Abstract
It was reported that aminochrome induces the formation of alpha synuclein (SNCA) oligomers during dopamine oxidation. We found that DT-diaphorase (NQO1) prevents the formation of SNCA oligomers in the presence of aminochrome determined by Western blot, transmission electron microscopy, circular dichroism, and thioflavin T fluorescence, suggesting a protective role of NQO1 by preventing the formation of SNCA oligomers in dopaminergic neurons. In order to test NQO1 protective role in SNCA neurotoxicity in cellular model, we overexpressed SNCA in both RCSN-3 cells (wild-type) and RCSN-3Nq7 cells, which have constitutive expression of a siRNA against NQO1. The expression of SNCA in RCSN-3SNCA and RCSN-3Nq7SNCA cells increased 4.2- and 4.4-fold, respectively. The overexpression of SNCA in RCSN-3Nq7SNCA cells induces a significant increase in cell death of 2.8- and 3.2-fold when they were incubated with 50 and 70 µM aminochrome, respectively. The cell death was found to be of apoptotic character determined by annexin/propidium iodide technique with flow cytometry and DNA laddering. A Western blot demonstrated that SNCA in RCSN-3SNCA is only found in monomer form both in the presence of 20 µM aminochrome or cell culture medium contrasting with RCSN-3Nq7SNCA cells where the majority SNCA is found as oligomer. The antioligomer compound scyllo-inositol induced a significant decrease in aminochrome-induced cell death in RCSN-3Nq7SNCA cells in comparison to cells incubated in the absence of scyllo-inositol. Our results suggest that NQO1 seems to play an important role in the prevention of aminochrome-induced SNCA oligomer formation and SNCA oligomers neurotoxicity in dopaminergic neurons.
Keywords: alpha synuclein, dopamine, aminochrome, Parkinsons’s disease, neuroprotection, oligomers, NQO1, dopaminergic neurons
Several genes have been linked to Parkinson’s disease (PD), but synuclein (SNCA) has attracted the most attention since it is a major constituent of Lewy bodies (Scarlata and Golebiewska, 2014; Spillantini et al., 1998). Given that SNCA assumes a fibrillar beta-pleated sheet structure in Lewy bodies in PD and related SNCApathies (Spillantini et al., 1998), the leading hypothesis for its pathogenicity is the formation of toxic oligomers. Several lines of evidence have shown that a prerequisite of SNCA pathology is its oligomerization into cytotoxic oligomers (Volles and Lansbury, 2003). These toxic oligomers induced rigidity, hypokinesia, and immobility (Gruden et al., 2014).
SNCA has been shown to bind synaptic vesicles, and oligomers can form pores that could lead to permeabilization of the vesicle membranes, thereby releasing dopamine into the cytosol (Lashuel et al., 2002). Formation of oligomers is enhanced and stabilized by dopamine quinones derived from the oxidation of dopamine, and this could account for the selective toxicity of SNCA oligomers in the substantia nigra (Conway et al., 2001; Li et al., 2005). Aminochrome (also called dopaminochrome) has been identified as the dopamine quinone that forms adducts with SNCA, inhibiting SNCA fibrillization by inducing structural changes that can occur through the interaction of aminochrome with the 125YEMPS129 motif of SNCA (Norris et al., 2005).
Dopamine oxidation to aminochrome in dopaminergic neurons that contain neuromelanin seems to be a normal process since this compound is the precursor of neuromelanin. Dopamine can autooxidize to aminochrome at physiological pH in the presence of oxygen or can be oxidized by transition metals or enzymes such as prostaglandin H synthase, cytochrome P450 isoforms, xanthine oxidase, tyrosinase, and dopamine monooxygenase (for review see Segura-Aguilar et al., 2014). Aminochrome can be reduced by flavoenzymes through a 1-electron reduction to leukoaminochrome o-semiquinone radical, which is extremely reactive with oxygen and hence neurotoxic (Segura-Aguilar et al., 1998). Alternatively, NQO1 (EC.1.6.99.2) can reduce aminochrome and it is the unique flavoenzyme that catalyzes 2-electron reduction of quinones (Segura-Aguilar and Lind, 1989). NQO1 has been reported to prevent the 1-electron reduction of aminochrome to leukoaminochrome o-semiquinone radical by catalyzing a 2-electron reduction of aminochrome to leukoaminochrome (Arriagada et al., 2004; Lozano et al., 2010). In the present work, we investigated the ability of NQO1 to prevent the formation of aminochrome-induced SNCA oligomers in vitro and its neurotoxic effects in a catecholaminergic model cell line.
MATERIALS AND METHODS
Chemicals
CM-Sephadex C50-100, DEAE Sephadex 50-120, Sephadex G-25, dopamine, IPTG, tyrosinase [EC 1.14.18.1] from mushroom were purchased from SigmaAldrich (St Louis, MO, USA). Lysozyme was from US Biological. NQO1 was purified from rat liver according to Segura-Aguilar et al. (1992).
Synthesis and purification of aminochrome
Dopamine (7.5 mmol) and 15 ng of tyrosinase were incubated in 1.5 ml of 25 mM phosphate buffer pH 6.0 for 10 min at 25°C. To purify aminochrome formed, the incubation solution was loaded on a column 17 × 0.7 cm resin CM-Sephadex C-50-120 which was eluted first with 30 ml of 25 mM phosphate buffer pH 6.0. The eluate from the column was recovered in 500 µl aliquots. Each aliquot was monitored spectrophotometrically by absorbance at 280, 478, and 600 nm wavelength. The recovered pure aminocromo 5–7 ml eluted with 25 mM of phosphate buffer pH 6.0; dopamine recovered only after 5 ml of 25 mM 4-Morpholineethanesulfonic acid sodium salt (MES) pH 6.0 (Paris et al., 2010).
SNCA purification
Recombinant human SNCA in the pRK172-αsyn plasmid was expressed in BL21(DE3)pLysS bacteria and purified according to Conway et al. (2001) with the following modifications. Plasmid expression was induced with 0.5 mM IPTG for 3 h. The cells were harvested, resuspended in 50 mM Tris-HCl, pH 7.5, 50 mM NaCl, and 5% glycerol, and lysed by repeatedly freezing in liquid nitrogen and thawing 3 times. The cells were incubated with 7.7 KU lysozyme per milliliter in 50 mM Tris-HCl, pH 7.5, 50 mM NaCl, and 5% glycerol for 20 min at room temperature. Before precipitation with 50% ammonium sulfate, the protein was boiled for 30 min. The 50% ammonium sulfate pellet was resuspended in 25 mM Tris-HCl, pH 8.0, and desalted on a G-25 Sephadex column (30 × 0.7 cm). The SNCA fraction was applied to a DEAE Sephadex 50–120 column (18 × 0.7 cm) equilibrated with 25mM Tris-HCl, pH 8.0, and eluted with 25 mM Tris-HCl, pH 8.0, containing 0.5 M NaCl. The fractions containing SNCA were determined by Western blot and SDS-polyacrylamide gel electrophoresis (PAGE) with silver staining. The plasmid wtSYNpRK172 that encodes human SNCA was a kind donation from Professor Ischiropoulos.
Aggregation assays
SNCA aggregation measurements were performed at 1-, 2-, and 3-day incubation with recombinant human SNCA (100 µM) under constant stirring (200 rpm) in 20 mM MES and 100 mM NaCl, pH 6.5 at 37°C. The degree of aggregation was determined using thioflavin T (ThT) fluorescence, circular dichroism (CD), and transmission electron microscopy.
CD spectra
CD spectra were recorded using a Jasco J-810 spectropolarimeter. Spectra were collected at 25°C in a 0.1 cm-long quartz cuvette containing the 20× protein dilution in 20 mM MES buffer, pH 6.5.
ThT fluorescence
ThT fluorescence was scanned between 460 and 600 nm using an excitation wavelength of 446 nm by diluting an aliquot 400 times in 50 mM MES, pH 6.5, and 5µM ThT and data were collected at different incubation times. For each sample, the ThT intensity at 480 nm was used to measure the formation of fibers.
Electron microscopy
Formvar-coated grids were inverted over 10 µl drops of prepared SNCA aggregate suspensions 1 min. The grids were dried with a filter paper, stained with 2% aqueous uranyl acetate for 5 min, and allowed to dry at room temperature for 15 min. The grids were examined at 80 kV, and representative fields were photographed at ×33 000 magnifications on a Zeiss EM900 transmission electron microscope.
Cell culture
The RCSN-3 cell line was grown in monolayers, with a doubling time of 52 h, a plating efficiency of 21% and a saturation density of 56 000 cells/cm2 in normal growth media composed of DME/HAM-F12 (1:1), 10% bovine serum, 2.5% fetal bovine serum. The cultures were kept in an incubator at 37°C with 100% humidity, and the cells grew well in atmospheres of both 5% or 10% CO2 (Lozano et al., 2010; Paris et al., 2008). RCSN-3 cells are derived from the substantia nigra of an adult rat. The cell line does not require differentiation to express catecholaminergic traits, such as tyrosine hydroxylase, dopamine transport, VMAT-2, norepinephrine transport, dopamine release, monoamine oxidase (MAO)-A expression but not MAO-B; formation of neuromelanin and DT-diaphorase is responsible for 94% of the total quinone reductase activity catalyzed by flavoenzymes (Paris et al., 2008).
Transfection of RCSN-3 and RCSN-3Nq7 cells
RCSN-3 and RCSN-3NQ7 cells were transduced with lentiviral plasmid pLvGFP-SNCA constructed in our laboratory in order to overexpress SNCA. Lentiviral particles were obtained by transfecting HEK-293 T cells with a mixture of 6 µg DNA of pLvGFP-SNCA coding for GFP-IRES-SNCA, 6 µg DNA of packing plasmid pMDG, and FUGENE HD (Roche) in a relationship (8:2 DNA:FUGENE HD) in 300 µl cell culture medium without serum. After 1 h serum was added to the cells and was incubated during 24, 48, and 72 h until the supernatant was collected and filtered with a 0.45-µm filter. As control pRetrosuper without DNA insert and pMDG were transfected by using FUGENE HD as explained above. Transduced cells were incubated in the presence of 40 mg/l gentamicin sulfate to select the cells that have the plasmid with a gene resistant to gentamicin sulfate.
Dot blot
Dot blots were performed by using a Bio-Rad Bio-Dot apparatus assembled with a nitrocellulose membrane that previously was immersed in 20 mM Tris pH 7.6 containing 136 mM NaCl. All the samples contained 50 µg protein. The nitrocellulose membrane was blocked by incubating them in 20 mM Tris pH 7.6 containing 136 mM NaCl, Tween 20 0.1%; low fat milk 5% during 3 h at room temperature with gently shaking and washed 3 times. The membrane was incubated in a solution of 20 mM Tris pH 7.6 containing 136 mM NaCl, Tween 20 0.1%, BSA 5%, and SNCA antibodies diluted 1:1000 (Everest Biotech, Oxfordshire, UK, EB1111714), polyclonal antibodies against DT-diaphorase diluted 1:1000 (SC-7012, Santa Cruz Biotechnology Inc). The membrane was incubated for 5 min with alkaline phosphatase substrate buffer and the staining solution with 5-bromo- 4-chloro-3'-indolyphosphate (BCIP) and nitro-blue-tetrazolium (NBT). The quantification of pixels was done by using Scion Image software. The membranes were scanned to obtain a high-quality image grayscale and produced a 1200 dpi resolution TIFF file. The pixels of area corresponding to the band and an area without bands were measured. Finally, the background value (area × mean intensity) was subtracted from the band value (area × mean intensity).
DNA laddering
The cells were grown to 95% confluence and after the treatment we added 1 ml of trypsin to the cell monolayer. The cells were centrifuged at 2500 rpm for 10 min and we added 100 µl of lysis buffer (1% NP-40, 20 mM EDTA, and 50 mM Tris-HCl, pH 7.5) for 10 s. Centrifuged at 3000 rpm for 5 min to obtain the supernatant. The sample with 1% SDS was treated with RNase for 2 h and 2.5 µg proteinase K added and incubated for 2 h at 37°C. The DNA was precipitated by adding 0.5 vol of 10 M ammonium acetate and 2.5 vol of cold ethanol and incubated at −80°C for 1 h. The sample was centrifuged for 20 min at 12 000 rpm and a white pellet was washed with 200 µl of cold 80% ethanol and dried for 10 min at room temperature. The pellet was dissolved in TE buffer and the concentration was determined. The samples were loaded on a gel of 2% agarose.
Immunofluorescence
The cells were grown to 70% confluence on glass coverslips. The cells are incubation with 100% MeOH for 30 min at −20°C to permeabilize the cell membrane. After washing with PBS the cells were incubated with 30 min at room temperature with blocking solution (1.5% skimmed milk in PBS plus Triton-X100 0.15%) and followed by the incubation with a solution containing the primary antibody at a dilution of 1/200 dissolved in PBS plus 1% BSA overnight at 4°C. After washing the incubation with secondary antibody diluted at 1/250 in PBS plus 1% BSA for 1.5 h protected from light at room temperature and washed 3 times with PBS. Incubated with diluted Hoechst solution in TE buffer to 1/10 000 for 5 min, washed with PBS, and mounted with Dako solution. The results are observed in a confocal microscope. We used oligomer polyclonal antibody (A11) AHB0052 from Invitrogen to detect SNCA oligomer formation in the cells (Kayed et al., 2003).
Flow cytometry
Annexin binding buffer (BUA ) was composed by 10 mM Hepes, 140 mM NaCl 2.5 mM CaCl2, pH 7.4. The cells were grown to 80% confluence and treated with 20 µM aminochrome and 3.14 µM staurosporine for 24 h. After the treatment was trypsinized and washed with cold 1 × PBS. The counting was performed in neubauer chamber cell density of 1 × 106 cells/ml and dilute. The annexin V antibody conjugate was added before propidium iodide. The cells were cultured at room temperature for 15 min and then added 400 µl of BUA mixed gently and kept in ice. Analyzed as quickly as possible by flow cytometry, measuring the fluorescence emission of each fluorophore at 530 and 575 nm.
Western blot
The samples of SNCA were separated by SDS-PAGE (10% wt/vol). The separated proteins were then transferred electrophoretically to a 0.2-µm nitrocellulose membrane. After blocking with a solution of 0.5% skim milk in 10 mM Tris-HCl pH 7.6, 150 mM NaCl, and 0.025% Tween 20 for at least 4 h, the membrane was incubated with a polyclonal antibody against human α/β synuclein (Santa Cruz Biotechnology) overnight at room temperature in the same buffer. The membrane was washed 3 times for 15 min with a solution of 10 mM Tris-HCl pH 7.6, 150 mM NaCl, and 0.025% Tween 20 and incubated for 2 h with an anti-goat alkaline phosphatase-linked antibody. After a final washing, SNCA was detected using BCIP/NBT (Zymed laboratories Inc).
RESULTS
The Role of NQO1 on SNCA Oligomers
CD was used to determine the possible conformational changes in SNCA to a beta-sheet structure after addition of aminochrome. The conformation of soluble SNCA is a random coil as it is indicated by the observed minimum at 200 nm. Incubation of 100µM SNCA with stirring (200 rpm) at 37°C did not show conformational changes after 24 h (Fig. 1A). However, incubation under the same conditions for 48 and 72 h caused a conformational change, since we can observe the typical signal of a beta-sheet structure (Figs. 1B and 1C). The addition of 100µM aminochrome to 100µM SNCA delayed but did not prevent the conformational changes to the typical beta-sheet structure, since the incubation of SNCA with aminochrome for 48 h showed a transition state between the random coil conformation (Fig. 1B) and the beta-sheet structure observed at 72 h (Fig. 1C). Interestingly, NQO1, which catalyzes the 2-electron reduction of aminochrome preventing the formation of adducts between aminochrome and SNCA, should allow SNCA to form fibrils as we observed with SNCA alone. However, NQO1 completely prevented the conformational changes to a beta-sheet structure when 100 µM SNCA, 100 µM aminochrome, 1.2 µg NQO1, and 500µM NADH were incubated at 24 and 48 h (Figs. 1B and 1C). The addition of 500µM NADH and 1.2 µg NQO1 alone to the incubation mixture containing 100 µM SNCA had no effect on the typical conformational changes observed in SNCA aggregation to fibers (data not shown). The effect of aminochrome on the kinetics of SNCA fibril formation was monitored using the ThT assay at 37°C with stirring in the absence or presence of NQO1 (Fig. 1D). Untreated 100µM SNCA exhibited the characteristic sigmoidal curve, that is, an initial lag phase, a subsequent exponential growth phase between 20 and 40 h, and a final equilibrium phase after 48 h (t1/2 = 26 h). The exponential growth phase of SNCA (100µM) was inhibited in the presence of 100µM aminochrome (t1/2 = 37 h) and in the presence of 100µM aminochrome. However, SNCA fibril formation in the presence of 1.2 µg NQO1 with 500µM NADH was completely inhibited and therefore, it was not possible to determine the t1/2 (Fig. 1D). The addition of 500µM NADH alone to 100 µM SNCA had no effect on SNCA fibril formation (data not shown).
FIG. 1.
Conformational changes of SNCA in the presence of aminochrome and NQO1. A, The effect of NQO1 on aminochrome-induced conformational changes. Circular dichroism was used to determine the possible conformational changes in SNCA to a beta-sheet structure after addition of aminochrome. The circular dichroism spectra of 100 µM SNCA (1); 100 µM SNCA with 100 µM aminochrome (2); 100 µM SNCA, 100 µM aminochrome, 1.2 µg NQO1, and 500µM NADH (3) were obtained by incubated for 24 (A), 48 (B), and 72 h (C) at 37°C with stirring as described in Materials and Methods section. B, Fibrillization and beta-sheet formation kinetics of SNCA incubated with aminochrome in the presence of NQO1. Fibrillization and beta-sheet formation were determined with the ThT fluorescence assay. The incubation mixtures contained 100µM SNCA (•) show SNCA fibrils formation; 100µM SNCA together with 100µM aminochrome (○) show the formation of oligomers; 100µM SNCA, 100µM aminochrome, 1.2 µg NQO1, and 500µM NADH (▾) show that SNCA is in the monomer state. Measurements were performed at 37°C with agitation as described in the Experimental Procedures.
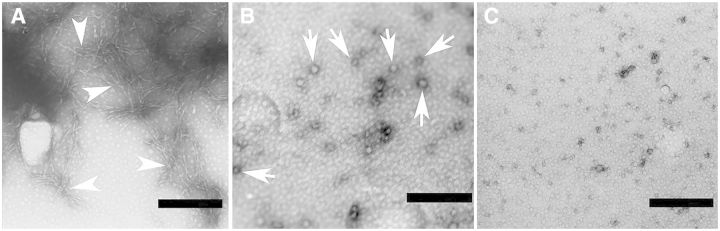
The aggregation of recombinant wild-type human SNCA incubated for 48 h at 37°C with stirring was determined by using transmission electron microscopy. We observed the formation of large aggregates of fibers at×33 000 magnification when 100µM SNCA was incubated alone (Fig. 2A). We observed a large number of small globular structures surrounded by a high-density dark circle corresponding SNCA globular aggregation at ×33 000 magnification when 100 µM SNCA was incubated with 100µM aminochrome (Fig. 2B). Interestingly, small globular structures or SNCA fibers were not detected at ×33 000 magnification when 100 µM SNCA and 100 µM aminochrome were incubated in the presence of 1.2 µg NQO1 with 500µM NADH (Fig. 2C).
FIG. 2.
Transmission electron microscopy of aminochrome-induced SNCA aggregation. A, Human recombinant SNCA (100µM) incubated 48 h at 37°C with stirring is shown. B, SNCA (100µM) incubated with 100µM aminochrome show the formation of small globular structures corresponding SNCA globular aggregation. C, SNCA (100µM) incubated with 100µM aminochrome in the presence of 1.2 µg NQO1 and 500µM NADH show the absence of small globular structures. The magnification was ×30 000 and the scale bar represents 0.25 µm. The white arrowheads show SNCA oligomers and the white arrows show SNCA globular aggregations.
NQO1 Prevents SNCA Oligomer-Induced Cell Death
Our in vitro data suggested that NQO1 may play an essential role in the prevention of SNCA-induced neurotoxicity by preventing the formation of neurotoxic SNCA oligomers and to test this idea we constructed the lentiviral plasmid pLvGFP-SNCA coding for green fluorescent protein (GFP) and SNCA (Fig. 3A) in order to generate a cell line overexpressing SNCA. We transduced the cell line RCSN-3 (control) and RCSN-3Nq7 (with constitutive expression of a siRNA against NQO1; Lozano et al., 2010) with pLvGFP-SNCA generating two cell lines, RCSN-3SNCA cells that has over expression of SNCA and normal level of expression of NQO1 and RCSN-3Nq7SNCA cells that has SNCA over expression and only 13 % of NQO1 expression. A dot blot analysis revealed that SNCA expression increased 4.2- (P < 0.05) and 4.4-fold (P < 0.05) in RCSN-3SNCA and RCSN-3Nq7SNCA, respectively (Figs. 3B and 3C). NQO1 activity in RCSN-3Nq7SNCA cells was found to be only a 29% of RCSN-3SNCA cells (Fig. 3D). We measured the mRNA expression of SNCA by using real-time PCR and we found SNCA overexpression both in RCSN-3SNCA (1.9 ± 0.4 relative quantity) and RCSN-3Nq7SNCA cells (1.7 ± 0.08 relative quantity) but we don’t found significant differences between these cells. A significant decrease in NQO1 expression was observed in RCSN-3Nq7SNCA cells (0.35 ± 0.09 relative quantity; P < 0.001) that correspond to 13% of the enzyme expression in RCSN-3SNCA cells (2.5 ± 0.3; Fig. 3E).
FIG. 3.
Overexpression of SNCA in RCSN-3 cells. A, We have constructed the lentiviral plasmid pLvGFP-SNCA that was transduced into RCSN-3 and RCSN-3Nq7 cells to overexpress SNCA. We generated 2 new cell lines RCSN-3SNCA (WT) and RCSN-3Nq7SNCA (Nq7SNCA) which have permanent expression of a siRNA against NQO1. The overexpression of SNCA was determined by using dot blot technique and immunostaining that show the total amount of alpha synuclein but you cannot see the different forms of SNCA such as monomer and oligomers (B). The result of dot blot was plotted by using the SNCA/GAPDH values (C). A significant decrease in enzymatic activity of NQO1 was observed in RCSN-3Nq7SNCA (D). Quantitative real-time PCR confirmed the over-expression of SNCA (SNCA) in both cell lines and the decrease of mRNA expression of NQO1 (NQO1) in RCSN-3Nq7SNCA cells (E). The statistical significance was assessed using analysis of variance (ANOVA) for multiple comparisons (*P < 0.05; **P < 0.01; ***P < 0.001).
We determined aminochrome toxicity in RCSN-3, RCSN-3SNCA, RCSN-3Nq7, and RCSN-3Nq7SNCA cell in the presence of different concentrations of aminochrome. The incubation of these cell lines with 0, 10, 30, 50, and 70 µM aminochrome at 24 h induced a concentration-dependent increase in cell death that it is statically significant from 30 µM (Fig. 1A). When we compare RCSN-3Nq7SNCA with respect to RCSN-3Nq7 we observed a 2.8- and 3.17-fold increase in cell death when RCSN-3Nq7SNCA cells are incubated with 50 µM (P < 0.001) or 70 µM aminochrome (P < 0.001) (Fig. 4A). We determined the cell death at very low concentration of aminochrome in order to have the majority of cells alive to study the formation of oligomers. The incubation of RCSN-3SNCA and RCSN-3Nq7SNCA cells with 20 µM aminochrome during 24 h resulted in 6.1 ± 1.5% and 13 ± 2% (P < 0.01) cell death, respectively (Fig. 4B). To determine whether aminochrome-induced cell death observed in RCSN-3SNCA and RCSN-3Nq7SNCA cell lines with overexpression of SNCA was an apoptotic cell death we determined DNA fragmentation with laddering technique. RCSN-3SNCA cells in the presence of cell culture medium or 20 µM aminochrome don’t show DNA laddering (Fig. 5A). However, RCSN-3Nq7SNCA cell line treated with 20 µM aminochrome shows a diffuse laddering that contrast with the cells exposed only to cell culture medium (Fig. 5B). We determined the possible role of apoptosis in aminochrome-induced cell death in RCSN-3SNCA and RCSN-3Nq7SNCA cells by using flow cytometry with Alexa Fluor 488 annexin V and propidium iodide. The majority of RCSN-3Nq7SNCA cells treated with 20 µM aminochrome (Fig. 5F) has similar localization than the RCSN-3Nq7SNCA cells incubated with 3.4 mM staurosporine which is a positive control of apoptosis (Fig. 5G). RCSN-3Nq7SNCA cells treated with 20 µM aminochrome or with 3.4 mM staurosporine have 74 ± 1% (P < 0.05) and 95 ± 4% (P < 0.05) of cells marked with both annexin V and propidium iodide, respectively. RCSN-3SNCA cells incubated with cell culture medium or 20 µM aminochrome and RCSN-3Nq7SNCA cells incubated with cell culture medium have very low number of cells marked with both annexin V and propidium iodide (1.0–0.03%, 1.7–0.1%, and 0.8 ± 0.05%, respectively) (Fig. 5H).
FIG. 4.
The effect of aminochrome on RCSN-3SNCA and RCSN-3Nq7SNCA cells. A, The effect of aminochrome at different concentrations (0, 10, 30, 50, and 70 µM) was determined on RCSN-3 (WT), RCSN-3SNCA (WT-SNCA), RCSN-3Nq7 (Nq7), and RCSN-3Nq7SNCA (Nq7-SNCA) cells at 24 h. B, The effect of 20 µM aminochrome was determined on RCSN-3SNCA (WT) and RCSN-3Nq7SNCA (Nq7SNCA) cells at 24 h. The values are the mean ± SD with n = 3. The statistical significance was assessed using ANOVA for multiple comparisons (*P < 0.05; **P < 0.01; ***P < 0.001).
FIG. 5.
Determination of the role of apoptosis in aminochrome-induced cell death in RCSN-3SNCA and RCSN-3Nq7SNCA cells. The possible role of apoptosis on aminochrome-induced cell death in RCSN-3SNCA and RCSN-3Nq7SNCA cells was determined by using DNA laddering (A and B) and flow cytometry with Alexa Fluor 488-annexin and propidium iodide technique (C–G). DNA fragmentation was only observed in RCSN-3Nq7SNCA cells (B) when the cells were treated with 20 µM aminochrome (AM) for 24 h contrasting with the lack of DNA fragmentation in RCSN-3SNCA cells treated under the same conditions (A). As positive control we used 3.4 mM staurosporine (C+) and as negative control the cells were incubated in the absence of aminochrome (C). The flow cytometry with Alexa Fluor 488 annexin V and propidium iodide revealed that only RCSN-3Nq7SNCA cells treated with 20 µM aminochrome (F) presented similar localization as cells treated with 3.4 mM staurosporine that is a positive control for apoptosis (G). RCSN-3SNCA (C) and RCSN-3Nq7SNCA (E) cells incubated in the absence of aminochrome or RCSN-3SNCA cells incubated with 20 µM aminochrome (D) for 24 h don’t have the same localization as cells treated with 3.4 mM staurosporine. The results were plotted in H. The values are the mean ± SD with n = 3. The statistical significance was assessed using ANOVA for multiple comparisons (*P < 0.05).
We determine the effect of aminochrome on oligomers formation in RCSN-3SNCA and RCSN-3Nq7SNCA cells by using Western blot technique; 20 µM aminochrome induces a significant increase in the formation of oligomers in RCSN-3Nq7SNCA cells (100 ± 3 pixels; P < 0.05) in comparison with the same cell line incubated with cell culture medium (47 ± 2 pixels). Interestingly, the amount of oligomers increased significantly also in the absence of aminochrome in RCSN-3Nq7SNCA cells (47 ± 2 pixels; P < 0.05) in comparison with RCSN-3SNCA (10 ± 1 pixels) incubated with cell culture medium alone (Figs. 6A and 6B). Consequently, with the results obtained with oligomers, the monomer significantly decreased both in RCSN-3Nq7SNCA cells incubated with 20 µM aminochrome (5.6 ± 0.8; P < 0.05) or when these cells were incubated with cell culture medium (8.1 ± 0.4 pixels; P < 0.05) compared with RCSN-3SNCA cells incubated with 20 µM aminochrome (28 ± 2 pixels) or with cell culture medium (34.7 ± 2 pixels), respectively (Figs. 6A and 6B). The formation of oligomers was also determined by using inmunofluorescence technique with antioligomer antibodies (Kayed et al., 2003). A significant increase in the number of oligomers was observed in RCSN-3Nq7SNCA cells treated with 20 µM aminochrome (45 ± 2 pixels; P < 0.01) in comparison with these cells incubated with cell culture medium (18 ± 2 pixels) or when we compare with RCSN-3SNCA cells treated with 20 µM aminochrome (Figs. 6C and 6D).
FIG. 6.
Aminochrome induces the formation of SNCA oligomers in RCSN-3Nq7SNCA cells and the effect of scyllo-inositol on aminochrome-induced cell death. The formation of oligomers were determined by using Western blot technique (A). In RCSN-3SNCA cells (WT-SNCA) SNCA is found in monomer state both in the presence or absence of aminochrome. However, SNCA monomer significantly decreases in RCSN-3Nq7SNCA cells both in untreated and treated with 20 µM aminochrome (B). SNCA oligomers were also detected by using inmunofluorescence (C) and the results were plotted in (D) where we can observe a significant increase in RCSN-3Nq7SNCA cells. (E) The incubation of RCSN-3Nq7SNCA (Nq7-SNCA) cells with 1.5 µM scyllo-inositol decreases aminochrome-induced cell death. No effect of scyllo-inositol was observed in RCSN-3SNCA (WT-SNCA) cells revealing the protective role of NQO1 against SNCA-aminochrome oligomers. The cells were incubated with 20 µM aminochrome during 24 h. The values are the mean ± SD with n = 3. The statistical significance was assessed using ANOVA for multiple comparisons (*P < 0.05; **P < 0.01; ***P < 0.001).
To study the role of oligomers formation in aminochrome-induced neurotoxicity in RCSN-3Nq7SNCA cells we used scyllo-inositol that it is an oligomer-stabilizing compound of both Aβ42 peptide and SNCA (Aytan et al., 2013; Vekrellis et al., 2009). A significant decrease in cell death was observed in RCSN-3Nq7SNCA cells treated with 20 µM aminochrome in the presence of 1.5 µM scyllo-inositol (8.2 ± 2% cell death; P < 0.005) compared with RCSN-3Nq7SNCA cells only treated with 20 µM aminochrome (14.7 ± 2% cell death). Interestingly, 1.5 µM scyllo-inositol has no effect on aminochrome-induced cell death (20 µM) in RCSN-3SNCA compared with cells treated only with aminochrome (11 ± 2% and 11 ± 2% cell death; Fig. 6E).
DISCUSSION
Aminochrome and SNCA
Mutations in SNCA have been associated with a familial form of PD (Puschmann, 2013) since they induce SNCA aggregation into cytotoxic oligomers that seems to be essential for its neurotoxic effects (Gruden et al., 2014; Lashuel et al., 2002; Volles and Lansbury, 2003). The question is how SNCA can generate neurotoxic oligomers in sporadic PD. It seems that neurotoxic SNCA oligomers are related to dopamine oxidation to o-quinones. Dopamine oxidizes to dopamine o-quinone that it is stable at pH lower than 2 (Segura-Aguilar et al., 1998) and at physiological pH this o-quinone undergoes intramolecular cyclization to aminochrome. Aminochrome can rearrange to 5,6-indolequinone that polymerize to form neuromelanin. The constant rate of dopamine o-quinone cyclization is 0.15 s−1 and 0.06 min−1 for aminochrome tautomerization to 5,6-indole quinone (Bisaglia et al., 2010), resulting in aminochrome accumulation (Segura-Aguilar et al., 2014). The 5,6-indolequinone forms adduct with SNCA but these adducts are only observed after 40 min of reaction (Bisaglia et al., 2007). Dopamine o-quinone has been reported to form adducts with mitochondrial complexes I, III, and V of electron transport chain and oxidative phosphorylation, UCHL-l, DJ-1, and parkin that it is a ligase 3 enzyme of the proteasome (LaVoie et al., 2005; Van Laar et al., 2009) but not with SNCA. Aminochrome is not only involved in the formation and stabilization of neurotoxic oligomers but also proposed to be involved in other mechanism in the degenerative process under PD such as mitochondria dysfunction by inactivation of complex I by aminochrome (Aguirre et al., 2012); protein degradation dysfunction by inhibiting autophagy/lysosomal system (Huenchuguala et al., 2014; Muñoz et al., 2012), as a consequence of α- and β-tubulin aggregation induced by aminochrome preventing microtubules formation (Paris et al., 2010), and also the proteasomal system (Zafar et al., 2006); inducing dysfunction of lysosome by increasing lysosome pH (Huenchuguala et al., 2014); and oxidative stress when aminochrome is 1-electron reduced, generating a redox cycling between aminochrome and this semiquinone with concomitant formation of superoxide and finally hydroxyl radicals (Arriagada et al., 2004). In this study, we used different concentrations of aminochrome. A high concentration of aminochrome (100 µM) was used in in vitro studies but to perform studies in cell lines we used a lower concentration (20 µM) where the constitutive expression of NQO1 is enough to prevent aminochrome toxicity while the silencing of 87% in RCSN-3 cells induce a significant cell death. The physiological concentration of aminochrome is still unknown due the difficulties to measure its intracellular concentration since aminochrome is not a stable product as we explained above. The results obtained in this study are relevant to understand the role of SNCA in PD. The link between SNCA and the sporadic form of the disease has been an open question but aminochrome seems to be the link between PD and SNCA since this compound induces and stabilizes the formation of neurotoxic SNCA oligomers (Conway et al., 2001; Norris et al., 2005). In addition, aminochrome is formed inside of dopaminergic neurons containing neuromelanin that are the neurons lost in the nigro-striatal system in PD, suggesting that the formation of SNCA oligomers induced by aminochrome generates a focalized cell death of a single dopaminergic neuron, suggesting a slow degenerative process that it is what it is in agreement with the observed in the disease. How much aminochrome is formed in the cytosol, that it is able to react with SNCA, is an interesting question but it is difficult to answer since although aminochrome is the most stable o-quinone formed during dopamine oxidation to neuromelanin this is not stable more than 40 min according to NMR experiments (Bisaglia et al., 2007). In the presence of NQO1 aminochrome will be reduced to leukoaminochrome preventing the formation of SNCA oligomers as a consequence of the formation of SNCA-aminochrome adducts and allowing the formation of SNCA fibrils. However, the formation of SNCA oligomers in the presence of aminochrome is possible when the level of expression of NQO1 is decreased or inhibited. The relevance of our findings is supported by the fact that SNCA oligomers that are able to bind synaptic vesicles, and oligomers form pores that could lead to permeabilization of the vesicle membranes, thereby releasing dopamine into the cytosol that will increase aminochrome formation (Lashuel et al., 2002). Overexpression of A30P SNCA mutation in mice displayed significantly increased cytosolic catecholamine levels (Mosharov et al., 2006), suggesting that SNCA pores resulted in the release of catecholamines into the cytosol. The increase in cytosolic dopamine will potentiate the formation of o-quinones and their neurotoxicity.
NQO1 Prevents Formation of SNCA Oligomers and Its Toxic Effects
In this work, we present evidences that NQO1, an enzyme that reduces aminochrome with 2 electrons, prevents the formation of aminochrome-induced SNCA oligomers and their neurotoxic effects since (i) we did not detect small globular structures similar to SNCA oligomers or fibers using transmission electron microscopy when SNCA and aminochrome were incubated in the presence of NQO1; (ii) CD spectra showed that the random coil conformation of SNCA did not change when it was incubated with aminochrome and NQO1 for 24 and 48 h suggesting that SNCA was in the monomer state; (iii) SNCA fibril formation, determined with the ThT assay, was completely inhibited in the presence of aminochrome and NQO1 suggesting that SNCA under these conditions is in the monomer state, contrasting with the formation of fibrils or oligomers when SNCA was incubated alone or in the presence of aminochrome, respectively; (iv) NQO1 prevented the formation of oligomers in RCSN-3-SNCA with normal expression of NQO1; and (v) NQO1 prevented SNCA-induced neurotoxic effects in these cell lines. SNCA oligomers induce an apoptotic cell death in RCSN-3NQ7SNCA cells due to the low expression of NQO1, which cannot reduce aminochrome and prevent the cell death. The overexpression of SNCA in RCSN-3-SNCA cells is not enough to induce DNA fragmentation when they are incubated with aminochrome since the expression of NQO1 is enough to prevent aminochrome-dependent cell death. These results suggest that the formation of neurotoxic SNCA oligomers depend on a competition between SNCA and NQO1 to react with aminochrome where NQO1 has higher affinity for aminochrome than SNCA. NQO1 seems to stabilize SNCA in the monomer state even under conditions that favor its aggregation to fibrils, suggesting that the product of 2-electron reduction (leukoaminochrome) plays a role in SNCA stabilization in the monomer state. Interestingly, the flavonoid baicalein was reported to inhibit SNCA fibrillation and disaggregate SNCA fibrils (Bae et al., 2010; Hong et al., 2008). The common between baicalein and leukoaminochrome structure is the existence of a catechol structure that probably may be important in the prevention of SNCA fibrillation. NQO1 is a unique flavoenzyme that catalyzes the 2-electron reduction of quinones to hydroquinones and can use both NADH and NADPH as electron donators. NQO1 is intracellular localized in the cytosol (95%) and about 5% of the enzyme is associated with mitochondria and the endoplasmic reticulum. NQO1 immunoreactivity has been found in neurons of the substantia nigra and ventral tegmental areas and colocalized with tyrosine hydroxylase-like immunoreactivity. The dense network of DT diaphorase-immunoreactive fibers in the striatum disappeared along with the dopaminergic innervations after 6-hydroxydopamine lesions. NQO1 immunoreactivity was also found in Bergmann glia, astrocytes, and tanycytes (Schultzberg et al., 1988).
Aminochrome-Induced Cell Death Is Dependent of SNCA Oligomers
Aminochrome-induced cell death in RCSN-3-Nq7SNCA cells was dependent on the formation of SNCA oligomers since scyllo-inositol inhibits aminochrome-induced cell death. Scyllo-inositol is a compound that stabilizes oligomers both from SNCA and also Aβ42 (Aytan et al., 2013; Vekrellis et al., 2009). It is important to mention that scyllo-inositol has no effect on aminochrome-induced cell death in RCSN-3SNCA that has normal expression of NQO1 and we have shown the absence of oligomers in this cell line. Therefore, it seems to be plausible that aminochrome-induced cell death in RCSN-3-Nq7SNCA cells is dependent on SNCA oligomer formation. The neurotoxic role of SNCA oligomers has extensively been reported where SNCA oligomers induce behavioral deficits by induced rigidity, hypokinesia, and immobility (Gruden et al., 2014). SNCA oligomers interact with membranes increasing the amount of α-helical structure, which induces small molecules release as a consequence of membrane permeability alteration (Fecchio et al., 2013). Phosphorylation of oligomers of SNCA at residue 87 also contributes to cell death (Ha et al., 2014). Large SNCA oligomers prevent neuronal SNARE-mediated vesicle docking by oligomers binding the N-terminal domain of synaptobrevin-2 (Choi et al., 2013). SNCA oligomers are secreted from the cell by exosome-mediated release when autophagic mechanisms are not enough to remove neurotoxic oligomers (Danzer et al., 2012). Dynamin GTPases have been reported to mediate SNCA uptake into neuronal and oligodendroglial cells and when alpha synuclein is inside the cells forms oligomers with high molecular weight (Konno et al., 2012). Alpha synuclein oligomers accumulate in endoplasmic reticulum and salubrinal, an anti-ER stress compound, reduces accumulation of SNCA oligomers in endoplasmic reticulum (Colla et al., 2012). Extracellular SNCA oligomers decreased cAMP response element-binding protein transcriptional activity, induced calcineurin activity, and increased intracellular Ca2+ levels (Martin et al., 2012).
Reactions Preventing Aminochrome-Induced SNCA Toxicity
The formation of SNCA oligomers is dependent on the availability of aminochrome, which can be removed both by 1- or 2-electron reduction reactions. However, there are 2 important mechanisms that prevent dopamine oxidation. (i) First, dopamine can be incorporated into monoamine transporter vesicles. The low pH inside of the vesicles prevents dopamine oxidation to aminochrome. It has been reported that neuromelanin synthesis is abolished by adenovirus-mediated overexpression of the synaptic vesicle catecholamine transporter VMAT2, which would decrease cytosolic dopamine by increasing vesicular accumulation of the neurotransmitter (Sulzer et al., 2000). (ii) Second, dopamine can be degraded by MAO. In this regard, overexpression of MAO induces protection against intracellular L-DOPA toxicity (Weingarten and Zhou, 2001).
Conclusions
In conclusion, the results presented in this study support the proposed neuroprotective role of NQO1 against SNCA aminochrome-induced neurotoxicity in dopaminergic neurons (Muñoz et al., 2012; Paris et al., 2009), since the enzyme prevents the formation of SNCA neurotoxic oligomers both in vitro and in RCSN-3SNCA cells (Fig. 7). In dopaminergic neurons containing neuromelanin we have dopamine oxidation to aminochrome since these cells contain neuromelanin but the presence of NQO1 prevents a neurotoxic pathway of aminochrome that end with the loss of these neurons as happened in PD.
FIG. 7.
A possible mechanism for aminochrome-induced cell death. NQO1 prevents the formation of aminochrome-dependent SNCA oligomers by 2-electron reduction of aminochrome that it is a neuroprotective reaction. When NQO1 is silenced by siRNA aminochrome forms adducts with SNCA inducing the formation of SNCA oligomers that induce neurotoxicity, apoptosis, and finally cell death.
ACKNOWLEDGMENTS
The authors thank Gonzalo R. Lamberto and Andres Binolfi for helping them perform the CD and ThT fluorescence experiments.
FUNDING
National Fund for Scientific and Technological Development in Chile (FONDECTYT 1061083, 1100165) and University of Chile (ENL014/14).
REFERENCES
- Aguirre P., Urrutia P., Tapia V., Villa M., Paris I., Segura-Aguilar J., Núñez M. T. (2012). The dopamine metabolite aminochrome inhibits mitochondrial complex I and modifies the expression of iron transporters DMT1 and FPN1. Biometals 25, 795–803. [DOI] [PubMed] [Google Scholar]
- Arriagada C., Paris I., Sanchez de las Matas M. J., Martinez-Alvarado P., Cardenas S., Castaneda P., Graumann R., Perez-Pastene C., Olea-Azar C., Couve E., et al. (2004). On the neurotoxicity mechanism of leukoaminochrome o-semiquinone radical derived from dopamine oxidation: mitochondria damage, necrosis, and hydroxyl radical formation. Neurobiol. Dis. 16, 468–477. [DOI] [PubMed] [Google Scholar]
- Aytan N., Choi J. K., Carreras I., Kowall N. W., Jenkins B. G., Dedeoglu A. (2013). Combination therapy in a transgenic model of Alzheimer’s disease. Exp. Neurol. 250, 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S. Y., Kim S., Hwang H., Kim H. K., Yoon H. C., Kim J. H., Lee S., Kim T. D. (2010). Amyloid formation and disaggregation of α-synuclein and its tandem repeat (α-TR). Biochem. Biophys. Res. Commun. 400, 531–536. [DOI] [PubMed] [Google Scholar]
- Bisaglia M., Mammi S., Bubacco L. (2007). Kinetic and structural analysis of the early oxidation products of dopamine: analysis of the interactions with alpha-synuclein. J. Biol. Chem. 282, 15597–15605. [DOI] [PubMed] [Google Scholar]
- Bisaglia M., Soriano M. E., Arduini I., Mammi S., Bubacco L. (2010). Molecular characterization of dopamine-derived quinones reactivity toward NADH and glutathione: implications for mitochondrial dysfunction in Parkinson disease. Biochim. Biophys. Acta 1802, 699–706. [DOI] [PubMed] [Google Scholar]
- Choi B. K., Choi M. G., Kim J. Y., Yang Y., Lai Y., Kweon D. H., Lee N. K., Shin Y. K. (2013). Large α-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc. Natl. Acad. Sci. U.S.A. 110, 4087–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colla E., Jensen P. H., Pletnikova O., Troncoso J. C., Glabe C., Lee M. K. (2012). Accumulation of toxic α-synuclein oligomer within endoplasmic reticulum occurs in α-synucleinopathy in vivo. J. Neurosci. 32, 3301–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway K. A., Rochet J. C., Bieganski R. M., Lansbury P.T., Jr (2001). Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science 294, 1346–1349. [DOI] [PubMed] [Google Scholar]
- Danzer K. M., Kranich L. R., Ruf W. P., Cagsal-Getkin O., Winslow A. R., Zhu L., Vanderburg C. R., McLean P. J. (2012). Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol. Neurodegener. 24, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecchio C., De Franceschi G., Relini A., Greggio E., Dalla Serra M., Bubacco L., Polverino de Laureto P. (2013). α-Synuclein oligomers induced by docosahexaenoic acid affect membrane integrity. PLoS One, 8, e82732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruden M. A., Davydova T. V., Narkevich V. B., Fomina V. G., Wang C., Kudrin V. S., Morozova-Roche L. A., Sewell R. D. (2014). Intranasal administration of alpha-synuclein aggregates: a Parkinson’s disease model with behavioural and neurochemical correlates. Behav. Brain Res. 263, 158–168. [DOI] [PubMed] [Google Scholar]
- Ha Y., Yang A., Lee S., Kim K., Liew H., Suh Y. H., Park H. S., Churchill D. G. (2014). Facile “stop codon” method reveals elevated neuronal toxicity by discrete S87p-α-synuclein oligomers. Biochem. Biophys. Res. Commun. 443, 1085–1091. [DOI] [PubMed] [Google Scholar]
- Hong D. P., Fink A. L., Uversky V. N. (2008). Structural characteristics of alpha-synuclein oligomers stabilized by the flavonoid baicalein. J. Mol. Biol. 383, 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huenchuguala S., Muñoz P., Zavala P., Villa M., Cuevas C., Ahumada U., Graumann R., Nore B. F., Couve E., Mannervik B., et al. (2014). Glutathione transferase mu 2 protects glioblastoma cells against aminochrome toxicity by preventing autophagy and lysosome dysfunction. Autophagy 10, 618–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R., Head E., Thompson J. L., McIntire T. M., Milton S. C., Cotman C. W., Glabe C. G. (2003). Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489. [DOI] [PubMed] [Google Scholar]
- Konno M., Hasegawa T., Baba T., Miura E., Sugeno N., Kikuchi A., Fiesel F. C., Sasaki T., Aoki M., Itoyama Y., Takeda A. (2012). Suppression of dynamin GTPase decreases α-synuclein uptake by neuronal and oligodendroglial cells: a potent therapeutic target for synucleinopathy. Mol. Neurodegener. 7, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashuel H. A., Hartley D., Petre B. M., Walz T., Lansbury P.T., Jr (2002). Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature 418, 291. [DOI] [PubMed] [Google Scholar]
- LaVoie M. J., Ostaszewski B. L., Weihofen A., Schlossmacher M. G., Selkoe D. J. (2005). Dopamine covalently modifies and functionally inactivates parkin. Nat. Med. 11, 1159–1161. [DOI] [PubMed] [Google Scholar]
- Li H. T., Lin D. H., Luo X. Y., Zhang F., Ji L. N., Du H. N., Song G. Q., Hu J., Zhou J. W., Hu H. Y. (2005). Inhibition of alpha-synuclein fibrillization by dopamine analogs via reaction with the amino groups of alpha-synuclein. Implication for dopaminergic neurodegeneration. FEBS J. 272, 3661–3672. [DOI] [PubMed] [Google Scholar]
- Lozano J., Muñoz P., Nore B. F., Ledoux S., Segura-Aguilar J. (2010). Stable expression of short interfering RNA for DT-diaphorase induces neurotoxicity. Chem. Res. Toxicol. 23, 1492–1496. [DOI] [PubMed] [Google Scholar]
- Martin Z. S., Neugebauer V., Dineley K. T., Kayed R., Zhang W., Reese L. C., Taglialatela G. (2012). α-Synuclein oligomers oppose long-term potentiation and impair memory through a calcineurin-dependent mechanism: relevance to human synucleopathic diseases. J. Neurochem. 120, 440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov E. V., Staal R. G., Bové J., Prou D., Hananiya A., Markov D., Poulsen N., Larsen K. E., Moore C. M., Troyer M. D., et al. (2006). Alpha-synuclein overexpression increases cytosolic catecholamine concentration. J. Neurosci. 26, 9304–9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz P., Huenchuguala S., Paris I., Segura-Aguilar J. (2012). Dopamine oxidation and autophagy. Parkinsons Dis. 2012, 920953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris E. H., Giasson B. I., Hodara R., Xu S., Trojanowski J. Q., Ischiropoulos H., Lee V. M. (2005). Reversible inhibition of alpha-synuclein fibrillization by dopaminochrome-mediated conformational alterations. J. Biol. Chem. 280, 21212–21219. [DOI] [PubMed] [Google Scholar]
- Paris I., Lozano J., Cardenas S., Perez-Pastene C., Saud K., Fuentes P., Caviedes P., Dagnino-Subiabre A., Raisman-Vozari R., Shimahara T., et al. (2008). The catecholaminergic RCSN-3 cell line: a model to study dopamine metabolism. Neurotox. Res. 13, 221–230. [DOI] [PubMed] [Google Scholar]
- Paris I., Lozano J., Perez-Pastene C., Muñoz P., Segura-Aguilar J. (2009). Molecular and neurochemical mechanisms in PD pathogenesis. Neurotox. Res. 16, 271–279. [DOI] [PubMed] [Google Scholar]
- Paris I., Perez-Pastene C., Cardenas S., Iturriaga-Vasquez P., Muñoz P., Couve E., Caviedes P., Segura-Aguilar J. (2010). Aminochrome induces disruption of actin, alpha-, and beta-tubulin cytoskeleton networks in substantia-nigra-derived cell line. Neurotox. Res. 18, 82–92. [DOI] [PubMed] [Google Scholar]
- Puschmann A. (2013). Monogenic Parkinson’s disease and parkinsonism: clinical phenotypes and frequencies of known mutations. Parkinsonism Relat. Disord. 19, 407–415. [DOI] [PubMed] [Google Scholar]
- Scarlata S., Golebiewska U. (2014). Linking alpha-synuclein properties with oxidation: a hypothesis on a mechanism underling cellular aggregation. J. Bioenerg. Biomembr. 46, 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultzberg M., Segura-Aguilar J., Lind C. (1988). Distribution of DT diaphorase in the rat brain: biochemical and immunohistochemical studies. Neuroscience 27, 763–766. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J., Paris I., Muñoz P., Ferrari E., Zecca L., Zucca F. A. (2014). Protective and toxic roles of dopamine in Parkinson’s disease. J. Neurochem. 129, 898–915. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J., Lind C. (1989). On the mechanism of the Mn3(+)-induced neurotoxicity of dopamine: prevention of quinone-derived oxygen toxicity by DT diaphorase and superoxide dismutase. Chem. Biol. Interact. 72, 309–324. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J., Kajser R., Lind C. (1992). Separation and characterization of isoforms of DT-diaphorase from rat liver cytosol. Biochim. Biophys. Acta 1120, 33–42. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J., Metodiewa D., Welch C. J. (1998). Metabolic activation of dopamine o-quinones to o-semiquinones by NADPH cytochrome P450 reductase may play an important role in oxidative stress and apoptotic effects. Biochim. Biophys. Acta 1381, 1–6. [DOI] [PubMed] [Google Scholar]
- Spillantini M. G., Crowther R. A., Jakes R., Hasegawa M., Goedert M. (1998). Alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. U.S.A. 95, 6469–6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D., Bogulavsky J., Larsen K. E., Behr G., Karatekin E., Kleinman M. H., Turro N., Krantz D., Edwards R. H., Greene L. A., Zecca L. (2000). Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc. Natl. Acad. Sci. U.S.A. 97, 11869–11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laar V. S., Mishizen A. J., Cascio M., Hastings T. G. (2009). Proteomic identification of dopamine-conjugated proteins from isolated rat brain mitochondria and SH-SY5Y cells. Neurobiol. Dis. 34, 487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekrellis K., Xilouri M., Emmanouilidou E., Stefanis L. (2009). Inducible over-expression of wild type alpha-synuclein in human neuronal cells leads to caspase-dependent non-apoptotic death. J. Neurochem. 109, 1348–1362. [DOI] [PubMed] [Google Scholar]
- Volles M. J., Lansbury P.T., Jr (2003). Zeroing in on the pathogenic form of alpha-synuclein and its mechanism of neurotoxicity in Parkinson’s disease. Biochemistry 42, 7871–7878. [DOI] [PubMed] [Google Scholar]
- Weingarten P., Zhou Q. Y. (2001). Protection of intracellular dopamine cytotoxicity by dopamine disposition and metabolism factors. J. Neurochem. 77, 776–785. [DOI] [PubMed] [Google Scholar]
- Zafar K. S., Siegel D., Ross D. (2006). A potential role for cyclized quinones derived from dopamine, DOPA, and 3,4-dihydroxyphenylacetic acid in proteasomal inhibition. Mol. Pharmacol. 70, 1079–1086. [DOI] [PubMed] [Google Scholar]