Abstract
Several reports demonstrated that maternal lipopolysaccharide (LPS) exposure at middle gestational stage caused neural tube defects (NTDs). This study investigated the effects of supplementation with vitamin D3 (VitD3) during pregnancy on LPS-induced NTDs. Pregnant mice except controls were ip injected with LPS (25 μg/kg) daily from gestational day (GD)8 to GD12. In LPS+VitD3 group, pregnant mice were orally administered with VitD3 (25 μg/kg) before LPS injection. As expected, a 5-day LPS injection resulted in 62.5% (10/16) of dams and 20.3% of fetuses with NTDs. Additional experiment showed that a 5-day LPS injection downregulated placental proton-coupled folate transporter (pcft) and reduced folate carrier 1 (rfc1), 2 major folate transporters in placentas. Consistent with downregulation of placental folate transporters, folate transport from maternal circulation into embryos was disturbed in LPS-treated mice. Interestingly, VitD3 not only inhibited placental inflammation but also attenuated LPS-induced downregulation of placental folate transporters. Correspondingly, VitD3 markedly improved folate transport from maternal circulation into the embryos. Importantly, supplementation with VitD3 during pregnancy protected mice from LPS-induced NTDs. Taken together, these results suggest that supplementation with VitD3 during pregnancy prevents LPS-induced NTDs through inhibiting placental inflammation and improving folate transport from maternal circulation into the embryos.
Keywords: vitamin D3, lipopolysaccharide, neural tube defects, folic acid, proton-coupled folate transporter, reduced folate carrier
Lipopolysaccharide (LPS) is a toxic component of cell walls in gram-negative bacteria and is widely present in the digestive tracts of humans and animals (Jacob et al., 1997). Humans are constantly exposed to a low concentration of LPS through infection. Gastrointestinal inflammatory diseases and excess alcohol intake are known to increase permeability of LPS from gastrointestinal tract into blood (Zhou et al., 2003). Mimicking maternal infection by exposing pregnant rodents to LPS at different gestational stages has been associated with adverse pregnant outcomes. According to an earlier report, maternal LPS exposure at early gestational stage caused embryonic resorption (Ogando et al., 2003). Recently, we showed that maternal LPS exposure at middle gestational stage caused neural tube defects (NTDs) (Rivera et al., 1998; Zhao et al., 2014). We and others found that maternal LPS exposure at late gestational stage resulted in fetal death, intrauterine growth restriction (IUGR), skeletal development retardation, and preterm delivery (Buhimschi et al., 2003; Rivera et al., 1998; Xu et al., 2005, 2006a, 2007). In addition, maternal LPS exposure at late gestational stage caused impairments of neurobehavioral development in offspring (Wang et al., 2010).
The mechanism of LPS-induced adverse pregnant outcomes has been extensively studied in rodent animals. Previous reports have demonstrated that excess reactive oxygen species partially contributes to LPS-induced fetal demise, IUGR, and NTDs (Xu et al., 2006a; Zhao et al., 2008). Inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), are involved in LPS-induced adverse pregnant outcomes including preterm delivery, fetal demise, and IUGR (Leazer et al., 2002; Xu et al., 2006b). In addition, eicosanoids are also important mediators of LPS-induced fetal demise and preterm delivery. Indeed, maternal LPS exposure during pregnancy showed an increase in cyclooxygenase (COX)-2 expression and eicosanoid production, followed by a dose-dependent elevation of embryo demise (Silver et al., 1995). COX-2 suppressors markedly alleviated LPS-induced fetal demise and preterm delivery (Gross et al., 2000; Sakai et al., 2001).
Vitamin D, a secosteroid hormone, is known for its classical functions in calcium uptake and bone metabolism (Wagner and Greer, 2008). It is increasingly recognized that vitamin D has antiinflammatory effects (Krishnan and Feldman, 2011). A recent report demonstrates that vitamin D regulates placental inflammation (Liu et al., 2011). Nevertheless, whether vitamin D protects against LPS-induced adverse developmental outcomes remains to be determined. In this study, we investigated the effects of supplementation with vitamin D3 (VitD3) during pregnancy on LPS-induced NTDs in mice. We demonstrated for the first time that supplementation with VitD3 during pregnancy prevents LPS-induced NTDs through inhibiting placental inflammation and improving folate transport from maternal circulation into the embryos.
MATERIALS AND METHODS
Chemicals and reagents
LPS (Escherichia coli LPS, serotype 0127:B8) and VitD3 (Cholecalciferol, C9756) were purchased from Sigma Chemical Co. (St. Louis, MO). TRI reagent was from Molecular Research Center (Cincinnati, Ohio). RNase-free DNase was from Promega Corporation (Madison, WI). All the other reagents were from Sigma or as indicated in the specified methods.
Animals and treatments
The ICR mice (8- to 10-week-old; male mice: 28–30 g; female mice: 24–26 g) were purchased from Beijing Vital River whose foundation colonies were all introduced from Charles River Laboratories. The animals were allowed free access to food and water at all times and were maintained on a 12-h light/dark cycle in a controlled temperature (20°C–25°C) and humidity (50% ± 5%) environment for a period of 1 week before use. For mating purposes, 4 females were housed overnight with 2 males starting at 9 pm. Females were checked by 7 am the next morning, and the presence of a vaginal plug was designated as gestational day (GD) 0.
All pregnant mice except controls were ip injected with LPS (25 μg/kg) daily from GD8 to GD12. In LPS+VitD3 group, pregnant mice were pretreated with VitD3 (25 μg/kg) by gavage daily 2 days before LPS injection. The doses of LPS used in this study referred to our previous study with minor modification (Zhao et al., 2008). The doses of VitD3 used in this study referred to others (Pitcher et al., 1994). All dams were sacrificed on GD18, and gravid uterine weights were recorded. For each litter, the numbers of live fetuses, dead fetuses, and resorption sites were counted. Live fetuses were weighed and examined for external malformations. All fetuses were then stored in ethanol a minimum of 2 weeks for subsequent skeletal evaluation. To investigate the effects of VitD3 on the transport of folate through placentas into the embryos, all pregnant mice except controls were ip injected with LPS (25 μg/kg) daily from GD8 to GD12. In LPS+VitD3 group, pregnant mice were pretreated with VitD3 (25 μg/kg) by gavage daily 2 days before LPS injection. All pregnant mice were sacrificed 12 h after last LPS injection. Maternal sera and embryos were collected for folate measurements. To investigate the effects of VitD3 on the expression of placental inflammatory cytokines and folate transporters, all pregnant mice except controls were ip injected with a single dose of LPS (25 μg/kg) on GD10. In LPS+VitD3 group, pregnant mice were pretreated with VitD3 (25 μg/kg) by gavage daily 2 days before LPS injection. Pregnant mice were sacrificed at either 2 or 12 h after LPS injection. Maternal sera and amniotic fluid were collected for measurement of inflammatory cytokines. Placentas were collected for real-time RT-PCR.
This study was approved by the Association of Laboratory Animal Sciences and the Center for Laboratory Animal Sciences at Anhui Medical University (Permit Number: 13-0012). All procedures on animals followed the guidelines for humane treatment set by the Association of Laboratory Animal Sciences and the Center for Laboratory Animal Sciences at Anhui Medical University.
Skeletal examination and evaluation
The fetuses stored in ethanol were cleared of skin, viscera, and adipose tissue. Fetuses were then incubated in acetone overnight and subsequently macerated and stained with Alizarin red S for 2 days. After an overnight incubation in 70% ethanol/glycerol/benzyl alcohol, the fetuses were stored in glycerol until examination. Skeletal evaluation included determination of the degree of ossification of the phalanges, metacarpals, vertebrae, sternatrae, and skull. The size of the anterior fontanel and ossification of the supraoccipital was scored.
Isolation of total RNA and real-time RT-PCR
Total RNAs in mouse placentas were extracted using TRI reagent. RNase-free DNase-treated total RNA (1.0 μg) was reverse-transcribed with AMV (Pregmega). Real-time RT-PCR was performed with a LightCycler 480 SYBR Green I kit (Roche Diagnostics GmbH) using gene-specific primers listed in Table 1. The amplification reactions were carried out on a LightCycler 480 Instrument (Roche Diagnostics GmbH) with an initial hold step (95°C for 5 min) and 50 cycles of a 3-step PCR (95°C for 15 sec, 60°C for 15 sec, 72°C for 30 sec).
TABLE 1.
Primers for Real-Time RT-PCR
| Genes | Sequences | Sizes (bp) |
|---|---|---|
| Gapdh | Forward: 5′- ACCCCAGCAAGGACACTGAGCAAG -3′ | 109 |
| Reverse: 5′- GGCCCCTCCTGTTATTATGGGGGT -3′ | ||
| tnf-α | Forward: 5′- CCCTCCTGGCCAACGGCATG -3′ | 109 |
| Reverse: 5′- TCGGGGCAGCCTTGTCCCTT -3′ | ||
| il-1β | Forward: 5′- GCCTCGTGCTGTCGGACCCATAT -3′ | 143 |
| Reverse: 5′- TCCTTTGAGGCCCAAGGCCACA -3′ | ||
| il-6 | Forward: 5′- AGACAAAGCCAGAGTCCTTCAGAGA -3′ | 146 |
| Reverse: 5′- GCCACTCCTTCTGTGACTCCAGC -3′ | ||
| mip-2 | Forward: 5′- TTGCCTTGACCCTGAAGCCCCC -3′ | 175 |
| Reverse: 5′- GGCACATCAGGTACGATCCAGGC -3′ | ||
| kc | Forward: 5′-ACTCAAGAATGGTCGCGAGG-3′ | 123 |
| Reverse: 5′-GTGCCATCAGAGCAGTCTGT-3′ | ||
| mcp-1 | Forward: 5′- GGCTGGAGAGCTACAAGAGG-3′ | 93 |
| Reverse: 5′-GGTCAGCACAGACCTCTCTC-3′ | ||
| pcft | Forward: 5′- CTACCCTACCTCACCAGCCT-3′ | 119 |
| Reverse: 5′- GCAAACGCAAAGACCACCAT-3′ | ||
| frα | Forward: 5′-GTGGAGACAAAGAAGCCCGA-3′ | 104 |
| Reverse: 5′-CTCCACTCCCTGCTTAGGGT-3′ |
Enzyme-linked immunosorbent assay
Commercial enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) kits were used to determine the levels of TNF-α and IL-6 in maternal sera and amniotic fluid according to the manufacturer’s protocol.
Measurement of folate
The blood samples taken for the measurement of folate levels were collected. The samples were then centrifuged at 4000 rpm for 10 min and stored at − 80°C until the measurement of folate. The levels of folate were measured by electrochemiluminescence immunoassay, using a kit from Roche (Roche Diagnostics GmbH, Mannheim, Germany) following manufacturer’s instructions.
Statistical analysis
The litter was considered the unit for statistical comparison among different groups. Fetal mortality was calculated per litter and then averaged per group. The incidence of fetal malformation with NTDs were calculated in litter and then averaged in each group. For fetal weight, crown-rump length, and skeletal evaluation, the means were calculated per litter and then averaged per group. Quantified data were expressed as means ± SEM at each point. P < .05 was considered statistically significant. ANOVA and the Student-Newmann-Keuls post hoc test were used to determine differences between the treated animals and the control and statistical significance. Fisher’s exact test was used to determine the significance of the categorical data between the 2 groups.
RESULTS
Effects of Maternal VitD3 Supplementation on LPS-Induced Fetal Death and IUGR
VitD3 alone had no effect on fodder consumption and weight gain of the pregnant mice (data not shown). No dams died throughout the pregnancy and no abortion was observed. No preterm delivery was observed in dams that completed the pregnancy. The number of litters, live fetuses per litter, and dead fetuses per litter were analyzed. As shown in Table 2, injection with a low dose of LPS (25 μg/kg) daily from GD8 to GD12 slightly increased the number of dead fetuses per litter. Of interest, pretreatment with VitD3 couldn’t protect against LPS-induced fetal demise. The effects of VitD3 on LPS-induced IUGR are presented in Table 2. As expected, injection with a low dose of LPS (25 μg/kg) daily from GD8 to GD12 slightly reduced fetal weight and crown-rump length. Interestingly, fetal weight rose from 1.30 ± 0.02 g in the LPS group to 1.36 ± 0.02 g in the LPS+VitD3 group, whereas VitD3 had little effect on LPS-induced reduction of fetal crown-rump length (P > .05). The effects of VitD3 on placenta weight were also analyzed. As shown in Table 2, placenta weight rose from 0.089 ± 0.002 g in the LPS group to 0.094 ± 0.002 g in the LPS+VitD3 group.
TABLE 2.
Fetal Outcomes Among Different Groups
| Control | VitD | LPS | LPS+VitD | |
|---|---|---|---|---|
| Number of dams (n) | 15 | 15 | 16 | 15 |
| Number of dams with NTDs (n[%]) | 0 | 0 | 10 (62.5) ** | 2 (13.3) †† |
| Resorptions per dam (n) | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.1 |
| Dead fetuses per dam (n) | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.8 ± 0.3* | 0.3 ± 0.2 |
| Numbers of live fetuses (n) | 203 | 207 | 208 | 202 |
| Live fetuses per dam (n) | 13.5 ± 0.7 | 13.8 ± 0.8 | 13.0 ± 0.8 | 13.5 ± 0.3 |
| Fetal weight(g) | 1.41 ± 0.02 | 1.40 ± 0.01 | 1.30 ± 0.02** | 1.36 ± 0.02† |
| Crown-rump length (cm) | 2.49 ± 0.01 | 2.48 ± 0.01 | 2.32 ± 0.02** | 2.36 ± 0.03 |
| Placenta weight (g) | 0.100 ± 0.001 | 0.101 ± 0.004 | 0.089 ± 0.002** | 0.094 ± 0.002† |
All data were expressed as means ± SEM. *P < .05, **P < .01 as compared with the control. †P < .05, ††P < .01 as compared with LPS group.
Effects of Maternal Vitamin D3 Supplementation on LPS-Induced NTDs
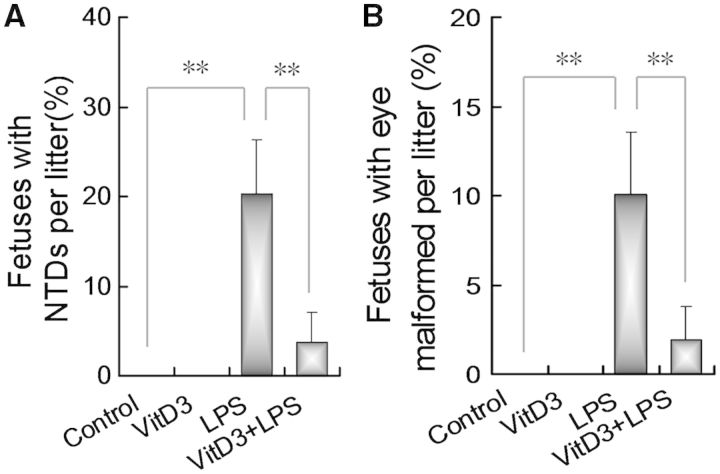
The effects of VitD3 on LPS-induced NTDs were analyzed. As shown in Table 2, a 5-day LPS injection resulted in 62.5% of litters (10/16) with NTDs. As expected, anencephaly, encephalomeningocele, and exencephaly were the most common NTDs. The incidence of fetuses with NTDs was significantly increased in fetuses from dams injected daily with LPS from GD8 to GD12, in which 20.3% of fetuses were with NTDs (Fig. 1A). In addition, 10.1% of fetuses were with eye malformed in LPS group (Fig. 1B). Interestingly, the rate of dams with NTDs was reduced to 13.3% (2/15) in LPS+VitD3 group (Table 2). Correspondingly, the incidence of fetuses with NTDs dropped to 3.8% in LPS+VitD3 group (Fig. 1A). The incidence of fetuses with eye malformed dropped to 1.9% in LPS+VitD3 group (Fig. 1B).
FIG. 1.
Vitamin D3 prevents lipopolysaccharide (LPS)-induced neural tube defects (NTDs). All pregnant mice except controls were ip injected with LPS (25 μg/kg) daily from gestational day (GD)8 to GD12. In LPS+VitD3 group, pregnant mice were pretreated with vitamin D3 (25 μg/kg) by gavage daily 2 days before LPS injection. All pregnant mice were sacrificed on GD18. Live fetuses were examined for NTDs. Fetal malformations were calculated in litter and then averaged in each group. Data were presented as means ± SEM **P < .01.
Effects of Maternal VitD3 Supplementation on LPS-Induced Skeletal Malformation
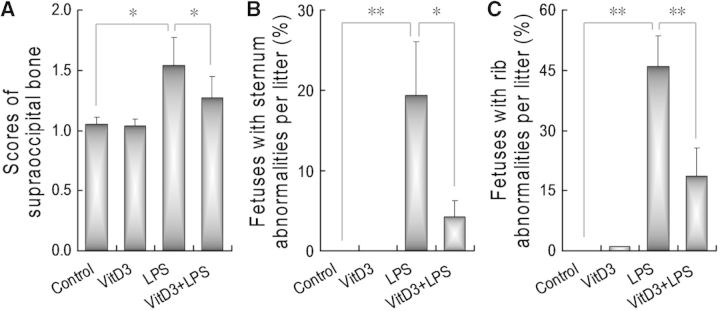
As shown in Figure 2A, an obviously retarded fetal supraoccipital ossification was observed in LPS-treated mice. Interestingly, supplementation with VitD3 during pregnancy significantly alleviated LPS-induced supraoccipital ossification retardation. The effects of VitD3 on LPS-induced sternum malformation were analyzed. As expected, 19.37% of fetuses were with sternum malformation in LPS group. The incidence of fetuses with sternum malformation declined to 4.22% in LPS+VitD3 group (Fig. 2B). The effects of VitD3 on LPS-induced rib malformation are presented in Figure 2C. As expected, 46.09% of fetuses were with rib malformation in LPS group. The incidence of fetuses with rib malformation declined to 18.74% in LPS+VitD3 group.
FIG. 2.
Vitamin D3 protects against LPS-induced skeletal malformations. All pregnant mice except controls were ip injected with LPS (25 μg/kg) daily from GD8 to GD12. In LPS+VitD3 group, pregnant mice were pretreated with vitamin D3 (25 μg/kg) by gavage daily 2 days before LPS injection. All pregnant mice were sacrificed on GD 18. Live fetuses were examined for skeletal malformations. A, Supraoccipital bone scores: 1, well ossified; 5, completely unossified. B, The incidence of fetus with sternum malformations. C, The incidence of fetus with rib malformations. Data were presented as means ± SEM *P < .05, **P < .01.
Effects of Maternal VitD3 Supplementation on LPS-Induced Impairment of Placental Folates Transport Into Embryos
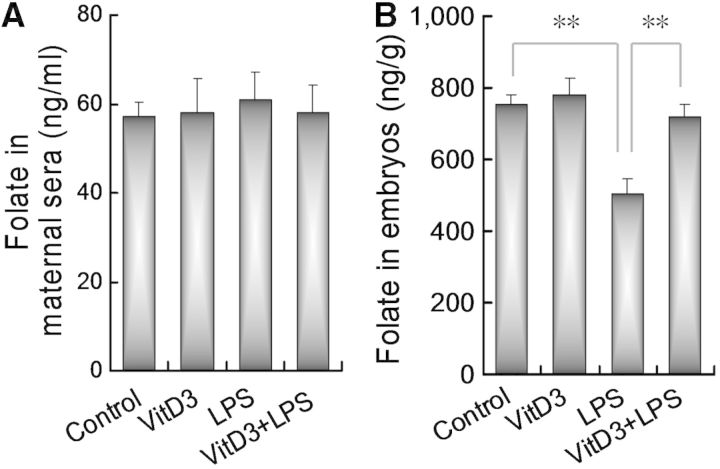
To test whether maternal LPS exposure disturbs placental folate transport from maternal circulation into the embryos, folate contents in maternal sera and the embryos were measured. As shown in Figure 3A, there was no significant difference on folate contents in maternal sera between LPS-treated mice and controls. Of interest, embryonic folate contents were obviously reduced in LPS group (Fig. 3B). We then test whether supplementation with VitD3 counteracts LPS-induced reduction of embryonic folate contents. As expected, VitD3 alone did not affect embryonic folate contents. Interestingly, supplementation with VitD3 during pregnancy significantly attenuated LPS-induced reduction of embryonic folate contents (Fig. 3B).
FIG. 3.
Vitamin D3 counteracts LPS-induced impairment of placental folate transfort into the embryos. All pregnant mice except controls were ip injected with LPS (25 μg/kg) daily from GD8 to GD12. In LPS+VitD3 group, pregnant mice were pretreated with vitamin D3 (25 μg/kg) by gavage daily 2 days before LPS injection. All pregnant mice were sacrificed 12 h after the last LPS injection. A, Folate contents in maternal sera. B, Folate contents in embryos. Data were presented as means ± SEM of 12 samples from 12 different pregnant mice. **P < .01.
Effects of Maternal VitD3 Supplementation on LPS-Induced Downregulation of Placental Folate Transporters
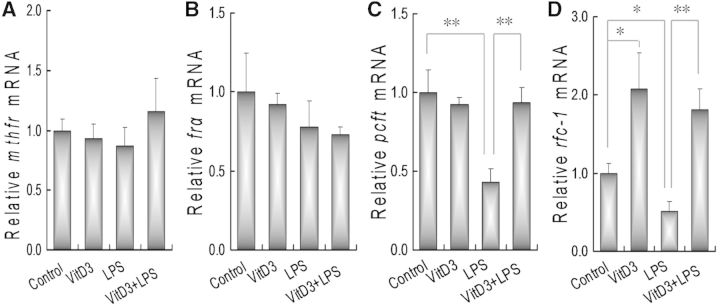
The effects of VitD3 on the expression of placental folate metabolizing enzyme were analyzed. As shown in Figure 4A, a 5-day LPS injection did not affect mRNA level of placental methylenetetrahydrofolate reductase (mthfr). In addition, VitD3 alone had no effect on the expression of placental mthfr. The effects of VitD3 on the expression of placental folate receptor alpha (frα) are shown in Figure 4B. As expected, a 5-day LPS injection did not affect the expression of placental frα. In addition, VitD3 alone had little effect on placental frα mRNA level. The effects of VitD3 on the expression of placental proton-coupled folate transporter (pcft) were then analyzed. As shown in Figure 4C, mRNA level of placental pcft was markedly decreased 12 h after LPS injection. Although VitD3 alone did not affect the expression of placental pcft, supplementation with VitD3 during pregnancy significantly attenuated LPS-induced downregulation of placental pcft. The effects of VitD3 on the expression of placental reduced folate carrier 1 (rfc1) are presented in Figure 4D. As expected, the expression of placental rfc1 was downregulated 12 h after LPS injection. By contrast, the expression of placental rfc1 mRNA was upregulated in VitD3-pretreated mice. Moreover, supplementation with VitD3 significantly attenuated LPS-induced downregulation of rfc1 in mouse placenta.
FIG. 4.
Vitamin D3 inhibits LPS-induced downregulation of placental folate transporters. All pregnant mice except controls were ip injected with a single dose of LPS (25 μg/kg) on GD10. In LPS+VitD3 group, pregnant mice were pretreated with vitamin D3 (25 μg/kg) by gavage 2 days before LPS injection. All pregnant mice were sacrificed 12 h after LPS injection. Placental mthfr, frα, pcft, and rfc1 mRNAs were measured using real-time RT-PCR. A, mthfr; B, frα; C, pcft; D, rfc1. Data were presented as means ± SEM of 6 samples from 6 different pregnant mice. *P < .05, **P < .01.
Effects of Maternal VitD3 Supplementation on LPS-Induced Release of Inflammatory Cytokines in Maternal Sera and Amniotic Fluid
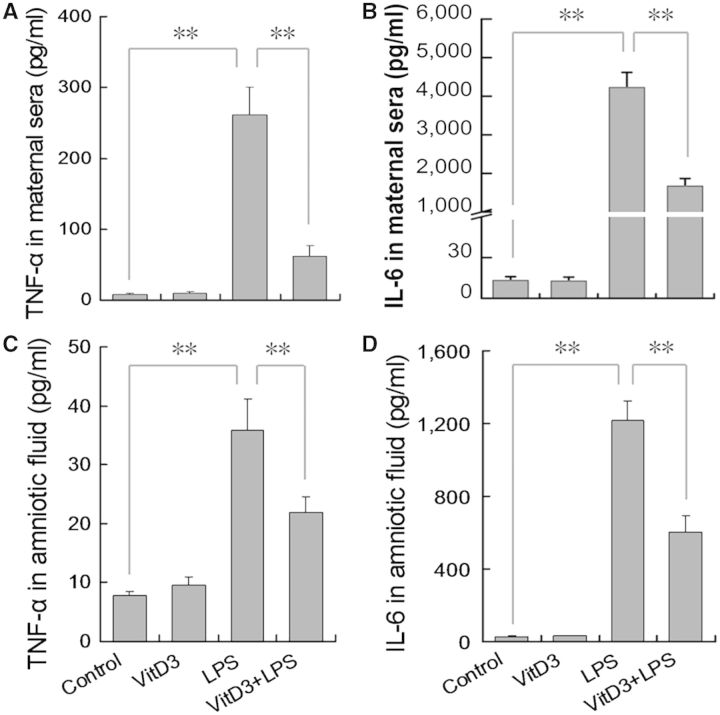
The effects of VitD3 on LPS-induced inflammatory cytokines in maternal sera and amniotic fluid are presented in Figure 5. As expected, the level of TNF-α and IL-6 in maternal sera was significantly increased 2 h after LPS injection (Figs. 5A and B). Consistent with elevation of inflammatory cytokines in maternal sera, the level of TNF-α and IL-6 in amniotic fluid was increased 2 h after LPS injection (Figs. 5C and D). Of interest, supplementation with VitD3 significantly repressed LPS-induced elevation of TNF-α and IL-6 in maternal sera. In addition, supplementation with VitD3 significantly attenuated LPS-induced release of TNF-α and IL-6 in amniotic fluid.
FIG. 5.
Vitamin D3 inhibits LPS-induced inflammatory cytokines in maternal sera and amniotic fluid. All pregnant mice except controls were ip injected with LPS (25 μg/kg) on GD10. In LPS+VitD3 group, pregnant mice were pretreated with vitamin D3 (25 μg/kg) by gavage daily 2 days before LPS injection. All pregnant mice were sacrificed 2 h after LPS injection. TNF-α and IL-6 in maternal sera and amniotic fluid were measured using enzyme-linked immunosorbent assay. A, TNF-α in maternal sera; B, IL-6 in maternal sera; C, TNF-α in amniotic fluid; D, IL-6 in amniotic fluid. All data were expressed as means ± SEM of 6 samples from 6 different pregnant mice. **P < .01.
Effects of Maternal VitD3 Supplementation on LPS-Induced Upregulation of Placental Inflammatory Cytokines and Chemokines
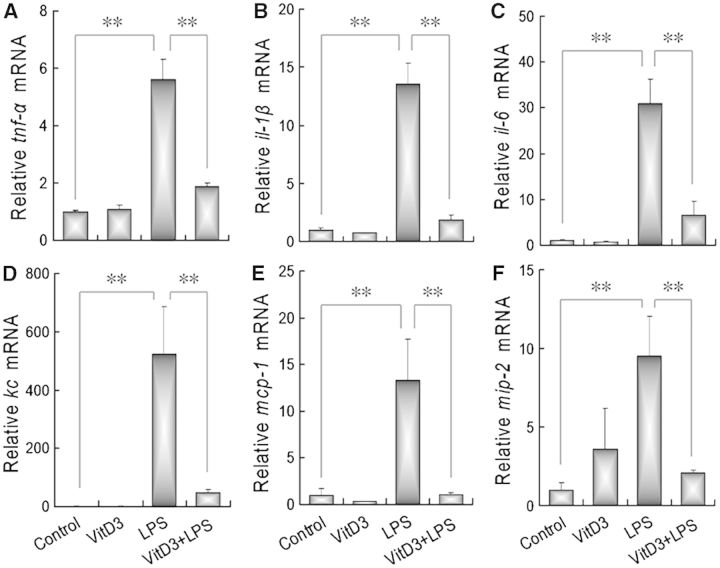
The effects of VitD3 on LPS-induced upregulation of placental inflammatory cytokines were analyzed. As expected, the levels of placental TNF-α, IL-1β, and IL-6 mRNAs were significantly increased 2 h after LPS injection (Figs. 6A–C). Of interest, supplementation with VitD3 almost completely inhibited LPS-induced upregulation of placental TNF-α, IL-1β, and IL-6 mRNAs. The effects of VitD3 on LPS-induced upregulation of placental inflammatory chemokines were then analyzed. As shown in Figures 6D–F, placental KC, MCP-1, and MIP-2 mRNAs were significantly increased 2 h after LPS injection. Of interest, supplementation with VitD3 during pregnancy almost completely inhibited LPS-induced upregulation of placental KC, MCP-1, and MIP-2 mRNAs.
FIG. 6.
Vitamin D3 inhibits LPS-induced placental inflammatory cytokines and chemokines. All pregnant mice except controls were ip injected with LPS (25 μg/kg) on GD10. In LPS+VitD3 group, pregnant mice were pretreated with vitamin D3 (25 μg/kg) by gavage daily 2 days before LPS injection. All pregnant mice were sacrificed 2 h after LPS injection. Placental tnf-α, il-1β, il-6, kc, mcp-1, and mip-2 mRNAs were measured using real-time RT-PCR. A, tnf-α; B, il-1β; C, il-6; D, kc; E, mcp-1; F, mip-2. All data were expressed as means ± SEM of 6 samples from 6 different pregnant mice. **P < .01.
DISCUSSION
Several studies demonstrated that maternal LPS exposure at middle gestational stage caused NTDs (Zhao et al., 2008, 2014). This study investigated the effect of supplementation with VitD3 during pregnancy on LPS-induced NTDs. Our results showed that the rate of litters with NTDs was reduced from 62.5% in LPS group to 13.3% in LPS+VitD3 group. The incidence of fetuses with NTDs dropped from 20.3% in LPS group to 3.8% in LPS+VitD3 group. These results suggest that supplementation with VitD3 during pregnancy prevents LPS-induced NTDs, which expands the protective effects of VitD3 against LPS-induced fetal developmental impairment.
Folate plays a crucial role as 1-carbon donors required for de novo synthesis of cellular DNA (Fox and Stover, 2008). As diets are the main source of folate, adequate dietary folate is especially important for the embryonic development during pregnancy. Increasing evidence has demonstrated that folate deficiency during pregnancy is major etiology of NTDs (Fleming and Copp, 1998). A daily supplementation with physiological dose of folate in the pregnant women is recommended to reduce the risk of NTDs (US Preventive Services Task Force, 2009). The availability of diet-derived folate is primarily determined by the rate of their uptake into the epithelial cells of the intestine. According to a recent study, treatment with VitD3 elevates the uptake of folic acid into Coca-2 cells (Eloranta et al., 2009). In this study, we investigated whether maternal LPS exposure or VitD3 supplementation influences folate uptake through enterocytes into maternal circulation. Our results showed that no significant difference on sera folate concentration was observed among different groups. Of interest, embryonic folate content was markedly reduced in LPS group, indicating that maternal LPS exposure during pregnancy disturbs folate transport from maternal circulation into the fetuses. Indeed, the placentas mediate uptake of folates from maternal circulation into the developing embryos, which are critically important for cell division and the development of critical structures, such as the embryonic neural tube (Farkas et al., 2013). Thus, we further test whether VitD3 influences embryonic folate transport from maternal circulation into the fetuses. Although VitD3 alone had no effect on embryonic folate content, supplementation with VitD3 during pregnancy significantly attenuated LPS-induced reduction of embryonic folate content. These results suggest that supplementation with VitD3 during pregnancy improves placental folate transport from maternal circulation into the fetuses.
The transport of folate from maternal circulation through the placentas into the fetus is mediated by folate receptors (mainly FR-α) and folate transporters (PCFT and RFC1) (Zhao et al., 2011). Several recent reports demonstrate that folate receptors and folate transporters are highly expressed in human and rodent placentas during early embryonic development (Cherukad et al., 2012; Solanky et al., 2010). This study investigated the effects of maternal LPS exposure on the expression of placental folate receptors and folate transporters. We showed that there was no significant difference on the expression of placental fr-α among different groups. As expected, placental pcft and rfc1, 2 major folate transporter genes, was markedly downregulated in LPS-treated mice. Interestingly, LPS-induced downregulation of placental pcft and rfc1 was obviously attenuated when pregnant mice were supplemented with VitD3. These results suggest that maternal LPS exposure disturbs folate transport from maternal circulation into the fetuses through downregulating placental folate transporters. Supplementation with VitD3 during pregnancy counteracts LPS-induced downregulation of placental folate transporters.
It remains obscure whether VitD3 can directly regulate folate transporters. An in vitro study showed that VitD3 upregulated intestinal PCFT expression in a vitamin D receptor (VDR)-dependent manner (US Preventive Services Task Force, 2009). By contrast, an in vivo study found that vitamin D and its receptor are not linked to intestinal pcft expression in rodent animals (Brandsch et al., 2014). This study found that VitD3 alone had no effect on the expression of placental folate transporters. Thus, we hypothesize that VitD3 indirectly regulates placental folate transporters through other unknown mechanisms. A recent study showed that TNF-α, an important inflammatory cytokine, repressed the uptake of folate by human syncytiotrophoblast (Araújo et al., 2013). Indeed, this study showed that supplementation with VitD3 significantly alleviated LPS-evoked releases of TNF-α and IL-6 in maternal sera and amniotic fluid. In addition, supplementation with VitD3 repressed LPS-induced placental inflammation. Additional experiment is necessary to demonstrate whether VitD3 counteracts LPS-induced downregulation of placental folate transporters through its antiinflammatory activity.
In summary, the present results allow us to reach the following conclusions. First, VitD3 not only inhibits placental inflammation but also attenuates LPS-induced downregulation of placental folate transporters. Second, VitD3 improves placental folate transport from maternal circulation into the developing embryos. Third, supplementation with VitD3 during pregnancy prevents LPS-induced NTDs. Indeed, vitamin D deficiency is common in pregnant women and is increasingly recognized as a public health problem (Holmes et al., 2009). Recently, vitamin D deficiency has been linked with adverse pregnant outcomes, including gestational diabetes mellitus and preeclampsia (Cho et al., 2013; Ma et al., 2012). According to several earlier reports, supplementation with VitD3 during late pregnancy reduced the risks of IUGR infants (Brooke et al., 1980; Marya et al., 1981). Therefore, VitD3 may have a potential preventive utility for protecting against LPS-induced developmental toxicity.
FUNDING
National Natural Science Foundation of China (81471467, 81172711, 30901617, 81473016).
REFERENCES
- Araújo J. R., Correia-Branco A., Moreira L., Ramalho C., Martel F., Keating E. (2013). Folic acid uptake by the human syncytiotrophoblast is affected by gestational diabetes, hyperleptinemia, and TNF-α. Pediatr. Res. 73, 388–394. [DOI] [PubMed] [Google Scholar]
- Brandsch C., Zibolka J., Frommhagen M., Lehmann U., Dierkes J., Kühne H., Hirche F., Stangl G. I. (2014). Vitamin D is not linked to folate status and mRNA expression of intestinal proton-coupled folate transporter. Eur. J. Nutr. 53, 1115–1122. [DOI] [PubMed] [Google Scholar]
- Brooke O. G., Brown I. R., Bone C. D., Carter N. D., Cleeve H. J., Maxwell J. D., Robinson V. P., Winder S. M. (1980). Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br. Med. J. 280, 751–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhimschi I. A., Buhimschi C. S., Weiner C. P. (2003). Protective effect of N-acetylcysteine against fetal fetal death and preterm labor induced by maternal inflammation. Am. J. Obstet. Gynecol. 188, 203–208. [DOI] [PubMed] [Google Scholar]
- Cherukad J., Wainwright V., Watson E. D. (2012). Spatial and temporal expression of folate-related transporters and metabolic enzymes during mouse placental development. Placenta 33, 440–448. [DOI] [PubMed] [Google Scholar]
- Cho G. J., Hong S. C., Oh M. J., Kim H. J. (2013). Vitamin D deficiency in gestational diabetes mellitus and the role of the placenta. Am. J. Obstet. Gynecol. 209, 560.e1–8. [DOI] [PubMed] [Google Scholar]
- Eloranta J. J., Zaïr Z. M., Hiller C., Häusler S., Stieger B., Kullak-Ublick G. A. (2009). Vitamin D3 and its nuclear receptor increase the expression and activity of the human proton-coupled folate transporter. Mol. Pharmacol. 76, 1062–1071. [DOI] [PubMed] [Google Scholar]
- Farkas S. A., Böttiger A. K., Isaksson H. S., Finnell R. H., Ren A., Nilsson T. K. (2013). Epigenetic alterations in folate transport genes in placental tissue from fetuses with neural tube defects and in leukocytes from subjects with hyperhomocysteinemia. Epigenetics 8, 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A., Copp A. J. (1998). Embryonic folate metabolism and mouse neural tube defects. Science 280, 2107–2109. [DOI] [PubMed] [Google Scholar]
- Fox J. T., Stover P. J. (2008). Folate-mediated one-carbon metabolism. Vitam. Horm. 79, 1–44. [DOI] [PubMed] [Google Scholar]
- Gross G., Imamura T., Vogt S. K., Wozniak D. F., Nelson D. M., Sadovsky Y., Muglia L. J. (2000). Inhibition of cyclooxygenase-2 prevents inflammation-mediated preterm labor in the mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R1415–R1423. [DOI] [PubMed] [Google Scholar]
- Holmes V. A., Barnes M. S., Alexander H. D., McFaul P., Wallace J. M. (2009). Vitamin D deficiency and insufficiency in pregnant women: a longitudinal study. Br. J. Nutr. 102, 876–881. [DOI] [PubMed] [Google Scholar]
- Jacob A. L., Goldberg P. K., Bloom N., Degenshein G. A., Kozinn P. J. (1997). Endotoxin and bacteria in portal blood. Gastroenterology 72, 1268–1270. [PubMed] [Google Scholar]
- Krishnan A. V., Feldman D. (2011). Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu. Rev. Pharmacol. Toxicol. 51, 311–336. [DOI] [PubMed] [Google Scholar]
- Leazer T. M., Barbee B., Ebron-McCoy M., Henry-Sam G. A., Rogers J. M. (2002). Role of the maternal acute phase response and tumor necrosis factor alpha in the developmental toxicity of lipopolysaccharide in the CD-1 mouse. Reprod. Toxicol. 16, 173–179. [DOI] [PubMed] [Google Scholar]
- Liu N. Q., Kaplan A. T., Lagishetty V., Ouyang Y. B., Ouyang Y., Simmons C. F., Equils O., Hewison M. (2011). Vitamin D and the regulation of placental inflammation. J. Immunol. 186, 5968–5974. [DOI] [PubMed] [Google Scholar]
- Ma R., Gu Y., Zhao S., Sun J., Groome L. J., Wang Y. (2012). Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am. J. Physiol. Endocrinol. Metab. 303, E928–E935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marya R. K., Rathee S., Lata V., Mudgil S. (1981). Effects of vitamin D supplementation in pregnancy. Gynecol. Obstet. Invest. 12, 155–161. [DOI] [PubMed] [Google Scholar]
- Ogando D. G., Paz D., Cella M., Franchi A. M. (2003). The functional role of increased production of nitric oxide in lipopolysaccharide-induced embryonic resorption in mice. Reproduction 125, 95–110. [DOI] [PubMed] [Google Scholar]
- Pitcher T., Pettifor J. M., Buffenstein R. (1994). The effect of dietary calcium content and oral vitamin D 3 supplementation on mineral homeostasis in a subterranean mole-rat Cryptomys damarensis. Bone Miner 27, 145–157. [DOI] [PubMed] [Google Scholar]
- Rivera D. L., Olister S. M., Liu X., Thompson J. H., Zhang X. J., Pennline K., Azuero R., Clark D. A., Miller M. J. (1998). Interleukin-10 attenuates experimental fetal growth restriction and demise. FASEB J. 12, 189–197. [DOI] [PubMed] [Google Scholar]
- Sakai M., Tanebe K., Sasaki Y., Momma K., Yoneda S., Saito S. (2001). Evaluation of the tocolytic effect of a selective cyclooxygenase-2 inhibitor in a mouse model of lipopolysaccharide-induced preterm delivery. Mol. Hum. Reprod. 7, 595–602. [DOI] [PubMed] [Google Scholar]
- Silver R. M., Edwin S. S., Trautman M. S., Simmons D. L., Branch D. W., Dudley D. J., Mitchell M. D. (1995). Bacterial lipopolysaccharide-mediated fetal death. Production of a newly recognized form of inducible cyclooxygenase (COX-2) in murine decidua in response to lipopolysaccharide. J. Clin. Invest. 95, 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanky N., Requena Jimenez A., D'Souza S. W., Sibley C. P., Glazier J. D. (2010). Expression of folate transporters in human placenta and implications for homocysteine metabolism. Placenta 31, 134–143. [DOI] [PubMed] [Google Scholar]
- US Preventive Services Task Force. (2009). Folic acid for the prevention of neural tube defects: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 150, 626–631. [DOI] [PubMed] [Google Scholar]
- Wagner C. L., Greer F. R. (2008). Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 122, 1142–1152. [DOI] [PubMed] [Google Scholar]
- Wang H., Meng X. H., Ji Y. L., Ning H., Zhao X. F., Wang Q., Liu P., Zhang H., Zhang C., Chen G. H., et al. (2010). Age- and gender-dependent impairments of neurobehaviors in mice whose mothers were exposed to lipopolysaccharide during pregnancy. Toxicol. Lett. 192, 242–251. [DOI] [PubMed] [Google Scholar]
- Xu D. X., Chen Y. H., Wang H., Zhao L., Wang J. P., Wei W. (2005). Effect of N-acetylcysteine on lipopolysaccharide-induced intra-uterine fetal death and intra-uterine growth retardation in mice. Toxicol. Sci. 88, 525–533. [DOI] [PubMed] [Google Scholar]
- Xu D. X., Chen Y. H., Zhao L., Wang H., Wei W. (2006a). Reactive oxygen species are involved in lipopolysaccharide-induced intra-uterine growth restriction and skeletal development retardation in mice. Am. J. Obstet. Gynecol. 195, 1707–1714. [DOI] [PubMed] [Google Scholar]
- Xu D. X., Chen Y. H., Wang H., Zhao L., Wang J. P., Wei W. (2006b). Tumor necrosis factor alpha partially contributes to lipopolysaccharide-induced intra-uterine fetal growth restriction and skeletal development retardation in mice. Toxicol. Lett. 163, 20–29. [DOI] [PubMed] [Google Scholar]
- Xu D. X., Wang H., Zhao L., Ning H., Zhang C., Chen Y. H. (2007). Effects of Low-dose lipopolysaccharide (LPS) pretreatment on LPS-induced intra-uterine fetal death and preterm labor. Toxicology 234, 167–175. [DOI] [PubMed] [Google Scholar]
- Zhao L., Chen Y. H., Wang H., Ji Y. L., Ning H., Wang S. F., Zhang C., Lu J. W., Duan Z. H., Wei L. Z., et al. (2008). Reactive oxygen species partially contribute to lipopolysaccharide (LPS)-induced teratogenesis in mice. Toxicol. Sci. 103, 149–157. [DOI] [PubMed] [Google Scholar]
- Zhao M., Chen Y. H., Chen X., Dong X. T., Zhou J., Wang H., Wu S. X., Zhang C., Xu D. X. (2014). Folic acid supplementation during pregnancy protects against lipopolysaccharide-induced neural tube defects in mice. Toxicol. Lett. 224, 201–208. [DOI] [PubMed] [Google Scholar]
- Zhao R., Diop-Bove N., Visentin M., Goldman I. D. (2011). Mechanisms of membrane transport of folates into cells and across epithelia. Annu. Rev. Nutr. 31, 177–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Wang L., Song Z., Lambert J. C., McClain C. J., Kang Y. J. (2003). A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-alpha production. Am. J. Pathol. 163, 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]