Abstract
Resting-state functional magnetic resonance imaging (Resting-state fMRI) is a tool for investigating the functional networks that arise during the resting-state of the brain. Recent advances of the resting-state fMRI analysis suggest its feasibility for evaluating language function. The most common clinical application is for presurgical mapping of cortex for a brain tumor or for resective epilespy surgery. In this article, we review the techniques and presurgical applications of resting-state fMRI analysis for language evaluation, and discuss the use in the clinical setting, focusing on planning for neurosurgery.
Keywords: fMRI, resting-state fMRI, functional connectivity, language, presurgical mapping
Introduction
Evaluating and mapping the language function of the brain is critical for planning neurosurgery in patients with neurological diseases, such as brain tumors and epilepsy, and for avoiding postsurgical functional deficits. Traditionally, the intracarotid amobarbital procedure (Wada test) has been used for determining language lateralization (1-3); however, recent advances in functional magnetic resonance imaging (fMRI) have made it possible to make this determination though the use of non-invasive neuroimaging techniques (4,5) and magnetoencephalography (MEG) (6,7). Currently, the standard technique requires acquiring functional neuroimaging data during a language task, which requires complicated paradigms and specifically trained personnel (see chapter in this volume by Brennan et al).
Resting-state fMRI (RS-fMRI) maps functional connections in the brain by analyzing the correlations of blood oxygenation level dependent (BOLD) signals throughout the brain, resulting in several resting-state networks (8). It is acquired at a resting state of the subject without requiring any tasks. Over a couple of decades, there have been increasing interests in the application of this method for understanding the brain networks in both healthy subjects and patients with neuropsychiatric diseases, e.g., Alzheimer's disease, schizophrenia and epilepsy (9,10,11). Here, we review the technique and the applications of RS-fMRI for evaluating the language function, and discuss combining functional and structural connectivity analyses.
Language mapping in presurgical evaluation
In epilepsy surgery, hemispheric language dominance, called the language lateralization, has been a major issue especially when a unilateral temporal lobectomy is considered as a surgical option. The Wada test, which administers a short-acting anesthetic to each hemisphere with amobarbital through a catheter in the intracarotid arteries, is the standard technique for determining the language lateralization; however, as an invasive technique, this test is not without risk to the patient. (1-3). Non-invasive neuroimaging techniques, including fMRI (4,5) and MEG (6,7), are now frequently used for language lateralization, and have successfully reduced the need for the Wada test (12). Typically these studies investigate the subject's neuronal responses generated by performing a language-related tasks such as semantic word-processing (5). The advantage of these neuroimaging techniques is the ability of localization of language function at the lobar or sublobar level in the brain, whereas the Wada test provides only lateralization information at the hemispheric level, i.e., the left or the right.
Classically, Wernicke and Broca areas are considered essential for representing receptive and expressive language function, thus, previous studies investigated the activation in these areas consisting of posterior part of superior/middle temporal gyrus, supramarginal gyrus as well as opercular and triangular parts of inferior frontal gyrus (pars opercualris and pars triangualris) (12,13). These anatomical regions are used as the region of interests (ROIs) for determining the lateralization. Laterality index (LI) is calculated as a ratio of the activation amplitude or the number of voxels of the activated cortex in the ROIs between both hemispheres (4-7). Several researchers have reported that various other regions, such as dorsolateral prefrontal cortex and primary motor cortex, also participate in language processing (12). Presurgical evaluation usually requires mapping the essential language areas, not just participating, therefore employment of optimal ROIs is critical for clinical purposes.
One of the limitations of this approach is that it requires the subject to actively participate and correctly perform complicated language tasks. These tasks are sometimes difficult to perform for children or patients with cognitive deficits, distorted fine motor skills and altered consciousness. Although there is a clinical demand, evaluating the language function by using task-based fMRI is still challenging in these patients.
Seed-based resting-state fMRI and language mapping
Resting-state fMRI measures intrinsic functional connectivity by calculating the temporal correlations of low-frequency BOLD signals (8). Images are acquired at resting condition, thus requiring no tasks. The intrinsic connectivity between functionally related parts of the brain allows for the mapping of neuronal networks. (8,9). Previous studies have revealed the altered connectivity in patients with epilepsy (11) compared with healthy controls; however, the relationship between focal epileptogenicity and abnormal connectivity is unclear. (14).
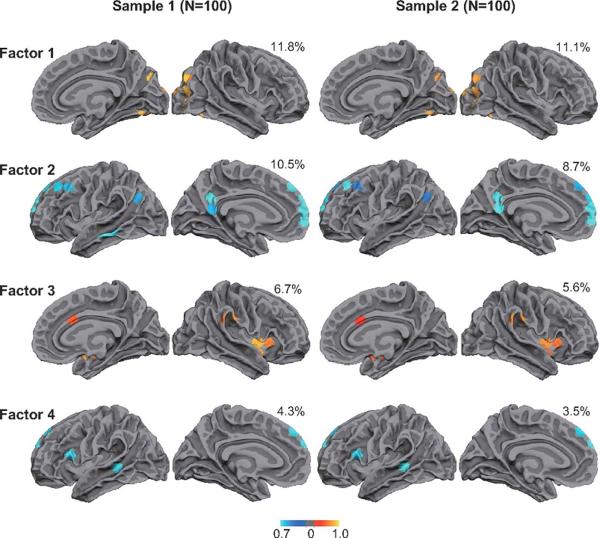
Resting-state fMRI has been studied for the ability to map the language network in healthy subjects (15) and patients with temporal lobe epilepsy (16). Recently several researchers have reported that the intrinsic connectivity of the whole brain demonstrates asymmetry between both hemispheres (17,18). Liu et al. (17) estimated the laterality of the intrinsic connectivity by deploying 200 seed/target regions in each hemisphere in 300 healthy subjects. They estimated the intensitic laterality (iLI) of the whole brain by calculating the correlation between seeds and targets throughout the cortex, and found the most lateralized regions from the resting state fMRI volumes. Multi-factorial analysis found four major clusters (Fig. 1), each of which represents the visual system (Factor 1), default mode network (Factor 2), attention system (Factor 3) and language network (Factor 4). Moreover, they found that LI of the intrinsic connectivity in the language network was significantly correlated with the LI obtained from the task-based language fMRI. Similarly, Wang et al. investigated the hemispheric differences of the connectivity by estimating the within-hemisphere and cross-hemisphere correlation (18). The laterality was measured by comparing the number of voxels correlated to within-hemisphere and cross-hemisphere seed ROIs distributed in both hemispheres. Again, they found that the laterality measurement of the intrinsic connectivity was correlated with the LI obtained from the task-based fMRI. These results strongly suggest that resting-state fMRI could be a potential tool for determining the language lateralization in clinical settings.
Fig. 1.
Factor analysis derived from the resting-fMRI intrinsic connectivity shows four significant clusters, each of which represents the visual system (Factor 1), default mode network (Factor 2), attention system (Factor 3) and language network (Factor 4). Used with permission (17)
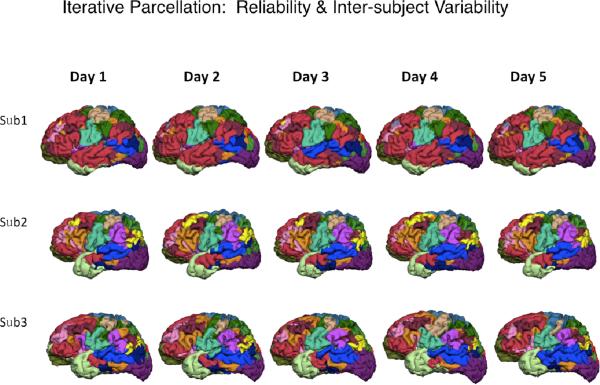
In addition to the language network, several different networks are robustly found by the resting-fMRI analysis using a variety of methods. Yeo et al. (19), for example, analyzed resting-fMRI data obtained from 1000 healthy subjects by using a clustering algorithm, and demonstrated the parcellation of these networks, including dorsal attention, ventral attention, frontoparietal control, and default networks, which are derived from the intrinsic functional connectivity. The population-based parcellation provides a robust atlas of the functional networks. Liu et al. (unpublished) have developed a procedure for parcellating the functional network of the individual brain by iterative application of templates generated from the population-based network atlas. This method successfully provides a stable atlas of individual functional networks obtained from the resting-fMRI data scanned on different days in the same subjects, i.e., showing the intra-subject stability as well as emphasizing the inter-subject differences of the intrinsic functional networks (Fig. 2). These results would be useful for establishing the reliability of resting-sate fMRI as a clinical testing.
Fig. 2.
The parcellation atlas of individuals show the intra-subject reliability and inter-subject variability of the functional networks. (Courtesy of Hesheng Liu, Ph. D)
Alternative approach: Independent component analysis
Previous studies analyzed the resting-state fMRI data by using a seed-based approach based on a priori knowledge of the language network (15,16) or distributed in the whole brain (17). The seed-based approach is highly interactive to determine the optimal placement of the seeds, however, employment of seeds may require significant experience to extract the language network from the whole brain connections. Independent component analysis (ICA) is an alternative approach, which decomposes the BOLD time series into a number of spatially independent components (20). These components provide identical maps, which may represent different networks. Several studies have reported successful extraction of neural networks compared to seed-based approaches, including the sensorimotor and visual networks (21,22).
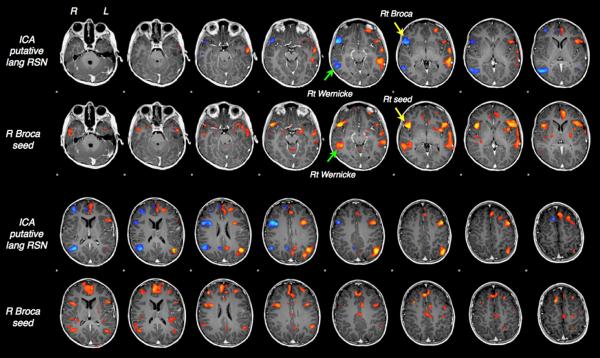
Application of ICA for demonstrating the language network was first reported by analyzing the task-based fMRI data (23), and later in resting-state fMRI (24), demonstrating lateralized connectivity in the language network determined by ICA. ICA is a model-free approach and does not require any a priori information of estimation for decomposition. On the other hand, it needs an optimized template to identify language-related components from other components. Fig. 3 represents an example of ICA-based mapping of the language network, showing the Wernicke and Broca areas which correlate well with the results of seed-based approach. A semi-automated protocols of the language networks and others, have suggested clinical value of ICA for determining the language lateralization in presurgical evaluation (21).
Fig.3.
ICA-based mapping of the language network, showing the Wernicke and Broca areas consistently with the results of seed-based approach. (Courtesy of Brad Buchbinder, M.D)
Future directions - Interactions with structural connectivity
The analysis of resting-state fMRI generally focus on the temporal correlation of the BOLD signals acquired at each voxel for calculating the functional connectivity networks. It does not incorporate the structural connections in the brain therefore lacks the structural basis. On the other hand, the structural connectivity networks have been investigated by using MR tractography derived from diffusion tensor imaging (DTI) or diffusion spectrum imaging (DSI) (25,26). The altered structural networks have been reported by analyzing the whole brain structural connectivity with graph theory in patients with temporal lobe epilepsy (27).
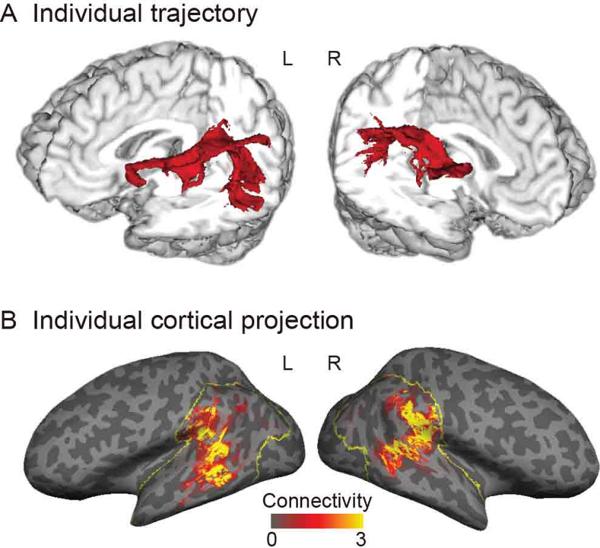
Structural connectivity has also been investigated in the context of understanding the language network. The arcuate fasciculus plays a critical role in this network by connecting Wenicke and Broca areas, and several researchers have reported the hemispheric asymmetry of arcuate fasciculus, showing that the white matter volume of the parietal-frontal pathway is larger in the right hemisphere, while that of the temporal-frontal pathway is larger in the left hemisphere in human subjects (28). Takaya et al. evaluated the tractography and its cortical projections by using surface-based analysis in healthy subjects (Fig. 4), and showed the asymmetric distribution of the arcuate fasciculus projections in the inferior parietal and lateral temporal cortices, extended more in the left than the right hemisphere (29).
Fig. 4.
A. Tractography represents the arcuate fasciculus. B. Cortical projection of tractography calculated by the surface-based analysis. Used with permission (29).
Recently the association between the functional and structural connectivity networks has been of interest in both healthy subjects and patients with neurological diseases. Previous studies have demonstrated that the structural connectivity networks are associated with or predictive of the functional connectivity networks in healthy subjects (26) and patients with temporal lobe epilepsy (30). Takaya et al. compared the structural, functional connectivity and regional BOLD activation, derived from DTI tractography, resting-state fMRI and task-based fMRI, respectively, and demonstrated the asymmetry overlaps in the left posterior temporal and parietal region in the right-handed healthy subjects (29). Integrated estimation incorporating both structural and functional aspects of the language network may provide reliable lateralization information in presurgical evaluation of epileptic patients.
Conclusions
Resting-state fMRI appears to provide useful information of the language network in both healthy population and patients with neuropsychiatric disease. Language lateralization and language mapping using the whole brain connectivity analysis or ICA may be useful in planning of surgery, particularly when operating on the language dominant hemisphere. Resting-state functional connectivity has now been established to have clinical relevance in the mapping of language function, especially in patient populations that might have difficulty performing task-based fMRI.
References
- 1.Dion JE, Gates PC, Fox AJ, et al. Clinical events following neuroangiography: a prospective study. Stroke. 1987;18:997–1004. doi: 10.1161/01.str.18.6.997. [DOI] [PubMed] [Google Scholar]
- 2.Snyder PJ, Harris LJ. The intracarotid amobarbital procedure: an historical perspective. Brain Cogn. 1997;33:18–32. doi: 10.1006/brcg.1997.0882. [DOI] [PubMed] [Google Scholar]
- 3.De Paola L, Mader MJ, Germiniani FM, et al. Bizarre behavior during intracarotid sodium amytal testing (WADA test): are they predictable? Arq Neuropsiquiatr. 2004;62:444–8. doi: 10.1590/s0004-282x2004000300012. [DOI] [PubMed] [Google Scholar]
- 4.Dym RJ, Burns J, Freeman K, et al. Is functional MR imaging assessment of hemispheric language dominance as good as the Wada test?: a meta-analysis. Radiology. 2011;261:446–55. doi: 10.1148/radiol.11101344. [DOI] [PubMed] [Google Scholar]
- 5.Janecek JK, Swanson SJ, Sabsevitz DS, et al. Language Lateralization by fMRI and Wada Testing in 229 Epilepsy Patients: Rates and Predictors of Discordance. Epilepsia. 2013;54:314–22. doi: 10.1111/epi.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papanicolaou AC, Simos PG, Breier JI, et al. Magnetoencephalographic mapping of the language-specific cortex. J Neurosurg. 1999;90:85–93. doi: 10.3171/jns.1999.90.1.0085. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka N, Liu H, Reinsberger C, et al. Language lateralization represented by spatiotemporal mapping of magnetoencephalography. AJNR Am J Neuroradiol. 2013;34:558–63. doi: 10.3174/ajnr.A3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 9.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Liang M, Zhou Y, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–61. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- 11.Douw L, DeSalvo MN, Tanaka N, et al. Dissociated multimodal hubs and seizures in temporal lobe epilepsy. Ann Clin Transl Neurol. 2015;2:338–52. doi: 10.1002/acn3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Démonet JF, Thierry G, Cardebat D. Renewal of the neurophysiology of language: functional neuroimaging. Physiol Rev. 2005;85:49–95. doi: 10.1152/physrev.00049.2003. [DOI] [PubMed] [Google Scholar]
- 13.Stufflebeam SM. Clinical magnetoencephalography for neurosurgery. Neurosurg Clin N Am. 2011;22:153–67. doi: 10.1016/j.nec.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stufflebeam SM, Liu H, Sepulcre J, et al. Localization of focal epileptic discharges using functional connectivity magnetic resonance imaging. J Neurosurg. 2011;114:1693–7. doi: 10.3171/2011.1.JNS10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu L, Fan Y, Zou Q, et al. Temporal reliability and lateralization of the resting-state language network. PLoS One. Jan 24. 2014;9(1):e85880. doi: 10.1371/journal.pone.0085880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doucet GE, Pustina D, Skidmore C, et al. Resting-state functional connectivity predicts the strength of hemispheric lateralization for language processing in temporal lobe epilepsy and normals. Hum Brain Mapp. 2015;36:288–303. doi: 10.1002/hbm.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Stufflebeam SM, Sepulcre J, et al. Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proc Natl Acad Sci U S A. 2009;106:20499–503. doi: 10.1073/pnas.0908073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Buckner RL, Liu H. Functional specialization in the human brain estimated by intrinsic hemispheric interaction. J Neurosci. 2014;34:12341–52. doi: 10.1523/JNEUROSCI.0787-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeo BTT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKeown MJ, Makeig S, Brown GG, et al. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 1998;6:160–88. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pravatà E, Sestieri C, Mantini D, et al. Functional connectivity MR imaging of the language network in patients with drug-resistant epilepsy. AJNR Am J Neuroradiol. 2011;32:532–40. doi: 10.3174/ajnr.A2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tie Y1, Rigolo L, Norton IH, et al. Defining Language Networks From Resting-State fMRI for Surgical Planning—A Feasibility Study. Hum Brain Mapp. 2014;35:1018–30. doi: 10.1002/hbm.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagmann P, Cammoun L, Gigandet X, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honey CJ, Sporns O, Cammoun L, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA. 2009;106:2035–40. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeSalvo MN, Douw L, Tanaka N, et al. Altered Structural Connectome in Temporal Lobe Epilepsy. Radiology. 2014;270:842–8. doi: 10.1148/radiol.13131044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catani M, Allin MP, Husain M, et al. Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA. 2007;104:17163–8. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takaya S, Kuperberg GR, Liu H, et al. Asymmetric projections of the arcuate fasciculus to the temporal cortex underlie lateralized language function in the human brain. Front Neuroanat. 2015;9:119. doi: 10.3389/fnana.2015.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douw L, DeSalvo MN, Tanaka N, et al. Dissociated multimodal hubs and seizures in temporal lobe epilepsy. Ann Clin Transl Neurol. 2015;2:338–352. doi: 10.1002/acn3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]