mouse models regarding the effect of vitamin C shows a positive effect on bone health. Overall, vitamin C exerts a positive effect on trabecular bone formation by influencing expression of bone matrix genes in osteoblasts. Recent studies on the molecular pathway for vitamin C actions that include direct effects of vitamin C on transcriptional regulation of target genes by influencing the activity of transcription factors and by epigenetic modification of key genes involved in skeletal development and maintenance are discussed. With an understanding of mechanisms involved in the uptake and metabolism of vitamin C and knowledge of precise molecular pathways for vitamin C actions in bone cells, it is possible that novel therapeutic strategies can be developed or existing therapies can be modified for the treatment of osteoporotic fractures.
Keywords: Osteoporosis, Bone fracture, Osteoblasts, epigenetics, Prolyl Hydroxylase Domain-containing Protein 2, Endochondral Bone Formation, Hypoxia-inducible Factors
Introduction
Reports of severe bone pain in maritime explorers suffering from scurvy during the 15th century implied that vitamin C deficiency affects bone health. However, the significance of vitamin C effects on bone metabolism have only become evident during the past several decades based on findings in animal models and from epidemiological studies. Initial studies identified vitamin C as a critical modulator of the production of collagen, and that the reduced form, ascorbic acid (AA), had potent antioxidant effects. It has been well established that AA is an important cofactor for many prolyl and lysyl hydroxylases which actively participate in collagen maturation (1,2). More recently, studies using genetically modified mouse and rat models of vitamin C deficiency, have demonstrated a direct role for vitamin C in regulating gene transcription in bone. Specifically, these models reveal that subtle changes in one or more genes in vitamin C signaling pathways can significantly impair the transcription of genes involved in osteoblast maturation and function. Furthermore, recent epidemiological studies have provided convincing evidence for the increased risk of osteoporosis and fractures caused by decreased bone formation in patients with vitamin C deficiency. In this article, we will present a brief overview of vitamin C including its history, physiologic role in skeletal and non-skeletal tissues, and the epidemiological studies that have evaluated the relationship between vitamin C, and bone mineral density (BMD), and fracture. Our main discussion will focus on the genetically modified mouse and rat models of vitamin C deficiency, their skeletal phenotypes, and contributions to our knowledge of the molecular mechanism of vitamin C action in bone. Finally, we will discuss future research studies that are needed in this area.
Historical Perspective
Vitamin C deficiencies can cause a variety of medical conditions, which have been documented as early as 1500 B.C. in Ebers papyrus by an Egyptian medical herbalist. Scurvy, a disorder caused by AA deficiency, can lead to multiple complications including lethargy, bone pain, gingivitis, impaired wound healing, myalgia, impaired bone growth and pseudoparalysis. In the 18th century, in what is considered to be one of the first documented clinical trials, Dr. James Lind, a naval physician, discovered that scurvy could be treated with the ingestion of citrus fruits (e.g., lemons and oranges), leading to a reduction of symptoms. A recent study examined 970 human skeletons from mass graves dating to the great potato famine of Ireland (1845–1852). The major cause of death was determined to be infections related to reduced immunity influenced by scurvy, which was attributed to a lack of vitamin C from consumption of potatoes, typically the only source of food for the poor population of Ireland at the time(3). It was not until 1931 that the effective agent in citrus fruit was identified; when Albert Szent-Gyorgyi isolated the molecule that he named hexuronic acid. Subsequently, it was determined to be a form of vitamin C and was later renamed ascorbic acid (AA)(4). Today scurvy is a rare condition in developed countries, found primarily among individuals in circumstances related to malnourishment (e.g. homelessness, isolation) or in persons with allergies to foods which typically contain vitamin C(5).
The Role of Vitamin C in Non-skeletal Tissues
Vitamin C affects multiple tissues due to its global functioning as an essential component of collagen synthesis and as an antioxidant. AA is involved in the synthesis of collagen by influencing the function of prolyl hydroxylase domain protein (PHD), which hydroxylates prolines that are important for collagen assembly(6,7). Vitamin C is involved in vascular remodeling as well as in the maintenance of vascular cell integrity by influencing vascular smooth muscle cell differentiation and the expression of connective tissue proteins(8,9). In a recent meta-analysis of randomized controlled trials involving vitamins C and E, it was found that supplementation with either vitamin C or vitamin E alone improved endothelial function(10). In the central nervous system, adequate AA concentrations are important for the formation of myelin sheaths, peptide amidation, and protection against glutamate toxicity. Deletions of sodium-dependent vitamin C transporter-1 (SVCT2), an ascorbate transporter and regulator of AA concentration, result in cerebral hemorrhaging and death(11). AA has additionally been suggested as a preventative agent against Alzheimer’s disease through its function in reducing oxidative stress which often leads to age-related disorders such as Alzheimer’s(12). In keratinocytes of the epidermis, AA plays a role in collagen synthesis and wound repair. AA also reduces reactive oxygen species by scavenging factors that promote inflammation in the dermis; as such, AA can function as a therapeutic agent against UV induced skin cancers(13). In many of these examples, it seems that the primary method of function for AA is either by regulating biosynthesis of connective tissue or by acting as an antioxidative agent.
Epidemiology and Human Studies in Vitamin C
The current recommended dietary allowance (RDA) for vitamin C for healthy individuals is 90mg/day for adult males, and 75mg/day for adult females, 15–65mg/day for children depending on age and gender, and 40–50mg/day for infants(14). In adults, intakes above RDA are recommended during pregnancy, if lactating, and in smokers (additional 15, 50 and 35mg/day, respectively)(14). Based on data from the most recent National Health and Nutrition Examination Survey (NHANES) 2003–2006(4), the Center for Disease Control reported the overall prevalence of vitamin C deficiency in the U.S. to be 6%. Defined as serum AA levels <11.4 µmol/L, deficiency was significantly more prevalent in males (7%) than females (5%), Mexican Americans (12%) and Non-Hispanic Blacks (31%) compared to Non-Hispanic White (3%). Deficiency was less commonly observed in children (aged 6–11y) and older adults (aged ≥ 60 y) compared to young adults (aged 20–39y).(3)
For purposes of this review, we present only a broad overview of the work. For an in-depth discussion of the field, we refer the reader to the recently published review by Finck, et. al.(15). Human studies examining vitamin C effects on bone are relatively sparse and the findings are somewhat inconsistent. This inconsistency is likely the result of differences in study design, and the methodologies used.
The bulk of studies assessing vitamin C and bone have been comprised of elderly subjects of both genders (aged >60 years) and/or postmenopausal females. This is not surprising in light of the fact that the prevalence of osteoporosis and the incidence of osteoporotic fracture are highest within these groups. However, there is significant variability in the approaches used to measure vitamin C exposure and assess skeletal outcomes, as well as defining stratification cutoff points (e.g. use of tertiles, quartiles, high vs. low intake), which limits direct comparisons between studies. In addition, studies differ widely in the inclusion/exclusion criteria and examination of covariates such as smoking status, calcium or other nutrient intake and use of estrogen in female subjects.
The vast majority of the human studies have been observational, and have assessed the bone mineral density (BMD) level or change in BMD over time, as a skeletal outcome. The most widely used observational study design has been cross-sectional. These studies assessed vitamin C exposure via self-reported dietary(5,16–19), supplemental(19–22) and/or total (dietary + supplemental) intake(18–25). The instruments used to determine intake have differed from food frequency questionnaires, 24-hour diet recall(24,26,27), and 3-day(23) or 7-day dietary diary(28). Only 1 of the 13 cross-sectional studies measured vitamin C by the more objective method of serum AA concentration(22). In regards to BMD outcomes, although most cross-sectional studies examined BMD, the reported skeletal sites varied from the more conventionally-used clinical sites (lumbar spine (16–18,20,25,27–31), femoral neck(16–20,23,25,27–29,31), total hip(16,20,22,23,25,28,30,32)), to less conventional bony sites (trochanter(17,18,23,25,27,28,31), Ward’s triangle(17,18,23), whole body(21,23,25,28), forearm(20,23,24,27,31,33) and hand(23)). Three of the cross-sectional assessed skeletal outcomes in addition to BMD; either self-reported fractures(22) or biochemical bone markers(18,21)
Because of the wide variety of methodologies employed in the cross-sectional studies, comparisons of results are difficult and the findings have been inconsistent. For example, several studies have examined the relationship between dietary vitamin C intake and BMD in postmenopausal women using multivariate linear regression modeling. We(16) and others(17,23,33) observed significant positive correlations with dietary vitamin C and BMD at one or more bony sites. In contrast, other studies(18,19,22,25) reported positive associations between vitamin C and BMD, but effect sizes were smaller and not significant. One study reported negative, non-significant correlations(28). Similar study inconsistencies exist when comparing cross-sectional studies that measured other vitamin C exposure variables (e.g. supplemental and total vitamin C intake or blood concentrations of vitamin C), studied male populations, and performed different co-variate analyses. As above, most studies reported positive trends but differed in effect size and statistical significance.
The case-control study design has been employed in 5 studies. Three of these defined a case based on fracture (hip(26,34) or any fragility fracture(35) and measured blood levels of AA. Falch, et. al.(34) and Martinez-Ramirez, et. al.(35) both examined elderly men and women, and reported significantly higher serum vitamin C level in controls compared to subjects who had suffered hip fractures or fragility fractures, respectively. In contrast, Lumbers, et al.(26) studied elderly women only and observed the opposite scenario, in that, plasma concentrations of vitamin C were higher in hip fracture cases than controls. The other 2 case-control studies identified cases of osteoporosis (T-scores ≤ −3.5(36) or ≤ −2.5(37). One study assessed plasma AA levels(36) while the other assessed dietary vitamin C intake by FFQ(37). Both studies involved older women and both indicated that osteoporotic cases had lower vitamin C level status than controls.
Three longitudinal studies have evaluated vitamin C and bone health in the elderly(27,31,32). One study conducted by Sahni and colleagues used data derived from the US Framingham Study cardiac outcomes study(38), focused on incidence of fracture as the skeletal outcome and encompassed a long follow-up period (15–17 years)(27). This Framingham sub-study included US men and women ((N=918; mean age ∼75 years), and assessed the relationships between vitamin C consumption (dietary, supplemental, and total) as measured by FFQ. Significant, inverse dose-dependent trends were observed between total vitamin C intakes, as well as supplement use, and fracture risk. Hazard ratios for medical record-documented hip and self-reported non-vertebral fractures were significantly lower in the highest tertile of total vitamin C intake (median total intake = ∼300mg/day) compared to the lowest tertile (median total intake = ∼95g/day). Similarly, the risk of hip fracture was significantly reduced in subjects taking vitamin C supplements (median 260mg/day) compared to non-supplemented subjects. Non-significant trends suggesting protective effects were also observed with higher vitamin C supplementation and non-vertebral fractures, and with dietary intake and hip and non-vertebral fractures. A second longitudinal study also performed by Sahni, et al. using the Framingham data, examined 4-year changes in BMD at various sites as the primary outcome and reported mixed results in elderly adults (N=606)(31). No significant associations were reported in women. In men, beneficial effects were observed with some, but not all, vitamin C intake variables, at some, but not all, measured bone sites. Results also varied between groups of men stratified by calcium intake. A third longitudinal study, also done in elderly men and women (N=944; mean age ∼72 years), assessed vitamin C dietary intake by 7-day food diary and looked at bone loss between two BMD measurements at the total hip taken 2–5 years apart(32). Results pointed to beneficial effects of vitamin C in women, in that, vitamin C was significantly associated with more rapid bone loss in a linear fashion across increasing tertiles of vitamin C intake. In men, a similar linear trend was observed, but the effect was smaller and non-significant.
To date, there have been no placebo-controlled randomized clinical trials (RTCs) evaluating the effect of vitamin C exposure alone on skeletal dietary and supplemented nutrient effects. There have been two small (N=30 or N=7–11 per group), randomized placebo-controlled studies conducted examining change in BMD and use of combination dosing of vitamin C and vitamin E(29,30). Significant protective bone effects of supplementation were reported in both studies at some skeletal sites but not others. A third small (N=13) interventional study using a before-and-after study design reported a decrease in bone-specific alkaline phosphatase after subjects received daily vitamin C and vitamin E supplementation for 8 weeks.(39)
Based on the wide variety of methodologies used to study the clinical effects of vitamin C effects on bone, it is not surprising that there are inconsistencies in the reported results.
Nevertheless, when taken as a whole, the majority of studies have observed either significant beneficial effects of vitamin C or positive but non-significant effects on one or more of the skeletal outcomes. Furthermore, differences observed in results based on different covariate analyses suggest that vitamin C effects are likely influenced by other factors. In conclusion, these observations point to the likelihood that vitamin C effects on bone are beneficial in nature but highly complex.
Vitamin C Biosynthesis and Animal Models of Vitamin C Deficiencies and Their Effects on Bone
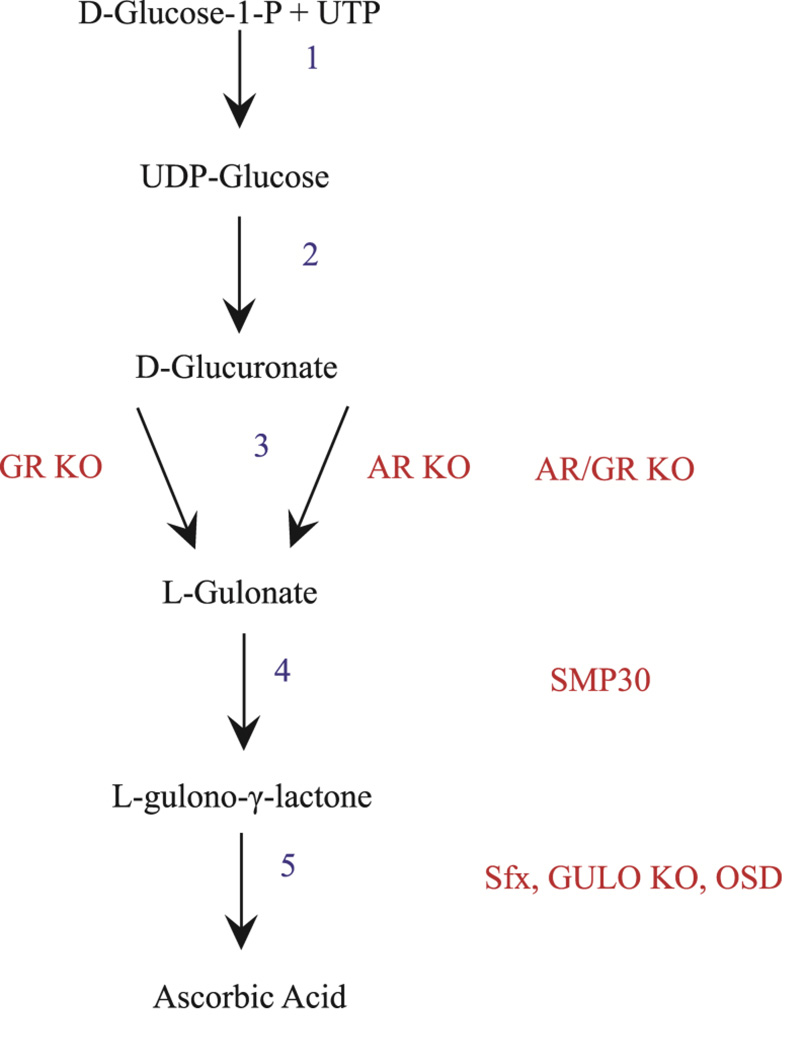
Most animals, including rats and mice, are capable of de novo synthesis of Vitamin C. In these species, production of vitamin C begins with the formation of UDP-glucose from D-glucose-1-phosphate and uridine triphosphate (UTP). UDP-glucose is then dehydrogenated and loses its uridine diphosphate to become D-glucuronate (Fig. 1). Next, D-glucuronate is reduced to L-gulonate at which point, it can either be used in the vitamin C synthesis pathway or the pentose phosphate pathway. In vitamin C biosynthesis, L-gulonate is enzymatically converted to L-gulono-γ-lactone via gulonolactonase. A mutation in senescence marker protein 30 (SMP30), which is a gulonolactonase, prevents the conversion of L-gulonate to L-gulono-γ-lactone. Mice with this mutation are often used for vitamin C deficiency studies. In the final step of the synthesis pathway, L-gulono-γ-lactone is converted to AA via L-gulonolactone oxidase (GULO). Although many mammals produce vitamin C, de novo, this ability has been evolutionarily lost in some species, such as guinea pigs, capybaras, certain bats, and some primates including humans(40,41).
Fig. 1. Vitamin C synthesis and mutation map.
The AR (Aldose Reductase) and GR (Aldehyde Reductase) mutations affect two separate portions of the third step of the vitamin C synthesis pathway. The AR/GRKO mutation is a double knockout preventing the conversion of L-glucuronate to L-gulonate. SMP30 is a mutation in the fourth step of synthesis affecting gulonolactonase, preventing the conversion of L-gulonate to L-gulono-γ-lactone. Sfx, GULOKO and OSD affect the fifth step of synthesis and are distinct mutations of L-gulono-γ-lactoneoxidase, which converts L-gulono-γ-lactone to ascorbic acid. Mutation names are denoted in red, intermediates are denoted in black, step numbers are denoted in blue.
One of the earliest model organisms for studying the effects of vitamin C on bone was the guinea pig, as this vertebrate species lacks the GULO gene and, therefore, is incapable of catalyzing the final step in vitamin C biosynthesis. Gene structure and phylogenetic analyses revealed that the vertebrate GULO genes exhibit 64–95% amino acid identity and consist of 11 conserved exons(42). However, in guinea pigs and in higher primates, the GULO gene is inactive due to mutations that occurred roughly 20 million years ago(43,44). Early guinea pig studies revealed that AA deficiencies inhibited proper collagen synthesis(45), and moreover that dysfunctional femoral bone collagen synthesis was the result of reduced hydroxyproline content(46). This lead to further studies where it was shown that AA deficient guinea pigs had reduced bone trabecular number and thickness, but increased trabecular spacing in the tibia. Femur length and bone volume density were also decreased in scorbutic animals, though, bone strength remained unaffected(47). Scorbutic guinea pigs display greatly reduced ALP activity in both bone and serum compared to those on a diet supplemented with AA(48).
In contrast to the guinea pig, rats and mice are able to synthesize vitamin C and do not require dietary vitamin C ingestion. Thus, gene knock-out animals involving specific molecular components of the vitamin C synthesis and cellular transport systems have been developed as a means to study skeletal and non-skeletal effects of vitamin C deficiency (Table 1). Three rodent models, the osteogenic disorder Shionogi (ODS) rat, the GULO KO mouse and the spontaneous fracture (sfx) mouse, target the GULO gene which is required for the conversion of L-gulono-γ-lactone to AA, and which is lacking in humans. The sfx mouse is a consequence of an entire gene deletion(49,50), whereas the ODS rat and the GULO KO mouse models are the result of a missense mutation ( G->A nucleotide 182, Cys->Tyr amino acid 61)(51) and insertional mutations of exons 3–7 (i.e. gene trapping)(52), respectively. These models share similar skeletal phenotypes including spontaneous fracturing, decreased cortical thickness, and reduced metaphyseal trabeculae number and weight loss compared to wild type animals(49,53–55).
Table 1.
Vitamin C transgenic animals.
| Mutant | Animal | Gene | Mutant Type | Phenotype | Source |
|---|---|---|---|---|---|
| ODS | Rat | L-gulono-γ- lactoneoxidase |
Missense Mutant Cys 61-Tyr 61 |
Spontaneous fractures, missing trabeculae, reduced cortical zone |
(Kawai et al., 1992; Mizushima et al., 1984)(51,103) |
| GULO KO | Mouse | L-gulono-γ- lactoneoxidase |
Gene Trap Exons 3–7 |
Spontaneous fractures, missing trabeculae, reduced cortical zone |
(Maeda et al., 2000)(52) |
| Sfx | Mouse | L-gulono-γ- lactoneoxidase |
Gene Deletion | Spontaneous fractures, missing trabeculae, reduced cortical zone |
(Mohan et al., 2005; Jiao et al., 2005; Beamer et al., 2000)(49,50,57) |
| AR KO | Mouse | Aldose Reductase | Exon 9 Deletion |
No skeletal phenotype | (Gabbay et al., 2010)(40) |
| GR KO | Mouse | Aldehyde Reductase |
Gene Trap Intron 1 |
Susceptible to osteoporosis under oxidative stress/low AA |
(Gabbay et al., 2010)(40) |
| AR/GR KO | Mouse | AR/GR | AR/GR Cross | Spontaneous fractures, missing trabeculae, reduced cortical zone |
(Gabbay et al., 2010)(40) |
| SMP-30 | Mouse | Gulonolactonase | Gene Trap Exon 3 |
Spontaneous fractures, missing trabeculae, reduced cortical zone |
(Ishigami et al., 2002)(104) |
All relevant vitamin C mutations in mouse and rats organized by gene, mutations in the gene and the effects of said mutation.
For unknown reasons, tail vertebrae in ODS rats are seemingly unaltered, suggesting that the AA deficiency caused by the GULO mutation has minimal or no effect on vertebral maintenance and repair(56). ODS rats do, however, have deformations in the curvature of the spinal column as well as stunted growth along the body axis and limb buds(55). The epiphyseal growth plate is reduced in size and chondrocyte number is decreased in ODS rats(56). Osteoblasts do not show cuboidal profiles, instead they are rounded and localized on the bone surface which does lack an osteoid layer. There is no increase in osteoclasts, indicating that stunted growth likely occurs as a result of dysfunctional or reduced osteoblast populations(55).
GULO KO mice also appear to have impaired osteogenesis as evidenced by reduced circulating osteocalcin levels further implying deficiency in the bone formation process(52,54). In addition to the reduced trabecular bone volume, GULO KO mice exhibit aortic wall damage(52,54). While there are not many studies describing the skeletal phenotypes of GULO KO mice, we and others showed that deletion of the entire GULO gene is the cause for impaired osteoblast differentiation, attenuated bone formation and spontaneous fractures in sfx mice(49,57). Sfx homozygous mice weigh significantly less than their wild type counterparts, have virtually no osteoid layer or trabecular bone by the growth plate, as well as a very thin growth plate, an indication that hypertrophic chondrocytes are virtually inactive(57). Femur periosteal bone formation rate (BFR) and mineral apposition rate (MAR) fall by a considerable amount in sfx mutants when compared to wild type mice (BFR 10−3 mm/day [sfx 1.137 ± 0.692, wild type 14.951 ± 3.552], MAR µm/day [sfx 1.128 ± 0.318, wild type 5.170 ± 1.782]). Accordingly, BMD is reduced in the tibia (64% of wt BMD) and femur (73% of wt BMD) at 35 days post-natal(49). Sfx mice have attenuated expression of insulin-like growth factor I (IGF-I) and a minor decrease in ALP, but very much like GULO KO mice exhibit a very large decrease in osteocalcin levels in the serum(57).
Another rodent model of vitamin C deficiency involves mutating the gene for senescence marker protein 30 (SMP30), the gulonolactonase responsible for the conversion of L-gulonate to L-gulono-γ-lactone, the penultimate step in the vitamin C biosynthesis pathway. SMP30 mice can alternatively convert D-glucuronate to D-gulono-γ-lactone, which can then undergo an isomeric change to L-gulono-γ-lactone, affectively bypassing the L-gulonate intermediate or the need for SMP30. Moreover, treating SMP30 mice with D-gulono-γ-lactone increases the urine concentration of AA at least 30 fold. This secondary pathway, however, does not endogenously yield adequate levels of L-gulono-γ-lactone to abrogate the need for SMP30(58,59). Very much like the ODS rats, GULO KO mice and sfx mice, SMP30 KO mice display the scurvy-like skeletal symptoms, including spontaneous limb fractures, reduced cortical thickness and trabecular number compared to wild type mice(60). SMP30 mice also display signs of rachitic rosary and lower subcranial BMD when compared to wild type (∼80% of wt BMD)(58). In addition, receptor activator of nuclear factor kappa-B ligand (RankL), an important mediator of osteoclast differentiation, is increased in SMP30 KO mice while osteoblast numbers are decreased(60).
Three additional KO mouse models that have been developed involve the aldehyde reductase (GR) and aldose reductase (AR) enzymes of the vitamin C synthesis pathway. Mutation of the GR gene via gene trapping of intron 1 produces mice (GR KO) that grow normally but are susceptible to developing osteoporosis when subjected to oxidative stress or reduced AA intake. Mice with deletion of exon 9 in AR (AR KO) display mildly reduced AA production (∼15%) but no visible skeletal phenotype. In contrast, double KO mice (AR/GR KO) develop signs of scurvy with bony features similar to that of the sfx, GULO KO, SMP30 mice and ODS rats(40).
It is important to note that these phenotypes can be rescued with ingestion of vitamin C, suggesting that these phenotypes are not a result of secondary effects of the ODS, SMP30, GULO KO or AR/GR KO mutations, but rather, are primarily due to vitamin C deficiency alone. In each of these KO models, it appears that bone formation is more affected than bone resorption. Together, these data suggest that impairment in bone formation caused by defective osteoblast function is the major cause for low BMD, trabecular number and cortical thickness resulting from AA deficiency. This leads to osteoporosis and increased fracture risk.
Vitamin C Effects on Bone and Cartilage Cells
AA has effects on various types of bone cells in vitro as well as in vivo. A number of in vitro studies have shown that vitamin C plays an important role in promoting expression of genes involved in differentiation of chondrocytes(61,62). In terms of mechanisms by which AA induces chondrocyte differentiation, studies using ATDC5 chondrogenic cells have shown evidence for involvement of ERK signaling. AA treatment induced ERK activation, while ERK inhibition attenuated vitamin C-induced chondrocyte differentiation(63). These data, taken together with the in vivo data, imply that AA is an important regulator of chondrocyte fate determination.
AA seems to be an important regulator for osteoblast fate determination as well as proliferation. Several studies have shown that addition of AA to cultured osteoblast-like cells stimulates initial deposition of collagenous extracellular matrix(7,64) followed by induction of specific genes associated with the osteoblast phenotype, such as alkaline phosphatase (ALP)(64,65) and osteocalcin(64,66), as well as osteopontin (OPN), osteonectin, and runt-related transcription factor 2 (Runx2) from undifferentiated mononuclear cells(67). AA treatment along with dexamethasone and β-glycerophosphate has been shown to exert a positive effect on differentiating mouse embryonic stem (ES) cell cultures to become osteoblasts(68). The mouse bone marrow stromal cell line, ST2 can develop into osteoblast lineage cells under the influence of AA(69). Treatment of MC3T3-E1 mouse calvaria-derived cells with AA increases proliferation as well as type I collagen synthesis(64,70). MG-63 osteoblast-like cells display similar results when treated with AA as well as its derivative AA 2-phosphate. These effects are attenuated in both MC3T3-E1 and MG-63 cell lines when treated with collagen synthesis inhibitors, indicating that collagen somehow influences the effects of AA on osteoblast proliferation/differentiation(64,66,70,71).
The biological effects of AA on osteoclasts have also been demonstrated using in vitro studies. Both stimulatory and inhibitory effects of AA on osteoclastogenesis in vitro have been demonstrated(72,73). In a recent study, Le Nihouannen et al.(74) have reported that treatment with AA significantly increased osteoclast number, size and nucleation in primary mouse bone marrow cultures as well as monocytic RAW 264.7 cells. Consistent with this data, treatment of the clonal stromal cell line, ST2 with AA and 1α, 25-dihydroxy-vitamin D3 caused a 5-fold increase in RANKL expression and induced formation of tartrate-resistant acid phosphatase-positive osteoclasts when co-cultured with mouse bone marrow cells(75). Interestingly, late stage osteoclasts treated with AA in culture initiated cell death(74). This dual function of AA may explain the attenuation of bone resorption observed by AA treatment in ovariectomized animals in vivo(76,77).
Molecular Pathways for Vitamin C Action on Bone Cells
AA is known as an antioxidant and important co-factor for catalyzing many biochemical reactions(78). Studies performed on smoking and its reductive effect on BMD as a result of increased free radicals have suggested that antioxidant vitamins such as vitamin C and vitamin E play a role in minimalizing such deleterious effects(79). Furthermore, overiectomized rats reduce thiol antioxidants such as glutathione and thioredoxin reductases due to a lack of estrogen. This in turn increases the concentration of reactive oxygen species (ROS),which results in increased osteoclastic differentiation and bone resorption. This osteoclastic effect can be reversed by treating rats with AA, which increases glutathione concentrations(76). There are many studies showing that antioxidants can minimize bone loss by preventing osteoclastic differentiation; however, antioxidizing functions are not enough to promote osteoblast differentiation. There have been no studies suggesting that antioxidants such as vitamin E can promote de novo bone regeneration. This suggests that when vitamin C functions as an antioxidant, it is to prevent osteoclastic differentiation but not osteoblastic activity.
Recent studies have demonstrated mechanisms for vitamin C stimulation of osteoblast differentiation in addition to its antioxidant effects on osteoclastogenesis. Several growth factors and hormones play a role in the differentiation and proliferation of chondrogenic and osteogenic cells(80). The actions of one or more of these osteogenic growth factors are subject to regulation by AA in bone cells. For example, AA-treated osteoblasts have increased expression of transforming growth factor (TGF)-β, estrogen receptor (ER)-α, and OPN, all of which are important regulators of bone formation(60). Additionally, in ovariectomized mice, AA can prevent the loss of osteoblast differentiation markers (Osterix, osteocalcin, Runx2, BMP-2) and attenuate bone loss, as well as stimulate bone formation(77). Much of the growth factor effect is regulated through increased collagen matrix production which is stimulated by AA. Vitamin C treatment upregulates sonic hedgehog (Shh) signaling by promoting expression of Gli1 and Ptc1, target genes of the Shh pathway in MC3T3-E1 osteoblasts(81). Similarly, AA modulates MapK/ERK signaling as well as Indian hedgehog (Ihh), bone morphogenetic protein 2 (BMP-2), and SOX9 expression via induction of a collagen matrix(63). The issue of whether the changes in expression or actions of these growth factors are due to direct or indirect effects of AA in bone cells remain to be examined.
In our studies on the mechanisms for AA effects on the differentiation of bone marrow stromal cells into mature osteoblasts, we found that AA is essential for the increase in osterix expression that occurs during osteoblast differentiation. We found that the AA effect on osterix expression was specific since expression levels of other transcription factors such as Runx2 and Dlx5 were unaffected(82). In our effort to understand how AA regulates osterix expression by transcriptional regulation, we focused on the nuclear factor-E2-related factor (NFE2, also known as Nrf) pathway since Nrf regulation of antioxidant genes via antioxidant response elements (ARE) is well known(83). Studies in a number of laboratories have shown that to combat DNA damage by electrophiles and ROS, cells have developed elaborate defense mechanisms that involve coordinated function of genes encoding drug detoxification, GSH metabolism, and protection against oxidative damage(84–86). Furthermore, the transcriptional activation of many of these genes involves the binding of Nrf to AREs to induce transcriptional activation(87). Since the proximal promoter of the osterix gene contains AREs that are highly conserved among mice, rats and humans, we examined if osterix gene transcription was regulated by Nrf binding to its promoter. Our data demonstrate that the effects of AA on osterix gene expression at the early stage of osteoblast differentiation is due to the direct effect of AA on osterix gene transcription, in part via a pathway involving increased binding of Nrf1 to the ARE in the promoter. Consistent with a role for Nrf1 in regulating osteoblast differentiation and bone formation, conditional disruption of Nrf1 in osteoblasts, in vivo, using the Cre/loxP approach decreased osterix mRNA levels, femur length and trabecular bone volume(82). Since a number of other transcription factors besides Nrf1 are known to bind to AREs, it remains to be determined if AA regulation of osterix gene transcription is mediated via other ARE binding proteins, besides Nrf1.
It has been well established that AA is an important cofactor for many prolyl and lysyl hydroxylases which are involved in the modulation of many transcription factors, as well as the formation and maturation of collagen. Prolyl and lysyl hydroxylases have multiple functions within the cell. One such function is regulation of hydroxylation of specific proline and lysine residues within the collagen molecule, a process that is essential for collagen cross linking and maintenance of the normal mature collagen network (1,2,7). Besides regulating collagen cross linking, prolyl hydroxylase domain (PHD) proteins activated by AA are known to induce hydroxylation of proline residues of other proteins such as hypoxia-inducible factors (HIFs). Under normoxia, and in the presence of Fe2+, 2-oxoglutarate and AA, PHD proteins are activated to induce hydroxylation of certain conserved proline residues in HIFs. The hydroxylated HIFs interact with the β-domain of von Hippel-Lindau tumor suppressor protein (pVHL), and are subsequently ubiquitinated by the pVHL-E3 ligase complex, thereby marking HIFs for degradation by the 26S proteasome(88,89). Thus, AA-induced activation of PHDs may modulate functions of bone cells in part by regulating the protein level of HIFs and other transcription factors.
Consistent with an important role for PHD proteins in mediating AA effects on osterix expression and osteoblast differentiation, it has been demonstrated that treatment with inhibitors of PHDs result in a blockade of AA-induced osterix expression and osteoblast differentiation(89,90). In order to determine the role of PHD2 expressed in osteoblasts, we conditionally disrupted the PHD2 gene in type I collagen producing osteoblasts(91). In these studies, it was found that mice with the loss of Phd2 gene function in osteoblasts had significant reductions in bone size and trabecular bone volume that was caused by impaired bone formation and not bone resorption. The loss of PHD2 resulted in reduced expression of osterix, osteocalcin and bone sialoprotein in osteoblasts, thus suggesting that PHD2 plays an important role in regulating bone formation in part by modulating expression of osterix and bone formation marker genes. Inactivation of PHD2 is predicted to increase HIF1α protein levels due to its escape from proteasomal degradation. Accordingly, the serum levels of erythropoietin, a target of HIF1α, are increased in the osteoblast PHD2 conditional knockout mice, since conditional deletion of HIF1α in osteoblasts has shown that HIF1α is a positive regulator of bone formation(92). Furthermore, the HIF1α pathway has been shown to be activated during bone repair and manipulation of HIFα signaling has been used as a strategy to promote bone repair(93,94). These findings seem to argue against the involvement of HIF1α signaling in mediating the positive effects of PHD2 in osteoblast differentiation and bone formation.
In contrast to the positive effects of PHD2 in osteoblasts, recent studies in our laboratory involving mice with conditional disruption of PHD2 in chondrocytes have uncovered an opposing function of PHD2 in chondrocytes. A conditional knockout of PHD2 in collagen 2 positive (Col2+) chondrocytes leads to a dramatic increase in endochondral bone formation and trabecular bone volume at multiple skeletal sites. The increased bone formation is caused by increased chondrocyte and osteoblast functions as reflected by the marked increases in expression levels of osterix, ALP, and bone sialoprotein, as well as HIF1α and chondrogenic genes such as Col2 and Sox9(95). PHD2 inhibition in chondrocytes additionally increases the potential for cartilage repair by preventing degradation of HIF2α, which acts as an upstream enhancer of expression of the cartilage regulator Sox9 (96). Indeed, knockdown of von Hippel-Lindau (VHL) tumor suppressor protein, an E3 ubiquitin ligase which ubiquitinates HIF1α post PHD2 hydroxylation, results in a HIF1α mediated increase in Sox9 and subsequent chondrogenic differentiation(97). Taken together, it would seem that PHD2 negatively regulates endochondral ossification by inhibiting the HIF1α pathway. The issue of whether the PHD2-mediated HIF1α signaling pathway is involved in mediating AA effects on chondrocytes remains to be established.
Another exciting mechanism for vitamin C regulation of gene transcription is via epigenetic modification of gene activity. DNA methylation at the 5 position of cytosine is the major covalent modification of mammalian DNA that plays a critical role in regulating gene transcription. AA induces Ten-Eleven Translocation (Tet) 1/2 function by acting as an enzymatic cofactor for the hydroxylation of 5-methylcytosine (5-mC). By doing so, it upregulates transcription in genes that typically have hypermethylated regions; however, it does this independently of increasing Tet expression(98,99). Tet2 promotes hematopoietic differentiation and mice with Tet2 deficiencies develop myeloid malignancies and eventually die(99,100). Furthermore, Blaschke et al.(98) demonstrated that knockdown of Tet1/2 prevented AA-induced hydroxylation of methyl cytosine in mouse embryonic stem cells, thus suggesting that vitamin C is a direct regulator of Tet activity and DNA methylation fidelity. This novel function of vitamin C in promoting Tet-mediated generation of hydroxyl-methyl cytosine supports ascorbate as a critical mediator of the interface between the genome and the environment(99).
Future Research Considerations
Despite efforts to eradicate vitamin C deficiency by its supplementation with food products, vitamin C deficiency and insufficiency persists, and likely contributes to suboptimal bone health. Even with normal intakes of vitamin C, effects in any of the mechanisms (e.g. vitamin C transporters, reduction of DHAA to AA) can influence cellular levels of vitamin C. Furthermore, subtle genetic changes in any one of the genes involved in the vitamin C signaling pathway in target cell types could lead to alterations in bone formation (Fig. 2, Fig. 3). Thus, future elucidation of AA pathway gene alleles that are polymorphic and elucidation of a linkage between polymorphisms and bone formation could lead to a better understanding of how this pathway affects human skeletal maintenance.
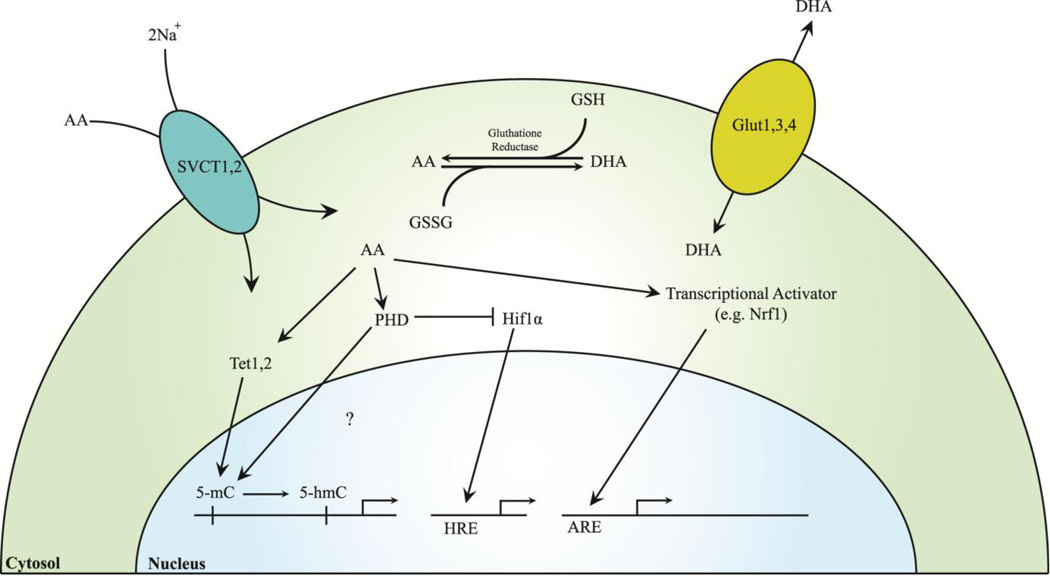
Fig. 2. Intracellular vitamin C mechanisms and transport.
SCVT 1/2 are responsible for ion mediated transport of AA into cells, while Glut 1/3/4 transport DHA. AA and DHA states are mediated via glutathione reductase. AA induces nuclear translocation of transcriptional activators such as Nrf1, which bind to AREs and activate transcription of bone proliferation/differentiation factors, and Tet 1/2, which hydroxylate 5-mCs, thereby removing epigenetic silencing. AA activates PHDs, which activate degradation of HIFs and may hydroxylate 5-mCs. HIFs bind to HREs, which can act as transcriptional activators of bone differentiation or apoptosis. Abbreviations: 5-mC, 5-methylcytosine; 5-hmC, 5-hydroxymethylcytosine; AA, ascorbic acid; ARE, antioxidant response element; DHA, dehydroascorbic acid; Glut, glucose transporter; GSH, glutathione; GSSG, glutathione disulfide; HIF, hypoxia-inducible factor; HRE, hypoxia response element; Nrf1, nuclear factor-E2-related factor; PHD, prolyl hydroxylase domain protein; SVCT, sodium-dependent vitamin C transporter-1; Tet, ten-eleven translocation protein.
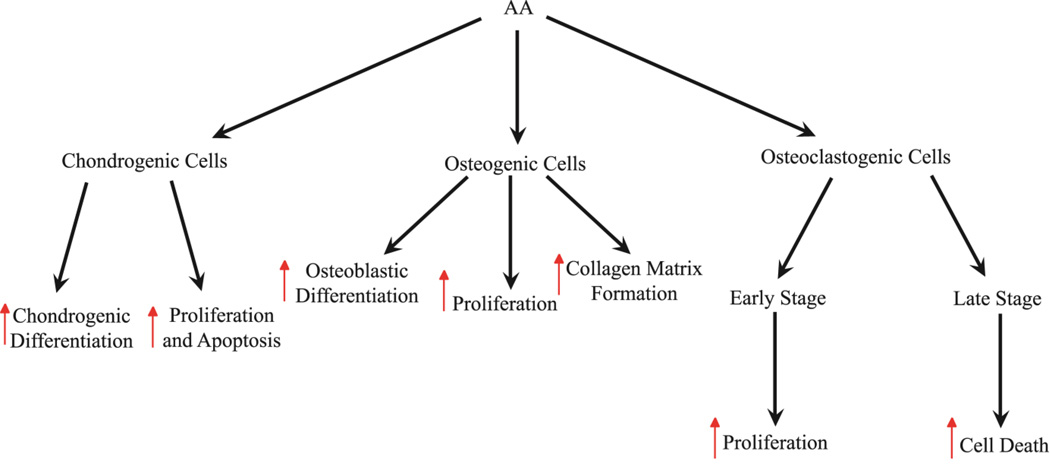
Fig. 3. Differential effects of vitamin C on different cell types.
AA induces chondrogenic differentiation and cell maintenance (Upregulated proliferation and apoptosis) in chondrogenic cells. AA promotes collagen matrix formation, as well as osteoblastic differentiation and proliferation in osteogenic cells. In early stage osteoclasts, AA induces proliferation, whereas in late stage osteoclasts, AA induces the upregulation of cell death. Red arrows indicate upregulation of the cell property.
Vitamin C deficiency caused by mutations in the GULO gene caused a dramatic reduction in bone mass of long bones but not in the vertebra(49). Accordingly, spontaneous fractures were mainly identified in the long bones of spontaneous fracture mutant mice caused by deletion of the GULO gene(57). Interestingly, deletion of PHD2 in type I collagen expressing osteoblasts also exerted differential effects on long bones versus vertebra. Loss of PHD2 in osteoblasts reduced trabecular bone volume in the femur but not in the vertebra(91). Furthermore, genetic linkage studies show that genes that contribute to variation in peak bone mass are skeletal site dependent(101). These data imply that the vitamin C signaling pathway must be exploited towards identification of therapies to treat hip fractures, the most debilitating osteoporotic fractures in humans.
While AA is a known cofactor for PHD enzymes, deletion of PHD2 in osteoblasts versus chondrocytes produced opposite skeletal phenotypes. To better understand the mechanism of its action, it will be important to identify more targets (transcriptional modulators) of PHD2 hydroxylation and ubiquitination. Knockdown of PHD2 resulted in dramatic increase in the expression of PHD3 in both osteoblasts and chondrocytes(91). However, the role of PHD3 in regulating the functions of these two cell types is unknown. AA has also been shown to exert different effects on osteoclasts depending on its differentiation status. Besides bone cells, vitamin C is also known to regulate differentiation of other cell types including neurons, myoblasts and keratinocytes. The issue of whether the PHD-mediated mechanism is involved in mediating vitamin C effects in these other cell types should be the subject of future investigation.
Vitamin C regulation of Tet is important for gene regulation in embryonic stem cells and represents a key exciting discovery in this area. Vitamin C regulation of hydroxylation of methyl cytosine provides an interesting avenue to study AA regulation of transcription at the whole genome level. While AA regulation of hydroxylation of proteins via PHD has been well studied, the issue of whether PHDs are also involved in the hydroxylation of methyl cytosine remains an interesting possibility. In this regard, there is evidence that PHDs are localized in the nucleus(102). Thus, AA regulation of gene transcription via epigenetic modification of genome activity involving PHDs provides another mechanism for regulation of vitamin C target genes, a concept that needs to be examined experimentally in future investigations.
Finally, further epidemiological studies are required to establish a better understanding of the vitamin C effect on human bone. As illustrated by Finck et al.(15), while many different varieties of studies exist, many of them lack the proper controls (i.e. placebo controlled randomized clinical trials, proper sample sizes, etc.) to obtain consistent results. Therefore, epidemiological studies with stringent variable controls must be considered.
Conclusions
AA has been shown to be a vital modulator of osteogenic and chondrogenic differentiation. Vertebrate organisms deficient in normal physiological levels of AA develop bone disorders such as spontaneous fracturing, impaired bone growth and impaired bone healing. Therefore, the effect of AA on bone health is vital and has proven to be regulated through a series of complex mechanisms of interaction. Although there is some inconsistency in studies conducted in humans, most point to the conclusion that reduced serum vitamin C levels or intake may be associated with the development of osteoporosis and increased risk of fracture. In order to develop novel therapeutics for vitamin C deficiencies, it is important to understand all of the methods by which it is absorbed and manipulated to be functionally relevant within the cell. This includes its functions as an antioxidant, but also as a cofactor that is involved in gene regulation that can influence bone development/regeneration. Identifying downstream effectors of vitamin C and understanding the mechanisms by which they interact will provide more targets for the treatment of bone related illnesses. Additionally, bone health is not entirely mediated via AA dependent mechanisms, and the existence of other mechanisms provides an open door to worthwhile studies on the synergistic impact of multiple bone modulators.
Acknowledgments
This study was supported by funding from a Veterans Administration BLR&D merit review grant 1-101-BX-001396 to SM and the National Institutes of Arthritis and Musculoskeletal Diseases R01 grant AR048139 to SM. The authors would like to thank Dr. Donna Strong for proof reading the manuscript.
Footnotes
Disclosure
All authors state that they have no conflicts of interest.
Authors roles: Drafting manuscript: PA, SH, and SM. Revising manuscript content: PA, SH, MW, and SM. Approving final version of manuscript: SM
References
- 1.Walmsley AR, Batten MR, Lad U, Bulleid NJ. Intracellular retention of procollagen within the endoplasmic reticulum is mediated by prolyl 4-hydroxylase. The Journal of biological chemistry. 1999;274(21):14884–14892. doi: 10.1074/jbc.274.21.14884. [DOI] [PubMed] [Google Scholar]
- 2.Marini JC, Cabral WA, Barnes AM, Chang W. Components of the collagen prolyl 3-hydroxylation complex are crucial for normal bone development. Cell cycle. 2007;6(14):1675–1681. doi: 10.4161/cc.6.14.4474. [DOI] [PubMed] [Google Scholar]
- 3.Geber J, Murphy E. Scurvy in the Great Irish Famine: evidence of vitamin C deficiency from a mid-19th century skeletal population. American journal of physical anthropology. 2012;148(4):512–524. doi: 10.1002/ajpa.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pimentel L. Scurvy: historical review and current diagnostic approach. The American journal of emergency medicine. 2003;21(4):328–332. doi: 10.1016/s0735-6757(03)00083-4. [DOI] [PubMed] [Google Scholar]
- 5.Levavasseur M, Becquart C, Pape E, et al. Severe scurvy: an underestimated disease. European journal of clinical nutrition. 2015 doi: 10.1038/ejcn.2015.99. [DOI] [PubMed] [Google Scholar]
- 6.Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(5):2879–2882. doi: 10.1073/pnas.78.5.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franceschi RT, Iyer BS, Cui Y. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1994;9(6):843–854. doi: 10.1002/jbmr.5650090610. [DOI] [PubMed] [Google Scholar]
- 8.Villacorta L, Azzi A, Zingg JM. Regulatory role of vitamins E and C on extracellular matrix components of the vascular system. Molecular aspects of medicine. 2007;28(5–6):507–537. doi: 10.1016/j.mam.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 9.May JM, Harrison FE. Role of vitamin C in the function of the vascular endothelium. Antioxidants & redox signaling. 2013;19(17):2068–2083. doi: 10.1089/ars.2013.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashor AW, Siervo M, Lara J, Oggioni C, Afshar S, Mathers JC. Effect of vitamin C and vitamin E supplementation on endothelial function: a systematic review and meta-analysis of randomised controlled trials. The British journal of nutrition. 2015;113(8):1182–1194. doi: 10.1017/S0007114515000227. [DOI] [PubMed] [Google Scholar]
- 11.May JM. Vitamin C transport and its role in the central nervous system. Sub-cellular biochemistry. 2012;56:85–103. doi: 10.1007/978-94-007-2199-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison FE, Bowman GL, Polidori MC. Ascorbic acid and the brain: rationale for the use against cognitive decline. Nutrients. 2014;6(4):1752–1781. doi: 10.3390/nu6041752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catani MV, Savini I, Rossi A, Melino G, Avigliano L. Biological role of vitamin C in keratinocytes. Nutrition reviews. 2005;63(3):81–90. doi: 10.1111/j.1753-4887.2005.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 14.Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. Journal of the American Dietetic Association. 2000;100(6):637–640. doi: 10.1016/S0002-8223(00)00189-9. [DOI] [PubMed] [Google Scholar]
- 15.Finck H, Hart AR, Jennings A, Welch AA. Is there a role for vitamin C in preventing osteoporosis and fractures? A review of the potential underlying mechanisms and current epidemiological evidence. Nutr Res Rev. 2014;27(2):268–283. doi: 10.1017/S0954422414000195. [DOI] [PubMed] [Google Scholar]
- 16.Hall SL, Greendale GA. The relation of dietary vitamin C intake to bone mineral density: results from the PEPI study. Calcified tissue international. 1998;63(3):183–189. doi: 10.1007/s002239900512. [DOI] [PubMed] [Google Scholar]
- 17.New SA, Bolton-Smith C, Grubb DA, Reid DM. Nutritional influences on bone mineral density: a cross-sectional study in premenopausal women. The American journal of clinical nutrition. 1997;65(6):1831–1839. doi: 10.1093/ajcn/65.6.1831. [DOI] [PubMed] [Google Scholar]
- 18.New SA, Robins SP, Campbell MK, et al. Dietary influences on bone mass and bone metabolism: further evidence of a positive link between fruit and vegetable consumption and bone health? The American journal of clinical nutrition. 2000;71(1):142–151. doi: 10.1093/ajcn/71.1.142. [DOI] [PubMed] [Google Scholar]
- 19.Leveille SG, LaCroix AZ, Koepsell TD, Beresford SA, Van Belle G, Buchner DM. Dietary vitamin C and bone mineral density in postmenopausal women in Washington State, USA. Journal of epidemiology and community health. 1997;51(5):479–485. doi: 10.1136/jech.51.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morton DJ, Barrett-Connor EL, Schneider DL. Vitamin C supplement use and bone mineral density in postmenopausal women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001;16(1):135–140. doi: 10.1359/jbmr.2001.16.1.135. [DOI] [PubMed] [Google Scholar]
- 21.Pasco JA, Henry MJ, Wilkinson LK, Nicholson GC, Schneider HG, Kotowicz MA. Antioxidant vitamin supplements and markers of bone turnover in a community sample of nonsmoking women. Journal of women’s health. 2006;15(3):295–300. doi: 10.1089/jwh.2006.15.295. [DOI] [PubMed] [Google Scholar]
- 22.Simon JA, Hudes ES. Relation of ascorbic acid to bone mineral density and self-reported fractures among US adults. American journal of epidemiology. 2001;154(5):427–433. doi: 10.1093/aje/154.5.427. [DOI] [PubMed] [Google Scholar]
- 23.Ilich JZ, Brownbill RA, Tamborini L. Bone and nutrition in elderly women: protein, energy, and calcium as main determinants of bone mineral density. European journal of clinical nutrition. 2003;57(4):554–565. doi: 10.1038/sj.ejcn.1601577. [DOI] [PubMed] [Google Scholar]
- 24.Sowers MR, Wallace RB, Lemke JH. Correlates of mid-radius bone density among postmenopausal women: a community study. The American journal of clinical nutrition. 1985;41(5):1045–1053. doi: 10.1093/ajcn/41.5.1045. [DOI] [PubMed] [Google Scholar]
- 25.Wolf RL, Cauley JA, Pettinger M, et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: results from the Women’s Health Initiative. The American journal of clinical nutrition. 2005;82(3):581–588. doi: 10.1093/ajcn.82.3.581. [DOI] [PubMed] [Google Scholar]
- 26.Lumbers M, New SA, Gibson S, Murphy MC. Nutritional status in elderly female hip fracture patients: comparison with an age-matched home living group attending day centres. The British journal of nutrition. 2001;85(6):733–740. doi: 10.1079/bjn2001350. [DOI] [PubMed] [Google Scholar]
- 27.Sahni S, Hannan MT, Gagnon D, et al. Protective effect of total and supplemental vitamin C intake on the risk of hip fracture--a 17-year follow-up from the Framingham Osteoporosis Study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009;20(11):1853–1861. doi: 10.1007/s00198-009-0897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prynne CJ, Mishra GD, O’Connell MA, et al. Fruit and vegetable intakes and bone mineral status: a cross sectional study in 5 age and sex cohorts. The American journal of clinical nutrition. 2006;83(6):1420–1428. doi: 10.1093/ajcn/83.6.1420. [DOI] [PubMed] [Google Scholar]
- 29.Chuin A, Labonte M, Tessier D, et al. Effect of antioxidants combined to resistance training on BMD in elderly women: a pilot study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009;20(7):1253–1258. doi: 10.1007/s00198-008-0798-5. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Ramos M, Vargas LA, Fortoul Van der Goes TI, Cervantes-Sandoval A, Mendoza-Nunez VM. Supplementation of ascorbic acid and alpha-tocopherol is useful to preventing bone loss linked to oxidative stress in elderly. J Nutr Health Aging. 2010;14(6):467–472. doi: 10.1007/s12603-010-0099-5. [DOI] [PubMed] [Google Scholar]
- 31.Sahni S, Hannan MT, Gagnon D, et al. High vitamin C intake is associated with lower 4-year bone loss in elderly men. J Nutr. 2008;138(10):1931–1938. doi: 10.1093/jn/138.10.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaptoge S, Welch A, McTaggart A, et al. Effects of dietary nutrients and food groups on bone loss from the proximal femur in men and women in the 7th and 8th decades of age. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2003;14(5):418–428. doi: 10.1007/s00198-003-1391-6. [DOI] [PubMed] [Google Scholar]
- 33.Sugiura M, Nakamura M, Ogawa K, et al. Dietary patterns of antioxidant vitamin and carotenoid intake associated with bone mineral density: findings from post-menopausal Japanese female subjects. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011;22(1):143–152. doi: 10.1007/s00198-010-1239-9. [DOI] [PubMed] [Google Scholar]
- 34.Falch JA, Mowe M, Bohmer T. Low levels of serum ascorbic acid in elderly patients with hip fracture. Scand J Clin Lab Invest. 1998;58(3):225–228. doi: 10.1080/00365519850186616. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Ramirez MJ, Palma Perez S, Delgado-Martinez AD, Martinez-Gonzalez MA, De la Fuente Arrillaga C, Delgado-Rodriguez M. Vitamin C, vitamin B12, folate and the risk of osteoporotic fractures. A case-control study. Int J Vitam Nutr Res. 2007;77(6):359–368. doi: 10.1024/0300-9831.77.6.359. [DOI] [PubMed] [Google Scholar]
- 36.Maggio D, Barabani M, Pierandrei M, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88(4):1523–1527. doi: 10.1210/jc.2002-021496. [DOI] [PubMed] [Google Scholar]
- 37.Park HM, Heo J, Park Y. Calcium from plant sources is beneficial to lowering the risk of osteoporosis in postmenopausal Korean women. Nutr Res. 2011;31(1):27–32. doi: 10.1016/j.nutres.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations health. 1951;41(3):279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maimoun L, Simar D, Caillaud C, et al. Effect of antioxidants and exercise on bone metabolism. J Sports Sci. 2008;26(3):251–258. doi: 10.1080/02640410701501689. [DOI] [PubMed] [Google Scholar]
- 40.Gabbay KH, Bohren KM, Morello R, Bertin T, Liu J, Vogel P. Ascorbate synthesis pathway: dual role of ascorbate in bone homeostasis. The Journal of biological chemistry. 2010;285(25):19510–19520. doi: 10.1074/jbc.M110.110247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linster CL, Van Schaftingen E, Vitamin C. Biosynthesis, recycling and degradation in mammals. The FEBS journal. 2007;274(1):1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- 42.Yang H. Conserved or lost: molecular evolution of the key gene GULO in vertebrate vitamin C biosynthesis. Biochemical genetics. 2013;51(5–6):413–425. doi: 10.1007/s10528-013-9574-0. [DOI] [PubMed] [Google Scholar]
- 43.Nishikimi M, Kawai T, Yagi K. Guinea pigs possess a highly mutated gene for L-gulono-gamma-lactone oxidase, the key enzyme for L-ascorbic acid biosynthesis missing in this species. The Journal of biological chemistry. 1992;267(30):21967–21972. [PubMed] [Google Scholar]
- 44.Drouin G, Godin JR, Page B. The genetics of vitamin C loss in vertebrates. Current genomics. 2011;12(5):371–378. doi: 10.2174/138920211796429736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bates CJ. Vitamin C deficiency in guinea pigs: variable sensitivity of collagen at different sites. Int J Vitam Nutr Res. 1979;49(1):77–86. [PubMed] [Google Scholar]
- 46.Tsuchiya H, Bates CJ. Ascorbic acid deficiency in guinea pigs: contrasting effects of tissue ascorbic acid depletion and of associated inanition on status indices related to collagen and vitamin D. The British journal of nutrition. 1994;72(5):745–752. doi: 10.1079/bjn19940076. [DOI] [PubMed] [Google Scholar]
- 47.Kipp DE, McElvain M, Kimmel DB, Akhter MP, Robinson RG, Lukert BP. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone. 1996;18(3):281–288. doi: 10.1016/8756-3282(95)00481-5. [DOI] [PubMed] [Google Scholar]
- 48.Gould B, Schwachman H. Bone and Tissue Phosphatase in Experimental Scurvy. Am J Phys. 1941;135(2):485–491. [Google Scholar]
- 49.Mohan S, Kapoor A, Singgih A, et al. Spontaneous fractures in the mouse mutant sfx are caused by deletion of the gulonolactone oxidase gene, causing vitamin C deficiency. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005;20(9):1597–1610. doi: 10.1359/JBMR.050406. [DOI] [PubMed] [Google Scholar]
- 50.Jiao Y, Li X, Beamer WG, et al. A deletion causing spontaneous fracture identified from a candidate region of mouse Chromosome 14. Mammalian genome : official journal of the International Mammalian Genome Society. 2005;16(1):20–31. doi: 10.1007/s00335-004-2414-0. [DOI] [PubMed] [Google Scholar]
- 51.Kawai T, Nishikimi M, Ozawa T, Yagi K. A missense mutation of L-gulono-gamma-lactone oxidase causes the inability of scurvy-prone osteogenic disorder rats to synthesize L-ascorbic acid. The Journal of biological chemistry. 1992;267(30):21973–21976. [PubMed] [Google Scholar]
- 52.Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(2):841–846. doi: 10.1073/pnas.97.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim H, Bae S, Yu Y, et al. The analysis of vitamin C concentration in organs of gulo(−/−) mice upon vitamin C withdrawal. Immune network. 2012;12(1):18–26. doi: 10.4110/in.2012.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim W, Bae S, Kim H, et al. Ascorbic acid insufficiency induces the severe defect on bone formation via the down-regulation of osteocalcin production. Anatomy & cell biology. 2013;46(4):254–261. doi: 10.5115/acb.2013.46.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakamoto Y, Takano Y. Morphological influence of ascorbic acid deficiency on endochondral ossification in osteogenic disorder Shionogi rat. The Anatomical record. 2002;268(2):93–104. doi: 10.1002/ar.10122. [DOI] [PubMed] [Google Scholar]
- 56.Tsunenari T, Fukase M, Fujita T. Bone histomorphometric analysis for the cause of osteopenia in vitamin C-deficient rat (ODS rat) Calcified tissue international. 1991;48(1):18–27. doi: 10.1007/BF02555792. [DOI] [PubMed] [Google Scholar]
- 57.Beamer WG, Rosen CJ, Bronson RT, et al. Spontaneous fracture (sfx): a mouse genetic model of defective peripubertal bone formation. Bone. 2000;27(5):619–626. doi: 10.1016/s8756-3282(00)00369-0. [DOI] [PubMed] [Google Scholar]
- 58.Kondo Y, Inai Y, Sato Y, et al. Senescence marker protein 30 functions as gluconolactonase in L-ascorbic acid biosynthesis, and its knockout mice are prone to scurvy. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(15):5723–5728. doi: 10.1073/pnas.0511225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maruyama N, Ishigami A, Kondo Y. Pathophysiological significance of senescence marker protein-30. Geriatrics & gerontology international. 2010;10(Suppl 1):S88–S98. doi: 10.1111/j.1447-0594.2010.00586.x. [DOI] [PubMed] [Google Scholar]
- 60.Park JK, Lee EM, Kim AY, et al. Vitamin C deficiency accelerates bone loss inducing an increase in PPAR-gamma expression in SMP30 knockout mice. International journal of experimental pathology. 2012;93(5):332–340. doi: 10.1111/j.1365-2613.2012.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leboy PS, Vaias L, Uschmann B, Golub E, Adams SL, Pacifici M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. The Journal of biological chemistry. 1989;264(29):17281–17286. [PubMed] [Google Scholar]
- 62.Daniel JC, Pauli BU, Kuettner KE. Synthesis of cartilage matrix by mammalian chondrocytes in vitro. III. Effects of ascorbate. The Journal of cell biology. 1984;99(6):1960–1969. doi: 10.1083/jcb.99.6.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Temu TM, Wu KY, Gruppuso PA, Phornphutkul C. The mechanism of ascorbic acid-induced differentiation of ATDC5 chondrogenic cells. American journal of physiology Endocrinology and metabolism. 2010;299(2):E325–E334. doi: 10.1152/ajpendo.00145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1992;7(2):235–246. doi: 10.1002/jbmr.5650070216. [DOI] [PubMed] [Google Scholar]
- 65.Franceschi RT, Young J. Regulation of alkaline phosphatase by 1,25-dihydroxyvitamin D3 and ascorbic acid in bone-derived cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1990;5(11):1157–1167. doi: 10.1002/jbmr.5650051111. [DOI] [PubMed] [Google Scholar]
- 66.Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid-dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Molecular endocrinology. 1997;11(8):1103–1113. doi: 10.1210/mend.11.8.9955. [DOI] [PubMed] [Google Scholar]
- 67.Hadzir SN, Ibrahim SN, Abdul Wahab RM, et al. Ascorbic acid induces osteoblast differentiation of human suspension mononuclear cells. Cytotherapy. 2014;16(5):674–682. doi: 10.1016/j.jcyt.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 68.Buttery LD, Bourne S, Xynos JD, et al. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue engineering. 2001;7(1):89–99. doi: 10.1089/107632700300003323. [DOI] [PubMed] [Google Scholar]
- 69.Otsuka E, Yamaguchi A, Hirose S, Hagiwara H. Characterization of osteoblastic differentiation of stromal cell line ST2 that is induced by ascorbic acid. The American journal of physiology. 1999;277(1 Pt 1):C132–C138. doi: 10.1152/ajpcell.1999.277.1.C132. [DOI] [PubMed] [Google Scholar]
- 70.Harada S, Matsumoto T, Ogata E. Role of ascorbic acid in the regulation of proliferation in osteoblast-like MC3T3-E1 cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1991;6(9):903–908. doi: 10.1002/jbmr.5650060902. [DOI] [PubMed] [Google Scholar]
- 71.Takamizawa S, Maehata Y, Imai K, Senoo H, Sato S, Hata R. Effects of ascorbic acid and ascorbic acid 2-phosphate, a long-acting vitamin C derivative, on the proliferation and differentiation of human osteoblast-like cells. Cell biology international. 2004;28(4):255–265. doi: 10.1016/j.cellbi.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 72.Tsuneto M, Yamazaki H, Yoshino M, Yamada T, Hayashi S. Ascorbic acid promotes osteoclastogenesis from embryonic stem cells. Biochemical and biophysical research communications. 2005;335(4):1239–1246. doi: 10.1016/j.bbrc.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 73.Xiao XH, Liao EY, Zhou HD, Dai RC, Yuan LQ, Wu XP. Ascorbic acid inhibits osteoclastogenesis of RAW264.7 cells induced by receptor activated nuclear factor kappaB ligand (RANKL) in vitro. Journal of endocrinological investigation. 2005;28(3):253–260. doi: 10.1007/BF03345382. [DOI] [PubMed] [Google Scholar]
- 74.Le Nihouannen D, Barralet JE, Fong JE, Komarova SV. Ascorbic acid accelerates osteoclast formation and death. Bone. 2010;46(5):1336–1343. doi: 10.1016/j.bone.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 75.Otsuka E, Kato Y, Hirose S, Hagiwara H. Role of ascorbic acid in the osteoclast formation: induction of osteoclast differentiation factor with formation of the extracellular collagen matrix. Endocrinology. 2000;141(8):3006–3011. doi: 10.1210/endo.141.8.7597. [DOI] [PubMed] [Google Scholar]
- 76.Lean JM, Davies JT, Fuller K, et al. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. The Journal of clinical investigation. 2003;112(6):915–923. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu LL, Cao J, Sun M, et al. Vitamin C prevents hypogonadal bone loss. PloS one. 2012;7(10):e47058. doi: 10.1371/journal.pone.0047058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Padayatty SJ, Katz A, Wang Y, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. Journal of the American College of nutrition. 2003;22(1):18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 79.Melhus H, Michaelsson K, Holmberg L, Wolk A, Ljunghall S. Smoking, antioxidant vitamins, and the risk of hip fracture. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1999;14(1):129–135. doi: 10.1359/jbmr.1999.14.1.129. [DOI] [PubMed] [Google Scholar]
- 80.Baylink DJ, Finkelman RD, Mohan S. Growth factors to stimulate bone formation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1993;8(Suppl 2):S565–S572. doi: 10.1002/jbmr.5650081326. [DOI] [PubMed] [Google Scholar]
- 81.Zunich SM, Valdovinos M, Douglas T, Walterhouse D, Iannaccone P, Lamm ML. Osteoblast-secreted collagen upregulates paracrine Sonic hedgehog signaling by prostate cancer cells and enhances osteoblast differentiation. Molecular cancer. 2012;11:30. doi: 10.1186/1476-4598-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim J, Xing W, Wergedal J, Chan JY, Mohan S. Targeted disruption of nuclear factor erythroid-derived 2-like 1 in osteoblasts reduces bone size and bone formation in mice. Physiological genomics. 2010;40(2):100–110. doi: 10.1152/physiolgenomics.00105.2009. [DOI] [PubMed] [Google Scholar]
- 83.Oyake T, Itoh K, Motohashi H, et al. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Molecular and cellular biology. 1996;16(11):6083–6095. doi: 10.1128/mcb.16.11.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gallorini M, Petzel C, Bolay C, et al. Activation of the Nrf2-regulated antioxidant cell response inhibits HEMA-induced oxidative stress and supports cell viability. Biomaterials. 2015;56:114–128. doi: 10.1016/j.biomaterials.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 85.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free radical biology & medicine. 2009;47(9):1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pelin M, De Iudicibus S, Fusco L, et al. Role of Oxidative Stress Mediated by Glutathione-S-transferase in Thiopurines’ Toxic Effects. Chemical research in toxicology. 2015;28(6):1186–1195. doi: 10.1021/acs.chemrestox.5b00019. [DOI] [PubMed] [Google Scholar]
- 87.Niture SK, Kaspar JW, Shen J, Jaiswal AK. Nrf2 signaling and cell survival. Toxicology and applied pharmacology. 2010;244(1):37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fan L, Li J, Yu Z, Dang X, Wang K. The hypoxia-inducible factor pathway, prolyl hydroxylase domain protein inhibitors, and their roles in bone repair and regeneration. BioMed research international. 2014;2014:239356. doi: 10.1155/2014/239356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xing W, Pourteymoor S, Mohan S. Ascorbic acid regulates osterix expression in osteoblasts by activation of prolyl hydroxylase and ubiquitination-mediated proteosomal degradation pathway. Physiological genomics. 2011;43(12):749–757. doi: 10.1152/physiolgenomics.00229.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Minamishima YA, Moslehi J, Bardeesy N, Cullen D, Bronson RT, Kaelin WG., Jr Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood. 2008;111(6):3236–3244. doi: 10.1182/blood-2007-10-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng S, Xing W, Pourteymoor S, Mohan S. Conditional disruption of the prolyl hydroxylase domain-containing protein 2 (Phd2) gene defines its key role in skeletal development. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29(10):2276–2286. doi: 10.1002/jbmr.2258. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y, Wan C, Deng L, et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. The Journal of clinical investigation. 2007;117(6):1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ding H, Gao YS, Hu C, et al. HIF-1alpha transgenic bone marrow cells can promote tissue repair in cases of corticosteroid-induced osteonecrosis of the femoral head in rabbits. PloS one. 2013;8(5):e63628. doi: 10.1371/journal.pone.0063628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wan C, Gilbert SR, Wang Y, et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(2):686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng S, Xing W, Pourteymoor S, Schulte J, Mohan S. Conditional Deletion of Prolyl Hydroxylase Domain-containing Protein 2 (Phd2) Gene Reveals its Essential Role in Chondrocyte Function and Endochondral Bone Formation. Endocrinology. Forthcoming. 2015 doi: 10.1210/en.2015-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thoms BL, Murphy CL. Inhibition of hypoxia-inducible factor-targeting prolyl hydroxylase domain-containing protein 2 (PHD2) enhances matrix synthesis by human chondrocytes. The Journal of biological chemistry. 2010;285(27):20472–20480. doi: 10.1074/jbc.M110.115238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Riddle RC, Khatri R, Schipani E, Clemens TL. Role of hypoxia-inducible factor-1alpha in angiogenic-osteogenic coupling. Journal of molecular medicine. 2009;87(6):583–590. doi: 10.1007/s00109-009-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blaschke K, Ebata KT, Karimi MM, et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature. 2013;500(7461):222–226. doi: 10.1038/nature12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Minor EA, Court BL, Young JI, Wang G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. The Journal of biological chemistry. 2013;288(19):13669–13674. doi: 10.1074/jbc.C113.464800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ponnaluri VK, Maciejewski JP, Mukherji M. A mechanistic overview of TET-mediated 5-methylcytosine oxidation. Biochemical and biophysical research communications. 2013;436(2):115–120. doi: 10.1016/j.bbrc.2013.05.077. [DOI] [PubMed] [Google Scholar]
- 101.Sabsovich I, Clark JD, Liao G, et al. Bone microstructure and its associated genetic variability in 12 inbred mouse strains: microCT study and in silico genome scan. Bone. 2008;42(2):439–451. doi: 10.1016/j.bone.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pientka FK, Hu J, Schindler SG, et al. Oxygen sensing by the prolyl-4-hydroxylase PHD2 within the nuclear compartment and the influence of compartmentalisation on HIF-1 signalling. Journal of cell science. 2012;125(Pt 21):5168–5176. doi: 10.1242/jcs.109041. [DOI] [PubMed] [Google Scholar]
- 103.Mizushima Y, Harauchi T, Yoshizaki T, Makino S. A rat mutant unable to synthesize vitamin C. Experientia. 1984;40(4):359–361. doi: 10.1007/BF01952551. [DOI] [PubMed] [Google Scholar]
- 104.Ishigami A, Fujita T, Handa S, et al. Senescence marker protein-30 knockout mouse liver is highly susceptible to tumor necrosis factor-alpha- and Fas-mediated apoptosis. The American journal of pathology. 2002;161(4):1273–1281. doi: 10.1016/s0002-9440(10)64404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]