Abstract
The differential risk of exposure to fumonisin (FB), deoxynivalenol (DON), and zearalenone (ZEA) mycotoxins to the South African population, residing in the nine Provinces was assessed during a cross-sectional grain consumer survey. The relative per capita maize intake (g/day) was stratified by gender, ethnicity, and Province and the probable daily intake (PDI) for each mycotoxin (ng/kg body weight/day) calculated utilizing SPECIAL and SUPER dry milled maize fractions representing different exposure scenarios. Men consumed on an average more maize (173 g/day) than women (142 g/day) whereas the black African ethnic group had the highest intake (279 g/day) followed by the Colored group (169 g/day) with the Asian/Indian and White groups consuming lower quantities of 101 and 80 g/day, respectively. The estimated mean PDIs for the various subgroups and Provinces, utilizing the different dry milled maize fractions, were below the provisional maximum tolerable daily intake (PMTDI) for each mycotoxin. A distinct and more sensitive mycotoxin risk assessment model (MYCORAM) for exposure, stratified by Province and ethnicity were developed utilizing specific maize intake increments (g/kg body weight/day) that provides information on the percentage of the population exposed above the PMTDI for each mycotoxin. Evaluation of the MYCORAM utilizing commercial and experimentally derived SPECIAL milling fractions, containing predefined mycotoxins levels, predicts the percentage of maize consumers exposed above the respective PMTDI. Safety modeling using the MYCORAM could also predict a maximum tolerated level adequate to safeguard all South African maize consumers including the most vulnerable groups.
Keywords: mycotoxins exposure, probable daily intakes, maize consumers, risk assessment model, South Africa
Mycotoxins enter the human food chain via three possible routes: (1) directly via the diet by consuming cereals such as maize; (2) indirectly via food products derived from fungal contaminated commodities; and (3) consumption of meat or animal products from livestock fed contaminated feed (Turner et al., 2009). From a commercial perspective, most food products may contain mycotoxins below the maximum tolerated levels due to good agricultural practices, selective breeding, modern biotechnology strategies, improved storage, food preparation, and processing. However, the cumulative exposure to an unvaried diet and/or high intakes of contaminated food commodities is a concern to health authorities and the food industry. South Africa is an agricultural country with maize as an important commodity for both commercial and subsistence farming communities. Maize and maize-based products are consumed by the majority of the population (between 67 and 83%), and the average cooked maize consumption is estimated between 475 and 690 g/person/day (Nel and Steyn, 2002). The population's demographics together with rapid urbanization impact on dietary patterns and necessitate the assessment of maize intakes in order to determine the risk of mycotoxin exposure. South Africa is known for its population diversity, which is reflected in large differences in social-economic status and cultural traditions.
The mycotoxins relevant to human health include aflatoxins (AFs) produced by Aspergillus spp., ochratoxin A (OTA) produced by Aspergillus and Penicillium spp., deoxynivalenol (DON), zearalenone (ZEA), and fumonisins (FB) produced by Fusarium spp. (Binder et al., 2007). The suggested adverse health effects in humans include (1) hepatitis, liver cancer, stunting, and immune suppression by AF (IARC, 1993a, 2002a; Gong et al., 2004; Turneret al., 2002, 2003, 2007); (2) nephropathy by OTA (Coronel et al., 2010; IARC, 1993b); (3) gastrointestinal disorders, anorexia, nausea, emesis, headache, chills, giddiness, and convulsions by DON (Amuzie and Pestka, 2010; Maresca et al., 2002); (4) precocious pubertal changes in children, early menarche and possibly infertility due to the endocrine disruptive effect of ZEA (Massart et al., 2008; Warth et al., 2013); and (5) increased risk of esophageal and liver cancer, neural tube defects, and stunting by FB (IARC, 2002b; Kimanya et al., 2010; Marasas et al., 2001, 2004). The proposed health outcomes are expected to be further exacerbated by possible additive and/or synergistic effects of mycotoxins due to the co-occurrence of mycotoxins in a particular food commodity (Ariño et al., 2007). In South Africa, exposure to FB1 has been associated with a high incidence of esophageal cancer especially among rural maize subsistence farming communities (Rheeder et al., 1992; Shephard et al., 2013). Chronic exposure to mycotoxins known to contaminate South African commercial maize, even at low levels in an unvaried diet is currently unknown and a public health concern. The Joint FAO/WHO Expert Committee on Food Additives (JECFA) has established, for each mycotoxin, a provisional maximum tolerable daily intake (PMTDI), which includes (1) zero for AF since it is a potent genotoxin exhibiting a no threshold level of exposure; (2) 0.1 μg/kg body weight (bw)/week or ±14 ng/kg bw/day for OTA (JECFA, 2002); (3) 1 μg/kg bw/day for DON (JECFA, 2001); (5) 0.5 μg/kg bw/day for ZEA (JECFA, 2000) and 2 μg/kg bw/day for FB (JECFA, 2001,2012).
The purpose of this study was to determine the probable daily intakes (PDIs) of FBT (FB1 + FB2), DON, and ZEA among a representative sample covering the nine Provinces of South Africa utilizing dietary intakes of maize-based food products obtained during a national consumer survey. Aflatoxins (B1, B2, G1, and G2), ochratoxin A and T-2 toxin are only present at very low levels in South African commercial maize and were excluded from the present study (Burger et al., 2013). Based on defined dietary maize intake categories and relevant mycotoxin exposure levels, a sensitive mycotoxin risk assessment model (MYCORAM) was developed to determine the percentage of the population exposed above the PMTDI levels for each respective mycotoxin stratified by ethnicity and Province. Different experimental dry milled maize fractions, prepared from specific maize samples containing predefined mycotoxin levels as well as commercial fractions intended for human consumption were utilized to evaluate the MYCORAM at provincial level. Maximum tolerated levels (MTLs) for mycotoxin exposure of different ethnic groups were also estimated using the relevant MYCORAM. This is the first study to assess mycotoxin exposure in a broader South African maize consuming population.
MATERIALS AND METHODS
The current study was a collaborative effort between a reputable and leading grain-based South African manufacturing company, the Cape Peninsula University of Technology (CPUT) and the Nutrition Information Centre, University of Stellenbosch (NICUS), South Africa. Ethical approval was obtained from the Health Research Ethics Committee of Stellenbosch University, South Africa.
Study design
A national consumer survey was conducted by a private South African marketing research company specializing in consumer studies, utilizing a cross-sectional study design to ensure a representative sample of South African grain (including maize) consumers. In addition to the structured consumer questionnaire, a quantitative food frequency questionnaire (QFFQ) was included. This QFFQ was developed by including maize intake questions originating from a validated questionnaire used during the South African National Food Consumption Survey (Labadarios et al., 2008). After informed and signed consent, information on habitual maize intakes and body weight measurements were obtained from each participant. Demographic information from the consumer questionnaire was also collected and included ethnicity, age, gender, residential Province, household income, employment, and education. South Africa consists of heterogeneous populations with four main ethnic groups: black Africans, (ancestry from the African continent; 79% of total South African population); Colored (mixed ancestry, mainly of Khoi origin as well as mixed Caucasian, African Malay, and San origins; 8.9% of total population), White (Caucasian descendants from Europe; 9.6% of total population), and Indian/Asian (originating from India or other Asian countries, 2.5% of total population) (Byrnes, 1996, Marais, 1968; Thomas and Bendixen, 2000). These ethnic groups reside in nine different Provinces, namely the Eastern Cape (EC), Free State (FS), Gauteng (GP), KwaZulu-Natal (KZN), Limpopo (LP), Mpumalanga (MP), North Cape (NC), North West (NW), and Western Cape (WC) Provinces (Blaauw and Gilson, 2001). Professional interviewers were trained on the basics of scientific data collection, the sampling methodology, completion of the various questionnaires, and body weight measurements.
A sample size of 3000 was selected using the South African 2001 Census Household data (Statistics South Africa, 2001) and adult male and female (older than 16 years) consumers of maize from the different ethnic groups within the nine Provinces were eligible to participate. The sample was stratified according to Province, Metro, and District Municipality down to the level of suburb/township/village and systematic probability sampling was applied. Each household had an equal chance of being selected to participate in the survey consisting of face-to-face interviews.
Mean raw/uncooked maize intakes
The quantitative food frequency questionnaire, based on usual intakes of maize-based products over a month period and expressed as gram per person per day (g/day) was used to assess maize intakes. Photographic aids with pictures of various maize-based dishes such as crumbly-, soft-, and stiff-porridge, samp, combined dishes (samp and beans, spinach and maize meal, pumpkin and maize meal), and nonalcoholic maize fermented beverages, in four different portion sizes were developed to improve the accuracy of maize intake estimates. Individual total raw/uncooked maize intakes were estimated using recipes from FoodFinder 3, a dietary analysis computer software application (South African Medical Research Council, PO Box 19070, Tygerberg, South Africa). If a recipe was unavailable a validated culturally specific dietary assessment method, the Ratio And Portion size Photo (RAPP) tool was used to calculate the raw maize intake (Lombard et al., 2014). The RAPP tool was developed to determine the dietary habits and nutrient intakes of rural and urban Xhosa-speaking black Africans living in the EC. Numerical means were calculated for the raw maize intakes and stratified according to the gender, ethnicity, and provincial residence
Mycotoxin levels in maize samples for risk evaluation
Five maize samples selected to represent different mycotoxin levels of FBT (FB1 + FB2), DON, and ZEA, were dry milled under laboratory conditions and the different fractions normally intended for human consumption (SPECIAL and SUPER maize meal) collected (Burger et al., 2013). The mean mycotoxin levels (n = 5) of FBT, DON, and ZEA of the experimental SPECIAL and SUPER milling fractions were used to determine exposure to the different mycotoxins. The respective SUPER and SPECIAL fractions of nine commercial maize samples, representing industrial milling, were included for comparative purposes. In addition the SPECIAL and SUPER milling fractions of two selected maize samples (Maize 1 and Maize 2), representing worst-case contamination levels with either a high total FBT or low FBT, high DON, and ZEA (DON/ZEA) were used for the evaluation of the MYCORAM. Multi-mycotoxin analyses of the dry milled fractions utilizing a validated and standardized LC-MS/MS method, were conducted by the Southern African Grain Laboratory (SAGL, Pretoria, South Africa) and included FB1, FB2, DON, and ZEA (Burger et al., 2013).
Mycotoxin exposure stratified by gender, ethnicity, and Province
The PDIs (expressed as ng/kg bw/day) of the different mycotoxins were calculated using the experimental and commercial SPECIAL and SUPER fractions utilizing: (1) the total raw/uncooked maize intakes; (2) body weight of the maize consumers; and (3) mean mycotoxin (n = 5) levels for FBT, DON, ZEA. The PDIs for FBT, DON, and ZEA were stratified by gender, ethnicity, and Province, respectively.
Development and evaluation of the exposure mycotoxin risk assessment model (MYCORAM)
The MYCORAM for FBT, DON, and ZEA exposure were developed to assess the percentage of maize consumers that will be at risk of mycotoxin exposure above the PMTDI for each mycotoxin stratified by Province and ethnicity. The development of the MYCORAM was conducted in three stages using the data obtained during the cross-sectional maize consumer survey. Firstly, the daily individual raw/uncooked maize intakes were calculated in terms of body weight (g maize/kg bw/day). Specific maize intake categories of: (1) ≥1; (2) ≥2; (3) ≥4; (4) ≥10; (5) ≥20 g/kg bw/day were defined together with a specific contamination level for each mycotoxin to effect PDIs equal to or above the relevant PMTDIs. The mycotoxin contamination ranges included: 0–2000, 0–1000, and 0–500 μg/kg for FBT, DON, and ZEA, respectively. To illustrate this, people consuming 1 g/kg bw/day at a FBT contamination level of 2000 μg/kg will equal the PMTDI of FB (2 μg/kg bw/day). In the second stage, the number of maize consumers expressed as a percentage within each maize intake category was stratified either by Provinces or ethnicity. The final stage included the plotting of the percentage of consumers equal or above the PMTDI exposure for a specific mycotoxin against the selected mycotoxin contamination ranges.
The MYCORAM stratified by Province was evaluated using the mean mycotoxin levels of experimental and commercial SPECIAL and SUPER milling fractions. In addition, the corresponding milling fractions of two selected maize samples representing high levels of FBT (Maize 1) and DON/ZEA (Maize 2), respectively (Burger et al., 2013) were included. These contamination levels mimic mycotoxin levels reported in maize samples obtained from rural maize subsistence communities (Burger et al., 2010; Sydenham et al., 1992).
Safety modeling was also conducted using the MYCORAM stratified by ethnicity to predict maximum tolerated levels (MTLs) guarantee an exposure risk below 1% above the PMTDI for maize consumers.
STATISTICAL ANALYSIS
Analysis of covariance (ANCOVA) was used to determine numerical means and to test for normality. As the data failed to be normally distributed, log transformation was used to determine geometric means (GM) which were used for multiple comparisons utilizing the Tukey-Kramer test. All statistical analyses and the development of the exposure MYCORAM were performed using the NCSS statistical package version 8, released 25 July 2012 (Hintze, 2007).
RESULTS
Population Characteristics
Study compliance was 94% and comprehensive information on demographics, body weights, and raw maize intakes were obtained for 2809 participants. Ninety-nine percent of the total study population (n = 2809) were maize consumers (n = 2778) with only 30% of the maize consumer population representing men. According to the 2001 Census Household data gender distribution in South Africa was 48 and 52% for men and women, respectively (Statistics South Africa, 2001). The overall mean population age was 34 (range 16–88). Sixty-six percent of the population was employed, 9% self-employed, 8% unemployed, and the remainder varied between students (6%), housewives (5%), and pensioners (4%) with 2% being nonresponders. The monthly household income of the study population (in South African Rand) indicated that 34% had an income of between R500 and R6000, 33% had an income above R8000, and the rest (33%) were nonresponders. Twenty-four percent the study population had education of grade 1 to grade 11, 45% had a grade 12 or equivalent (National Qualifications Framework, NQF 4 level) education followed by 31% with a Technikon or University degree.
Mycotoxin Contamination of Maize Milling Fractions (Table 1)
TABLE 1. Mycotoxin Levels in Dry Milled Maize Fractions (SPECIAL and SUPER) from Different Maize Sourcesa for Determining Probable Daily Intake (PDI) and Evaluation of the MYCORAM.
| FBT (μg/kg) | DON (μg/kg) | ZEA (μg/kg) | |
|---|---|---|---|
| Experimental maize samples (n = 5) | |||
| SPECIAL | 338.4 (70–1161) | 127.8 (43–240) | 31.0 (0–81) |
| SUPER | 61.0 (0–221) | 27.2 (0–67) | 8.6 (0–18) |
| Commercial maize samples (n = 9) | |||
| SPECIAL | 67.1 (20–135) | 18.7 (4–26) | 0.0 |
| SUPER | 1.6 (0–5) | 5.8 (0–13) | 0.6 (0–3) |
| Maize 1 (high FBT) (n = 1) | |||
| SPECIAL | 1161.0 | 195.0 | 44.0 |
| SUPER | 221.0 | 28.0 | 13.0 |
| Maize 2 (high DON/ZEA) (n = 1) | |||
| SPECIAL | 107.0 | 240.0 | 81.0 |
| SUPER | 20.0 | 67.0 | 18.0 |
Note. Data expressed as numerical means with ranges in brackets. n = number of samples.
aAdapted from Burger et al. (2013).
The mycotoxin levels in experimental and commercial (SPECIAL and SUPER) dry milling fractions, intended for human consumption are summarized as numerical means with their respective ranges. Appreciably lower mycotoxin contamination levels were observed in the milling fractions of the commercial samples compared with the preselected experimental samples. The mycotoxin levels in the dry milling fractions obtained from the two selected maize samples were much higher with respect to FBT (Maize 1) and DON/ZEA (Maize 2), which as mentioned above, reflects contamination levels of raw maize utilized by rural subsistence farmers in South Africa (Burger et al., 2010).
Body Weight, Maize Intake Profiles, and Probable Mycotoxin Intake Parameters Stratified According to Gender, Ethnicity, and Province Differences between men, women, and ethnic groups (Table 2)
TABLE 2. Mean Intakes of Raw Maize, Body Weights and Probable Daily Intakes for FBT, DON, and ZEA Utilizing the Experimental SPECIAL and SUPER Dry Milled Fractions Stratified by Gender and Ethnicity.
| Group | Percentage of the maize consumer population (N) | Mean maize intakes (range) (g/day) | SPECIAL fraction Mean PDI (range) (ng/kg bw/day) | SUPER fraction Mean PDI (range) (ng/kg bw/day) | ||||
|---|---|---|---|---|---|---|---|---|
| FBT | DON | ZEA | FBT | DON | ZEA | |||
| Maize consumer population | 100% (2778) | 157 (0–3055) | 74 (0–2298) | 25.4 (0–870) | 6.7 (0–211) | 13.3 (0–414) | 5.9 (0–185) | 2 (0.0–58) |
| Men | 30% (776) | 173 a (1–2149) | 80.0 a (0–1070) | 30.2 a (0–403) | 7.3 a (0–100) | 14.4 a (0–192) | 6.4 a (0–86) | 2.0 a (0–27) |
| Women | 70% (2002) | 142 a (0–3055) | 67.2 a (0–2297) | 25.4 a (0–870) | 6.2 a (0–210) | 12.1 a (0–414) | 5.4 a (0–185) | 1.7 a (0–58) |
| Asian/Indian | 4% (123) | 101 c,d (1–537) | 47.3 c,d (0–240) | 17.8 c,d (0–90) | 4.3 c,d (0–22) | 8.5 c,d (0–43) | 4.0 c,d (0–19) | 1.2 c,d (0–6) |
| Black African | 63% (1750) | 279 b,c (0–2483) | 13.2 b,c (0–1070) | 50.0 b,c (0–403) | 12.0 b,c (0–100) | 23.7 b,c (0–1923) | 11.0 b,c (0–86) | 3.3 b,c (0–27) |
| Colored | 15% (407) | 169 b,d (3–3055) | 77.2 b,d (0–2297) | 29.2 b,d (0–870) | 7.1 b,d (0–210) | 13.9 b,d (0–414) | 6.2 b,d (0–185) | 2.0 b,d (0–58) |
| White | 18% (498) | 80 b (1–1408) | 38.2 b (0–822) | 14.4 b (0–310) | 3.5 b (0–75) | 7.0 b (0–148) | 3.1 b (0–66) | 1.0 b (0–21) |
Note. Data presented as numerical means with the range in brackets below. Statistical differences (p < 0.05) were determined using the log transformed means (geometrical means, GM). Means (within columns) with the same lowercase letter in bold are indicative of a significantly difference (p < 0.05) between the respective groups. n = sample size.
The total mean body weight of the maize consumer population was 74 kg (range 36–210 kg). The mean body weight for men of 76 kg (range 36–147) was significantly higher (p < 0.05) than the women (72 kg, range 39–210 kg). Body weight stratified by ethnicity was 71 kg (range 42–104 kg) for the Asians/Indian, 75 kg (range 36–210 kg) for the black Africans and 73 kg (range 40–170 kg) for the White group. The Colored ethnic group (15% of the maize consumer population) had the highest mean body weight (p < 0.05), that of 77 kg (range 39–135 kg) compared with the Asian/Indian (4%) and White (18%) groups, whereas the black African group (63%) had a significantly (p < 0.05) higher mean body weight then the Asian/Indian group. Raw maize intakes (g/day) among the men were statistically higher (p < 0.05) than the women. The mean intake stratified according to ethnicity indicated that the black Africans consumed the highest amount of maize, which was statistically higher (p < 0.05) than all the other ethnic groups. The Colored group had the second highest intakes that differed (p < 0.05) from the black Africans, Indian/Asian, and White groups, the latter two groups consuming the lowest but similar amounts. The large variation of maize intakes is indicative of the skewed data distribution. For instance, the black African group had significant higher (p < 0.05) mean raw maize intake than the Colored group, although the ranges varied between 0–2483 and 3–3055 g/day for the black African and Colored ethnic groups, respectively.
Probable daily intakes (ng/kg bw/day), utilizing the mycotoxin contamination levels of the SPECIAL and SUPER milling fractions obtained from the experimental and commercial maize samples (Table 1) represented different exposure scenarios. The SPECIAL experimental milling fraction with its overall higher levels of FBT, DON, and ZEA resulted in higher PDIs compared with the SUPER milling fraction. When stratified according to gender, men had a statistically significant (p < 0.05) higher PDI compared with the women (Table 2). Based on ethnicity, the black Africans had the highest PDI that differed significantly (p < 0.05) from the other ethnic groups. The Colored group had a significantly higher PDI compared with the Asian/Indian and White ethnic groups, which did not differ significantly.
Differences among maize consumers and mycotoxin exposure between the nine South African Provinces (Table 3)
TABLE 3. Mean Intakes of Raw Maize, Body Weight, and Probable Daily Intakes for FBT, DON, and ZEA Utilizing the Experimental SPECIAL and SUPER Dry Milled Fractions Stratified by Province.
| Province | EC | FS | GP | KZN | LP | MP | NC | NW | WC |
|---|---|---|---|---|---|---|---|---|---|
| n | 348 | 189 | 629 | 388 | 346 | 192 | 144 | 241 | 301 |
| Mean maize intake (range) (g/day) | 153 co (16–1037) | 157 bn (3–1119) | 168 ahi (1–3055) | 163 lm (1–1263) | 166 jk (3–2149) | 181 fg (9–2081) | 201 abcde (2–915) | 125 dfhjl (0–1347) | 103 egikmno (2–830) |
| Experimental SPECIAL milling fraction | Mean PDI (range) (ng/kg bw/day) | ||||||||
| FBT | 70.0 a,h (0–462) | 75.6 i (0–560) | 80.5 b,j (0–230) | 74.0 e,k (0–750) | 78.1 f,l (1–969) | 86.0 g,m (4–1067) | 87.3 a,b,c,d (1–369) | 64.3 c,e,f,g (0–742) | 47.0 d,h,i,j,k,l,m (1–390) |
| DON | 26.4 a,h (0–174) | 29.0 i (0–212) | 30.4 b,j (0–870) | 27.8 e,k (0–283) | 29.5 f,l (0–366) | 32.4 g,m (1–403) | 33.0 a,b,c,d (0–139) | 24.3 c,e,f,g (0–280) | 18.0 d,h,i,j,k,l,m (0–147) |
| ZEA | 6.4 a,h (0–42) | 6.9 i (0–51) | 7.4 b,j (0–210) | 6.7 e,k (0–70) | 7.2 f,l (0–90) | 8.0 g,m (0–100) | 8.0 a,b,c,d (0–34) | 6.0 c,e,f,g (0–70) | 4.3 d,h,i,j,k,l,m (0–36) |
| Experimental SUPER milling fraction | Mean PDI (range) (ng/kg bw/day) | ||||||||
| FBT | 12.6 a,h (0–83) | 13.6 i (0–101) | 14.5 b,j (0–414) | 13.3 e,k (0–135) | 14.1 f,l (0–175) | 15.5 g,m (0–192) | 15.7 a,b,c,d (0–66) | 12.0 c,e,f,g (0–134) | 8.4 d,h,i,j,k,l,m (0–70) |
| DON | 5.6 a,h (0–37) | 6.1 i (0–45) | 6.5 b,j (0–185) | 5.9 e,k (0–60) | 6.3 f,l (0–80) | 7.0 g,m (0–86) | 7.0 a,b,c,d (0–30) | 5.2 c,e,f,g (0–60) | 4.0 d,h,i,j,k,l,m (0–31) |
| ZEA | 2.0 a,h (0–12) | 1.9 I (0–14) | 2.0 b,j (0–58) | 2.0 e,k (0–19) | 2.0 f,l (0–25) | 2.2 g,m (0–27) | 2.2 a,b,c,d (0–9) | 1.6 c,e,f,g (0–19) | 1.2 d,h,i,j,k,l,m (0–10) |
Note. Data presented as numerical means with the range in brackets. Statistical differences (p < 0.05) were determined using the log transformed means (geometrical means, GM). Means (within rows) with the same lowercase letter in bold indicates a significantly difference (p < 0.05) between the different Provinces. n = sample size.
The mean body weight of the maize consumers in the Northern Cape (NC) was significantly higher (p < 0.05), 79 kg (range 50–147 kg) compared with those from the GP 74 kg (range 40–160), LP 73 kg (range 39–210 kg), WC 71 kg (range 49–112 kg), NW 71 kg (range 37–129 kg), and FS 71 kg (range 39–98 kg) Provinces. Maize consumers from KZN had a higher mean body weight (p < 0.05) that of 76 kg (range 42–170 kg) than those from the NW, whereas those of the consumers of the other Provinces MP, 74 kg (range 49–150 kg) and EC 75 kg (range 36–170 kg) did not differ. When considering the mean raw maize intake profiles, the NC consumers had significantly (p < 0.05) higher intakes when compared with those from the GP, FS, Eastern Cape, NW, and WC Provinces. Consumers residing in the MP, GP, LP, KZN, FS, and EC Provinces had similar maize intakes, however, the respective intakes for consumers in MP, GP, LP, and KZN were significantly higher (p < 0.05) when compared with the NW and WC consumers. The raw maize intakes from FS and EC consumers were significantly higher when compared with the WC.
Utilizing the various mycotoxin contamination levels obtained from the experimental dry milled fractions (SPECIAL and SUPER), the resultant PDIs across nine Provinces were far below the respective PMTDIs. When considering the SPECIAL milling fraction prepared from the experimental maize samples, maize consumers in the NC and MP Provinces had the highest (p < 0.05) mean PDIs (86.0 and 87.3 ng/kg bw/day, respectively) for FBT (338.4 μg/kg). Except for the NW and WC Provinces, which had the lowest PDIs (64.3 and 47.0 ng/kg bw/day, respectively), the other Provinces FS, GP, LP, EC, and KZN had similar PDIs ranging between 70.0 and 80.5 ng/kg bw/day. For DON (127.8 μg/kg), the PDIs ranged between 26.4 and 33.9 ng/kg bw/day compared with the NC and WC having the lowest PDIs (14.3 and 18.0 ng/kg bw/day, respectively). For ZEA (31.0 μg/kg), the PDIs followed the same pattern as other two mycotoxins with the highest in MP and NC (8.0 ng/kg bw/day) and with the lowest (6.0 and 4.3 ng/kg bw/day) observed in the NW and WC, respectively. For the experimental SUPER fraction with its low mycotoxin levels, the resultant mean PDIs were far lower (ranging between 1.2 and 15.7 ng/kg bw/day) and did not differ much between the Provinces.
MYCORAM Development According to Province and Ethnicity (Table 4 and Figs. 1A and 1B)
TABLE 4. Development of the Mycotoxin Risk Assessment Model (MYCORAM): Defined Maize Intakea Categories and Preselected Contamination Levelsb of FBT, DON, and ZEA Representing Exposure Equal or Above the Relevant PMTDI and the Percentage (%) of Maize Consumers Within an Intake Category Stratified by Province and Ethnicity.
| FBT levels (μg/kg) | DON levels (μg/kg) | ZEA levels (μg/kg) | Intake categories (g/kg bw/day) | Provinces | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC | FS | GP | KZN | LP | MP | NC | NW | WC | Total | ||||
| 100 | 50 | 25 | ≥20 | 0% | 0% | 1% | 1% | 1% | 4% | 0% | 0% | 0% | 1% |
| 200 | 100 | 50 | ≥10 | 2% | 4% | 4% | 3% | 3% | 5% | 1% | 2% | 1% | 3% |
| 500 | 250 | 125 | ≥4 | 26% | 31% | 29% | 36% | 33% | 29% | 23% | 24% | 14% | 28% |
| 1000 | 500 | 250 | ≥2 | 60% | 62% | 59% | 67% | 76% | 64% | 66% | 56% | 48% | 62% |
| 2000 | 1000 | 500 | ≥1 | 79% | 78% | 76% | 83% | 91% | 92% | 83% | 76% | 74% | 81% |
| Up to 1 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | |||
| FBT levels (μg/kg) | DON levels (μg/kg) | ZEA levels (μg/kg) | Intake categories (g/kg bw/day) | Ethnic groups | |||||||||
| Asians/Indians | Black African | Colored | White | Total | |||||||||
| 100 | 50 | 25 | ≥20 | 0 | 1% | 0 | 0 | 1% | |||||
| 200 | 100 | 50 | ≥10 | 0 | 4% | 1% | 1% | 3% | |||||
| 500 | 250 | 125 | ≥4 | 10% | 35% | 11% | 65 | 25% | |||||
| 1000 | 500 | 250 | ≥2 | 25% | 73% | 44% | 16% | 56% | |||||
| 2000 | 1000 | 500 | ≥1 | 37% | 90% | 65% | 31% | 73% | |||||
| Up to 1 | 100% | 100% | 100% | 100% | 100% | ||||||||
aMaize intake categories and percentages calculated using individual raw maize intakes and body weights from maize consumers (n = 2522).
bMycotoxin levels were selected to obtain the PMTDI level for each mycotoxin when multiplied by the specific maize intake category.
FIG. 1.
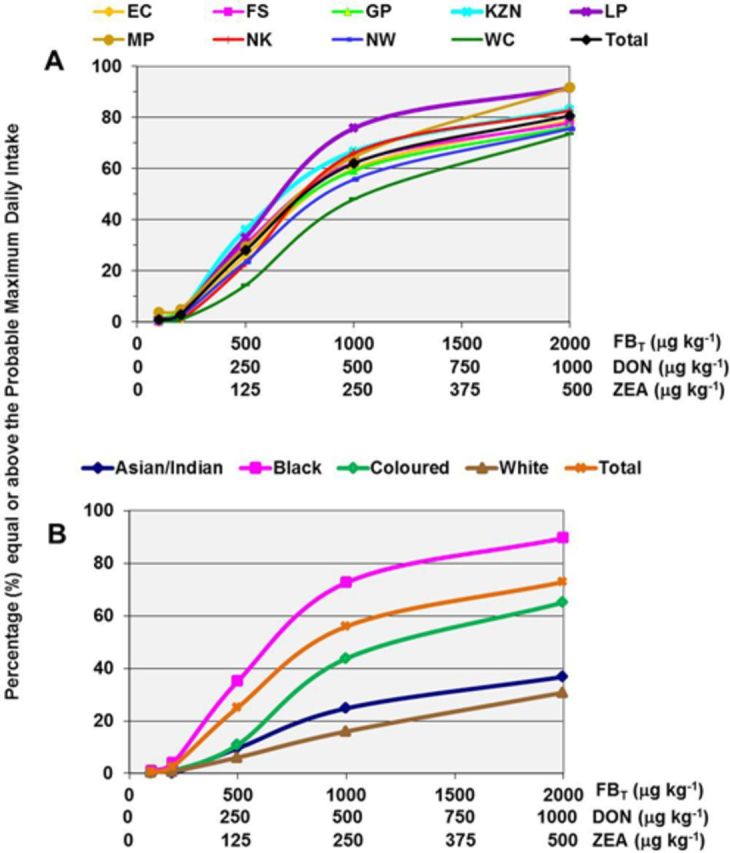
MYCORAM for FBT DON and ZEA. Percentages of the South African maize consumers stratified by Province (A) and ethnicity (B) equal or above the respective mycotoxin PMTDIs. The number of lines represent the different Provinces (nine in total) and ethnic groups (four in total). Each line represents the percentage equal to or exceeding the PMTDI of the three mycotoxins—the μg/kg contamination (x-axis) differs for each mycotoxin.
The percentage of the study population within a specific maize intake category was stratified across nine South African Provinces and ethnicity. For each maize intake category, the equivalent mycotoxin contamination level was selected such that the PDI produced by their product will equal the respective PMTDI for each mycotoxin. Based on these data, the MYCORAM for FBT, DON, and ZEA was developed by predicting the percentages (%) of maize consumers that will be equal or above the respective PMTDI for each mycotoxin, as a function of the selected mycotoxin contamination level: FBT, DON, and ZEA according to Province and ethnicity (Figs. 1A and 1B). The percentage of consumers above the limit is affected by the number of people within a specific maize intake category and provides a more informative assessment of exposure compared with the mean PDI.
MYCORAM Evaluation Stratified by Province (Table 5)
TABLE 5. Evaluation of the MYCORAM Using the Relevant Experimental and Commercial SPECIAL and SUPER Dry Milled Fractions Contaminated by FBT, DON, and ZEA, Stratified by Province.
| Maize milling fraction | n | Mycotoxin level (μg/kg)a | % Population above the PMTDI of 2 μg/kg bw/day for FBT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SPECIAL | EC | FS | GP | KZN | LP | MP | NK | NW | WC | ||
| Experimental | 5 | 338.4 | 12.7 | 16.2 | 16.0 | 18.2 | 17.0 | 16.3 | 11.0 | 12.1 | 7.0 |
| Commercial | 9 | 67.1 | 0.0 | 0.0 | 0.8 | 0.4 | 0.6 | 2.6 | 0.0 | 0.3 | 0.0 |
| SUPER | |||||||||||
| Experimental | 5 | 61.0 | 0.0 | 0.0 | 0.7 | 0.4 | 0.6 | 2.4 | 0.0 | 0.3 | 0.0 |
| Commercial | 9 | 1.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 |
| % Population above the PMTDI of 1 μg/kg bw/day for DON | |||||||||||
| SPECIAL | EC | FS | GP | KZN | LP | MP | NK | NW | WC | ||
| Experimental | 5 | 127.8 | 6.0 | 8.8 | 9.0 | 9.1 | 8.6 | 9.5 | 4.8 | 6.2 | 3.3 |
| Commercial | 9 | 18.7 | 0.0 | 0.0 | 0.4 | 0.2 | 0.4 | 1.5 | 0.0 | 0.2 | 0.0 |
| SUPER | |||||||||||
| Experimental | 5 | 27.2 | 0.0 | 0.0 | 0.6 | 0.3 | 0.5 | 2.1 | 0.0 | 0.3 | 0.0 |
| Commercial | 9 | 5.8 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.5 | 0.0 | 0.1 | 0.0 |
| % Population above the PMTDI of 0.5 μg/kg bw/day for ZEA | |||||||||||
| SPECIAL | EC | FS | GP | KZN | LP | MP | NK | NW | WC | ||
| Experimental | 5 | 31.0 | 0.4 | 0.9 | 2.0 | 1.2 | 1.4 | 4.2 | 0.2 | 1.0 | 0.2 |
| Commercial | 9 | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| SUPER | |||||||||||
| Experimental | 5 | 8.6 | 0.0 | 0.0 | 0.4 | 0.2 | 0.3 | 1.3 | 0.0 | 0.2 | 0.0 |
| Commercial | 9 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 |
Experimental and commercial dry milling fractions
The mycotohxin levels associated with (1) the SPECIAL and SUPER fractions obtained from experimental and commercial milling (2) and two respective maize samples (Maize 1 and Maize 2) with high levels of FB and DON/ZEA (Table 1) were utilized to evaluate the MYCORAM. The apparent “consumption” of the SPECIAL milling fraction obtained from the experimental samples [FB (338.4 μg/kg), DON (127.8 μg/kg), and ZEA (31.0 μg/kg)] resulted in higher percentage consumers exposed above the regulated levels. The Province with the highest number of consumers above the PMTDI for FBT was KZN (18.2%) with the other Provinces ranging between 16.0 and 17.0% (LP, MP, FS, and GP), and EC, NW, and NC ranging between 11.0 and 12.7% with WC (7.0%) having the lowest. For DON the highest percentage exposure above the PMTDI was observed in the MP (9.5%), KZN (9.1%), GP (9.0%), FS (8.8%), and LP (8.6%) followed by EC (6.0%), NC (4.8%), and WC (3.3%). The highest percentage above the PMTDI for ZEA was also observed in MP (4.2%) and GP (2.0%), followed by LP (1.4%), KZN (1.2%), NW (1.0%), and FS (0.9%), with the lowest percentages in EC (0.4%), NC (0.2%), and WC (0.2%).
The percentage of consumers above the limit of exposure using the commercial SPECIAL milling fraction was far less, reflecting lower mycotoxin levels. The highest percentage of consumers above the limit was observed in MP, 2.6% (FBT) and 1.5% (DON), respectively. None of the maize consumers residing in the different Provinces were exposed above the PMTDI limit for ZEA. The experimental SUPER fractions also produced similar patterns with much lower percentage, ranging between 0 and 2.4% (MP) for FBT, 0 and 2.1% (MP) for DON, and 0 and 1.3% (MP) for ZEA, respectively.
High FBT (Maize 1) and DON/ZEA (Maize 2) dry milling fractions (Table 6)
TABLE 6. Evaluation of the MYCORAM Utilizing Experimental SPECIAL and SUPER Milling Fractions Obtained from Two Selected Samples Reflecting Contamination Levels of Subsistent Maize, Stratified by Province.
| Maize milling fraction | Mycotoxin level (μg/kg)a | % Population above the PMTDI of 2 μg/kg bw/day for FBT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SPECIAL | EC | FS | GP | KZN | LP | MP | NK | NW | WC | |
| Maize 1 [H] | 1161.0 | 63.0 | 64.7 | 62.0 | 69.3 | 78.2 | 68.8 | 68.6 | 58.9 | 52.0 |
| Maize 2 [L] | 107.0 | 0.1 | 0.3 | 1.4 | 0.8 | 1.1 | 4.0 | 0.1 | 0.6 | 0.1 |
| SUPER | ||||||||||
| Maize 1 [H] | 221.0 | 3.2 | 5.8 | 6.2 | 5.3 | 5.2 | 6.7 | 2.3 | 3.8 | 1.8 |
| Maize 2 [L] | 20.0 | 0.0 | 0.0 | 0.2 | 0.1 | 0.2 | 0.8 | 0.0 | 0.1 | 0.0 |
| % Population above the PMTDI of 1 μg/kg bw/day for DON | ||||||||||
| SPECIAL | EC | FS | GP | KZN | LP | MP | NK | NW | WC | |
| Maize 2 [H] | 240.0 | 24.2 | 28.8 | 27.1 | 33.8 | 30.9 | 27.8 | 21.3 | 22.1 | 13.4 |
| Maize 1 [L] | 195.0 | 16.9 | 20.8 | 19.8 | 23.9 | 22.0 | 20.5 | 14.7 | 15.7 | 9.4 |
| SUPER | ||||||||||
| Maize 2 [H] | 67.0 | 0.0 | 0.0 | 1.6 | 0.8 | 1.2 | 5.2 | 0.0 | 0.6 | 0.0 |
| Maize 1 [L] | 28.0 | 0.0 | 0.0 | 0.7 | 0.3 | 0.5 | 2.2 | 0.0 | 0.3 | 0.0 |
| % Population above the PMTDI of 0.5 μg/kg bw/day for ZEA | ||||||||||
| SPECIAL | EC | FS | GP | KZN | LP | MP | NK | NW | WC | |
| Maize 2 [H] | 81.0 | 11.6 | 14.9 | 14.5 | 16.7 | 15.4 | 15.1 | 9.8 | 11.1 | 6.4 |
| Maize 1 [L] | 44.0 | 1.2 | 3.0 | 3.7 | 2.4 | 2.6 | 4.7 | 0.6 | 1.8 | 0.6 |
| SUPER | ||||||||||
| Maize 2 [H] | 18.0 | 0.0 | 0.0 | 0.8 | 0.4 | 0.7 | 2.8 | 0.0 | 0.3 | 0.0 |
| Maize 1 [L] | 13.0 | 0.0 | 0.0 | 0.6 | 0.3 | 0.5 | 2.0 | 0.0 | 0.2 | 0.0 |
A higher percentage of maize consumers were above the relevant PMTDI when modeling the milling fractions obtained from the high FBT and DON/ZEA experimental maize samples. When modeling the SPECIAL milling fraction of the high FBT (1161.0 μg/kg), exposure levels above the PMTDI ranged between the highest (78.2%) in LP and lowest (52.0%) in the WC. The SPECIAL milling fraction obtained from the high DON (240 μg/kg) maize sample, resulted in the highest percentage of consumers above the limit (33.8%) in KZN with lowest (13.4%) in WC. The high ZEA (81.0 μg/kg) containing SPECIAL milling fraction also followed the same pattern as DON with the highest (16.7%) level of exposure in KZN and the lowest in WC (6.4%). For the SUPER milling fraction derived from the high FBT maize sample (221.0 μg/kg), the percentage of consumers above the PMTDI ranged from the highest of 6.7% (MP) to the lowest in WC (1.8%). For the high DON (67.0 μg/kg) containing SUPER milling fraction, consumers above the limit ranged from 0.0% to the highest of 5.2% above the PMTDI in MP, whereas for high ZEA (18.0 μg/kg) containing SUPER milling fraction, it varied between 0 and 2.8% above the PMTDI in MP.
Predicted Safety Modeling According to Ethnicity (Table 7)
TABLE 7. Prediction of Maximum Tolerated Levels Using the MYCORAM Stratified by Ethnicity.
| Ethnic group | Mycotoxin | Maximum tolerated levels (μg/kg) | Percentage (%) equal or above the PMTDIa |
|---|---|---|---|
| Asian/Indian | FBT | 228 | 0.9 |
| DON | 114 | ||
| ZEA | 57 | ||
| Black African | FBT | 180 | 0.9 |
| DON | 90 | ||
| ZEA | 45 | ||
| Colored | FBT | 190 | 0.9 |
| DON | 95 | ||
| ZEA | 47 | ||
| White | FBT | 210 | 0.9 |
| DON | 104 | ||
| ZEA | 51 |
aPMTDI for FBT, 2 μg/kg bw/day; DON, 1 μg/kg bw/day, and for ZEA, 0.5 μg/kg bw/day.
Different maximum tolerable levels (MTLs) for FBT, DON, and ZEA stratified by ethnicity were modeled to affect <1% of the consumers above the PMTDI for each mycotoxin. Different MTLs for FBT, DON, and ZEA, were determined for each ethnic group as a function of the level of mycotoxin contamination and maize intake profiles depicted in the MYCORAM. The black African and Colored population groups required far lower MTLs of the different mycotoxins as compared with the Asia/Indian and White population groups within the defined risk paradigm.
DISCUSSION
The development and implementation of food safety parameters within a specific population requires valid risk assessment based on the estimation of the level of exposure, which requires accurate determination of food consumption data and the contamination levels in the specific food commodities. The process of exposure assessment is considered as a critical phase in risk assessment and relies on utilizing an appropriate model (FAO and WHO, 2006, Fryer et al., 2006). The choice of an appropriate model is by the aim of the assessment (screening, setting of standards or first-time exposure assessment), the food commodity affected, population characteristics (age, gender, cultural diversity, etc.), time frame of the exposure (acute or chronic), the study data available (accuracy and format), the scope of the problem (toxin characteristics), and resources (Fryer et al., 2006; Lambe, 2002, Parmar et al., 1997). The MYCORAM, developed in the current study, defined the percentage of people at risk of exposure using the full heterogeneity of the data such as maize intakes, body mass, population characteristics, the relevant tolerably daily intake, and mycotoxin levels. This model, based on data obtained from South African maize consumers, can then be used to determine MTLs in a specific food commodity to safeguard the affected population. The model could be further refined to develop MTLs for specific subgroups depending on their specific maize consumption profiles.
In most developed countries, national food consumption surveys provide detailed information to assess the level of exposure to a specific food contaminant. Food consumption data can also be formatted to provide information on specific food items consumed such as maize, e.g., cornflakes, ingredients (i.e., maize meal), or the raw agricultural commodity (Boon et al., 2009; Møller and Ireland 2008). As regular national dietary surveys and expensive dietary recording methods are lacking in South Africa, food frequency questionnaires are valuable and normally used to assess habitual intakes. However, dietary assessment methods are never without their inherent limitations when assessing exposure as the human diet is known for its complexities due to varied food choices and differences in the consumption patterns among individuals. Along with variability in dietary patterns, uncertainties regarding contaminant analyses, and sampling methodologies also exist (Hart et al., 2003). Most often, the association between intake of food contaminants, such as mycotoxins, is compromised due to the use of inadequate dietary assessment methods that are not validated or culturally specific (Kroes et al., 2002; Petersen, 2003). It is inevitable, therefore, that the process of risk assessment will differ within and between countries due to the approach followed, history, economy, cultural diversity, policy, and infrastructure available.
South African commercial maize is known to contain low levels of mycotoxins compared to home-grown maize cultivated in rural subsistence farming areas (Burger et al., 2010; Shephard et al., 2005, 2007). However, no regulation exists in South Africa for FB, DON, and ZEA, the major mycotoxins occurring in maize, and grain-based companies have to comply with international trading legislation. In the current study, the use of standardized methods to estimate maize intake, especially raw/uncooked maize as well as accurate mycotoxin analyses resulted in a valid and reliable outcome to define the risk of mycotoxin exposure. When considering the consumption of commercial maize, consumers are not directly exposed to the raw food commodity but to the various products obtained from maize milling. The low mycotoxin levels of two dry milled fractions relevant to human consumption (the SPECIAL and SUPER milling fraction) are due to the effective removal of maize kernel constituents vulnerable to fungal colonization and mycotoxin contamination (Burger et al., 2013). The SPECIAL maize milling fraction or maize flour contains maize kernel surface layers known to be more vulnerable to fungal penetration as compared with the SUPER milling fraction consisting of coarse grits, mainly derived from the endosperm, and containing far lower levels of mycotoxins (Burger et al., 2013; Castells et al., 2008; Scudamore and Patel, 2009). The mycotoxin levels associated with these milling fractions resulted in PDIs well below the respective mycotoxin PMTDI for each of the mycotoxins. As expected specific population groups with high maize intakes, such as men and the black African group reflected higher PDIs. However, due to the large variation in the maize consumption profiles, the risk of exposure of some individuals toward the higher end of consumption is masked when considering the mean values within a subgroup or subpopulation.
To address this, demographic distinct MYCORAM was developed to predict the percentage of consumers that will be at risk considering the respective PMTDIs of FBT, DON, and ZEA. Therefore, the percentage of consumers exposed above the PMTDI for the specific MYCORAM will depend on the maize intakes and body weights, including the specific subgroup or population size and gender characteristics. During the consumer survey, the sampling methodology was based on ethnic distribution according to the 2001 Census Household data and did not include gender distribution resulting in an under-representation of men. Therefore, the percentage of men at risk, normally consuming more maize may be masked within the respective MYCORAM.
Evaluation of the MYCORAM stratified by Provinces, using experimental and commercial milling fractions, the SPECIAL fractions with higher mycotoxin levels affected a larger percentage (5–8-fold) of consumers above the limit compared with the SUPER fractions. The highest percentage of consumers above the limit for FBT, DON, and ZEA were those residing in the MP, LP, GP, and KZN. The latter Provinces are known to have larger population sizes and therefore more consumers distributed across the different maize intake categories, whereas the NC had the smallest sample size.
Utilizing mycotoxin levels from two different experimental SPECIAL and SUPER fractions (Maize 1 and Maize 2), a higher percentage of the population is exposed above the limit for the three mycotoxins. The FB level in the experimental SPECIAL fraction (Maize 1 [H], 1161 μg/kg) was similar to the level reported in home-grown maize (1142 μg/kg) (Shephard et al., 2007). Similarly, the experimental SPECIAL fraction with the higher DON and ZEA (Maize 2 [H]) also showed higher percentages above the PMTDI across the nine Provinces. This milling fraction represents a “worst case scenario” relevant to human consumption and is also aligned with situations prevailing in rural subsistence communities. Regarding the DON level, a recent report by Shephard et al. (2010) showed similar levels (262.0 μg/kg) in South African commercial maize meal which favorable compared with the level in the SPECIAL milling fraction (240.0 μg/kg) of the high DON/ZEA maize sample. The FB exposure levels are in agreement with a study conducted among rural people living in the EC, where both home-grown and commercial maize are consumed in large quantities indicating a larger number of the study population with exposure above the PMTDI (Burger et al., 2010).
Based on these MYCORAM analyses, a MTL for each of the different mycotoxins can be projected for the South African population consuming maize and/or processed maize products such as maize meal. Total fumonisin levels of between 50 and 100, 20 and 50 for DON, and 20 and 30 μg/kg for ZEA, provides MTLs that is attainable in the milling industry while lowering the risk (<1%) of maize consumers residing in five of the nine Provinces. These predicted MTLs may still render certain South African maize consumers at risk with between 1.2 and 4.1% of the maize consumers in the four remaining Provinces (GP, KZN, LP, and MP) exposed above the relative PMTDIs, depending on the mycotoxin. In contrast, much lower levels of mycotoxins are required in order to ensure that the percentage of consumers at risk is <1% across the nine Provinces. The MTLs based on ethnicity, although realistic from an industry perspective, however, global harmonization and international trade may be unrealistic. In this regard, the lowest MTLs for the most vulnerable population (the black Africans) need to be considered.
Maize consumption remains an important part of the South African diet and ranges from the staple diets in some areas to maize-based snacks and side-dishes in more urban areas. The inclusion of more urban maize consumers, known to consume less maize or maize products in the present survey, is likely to affect the MYCORAM risk profile in a specific Province. Consumption of good quality commercial maize becomes evident; however, the unvaried diet of many South Africans consuming high levels of maize may increase the risk even at low levels of mycotoxin contamination. Food safety, being both an integral part as well as a contributor to food security remains a challenge in the context of changing socioeconomic realities that hinders basic food sufficiency and access. The MYCORAM provides the opportunity to identify populations groups of different ethnicity that will be at risk as the use of individual data during risk assessment is known to be more accurate than utilizing national averages (Kroes et al., 2002). This is especially applicable to the current population where the risk of exposure of vulnerable subgroups, is masked due to the inherent diversity in the maize intake of the population. The model is, therefore, far more sensitive approach to assess risk and address the large variation in the exposure data often encountered during epidemiological surveys. It provides an innovative and interactive way to assess the risk of exposure in maize consumers encompassing the three mycotoxins. In addition, it could also be useful in setting international standards for inferring risk in specific subpopulations or groups consuming maize. This could be of relevance for population groups consuming traditional diets such as polenta in Northern Italy; tortillas in Mexico, and people with gluten-free diet, e.g., celiac disease, dermatitis herpetiformis, or an allergy to wheat (Bolger et al., 2001; De Nijs et al., 1998; Pascale et al., 1995). Currently, very little is known about the risk of exposure among vulnerable subgroups such as children and the predicted risk is expected to be far greater than the South African adult population.
FUNDING
Cape Peninsula University of Technology.
Acknowledgments
The authors would like to acknowledge the reputable and prominent South African grain-based manufacturing company and its commitment to ensure safer food for all in South Africa. For the staff of this grain-based manufacturing company and the Southern African Grain Laboratory, a special word of appreciation and gratitude for their crucial contributions. The staff and fieldworkers of a South African award-wining market research company for conducting the consumer survey study and collection of data. Ms Cornelia Ownes, Nutrition Information Centre University of Stellenbosch (NICUS) for entering of the maize intake data and Prof. D.J. Van Schalkwyk for the statistical analysis. The authors are indebted to the participants of the national consumer survey.
REFERENCES
- Amuzie C.J., Pestka J.J. Suppression of insulin-like growth factor acid-labile subunit expression—A novel mechanism for deoxynivalenol-induced growth retardation. Toxicol. Sci. 2010;113:412–421. doi: 10.1093/toxsci/kfp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariño A., Juan T., Estopañan G., González-Cabo J.F. Natural occurrence of Fusarium species, fumonisin production by toxigenic strains, and concentrations of fumonisins B1, and B2 in conventional and organic maize grown in Spain. J. Food Protect. 2007;70:151–156. doi: 10.4315/0362-028x-70.1.151. [DOI] [PubMed] [Google Scholar]
- Binder E.M., Tan L.M., Chin L.J., Handl J., Richard J. Worldwide occurrence of mycotoxins in commodities feeds and feed ingredients. Anim. Feed Sci. Technol. 2007;137:265–282. [Google Scholar]
- Blaauw D., Gilson L. Johannesburg: Centre for Health Policy, University of Witwatersrand; 2001. Health and poverty reduction policies in South Africa. Report prepared for the World Health Organisation. [Google Scholar]
- Bolger M., Coker R.D., DiNovi M., Gaylor D., Gelderblom W., Olsen M., Paster N., Riley R.T., Shephard G., Speijers G.J.A. Prepared by the Fifty-sixth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), Safety Evaluation of Certain Mycotoxins in Food. Geneva, Switzerland: WHO; 2001. Fumonisins; pp. 103–279. WHO Food Additives Series No. 47, FAO Food and Nutrition Paper No. 74. [Google Scholar]
- Boon P.E., Ruprich J., Petersen A., Moussavian S., Debegnach F., Van Klaveren J.D. Harmonisation of food consumption data format for dietary exposure assessments of chemical analysed in raw agricultural commodities. Food Chem. Toxicol. 2009;47:2883–2889. doi: 10.1016/j.fct.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Burger H-M., Lombard M.J., Shephard G.S., Rheeder J.P., Van der Westhuizen L., Gelderblom W.C.A. Dietary fumonisin exposure in a rural population of South Africa. Food Chem. Toxicol. 2010;48:2103–2108. doi: 10.1016/j.fct.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Burger H-M., Shephard G.S., Louw W., Rheeder J.P., Gelderblom W.C.A. The mycotoxin distribution in maize milling fractions under experimental conditions. Int. J. Food Microbiol. 2013;165:57–64. doi: 10.1016/j.ijfoodmicro.2013.03.028. [DOI] [PubMed] [Google Scholar]
- Byrnes R.M., editor. South Africa: A Country Study. Washington, United States 532: Library of Congress; 1996. p. 532. Federal Research Division. [Google Scholar]
- Castells M., Marín S., Sanchis V., Ramos A.J. Distribution of fumonisin and Aflatoxins in corn fractions during industrial cornflake processing. Int. J. Food Microbiol. 2008;123:81–87. doi: 10.1016/j.ijfoodmicro.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Coronel M.B., Sanchis V., Ramos A.J., Marin S. Review. Ochratoxin A: Presence in human plasma and intake estimation. Food Sci. Technol. Int. 2010;16:0005–0018. doi: 10.1177/1082013209353359. [DOI] [PubMed] [Google Scholar]
- De Nijs M., Van Egmond H.P., Nauta M., Rombouts F.M., Notermans S.H. Assessment of human exposure to fumonisin B1. J. Food Protect. 1998;61:879–884. doi: 10.4315/0362-028x-61.7.879. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organisation of the United Nations (FAO) and the World Health Organisation (WHO) Rome: 2006. Food safety risk analysis: A guide for national food safety authorities. FAO Food and Nutrition Paper. Available at: http://www.who.int/foodsafety/micro/riskanalysis/en/#10.1093/toxsci/kfu134.html. Accessed May 2013. [PubMed] [Google Scholar]
- Fryer M., Collins C.D., Ferrier H., Colvile R.N., Nieuwenhuijsen M.J. Human exposure modelling for chemical risk assessment: A review of current approaches and research policy implications. Environ. Sci. Pol. 2006;9:261–274. [Google Scholar]
- Gong Y.Y., Hounsa A., Egal S., Turner P.C., Sutcliffe A.E., Hall A.J., Cardwell K., Wild C.P. Post weaning exposure to aflatoxin results in impaired child growth: A longitudinal study in Benin, West Africa. Environ. Health Perspect. 2004;112:1334–1338. doi: 10.1289/ehp.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A., Smith G.C., Macarthur R., Rose M. Application of uncertainty analysis in assessing dietary exposure. Toxicol. Lett. 2003. pp. 437–442. 140–141. [DOI] [PubMed]
- Hintze J. Kaysville, Utah, United States: NCSS, LLC.; 2007. NCSS. Available at: www.ncss.com.10.1093/toxsci/kfu134.html, http://www.sagl.co.za/Portals/0/Full%20Maize%20report%202.pdf10.1093/toxsci/kfu134.html. Accessed: January 2013. [Google Scholar]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. Vol. 56. Lyon, France: International Agency for Research on Cancer; 1993a. Aflatoxins; pp. 245–395. [Google Scholar]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans, Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxin. Vol. 56. Lyon, France: International Agency for Research on Cancer; 1993b. Ochratoxin A; pp. 489–521. [Google Scholar]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. Vol. 82. Lyon, France: International Agency for Research on Cancer; 2002a. Aflatoxins; pp. 171–366. [PMC free article] [PubMed] [Google Scholar]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. Vol. 82. Lyon, France: International Agency for Research on Cancer; 2002b. Fumonisin B1; pp. 301–366. [PMC free article] [PubMed] [Google Scholar]
- JECFA. Joint FAO/WHO Expert Committee on Food Additives (Ed.), Safety Evaluation of Certain Food Additives and Contaminants. Safety Evaluation of Certain Food Additives and Contaminants. Geneva, Switzerland: World Health Organisation; 2000. Zearalenone. WHO/FAO Food Additives Series 44. IPCS—International Programme on Chemical Safety. [Google Scholar]
- JECFA. Joint Expert Committee on Food Additives. Safety Evaluation of Certain Mycotoxins in Food. Geneva, Switzerland: World Health Organisation; 2001. Deoxynivalenol. FAO Food and Nutrition Paper 74 WHO Food Additives, Series 47 pp. 281–387 and 419–555. [Google Scholar]
- JECFA. Evaluation of Certain Mycotoxins in Food. 56th Report of the Joint FAO/WHO Expert Committee on Food Additives Technical Reports Series No. 906. Geneva, Switzerland: World Health Organisation; 2002. Ochratoxin A; pp. 27–35. Available at: http://whqlibdoc.who.int/trs/WHO_TRS_906.pdf. [Google Scholar]
- JECFA. Joint FAO/WHO Expert Committee on Food Additives (Ed.) 2012. Fumonisins. Safety Evaluation of Certain Food Additives and Contaminants. [Google Scholar]
- Kimanya M.E., De Meulenaer B., Roberfroid D., Lachat C., Kolsteren P. Fumonisin exposure through maize in complementary foods is inversely associated with linear growth of infants in Tanzania. Mol. Nutr. Food Res. 2010;54:1659–1667. doi: 10.1002/mnfr.200900483. [DOI] [PubMed] [Google Scholar]
- Kroes R., Muller D., Lambe J., Lowik M.R., Van Klaveren J., Kleiner J., Massey R., Mayer S., Urieta I., Verger P., et al. Assessment of intake from the diet. Food Chem. Toxicol. 2002;40:327–385. doi: 10.1016/s0278-6915(01)00113-2. [DOI] [PubMed] [Google Scholar]
- Labadarios D., Swart R., Maunder E.M.W., Kruger H.S., Gericke G.J., Kuzwayo P.M.N. Executive summary of the National Food consumption Survey Fortification Baseline (NFCS-FB-I) S. Afr. J. Clin. Nutr. 2008;21:245–300. [Google Scholar]
- Lambe J. The use of food consumption data in assessments of exposure to food chemicals including the application of probabilistic modelling. Proc. Nutr. Soc. 2002;61:11–18. doi: 10.1079/pns2001125. [DOI] [PubMed] [Google Scholar]
- Lombard M.J., Steyn N., Burger H-M., Charlton K., Senekal M., Gelderblom W.C.A. A proposed method to determine fumonisin exposure from maize consumption in a rural South African population using a culturally appropriate food frequency questionnaire. Public Health Nutr. 2014;17:131–138. doi: 10.1017/S1368980012004946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais J.S. The Cape Coloured People 1652 to 1932. South Africa: Witwatersrand University Press; 1968. pp. 1–31. [Google Scholar]
- Marasas W.F.O., Miller J.D., Riley R.T., Visconti A. Fumonisins-occurrence, toxicology, metabolism and risk assessment. In: Summerell B.A., Leslie J.F., Backhouse D., Bryden W.L., Burgess L.W., editors. Fusarium. St. Paul, Minnesota, United States 332-359: APS Press; 2001. pp. 332–359. Paul E. Nelson Memorial Symposium. [Google Scholar]
- Marasas W.F.O., Riley R.T., Hendricks K.A., Stevens V.L., Sadler T.W., Gelineau-van Waes J., Missmer S.A., Cabrera J., Torres O., Gelderblom W.C.A., et al. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo vulture and in vivo: A potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J. Nutr. 2004;134:711–716. doi: 10.1093/jn/134.4.711. [DOI] [PubMed] [Google Scholar]
- Maresca M., Mahfoud R., Garmy N., Fantini J. The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial cells. J. Nutr. 2002;132:2723–2731. doi: 10.1093/jn/132.9.2723. [DOI] [PubMed] [Google Scholar]
- Massart F., Meucci V., Saggese G., Soldani G. High growth rate of girls with precocious puberty exposed to estrogenic mycotoxins. J. Pediatr. 2008;152:690–695. doi: 10.1016/j.jpeds.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Møller A., Ireland J. How can the requirements of exposure assessment be realised by different categorisation system. 2008. Workshop on “Food Consumption Data and Dietary Exposure in the European Union” Berlin, 15–16 May 2008.
- Nel J.H., Steyn N.P. Pretoria, South Africa: 2002. Report on South African food consumption studies undertaken amongst different population groups (1983–2000): Average intakes of foods most commonly consumed. Available at: http://www.mrc.ac.za/chronic/foodstudies.htm10.1093/toxsci/kfu134.html. Accessed August 2012. [Google Scholar]
- Parmar B., Miller P.F., Burt R. Stepwise approaches for estimating the intakes of chemicals in food. Regul. Toxicol. Pharmacol. 1997;26:44–51. doi: 10.1006/rtph.1997.1117. [DOI] [PubMed] [Google Scholar]
- Pascale M., Doko M.B., Visconti A. Proceedings of the 2nd National Congress on Food Chemistry, Giardini-Naxos, 24–27 May 1995. Messina: La Grafica editorial; 1995. Detremination of fumonisin in polenta by high performance liquid chromatography; pp. 1067–1071. in Italian. [Google Scholar]
- Petersen B.J. Methodological aspects related to aggregate and cumulative exposure to contaminants with common mechanism of toxicity. Toxicol. Lett. 2003. pp. 140–141, 427–435. [DOI] [PubMed]
- Rheeder J.P., Marasas W.F.O., Thiel P.G., Sydenham E.W., Shephard G.S., Van Schalkwyk D.J. Fusarium moniliforme and fumonisins in corn in relation to human oesophageal cancer in Transkei. Phytopathology. 1992;82:353–357. [Google Scholar]
- Scudamore K.A., Patel S. Fusarium mycotoxins in milling streams from commercial milling maize imported to the UK, and relevance to current legislation. Food Addit. Contam. 2009;26:744–753. doi: 10.1080/02652030802688394. [DOI] [PubMed] [Google Scholar]
- Shephard G.S., Marasas W.F., Burger H-M., Somdyala N.I., Rheeder J.P., Van der Westhuizen L., Gatyeni P., Van Schalkwyk D.J. Exposure assessment for fumonisins in the former Transkei region of South Africa. Food Addit. Contam. 2007;24:621–629. doi: 10.1080/02652030601101136. [DOI] [PubMed] [Google Scholar]
- Shephard G.S., Van der Westhuizen L., Gatyeni P.M., Somdyala N.I., Burger H-M., Marasas W.F.O. Fumonisin mycotoxins in traditional Xhosa maize beer in South Africa. J. Agric. Food Chem. 2005;53:9634–9637. doi: 10.1021/jf0516080. [DOI] [PubMed] [Google Scholar]
- Shephard G.S., Van der Westhuizen L., Katerere D.R., Herbst M., Pineiro M. Preliminary exposure assessment of deoxynivalenol and patulin in South Africa. Myco. Res. 2010;26:181–185. doi: 10.1007/s12550-010-0052-9. [DOI] [PubMed] [Google Scholar]
- Shephard G.S., Kimanya M.E., Kpodo K.A., Gnonlonfin G.J.B., Gelderblom W.C.A. The risk management dilemma for fumonisin mycotoxins. Food Control. 2013. pp. 34,596–600.
- Statistics South Africa. 2001. http://www.statssa.gov.za/census01/html/CInbrief/CIB2001.pdf10.1093/toxsci/kfu134.html. Accessed August 2012.
- Sydenham E.W., Thiel P.G., Marasas W.F.O., Shephard G.S., Van Schalkwyk D.J., Koch K.R. Natural occurrence of some Fusarium mycotoxins in corn from low and high oesophageal cancer prevalence areas of the Transkei, Southern Africa. J. Agric. Food Chem. 1992;38:1900–1903. [Google Scholar]
- Thomas A., Bendixen M. The management implications of ethnicity in South Africa. J. Int. Bus. Stud. 2000;31:507–519. [Google Scholar]
- Turner N.W., Subrahmanyam S., Piletsky S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta. 2009;632:168–180. doi: 10.1016/j.aca.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Turner P.C., Sylla A., Diallo M.S., Castegnaro J.J., Hall A.J., Wild C.P. The role of aflatoxins and hepatis virus in the etiopathogenesis of hepatocellular carcinoma: A basis for primary prevention in Guinea-Conakry, West Africa. J. Gastroenterol. Hepatol. 2002;17:5441–5448. doi: 10.1046/j.1440-1746.17.s4.7.x. [DOI] [PubMed] [Google Scholar]
- Turner P.C., Moore S.E., Hall A.J., Prentice A.M., Wild C.P. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ. Health Perspect. 2003;111:217–220. doi: 10.1289/ehp.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P.C., Collinson A.C., Cheung Y.B., Gong Y.Y., Hall A.J., Prentice A.M., Wild C.P. Aflatoxin exposure in utero causes growth faltering in Gambian infants. Int. J. Epidemiol. 2007;36:1119–1125. doi: 10.1093/ije/dym122. [DOI] [PubMed] [Google Scholar]
- Warth B., Sulyok M., Berthiller F., Schuhmacher R., Krska R. New insights into the human metabolism of the Fusarium mycotoxins deoxynivalenol and zearalenone. Toxicol. Lett. 2013;220:88–94. doi: 10.1016/j.toxlet.2013.04.012. [DOI] [PubMed] [Google Scholar]


