Abstract
Endosulfan as a new member of persistent organic pollutants has been shown to induce reproductive dysfunction in various animal models. However, the action mechanism of endosulfan-produced reproductive toxicity remains largely unknown. This study was focused on investigating the reproductive toxicity induced by α-endosulfan and clarifying the role of mitochondria and genotoxic response genes in germ cell apoptosis of Caenorhabditis elegans. Our data showed that endosulfan induced a dose-dependent decrease of life span, fecundity, and hatchability, whereas the germ cell apoptosis was dose-dependently increased. The mitochondria membrane potential was disrupted by endosulfan, leading to a significant increase of germ cell apoptosis in mev-1(kn-1) mutant. However, the apoptotic effects of endosulfan were blocked in mutants of cep-1(w40), egl-1(n487), and hus-1(op241), indicating conserved genotoxic response genes played an essential role in endosulfan-induced germ cell apoptosis. Furthermore, exposure to endosulfan induced the accumulation of HUS-1::GFP foci and the germ cell cycle arrest. These findings provided clear evidence that endosulfan caused significant adverse effects on the reproduction system of C. elegans and increased germ cell apoptosis, which was regulated by mitochondrial dysfunction and DNA damage response genes. This study may help to understand the signal transduction pathways involved in endosulfan-induced reproductive toxicity.
Keywords: α-endosulfan, organochlorine pesticide, reproductive toxicity, germ cell apoptosis, C. elegans
Endosulfan, as an organochlorine pesticide (OCP), has been listed as a persistent organic pollutant (POP) by the Stockholm Convention and phased out termination of all manufactures and uses globally (UNEP-POP, 2011). However, the residues of endosulfan have been found ubiquitously in geographical regions ranging from temperate environments to the Arctic and various foods (Weber et al., 2010). These findings implied that a large number of ecosystem organisms and field workers may be potentially exposed to endosulfan via food chain and occupational routes.
There have been growing concerns about the reproductive toxicity of endosulfan to various species in recent years (Rastogi et al., 2014). Exposure to endosulfan significantly reduced the brood size and caused embryo abnormalities in Daphnia magna (Palma et al., 2009). It could cause an 80% reduction in progeny production and the change of sex ratio in fighting fish Betta splendens (Balasubramani and Pandian, 2014). The exposure of endosulfan to pregnant rats not only increases fetal resorption and induces gross fetal anomalies but also decreases spermatogenesis in offspring (Milesi et al., 2012). It should be noted that the reproductive toxicity of endosulfan has been variable in different experiments and may be the result of the types of endosulfan used, different experimental animal species, age at exposure, doses, duration of exposure, or the nature of the biological end points. Thus, there is an urgent demand for a comprehensive study to illustrate the reproductive dysfunction of endosulfan in intact organisms.
Apoptosis is a common programmed event in the development of reproductive system, which has critical functions in maintaining appropriate germ cell to Sertoli cell ratio, removing defective germ cells, and controlling the sperm production (Shukla et al., 2012). Endosulfan has been reported to induce apoptosis in Sertoli and Leyding cells of rabbit testis and cell death in Sertoli-germ cells in male rats (Rastogi et al., 2014). However, little is known about the underlying mechanism of germ cell apoptosis induced by endosulfan. Oxidative stress has been shown to play a pivotal role in endosulfan toxicity in multiple organs including the brain, liver, kidney, and testis (Wu et al., 2012). The antioxidant compounds, such as vitamins C and E, have shown to ameliorate the sperm and testicular toxicity of endosulfan in male rats (Takhshid et al., 2012). Moreover, endosulfan-induced mitochondrial membrane depolarization, resulting in the increase of reactive oxygen species (ROS) and apoptosis in rat testis (Aly and Khafagy, 2014; Sohn et al., 2004). These data suggest that ROS induced by endosulfan may further interact with the genetic material and cause germ cell DNA damage leading to abnormal transmission of genetic information.
Among in vivo animal models, Caenorhabditis elegans is a powerful genetic model to explore environmental toxicology and human biological mechanisms (Leung et al., 2008). The transparent cuticle allows observing and distinguishing germ cells at organism level. The genome of C. elegans showed a high level of conservation with vertebrates (Cutter et al., 2009); approximately 60–80% of C. elegans’ genes are conserved with humans (Kaletta and Hengartner, 2006). The C. elegans germline apoptosis during oogenesis is a fundamentally important reproductive process and is evolutionarily conserved from worms to mammals, including humans. The validity of using C. elegans to study genes and pathways related to human health and disease has been well established by dissecting the pathway controlling apoptosis (Lettre and Hengartner, 2006). In addition, C. elegans is an ideal animal model for the study of ecotoxicology due to its abundance in ecosystems and its properties of short life cycle, small size, plenty of offspring, and ease of handling in laboratory (Riddle et al., 1997).
Among the two types of isomers (α-: 64–67%; β-: 29–32%), α-endosulfan is supposed to be more poison and has higher soil permeability (Wan et al., 2005). In this study, the adverse effects of endosulfan on the reproductive system were assessed by the fecundity, the hatchability, germ cell apoptosis, and cell cycle arrest. Because oxidative stress has been implicated as a probable mechanism involved in the reproductive toxicity of endosulfan (Silva and Gammon, 2009), we speculated that the mitochondria which is the main source oxyradicals in vivo might mediate the germ cell apoptosis induced by endosulfan. To further explore the mechanism of endosulfan-induced toxicity, the highly conserved genotoxic stress response genes were determined by C. elegans mutants exposed to endosulfan. In the meantime, dichloro-diphenyl-trichloroethane (DDT) and lindane were included in the study for comparison with endosulfan as two other organochloride pesticides. Our results showed that endosulfan reduced the fecundity and hatchability in C. elegans. Moreover, the germ cell apoptosis induced by endosulfan was regulated by mitochondrial dysfunction and DNA damage response genes in a dose-dependent manner.
MATERIALS AND METHODS
Chemicals
α-endosulfan used in this study was purchased from Sigma-Aldrich (St. Louis, MO) and was of the highest purity available. DDT and lindane were purchased from AccuStandard (New Haven, CT). JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl benzimidalyl carbocyanide iodide) and 5-fluoro-2’-deoxyuridine (FudR) were purchased from Sigma-Aldrich (St. Louis, MO) as well. 4’,6’-diamidino-2-phenylindole (DAPI) and acridine orange (AO) were commercial products of Molecular Probes (Eugene, OR).
Worm strains and culture
All worms were cultured at 20°C in Petri dishes on nematode growth medium (NGM) seeded with Escherichia coli strain OP50 according to standard protocols. The wild-type strain used was Bristol N2. The following strains were provided by the Caenorhabditis Genetics Centre: hus-1::GFP(opIs34), ced-3(n717), ced-4(n1162), ced-9(n1950n), cep-1(w40), egl-1(n487), hus-1(op241), and mev-1(kn-1). The description of all mutants we used in the experiment was listed in Table 1. The double mutants hus-1(op241); mev-1(kn-1) were generated using standard methods.
TABLE 1.
Description of the Seven Mutants
| Gene Symbol and Allele | Gene Description | Phenotype |
|---|---|---|
| ced-3(n717) | Cell death abnormality | Absence of programmed cell death |
| ced-4(n1162) | Cell death abnormality | Absence of programmed cell death. |
| ced-9(n1950n) | Cell death abnormality | Enhanced level of germ cell corpses. |
| cep-1(w40) | C. elegans p53-like protein | Abnormality of DNA damage-induced apoptosis. |
| egl-1(n487) | Egg laying defective | Egg-laying serotinin sensitive and imipramine resistant. |
| hus-1(op241) | DNA damage checkpoint protein | Defective for DNA damage-induced germ cell death and cell cycle arrest. |
| mev-1(kn-1) | Methylviologen (paraquat) sensitive | Oxygen sensitive. Short life span. |
Worm exposure
Endosulfan, DDT, and lindane were dissolved in dimethyl sulfoxide (DMSO). Before using, the stock solutions were diluted by M9 Buffer (3 g KH2PO4, 6 g Na2HPO4, 5 g NaCl, 1 ml 1 M MgSO4) to the working concentrations. Young adult hermaphrodites were transferred into a Costar 24-well tissue plates with M9 buffer or test solutions for 6, 12, and 24 h, respectively. Escherichia coli strain OP50 was added as a food source. The maximum concentration of DMSO was 0.01% in the work solutions of endosulfan, DDT, and lindane, which showed no effects in C. elegans.
Life span assay
All life span assays were conducted at 20°C in 96-well plates containing M9 buffer. FudR at a concentration of 20 µg/ml was added to prevent progeny production. Our experiments illustrated that FudR does not significantly affect life span. We used the L4 molt as t = 0 for life span analysis. Briefly, 30 age-synchronized L4 hermaphrodites were transferred to a 96-well plate. Each well contained one worm in 200 µl M9 or test solutions. All worms were exposed to enduosulfan continuously until the worms were dead, which were identified when they did not move, pump, or respond to prodding.
Fecundity and hatchability assay
The fecundity and hatchability were measured as described (Middendorf and Dusenbery, 1993). Briefly, L1 stage worms were exposed to endosulfan for 60 h and then were moved to a new plate every 24 h until stop laying eggs. All eggs were cultured at 20°C for 24 h. The number of worms at all stages and the eggs was counted under a dissecting microscope. Offspring were counted every day and identified as the daily fecundity, whereas offspring in all 6 days were documented as the total fecundity. The ratio of eggs in all offspring (including worms and unhatched eggs) was enumerated as hatchability.
Apoptosis assay
Apoptotic germ cells were measured by AO vital staining as described (Kelly et al., 2000). Briefly, synchronized worms at the L4 stage of development were exposed to graded doses of endosulfan, DDT, or lindane and stained with 500 µl of 25 µg/ml AO and OP50. After incubation at 20°C for 60 min, the worms were transferred onto bacterial lawns for recovering. The worms were immobilized by levamisole, and the fluorescent staining was observed under an Olympus 1 × 71 microscope. The apoptotic cells with increased DNA fragmentation appeared yellow or yellow-orange, brighter than intact cells which were uniformly green in color.
Mitotic cell nuclei determination
The worms were fixed with Carnoy’s fixative (six parts ethanol, three parts chloroform, and one part glacial acid) and dried by air. The gonads were stained with DAPI at a concentration of 2 µg/ml (Gartner et al., 2000). The nuclei in the mitotic zone of the germline were counted under a fluorescence microscope.
Mitochondrial membrane potential assay
The florescent probe JC-1 was used to determine the potential of mitochondrial membrane as described (Iser et al., 2005). Briefly, JC-1 was dissolved in DMSO at a concentration of 5 mg/ml and then added into the M9 buffer with young adult animals. The worms were incubated with the dye for 2 h at 20°C and then transferred to NGM agar plates. The stained worms were paralyzed with levamisole and observed under a LSM 710 laser scanning confocal microscope (Carl Zeiss, Inc, Germany). The intensity of red- or green-channel fluorescence for mitochondria was measured by using Zen 2010 light edition (Carl Zeiss, Inc, Germany), and the red:green ratio was calculated.
Data analysis
All values were expressed as means ± standard error, statistical differences (P < 0.05) between various concentrations of different strains were tested using ANOVA followed by Tukey’s multiple comparison test. To compare the results for different strains, statistical analysis was performed with a two-way ANOVA with Dunnett’s t-test.
RESULTS
Effects of endosulfan on life span of worms
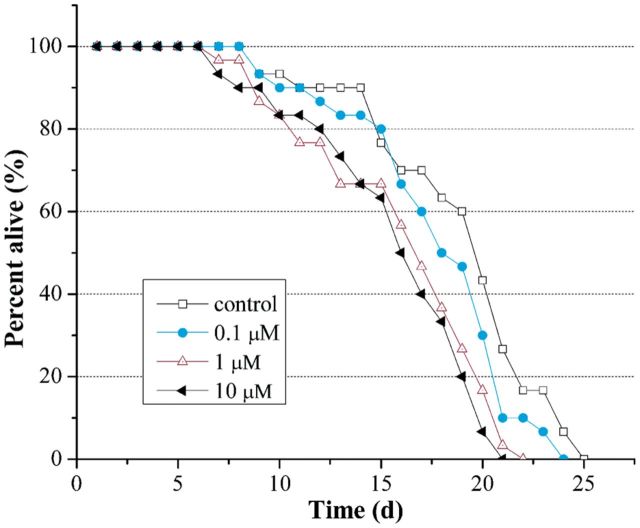
The average life span of N2 C. elegans used here was 19 d. As shown in Figure 1, there was a dose-dependent decrease of life span in endosulfan-exposed C. elegans. The life span was reduced to 18, 16, and 15 d when the concentrations of endosulfan were at 0.1, 1, and 10 μM, respectively. Although the life span was greatly decreased in C. elegans exposed to endosulfan at 10 µM, no acute lethal effect was observed in this study.
FIG. 1.
Effect of endosulfan on the life span of N2 worms. All worms were exposed in M9 buffer or test solutions for the whole life. Thirty worms were scored for each group.
Toxicity of endosulfan on fecundity and hatchability
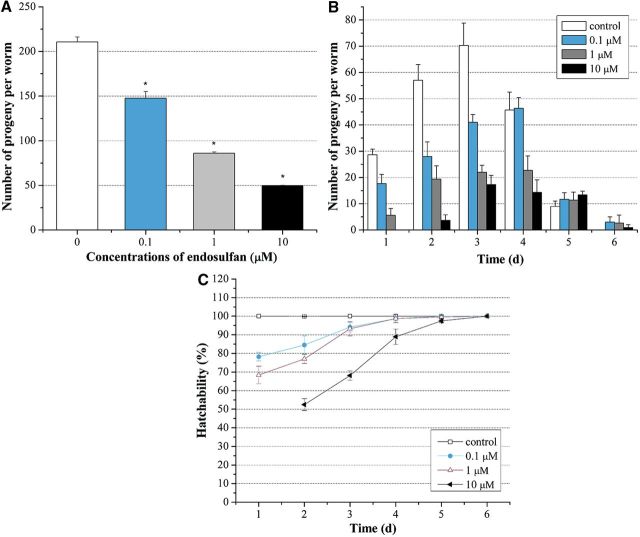
As shown in Figure 2A, exposure of L1 larvae to graded concentrations of endosulfan ranging from 0.1 to 10 µM caused a dose-dependent decrease in the fecundity. The total eggs laid during the 6 days were decreased dose dependently from 210.67 ± 5.51 in control group to 147.67 ± 7.52, 86 ± 1.41, and 49.67 ± 0.58 when the concentrations of endosulfan were at 0.1, 1, and 10 µM, respectively. The maximum fecundity of control group was reached on the third day, while it took one more day for worms exposed to endosulfan at 0.1 µM to reach the peak of fecundity and no egg was generated in the first day in worms exposed to endosulfan at 10 µM (Fig. 2B). Furthermore, the hatchability of eggs was decreased by exposure to endosulfan as well, especially in the initial 3 d (Fig. 2C). These results revealed that endosulfan induced the reproductive toxicity in C. elegans in a dose-dependent manner.
FIG. 2.
Effects of endosulfan on the fecundity and hatchability in N2 worms. A, Total fecundity during the whole life. All laid eggs were counted, including the unhatched ones. B, Daily fecundity. The number of laid eggs was counted day by day. C, Daily hatchability. The ratio represents hatched eggs in all laid eggs. Data were pooled from three independent experiments. Error bars indicate ± SD.
Role of core apoptotic machinery in endosulfan-induced germ cell death
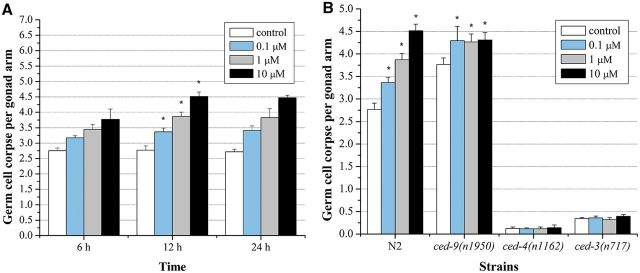
The average number of spontaneous germ cell corpses in WT worms used for this study was 2.77 ± 0.14. As shown in Figure 3A, there was a dose, time-dependent increase of germ cell death in C. elegans exposed to graded concentrations of endosulfan. A significant increase of germ cell death in the meiotic zone of the gonad was observed in worms exposed to endosulfan at concentrations of 0.1, 1, and 10 µM for 12 h, respectively. The germ cell corpses in worms exposed to 10 µM endosulfan was 4.52 ± 0.15, which was 1.6-fold higher than the control. Thus, 12-h exposure of endosulfan was used in the following experiments.
FIG. 3.
α-endosulfan-induced germline apoptotic cells in WT N2 and the mutants of core apoptosis signal transduction genes. A, The number of apoptotic germ cells in WT N2 strain. B, The number of apoptotic germ cells in ced-9(n1950n), ced-4(n1162) and ced-3(n717) mutant strains. Error bars indicate ± SD. *Significantly different at P < 0.05.
In C. elegans, germline apoptosis is regulated by the evolutionarily conserved core apoptotic machinery; the caspase protein CED-3 and apoptotic protease activation factor 1 (Apaf-1) homolog CED-4 are required for the killing process (Stergiou and Hengartner, 2004). The C. elegans CED-9 encodes a protein homologous to mammalian Bcl-2, which inhibit programmed cell death by sequestering CED-3 and CED-4 (del Peso et al., 2002). To confirm the germ cell corpses induced by endosulfan was under the core apoptosis machinery, loss-of-function mutants in ced-3(n717), ced-4(n1162), and gain-of-function mutants ced-9(n1950n) were exposed to graded doses of endosulfan. Few germline cell corpses were observed in the ced-3(n717) and ced-4(n1162) mutants, whereas the ced-9(n1950n) gain-of-function mutant showed a much higher induction of spontaneous cell death than the WT N2 worms. The endosulfan-induced germ cell corpses were greatly reduced to near the background in the ced-3(n717) and ced-4(n1162) mutants, respectively. In contrast, the induction of germ cell corpses was significantly increased in the ced-9(n1950n) mutant exposed to endosulfan, which was independent of the concentration (Fig. 3B). These data suggested that endosulfan elicited the programmed germ cell death in C. elegans. Similar dose-dependent increase trend in germ cell corpses exposed to other two organochloride pesticides: DDT and lindane were observed as well (Supplementary fig. S1A). After DDT and lindane exposure, the germline corpses in ced-3(n717) and ced-4(n1162) mutants were nearly absent, whereas in ced-9(n1950n) strain, dose-dependent manners were inhibited (Supplementary fig. S1B).
Contribution of mitochondrial dysfunction in endosulfan-induced apoptosis
To ascertain the contribution of mitochondria in germ cell apoptosis induced by endosulfan, mitochondria membrane potential was determined by JC-1 stain in WT worms. In worms with normal mitochondrial function, highly polarized mitochondrial membranes formed J-aggregates with intense red fluorescence as shown in control worms (Fig. 4A). With the enhancement of the concentrations of endosulfan, more JC-1 remained in a green fluorescent monomeric form (Figs. 4B–D). Quantification the ratio of red to green showed that endosulfan depressed the mitochondrial membrane potential in a dose-dependent way (Fig. 4E).
FIG. 4.
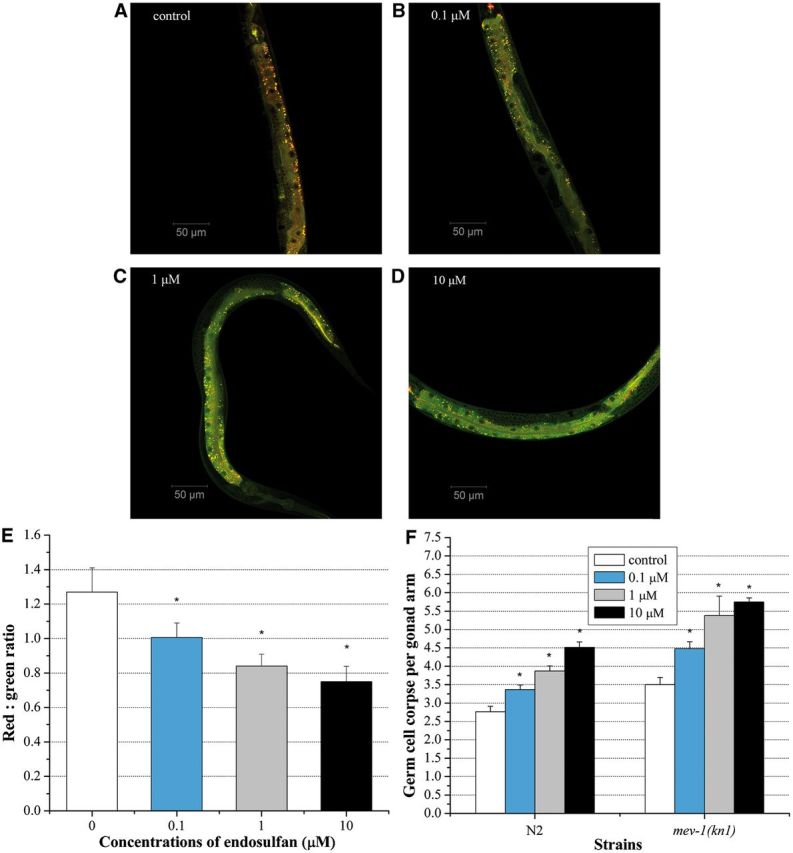
Mitochondrial membrane potential indicated by JC-1- specific fluorescence. Worms were treated with M9 buffer or graded doses of α-endosulfan (A–D). E, The red:green ratio of mitochondrial JC-1 fluorescence of four groups. Data were pooled from three independent experiments. F, The average number of α-endosulfan-induced germline apoptotic cells in mev-1(kn-1) mutant strain. For comparison convenience, data for N2 wild type in all figures herein were presented same as which shown in Figure 3A. Error bars indicate ± SD. *Significantly different at P < 0.05.
mev-1 encodes the C. elegans ortholog of the succinate dehydrogenase cytochrome b560 subunit, an integral membrane protein that is a subunit of mitochondrial respiratory chain complex II (ubiquinol-cytochrome c reductase) (Senoo-Matsuda et al., 2003). The spontaneous germ cell apoptosis in mev-1(kn-1) mutant was 3.5 ± 0.2, which was higher than that in the WT worm (2.77 ± 0.14). There was a dose-dependent increase of germ cell apoptosis in mev-1(kn-1) mutant exposed to endosulfan (Fig. 4F). Moreover, the germ cell corpses induced by endosulfan exposure in mev-1(kn-1) mutant were significant higher than that in WT worm at equal concentrations, indicating that the mev-1(kn-1) mutant are hypersensitive to endosulfan exposure.
We also used mev-1(kn-1) mutant to determine the contribution of mev-1 gene to germline apoptosis induced by DDT and lindane. As shown in Supplementary figures S2A and B, both DDT and lindane induced more apoptotic germ cell in mutant worms than that in N2 strain, which were similar to endosulfan.
Role of genotoxic response genes in endosulfan-induced germline apoptosis
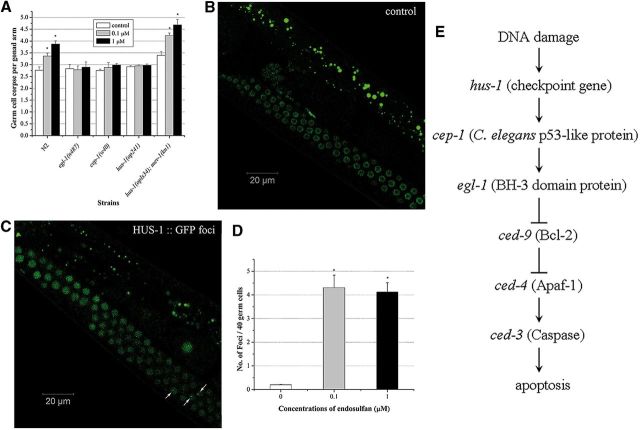
In C. elegans, CEP-1 is a homolog of the mammalian tumor suppressor gene p53, which promotes DNA damage-induced apoptosis (Derry et al., 2001). egl-1 encodes a BH3-only domain protein of Bcl-2 family that acts downstream of CED-9 (del Peso et al., 2002). These two genes are known to affect DNA damage-induced apoptosis under the environmental stress in C. elegans, whereas hus-1 gene is the DNA damage checkpoint gene required for DNA damage-induced germ cell death (Conradt and Horvitz 1998; Hofmann et al., 2002). To determine the activation of conserved genotoxic stress response genes in the germline cell apoptosis induced by endosulfan, loss-of-function mutants in egl-1(n487), hus-1(op241), and cep-1(w40) were exposed to graded concentrations of endosulfan, respectively. The spontaneous cell death in all mutants used here was similar to the WT worm. As shown in Figure 5, compared with WT worms, the egl-1(n487) loss-of-function mutant exhibited a significant reduction in germline cell apoptosis induced by endosulfan at concentrations ranging from 0.1 to 1 µM, indicating that egl-1 gene was indispensable for the endosulfan-induced apoptosis. The germline cell apoptosis also kept unchanged in cep-1(w40) loss-of-function mutant exposed to endosulfan. The mutant alleles in hus-1 blocked the germline cell apoptosis induced by endosulfan as well. These data suggested that the germline cell apoptosis induced by endosulfan was regulated by egl-1, cep-1, and hus-1 genes. Moreover, we constructed a double-mutant strain hus-1(op241); mev-1(kn-1) by cross breeding. hus-1(op241); mev-1(kn-1) was exposed to graded doses of endosulfan for 12 h. Even the worms carrying mutated alleles of checkpoint gene hus-1, germ cell corpses were increased significantly at all doses, compared to the untreated control (Fig. 5A). These findings implied that endosulfan-induced apoptosis was triggered by mitochondrial dysfunction and regulated by genotoxic stress response genes in a dose-dependent manner.
FIG. 5.
Roles of genotoxic response genes in α-endosulfan-induced germline apoptosis and subcellular relocalization of HUS-1::GFP. A, The average number of apoptotic cells in egl-1, hus-1, cep-1 and hus-1; mev-1 mutant strains. B, HUS-1:: GFP is diffuse in control. Images were acquired with Carl Zeiss LSM 710 laser scanning confocal microscope using ×63 (oil) magnifications. C, Relocalized HUS-1:: GFP is seen as bright foci. Arrow head implies the HUS-1:: GFP foci. D, Quantification of HUS-1:: GFP foci in control and graded doses of α-endosulfan exposure groups. E, DNA damage response genes and core apoptotic machinery signalings are involved in DNA damage-induced apoptosis in C. elegans. Error bars indicate ± SD. *Significantly different at P < 0.05.
Considering that the germ cell apoptosis induced by all the three organochloride pesticides were related to mitochondrial dysfunction, it is reasonable to investigate whether these genotoxic response genes were involved in DDT- and lindane-induced apoptosis. The results showed that the numbers of apoptotic cells in egl-1(n487), hus-1(op241), and cep-1(w40) mutants were similar to that in N2 worms after exposed to both DDT and lindane. Unsurprisingly, these two pesticides exhibited high apoptosis induction effects in hus-1(op241); mev-1(kn-1) double-mutant strain (Supplementary figs. S2C and D). The results indicated that different from endosulfan, genotoxic response genes did not play a key role in DDT- or lindane-induced germline apoptosis.
To confirm endosulfan-induced apoptosis was mediated by DNA damage, the localization of HUS-1 in germ cells was detected in a hus-1::GFP integrated line opIs34 exposed to graded concentrations of endosulfan. It has been reported that HUS-1 diffuses in proliferating germ nuclei under normal conditions, whereas in response to DNA damage, HUS-1 re-localizes in the nucleus and concentrates at distinct nuclear foci considered to be sites of DNA breaks (Hofmann et al., 2002). As shown in Figure 5B, limited number of HUS-1::GFP foci was occasionally observed in the pachytene cells of the germline, whereas endosulfan resulted in a dramatic increase in HUS-1::GFP foci in the germline (Fig. 5C). The number of foci induced by endosulfan at 0.1 and 1 µM was 4.3 ± 0.53 and 4.12 ± 0.4, respectively (Fig. 5D). The spontaneous foci in worms were 0.203 ± 0.013, which might be due to the normal oxidative metabolism such as mitochondrial respiration. These results demonstrated that endosulfan stimulated the cellular DNA damage sensing machinery in the germline as indicated by the formation of HUS-1 foci.
Endosulfan-induced germ cell cycle arrest
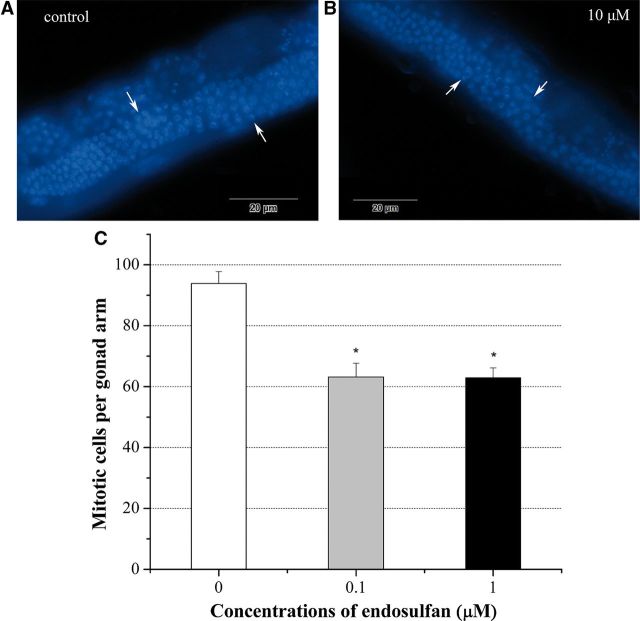
In the gonad of C. elegans hermaphrodites, mitotic nuclei located at the distal end of the gonad and can be easily distinguished from the crescent-shaped transition nuclei after DAPI staining. By exposing young adult hermaphrodites to different concentrations of endosulfan solutions for 24 h, the number of mitotic nuclei per gonad arm (63.15 ± 4.47 at 0.1 µM and 62.89 ± 3.25 at 1 µM) decreased significantly when compared with control (93.88 ± 3.89) (Fig. 6C), which indicated that endosulfan exposure could induce germline mitotic cell cycle arrest.
FIG. 6.
The average number of mitotic cells in control and graded doses of α-endosulfan exposure groups. A, C. elegans’ germline after DAPI staining in control group. Worms were cultured in M9 buffer. Image was taken by Olympus 1 × 71 microscope. B, Germline in C. elegans after exposed to 10 µM α-endosulfan for 24 h. Arrow head implies the transition zone. C, The average number of mitotic cells in germline. Data were pooled from three independent experiments. Arrow head implies the transition zone. Error bars indicate ± SD. *Significantly different at P < 0.05.
DISCUSSION
The prolonged persistence of endosulfan in the environment and its bioaccumulation through the food chain remains a global health concern affecting millions of people in both developed and developing countries (Silva and Beauvais, 2010). Although few data are available for subacute or chronic exposure to endosulfan in human being, experimental studies suggest that endosulfan could affect reproductive system, particularly when the exposure occurs during the developmental phase (Silva and Gammon, 2009). However, the underlying mechanisms of endosulfan-induced reproductive toxicity are still largely unknown and likely to involve multiple pathways. Caenorhabditis elegans with a short lifespan serves as a powerful model system for studying the molecular mechanisms underlying the reproductive toxicity in vivo.
As an organochlorine insecticide, endosulfan has adverse effects on target and non-target species and accumulates in various tissues (Naqvi and Vaishnavi, 1993). Although endosulfan exposure did not cause acute lethal effects in C. elegans, a dose-dependent decrease of lifespan was observed in young adult worms exposed to endosulfan (Fig. 1). The adverse impact of endosulfan on the prior-hatch development was evident by a significant delay of daily fecundity and hatchability, which was in concurrence with the observation in worms exposed to other pesticides (Salim and Rajini, 2014). Recent studies have indicated that the signals from the reproductive system influence the lifespan of C. elegans, which is highly conserved during evolution in mice and human (Berman and Kenyon, 2006). Thus, it is of interest to investigate the fate of germ cells in C. elegans exposed to endosulfan.
Apoptosis is an active and organized form of cell death in response to physiologic stimuli or environmental toxins. In C. elegans, germ cell apoptosis can be initiated by various environmental stresses, such as irradiation, heavy metals, and certain pathogenic bacteria (Wang et al., 2008). We found that endosulfan induced a significant increase of germ cell corpses (Fig. 3A), which required the core apoptosis signaling pathway composing of the anti-apoptotic Bcl-2 ortholog CED-9, the Apaf-1 ortholog CED-4, and the caspase CED-3 (Fig. 3B). DDT and lindane also exhibited the germ cell apoptosis induction effects in a dose-dependent manner. Core apoptotic machinery played an indispensable role in DDT- and lindane-induced apoptosis, which was similar to endosulfan. Germline apoptosis is an integral part of oogenesis in many animals including humans, and many of the regulators of C. elegans germline apoptosis are conserved. Our data indicated that the C. elegans germline might provide a valuable system for understanding the mechanisms involved in the apoptosis of reproductive system stimulated by endosulfan and other organochloride pesticides.
Mitochondria are known to be one of the major generators of ROS in vivo. Exposure to endosulfan is associated with mitochondrial energy metabolism dysfunction, structure disruption, and the abnormal of mitochondrial enzyme activity (Kalender et al., 2004). Our data showed that the ratio of red:green JC-1 fluorescence in mitochondria was modulated by endosulfan in a dose-dependent manner in the whole worms (Fig. 4E). MEV-1 as an integral membrane protein that is a subunit of mitochondrial respiratory chain complex II is required for oxidative phosphorylation in C. elegans (Senoo-Matsuda et al., 2003). In comparison to N2 worms, mev-1(kn-1) mutants which exhibits increased ROS level in vivo were more sensitive to endosulfan and generated a significant higher level of germ cell apoptosis (Fig. 4F). DDT and lindane also could induce more apoptotic germ cells in mev-1(kn-1) mutants (Supplementary figs. S2A and B). These results demonstrated that mitochondria played an essential role in the three OCPs-induced germ cell apoptosis.
Although endosulfan-induced genotoxic effects, such as chromosome alterations, micronuclei, DNA strand breaks, and gene mutations, have been reported to be mediated by ROS in exposed mammalian cells, Drosophila larvae, earthworm, zebrafish, and Channa punctatus, little information is available on its genotoxic stress in germ cells (Lu et al., 2000; Pandey et al., 2006). The C. elegans p53-1, CEP-1, is an ancient ortholog of p53 with a conserved DNA binding domain. CEP-1 induces germ cell death by activating the transcription of the target genes egl-1 and ced-13 (Derry et al., 2001). Our data obtained by scoring germ cell corpses in cep-1 and egl-1 mutants indicated that endosulfan-induced germ cell apoptosis in C. elegans through the genotoxic pathway (Fig. 5A), which was dependent on p53 and EGL-1. HUS-1 is a nuclear protein that is expressed in early embryos and the adult germlines which relocalizes to putative sites of DNA damage. Abnormal of hus-1 function abrogated endosulfan-induced germ cell apoptosis in this study (Fig. 5A). However, the levels of apoptosis induced by DDT and lindane in egl-1(n487), hus-1(op241), and cep-1(w40) mutants were similar to that in N2 strain (Supplementary figs. S2C and D). These findings implied that genotoxic pathway was not essential in DDT- and lindane-induced germline apoptosis, which was different to endosulfan. Moreover, an increased number of HUS-1:: GFP foci was observed in C. elegans exposed to endosulfan (Fig. 5D). These data provided a molecular explanation for how DNA damage checkpoint genes activated by endosulfan-induced apoptosis in germ cells of C. elegans. To determine the relationship between mitochondrial dysfunction and DNA damage checkpoint genes, we created double mutants of mev-1(kn-1) and hus-1(op241) and found that hus-1(op241); mev-1(kn-1) double mutants were more sensitive to endosulfan than hus-1(op241) single mutants (Fig. 5A), while exhibited similar levels of apoptosis as mev-1(kn-1) mutants exposed to endosulfan. This suggested that endosulfan-induced apoptosis was triggered by mitochondrial dysfunction and regulated by the activation of DNA damage response genes. The apoptosis induced by both DDT and lindane in hus-1(op241); mev-1(kn-1) double mutants and mev-1(kn-1) strain were at the same level (Supplementary figs. S2C and D). Combined with the data from egl-1(n487), hus-1(op241) and cep-1(w40) mutants above, mitochondrial dysfunction could be involved in these two OCPs induced germline apoptosis, while DNA damage response genes were not essential. Following DNA damage, checkpoints can induce not only apoptosis but also cell cycle arrest in the worm germline (Schumacher et al., 2001). A significant increase of cell cycle arrest was observed in the germline of C. elegans exposed to endosulfan (Fig. 6C).
As a new member of POPs, endosulfan has been detected ubiquitously in the environment. It is important to understand the action mechanisms of endosulfan-related biological effects. This study revealed that α-endosulfan-induced reproduction toxicity may be attributed to mitochondrial dysfunction and DNA damage response genes. It should be noted that in addition to DNA damage response in germline, the endocrine disruptive potential of endosulfan has been documented in various organisms and tissues including the mammalian reproductive system (Da Cuña et al., 2013). Recent evidence has shown that endosulfan tends to accumulate in the fatty tissues and can be transferred to the fetus through the placenta (Cerrillo et al., 2005). Thus, it is important to illustrate the role of hormone signaling pathway in DNA damage response to endosulfan. Moreover, the cyclic sulfite group of endosulfan can be oxidized to endosulfan sulfate in the environment. The impact of long-term exposure to low doses of endosulfan isomers and their metabolites should be taken into consideration in the future studies.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Major National Scientific Research Projects (2014CB932002); Strategic Leading Science & Technology Program (B) (XDB14030502); National Natural Science Foundation of China grants (U1232144) and (30570442).
Supplementary Material
REFERENCES
- Aly H. A., Khafagy R. M. (2014). Taurine reverses endosulfan-induced oxidative stress and apoptosis in adult rat testis. Food Chem. Toxicol. 64, 1–9. [DOI] [PubMed] [Google Scholar]
- Balasubramani A., Pandian T. J. (2014). Effect of commercial grade endosulfan on growth and reproduction of the fighting fish Betta splenden. Environ. Toxicol. 29, 1054–1062. [DOI] [PubMed] [Google Scholar]
- Berman J. R., Kenyon C. (2006). Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell 124, 1055–1068. [DOI] [PubMed] [Google Scholar]
- Cutter A. D., Dey A., Murray R. L. (2009). Evolution of the Caenorhabditis elegans genome. Mol. Biol. Evol. 26, 1199–1234. [DOI] [PubMed] [Google Scholar]
- Cerrillo I., Granada A., López-Espinosa M. J., Olmos B., Jiménez M., Caño A., Olea N., Fátima Olea-Serrano M. (2005). Endosulfan and its metabolites in fertile women, placenta, cord blood, and human milk. Environ. Res. 98, 233–239. [DOI] [PubMed] [Google Scholar]
- Conradt B., Horvitz H. R. (1998). The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like. Cell 93, 519–529. [DOI] [PubMed] [Google Scholar]
- Da Cuña R. H., Pandolfi M., Genovese G., Piazza Y., Ansaldo M., Lo Nostro F. L. (2013). Endocrine disruptive potential of endosulfan on the reproductive axis of Cichlasoma dimerus (Perciformes, Cichlidae). Aquat. Toxicol. 126, 299–305. [DOI] [PubMed] [Google Scholar]
- del Peso L., Gonzalez V. M., Inohara N., Ellis R. E., Nunez G. (2000). Disruption of the CED-9·CED-4 complex by EGL-1 is a critical step for programmed cell death in Caenorhabditis elegans. J. Biol. Chem. 275, 27205−27211. [DOI] [PubMed] [Google Scholar]
- Derry W. B., Putzke A. P., Rothman J. H. (2001). Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science 294, 591–595. [DOI] [PubMed] [Google Scholar]
- Gartner A., Milstein S., Ahmed S., Hodgkin J., Hengartner M. O. (2000). A conserved checkpoint pathway mediates DNA damage-induced apoptosis and cell cycle arrest in C. elegans. Mol . Cell. 5, 435–443. [DOI] [PubMed] [Google Scholar]
- Hofmann E. R., Milstein S., Boulton S. J., Ye M. J., Hofmann J. J., Stergiou L., Gartner A., Vidal M., Hengartner M. O. (2002). Caenorhabditis elegans HUS-1 is a DNA damage checkpoint protein required for genome stability and EGL-1-mediated apoptosis. Curr. Biol. 12, 1908–1918. [DOI] [PubMed] [Google Scholar]
- Iser W. B., Kim D., Bachman E., Wolkow C. (2005). Examination of the requirement for ucp-4, a putative homolog of mammalian uncoupling proteins, for stress tolerance and longevity in C. elegans. Mech . Ageing Dev. 126, 1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalender Y., Kalender S., Uzunhisarcikli M., Ogutcu A. E., Acikgoz F., Durak D. (2004). Effects of endosulfan on B cells of Langerhans islets in rat pancreas. Toxicology 200, 205–211. [DOI] [PubMed] [Google Scholar]
- Kaletta T., Hengartner M. O. (2006). Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 5, 387–398. [DOI] [PubMed] [Google Scholar]
- Kelly K. O., Dernburg A. F., Stanfield G. M., Villeneuve A. M. (2000). Caenorhabditis elegans msh-5 is required for both normal and radiation-induced meiotic crossing over but not for completion of meiosis. Genetics 156, 617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettre G., Hengartner M. O. (2006). Developmental apoptosis in C. elegans: a complex CED nario. Nat. Rev. Mol. Cell Biol. 7, 97–108. [DOI] [PubMed] [Google Scholar]
- Leung M. C., Williams P. L., Benedetto A., Au C., Helmcke K. J., Aschner M., Meyer J. N. (2008). Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol. Sci. 106, 5–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Morimoto K., Takeshita T., Takeuchi T., Saito T. (2000). Genotoxic effects of alpha-endosulfan and beta-endosulfan on human HepG2 cells. Environ. Health Perspect. 108, 559–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middendorf P. J., Dusenbery D. B. (1993). Fluoroacetic acid is a potent and specific inhibitor of reproduction in the nematode Caenorhabditis elegans. J. Nematol. 25, 573–577. [PMC free article] [PubMed] [Google Scholar]
- Milesi M. M., Varayoud J., Bosquiazzo V. L., Munoz-De-Toro M., Luque E. H. (2012). Neonatal exposure to low doses of endosulfan disrupts the expression of proteins regulating uterine development and differentiation. Reprod. Toxicol. 33, 85–93. [DOI] [PubMed] [Google Scholar]
- Naqvi S. M., Vaishnavi C. (1993). Bioaccumulative potential and toxicity of endosulfan insecticide to non-target animals. Comp. Biochem. Physiol. C 105, 347–361. [DOI] [PubMed] [Google Scholar]
- Palma P., Palma V. L., Fernandes R. M., Soares A. M., Barbosa I. R. (2009). Endosulfan sulphate interferes with reproduction, embryonic development and sex differentiation in Daphnia magna. Ecotoxicol. Environ. Saf. 72, 344–350. [DOI] [PubMed] [Google Scholar]
- Pandey S., Nagpure N. S., Kumar R., Sharma S., Srivastava S. K., Verma M. S. (2006). Genotoxicity evaluation of acute doses of endosulfan to freshwater teleost Channa punctatus (Bloch) by alkaline single-cell gel electrophoresis. Ecotoxicol. Environ. Saf. 65, 56–61. [DOI] [PubMed] [Google Scholar]
- Rastogi D., Narayan R., Saxena D. K., Chowdhuri D. K. (2014). Endosulfan induced cell death in Sertoli-germ cells of male wistar rat follows intrinsic mode of cell death. Chemosphere 94, 104–115. [DOI] [PubMed] [Google Scholar]
- Riddle D. L., Blumenthal T., Meyer B. J., Priess J. R. (1997). Introduction to C. elegans. In C elegans II (D. L. Riddle, T. Blumenthal, B. J. Meyer, and J. R. Priess, Eds.) Cold Spring Harbor, NY. [PubMed] [Google Scholar]
- Salim C., Rajini P. S. (2014). Glucose feeding during development aggravates the toxicity of the organophosphorus insecticide monocrotophos in the nematode, Caenorhabditis elegans. Physiol. Behav. 131, 142–148. [DOI] [PubMed] [Google Scholar]
- Schumacher B., Hofmann K., Boulton S., Gartner A. (2001). The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr. Biol. 11, 1722–1727. [DOI] [PubMed] [Google Scholar]
- Senoo-Matsuda N., Hartman P. S., Akatsuka A., Yoshimura S., Ishii N. (2003). A complex II defect affects mitochondrial structure, leading to ced-3- and ced-4-dependent apoptosis and aging. J. Biol. Chem. 278, 22031–22036. [DOI] [PubMed] [Google Scholar]
- Shukla K. K., Mahdi A. A., Rajender S. (2012). Apoptosis, spermatogenesis and male infertility. Front. Biosci. 4, 746–754. [DOI] [PubMed] [Google Scholar]
- Silva M. H., Beauvais S. L. (2010). Human health risk assessment of endosulfan. I: Toxicology and hazard identification. Regul. Toxicol. Pharmacol. 56, 4–17. [DOI] [PubMed] [Google Scholar]
- Silva M. H., Gammon D. (2009). An assessment of the developmental, reproductive, and neurotoxicity of endosulfan. Birth Defects Res. B Dev. Reprod. Toxicol. 86, 1–28. [DOI] [PubMed] [Google Scholar]
- Sohn H. Y., Kwon C. S., Kwon G. S., Lee J. B., Kim E. (2004). Induction of oxidative stress by endosulfan and protective effect of lipid-soluble antioxidants against endosulfan-induced oxidative damage. Toxicol. Lett. 151, 357–365. [DOI] [PubMed] [Google Scholar]
- Stergiou L., Hengartner M. O. (2004). Death and more: DNA damage response pathways in the nematode C. elegans. Cell Death Differ. 11, 21–28. [DOI] [PubMed] [Google Scholar]
- Takhshid M. A., Tavasuli A. R., Heidary Y., Keshavarz M., Kargar H. (2012). Protective effect of vitamins E and C on endosulfan-induced reproductive toxicity in male rats. Iran. J. Med. Sci. 37, 173–180. [PMC free article] [PubMed] [Google Scholar]
- UNEP (United Nations Environment Programme) (2011). Fifth Meeting of the Conference of the Parties to the Stockholm Convention. Available at: http://chm.pops.int/TheConvention/ConferenceoftheParties/Meetings/COP5/tabid/1267/mctl/ViewDetails/EventModID/870/EventID/109/xmid/4351/Default.aspx Accessed February 13, 2015. [Google Scholar]
- Wan M. T., Kuo J. N., Buday C., Schroeder G., Van Aggelen G., Pasternak J. (2005). Toxicity of alpha-, beta-, (alpha plus beta)-endosulfan and their formulated and degradation products to Daphnia magna, Hyalella azteca, Oncorhynchus mykiss, Oncorhynchus kisutch, and biological implications in streams. Environ. Toxicol. Chem. 24, 1146–1154. [DOI] [PubMed] [Google Scholar]
- Wang S. C., Tang M. L., Pei B., Xiao X., Wang J., Hang H. Y., Wu L. J. (2008). Cadmium-induced germline apoptosis in Caenorhabditis elegans: the roles of HUS1, p53, and MAPK signaling pathways. Toxicol. Sci. 102, 345–351. [DOI] [PubMed] [Google Scholar]
- Weber J., Halsall C. J., Muir D., Teixeira C., Small J., Solomon K., Hermanson M., Hung H., Bidleman T. (2010). Endosulfan, a global pesticide: a review of its fate in the environment and occurrence in the Arctic. Sci. Total. Environ. 408, 2966–2984. [DOI] [PubMed] [Google Scholar]
- Wu Y. H., Jia J., Li Y. B., Shi Z. X., Zhou X. Q., Sun Z. W. (2012). Crry receptor and oxidative stress involved in erythrocyte immune toxicity of mice caused by endosulfan and protective effects of vitamin E. J. Toxicol. Sci. 37, 1225–1237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.