Abstract
Numerous studies have reported on testicular toxicity of phthalates in different experimental paradigms and showed that Leydig cells (LCs) were one of the main targets of phthalate actions. Adverse effects of phthalates on LCs steroidogenesis have been attributed to their metabolites, monophthalates. This study focuses on investigation whether LCs responsiveness to monophthalates action is associated with their potential to produce androgens. We found that of 3 monophthalates investigated [ie, mono-2-ethylhexyl phthalate (MEHP), mono-n-butyl phthalate, and mono-n-benzyl phthalate] only MEHP caused biological effects on the mouse LCs function. This monophthalate stimulated basal steroidogenesis associated with upregulation of StAR protein expression with no effect on hCG-stimulated androgen production by LCs from CBA/Lac and C57BL/6j mouse genotypes were observed. Further, MEHP attenuated ATP production and increased superoxide generation by both phenotypes of mouse LCs that indicated on mitochondrial dysfunction induced by the monophthalate. All together, our data indicate that MEHP-mediated stimulation of steroidogenesis and perturbation in mitochondrial function are not associated with the capacity of the LCs to synthesize androgens. We suggest that this effect of MEHP observed in LCs of rodent origin needs to be taken into consideration in analysis of earlier start of puberty in boys and may highlight a possible influence of phthalates on reproductive health in males.
Keywords: Leydig cells, MEHP, monophthalates, steroidogenesis, StAR, mouse genotypes, mitochondria
Phthalates are widely used as plasticizers in the production of plastics and are present, among other locations, in food packaging, certain perfumes, and other cosmetics and medical devices such as tubings and catheters (Thomas and Thomas, 1984). Their ability to leak out from tubing during hemodialysis and blood transfusions represents a potential route of exposure of humans to relatively high concentrations of these environmental chemicals that can reach levels as high as 25 mg/kg/day in children during medical intensive care (Koch et al., 2006). It was reported that Europeans are predominantly exposed to the 2 major phthalates, di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP) in their day-to-day life (Wormuth et al., 2006). These phthalates may have negative influences on male reproduction (Gray et al., 2000). The harmful effects of DEHP and DBP on the male reproductive tract have been attributed to their monoester metabolites, mono-2-ethylhexyl phthalate (MEHP) and mono-n-butyl phthalate (MBP) (Albert and Jegou, 2014; Albro et al., 1989; Clewell et al., 2010). The monoester metabolites of the endocrinologically active phthalates have been shown to act directly on the testes, and more specifically the Leydig cell, suppressing Leydig cell capacity to produce androgens (Clewell et al., 2010). Leydig cells are the primary source of testosterone production in males, and differentiation of Leydig cells in the testes is one of the primary events in male sex differentiation, puberty, and fertility (Svechnikov et al., 2010).We and others have reported that MEHP inhibits LH/human chorionic gonadotropin (hCG)-stimulated androgen production by both isolated rat Leydig cells and MA-10 mouse tumor Leydig cells (Dees et al., 2001; Svechnikov et al., 2008; Zhou et al., 2013). However, currently little is known about the relationship between the steroidogenic potential of Leydig cells and their sensitivity to phthalate exposure. We recently identified 2 mouse genotypes, CBA/Lac and C57BL/6j, whose Leydig cells had high and low androgen production potential (Savchuk et al., 2013). In this study, we used this model of mouse Leydig cells to investigate possible links between the androgenic potency of Leydig cells and their responsiveness to 3 selected monophthalates; MEHP, MBP, and mono-n-benzyl phthalate (MBeP). We demonstrated for the first time that the sensitivity of mouse Leydig cells to these monophthalates was not associated to their capacity to produce androgens. MEHP was found to be the only phthalate that caused a biological effect on mouse Leydig cell steroidogenesis and mitochondrial function.
MATERIALS AND METHODS
Materials
Modified eagle’s medium (MEM), Hank’s balanced salts solution (HBSS) without Ca2+ or Mg2+ and penicillin-streptomycin were all obtained from Gibco/BRL (Life Technologies, Paisley, Scotland). Dulbecco’s MEM (DMEM)—Haḿs nutrient mixture F-12, bovine serum albumin (BSA) (fraction V), Percoll, HEPES, collagenase type I, and hCG were obtained from Sigma Chemical Co (St. Louis, Missouri). MEHP, MBP), and MBeP were purchased from TCI (TCI Europe, Belgium, Brussels).
Animals
Inbred 1-month-old males of CBA/Lac, C57BL/6j mouse strains were purchased from Charles River Laboratories International Inc (Charles-River, Germany) and fed with standard laboratory pellet diet and water ad libitum.
Ethics statement
Animal studies were approved by the local animal ethics committee (Stockholm North Animal Ethics Committee, permits number Dnr N311/12).
Isolation and primary culture of Leydig cells
Leydig cells were prepared from the testes of immature mice as described previously (Svechnikov et al., 2001). Briefly, after decapsulation and disruption by collagenase treatment (0.25 mg/ml for 20 min at 37°C), the seminiferous tubules were separated mechanically. The interstitial cells were collected by centrifugation at 300 × g for 7 min and washed in HBSS containing 0.1% (w/v) BSA. To obtain purified Leydig cells, this suspension was centrifuged through a continuous selfgenerated 60% Percoll gradient at 14 000 × g for 40 min at 4°C. The cell viability, as assessed by Trypan blue exclusion, was >90%. These purified Leydig cells were resuspended in DMEM-F12 supplemented with 15 mM HEPES (pH 7.4), 1 mg/ml BSA, 365 mg/l glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin.
For culturing, 100 μl of a suspension containing 1.5 × 105 cells/ml was plated in 96-well plate (Falcon, Franklin Lakes, New Jersey) and incubated with increasing concentrations of MEHP, MBP, and MBeP (1, 3, 10, 30, and 90 µM) alone or with combination with hCG (10 ng/ml) at the time of plating of the cells for 48 h at 34°C in 5% CO2. All compounds were dissolved in dimethyl sulfoxide (DMSO) and the final concentration of the solvent in the media did not exceed 0.1%.
Testosterone measurement
Culture medium samples were stored at −20°C prior to analysis of the concentrations of testosterone. The concentrations of testosterone were quantified employing the Coat-a-Count RIA kit (Diagnostic Products Corp, Los Angeles, California). Intraassay and interassay coefficients for testosterone were 6.4% and 4%, respectively.
Isolating RNA and producing cDNA
Total RNA was extracted from control and treatment groups by RNeasy Mini Kit (74104,Qiagen, Hilden, Germany), in accordance with manufactureŕs instructions. The RNA was pretreated with DNAse (RNase-free DNase Set, Qiagen) according to the manufacturer’s instructions. The amount of total RNA was measured by photometry (BioPhotometer, Hamburg, Germany). The RNA was kept at −80°C until analysis. Total RNA was further processed using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, California) as proposed in the manufacturer’s protocol.
Gene expression analysis by qPCR by quantitative PCR
The samples for qPCR were prepared using iQ SYBR Green Supermix (170-8882, Bio-Rad Laboratories, Hercules, California), and the PCR cycles were run at 95°C for 10 s, 60°C–62°C for 45 s, 95°C for 60 s, and 55°C for 60 s followed by a melting curve from 55°C–95°C in steps of 0.5°C and then held at 4°C (iCycler iQ, Bio-Rad Laboratories, Hercules, California) after having estimated the best reaction conditions by running a temperature gradient. All values were normalized to β-actin as a house keeping gene to balance possible irregularities in RNA concentration. To control the efficacy of the process, negative control (RT-) was always added to each qPCR assay. Before use all primers were run on a gel to check their validity. The 2−ΔΔCt method was used to calculate the fold changes in gene expression.
An overview of the used primers and the running conditions are described in Table 1 .
TABLE 1.
qPCR Primer Sequences and Running Conditions
| Oligo | Sequence | Prod. Length (bp) | Temp (°C) |
|---|---|---|---|
| Actb | F: 5′-acaacggctccggcatgtgcaaag-3′ | 107 | 55–65 |
| R: 5′-tcccaccatcacaccctggtgccta-3′ | |||
| Cyp 11a1 | F: 5′-ggggcaacaagctgcccttcaa-3′ | 88 | 60 |
| R: 5′-tgcagggtcatggaggtcgtgt-3′ | |||
| Cyp17a1 | F: 5′-ttcgcctgggtaccacaactgc-3′ | 110 | 60 |
| R: 5′-tagagtcaccatctggggccga-3′ | |||
| Tspo | F: 5′-agaaaccctcttggcatccg-3′ | 77 | 60 |
| R: 5′-gccataccccatggctgaata-3′ | |||
| Nr5a1 | F: 5′-ttctgagagcccgctagccact-3′ | 116 | 60 |
| R: 5′-cgtccgctgaacggaaggagaa-3′ | |||
| Cyp19a1 | F: 5′-tcggcatgcatgagaacggca-3′ | 93 | 57 |
| R: 5′-cagggcccgtcagagctttcat-3′ | |||
| Srd5a2 | F: 5′-gcggtttagcgtcggtgtct-3′ | 71 | 60 |
| R: 5′-gcagcatgcagtcactgtgga-3′ | |||
| 3aHSD | F: 5′-ggaaaactgttggctgaagca-3′ | 76 | 60 |
| R: 5′-ccagtccggcatctttacactt-3′ | |||
| Lipe | F: 5′-ctgcccaggattggatggtt-3′ | 230 | 60 |
| R: 5′-cgctgaggctttgatcttgc-3′ | |||
| Hsd17b3 | F: 5′-aagtgcatgaggttctcgca-3′ | 151 | 64 |
| R: 5′-gtccatgtctggccaactca-3′ | |||
| Vdac3 | F: 5′-aacactcgttttggcatcgc-3′ | 124 | 60 |
| R: 5′-gtttgactcctggtcggagg-3′ | |||
| Star | F: 5′-aaagccagcaggagaacgggga-3′ | 133 | 60 |
| R: 5′-gcctccatgcggtccacaagtt-3′ |
bp, base pair.
Wes automated Western blotting and analysis
All reagents were prepared and used according to manufacturer’s recommendations for use on Wes (ProteinSimple, San Jose, California, www.proteinsimple.com/simon.html). Reagents included: biotinylated molecular weight ladder, streptavidin-HRP, fluorescent standards, luminol-S, hydrogen peroxide, sample buffer, DTT, stacking matrix, separation matrix, running buffer, wash buffer, matrix removal buffer, capillaries, containing a proprietary UV-activated chemical linked reagent, and antibody diluent and antibodies (goat-anti rabbit secondary antibody). After incubation with MEHP for 48 h, mouse Leydig cells were lysed in CelLytic cell lysis reagent (Sigma Chemical Co St. Louis, Missouri). Samples were diluted to adjust protein concentration to 0.8 μg in 4 μl with sample buffer and further diluted 4:1 by adding 1 μl of the 5 × master mix. The final samples of 5 μl each were boiled 5 min, placed on ice for 5 min, briefly centrifuged and applied to proper wells. Because Wes is capable of performing loading, separation, washing, blocking, immunodetection, and analysis on 24 samples simultaneously, the expression of all steroidogenic enzymes interested (ie, StAR, Cyp11a1, 3βHSDI, and Cyp17) from 1 separate experiment was analyzed in one 25-capillaries cartridge. After plate loading, the separation of proteins by electrophoresis and immunodetection took place in the capillary system and were fully automated. Simple Western analysis was carried out at room temperature, and instrument default settings were used. StAR (1:50 dilution), Cyp11a1 (1:50 dilution), 3βHSDI (1:50 dilution), and CYP17 (1:50) primary antibody were diluted with antibody diluent (ProteinSimple). The digital images were analyzed with Compass software (ProteinSimple) on Wes. Band density differences were expressed as percent of control values. Data were collected from 3 to 4 independent experiments.
Measurement of extracellular superoxide production
Tetrazolium salt WST-1 (Roche, Mannheim, Germany) was used to assay a superoxide anion production by mouse Leydig cells. WST-1 is a cell-impermeable compound which is reduced to formazan by superoxide generated by specific trans-plasma membrane oxidoreductases (Berridge et al., 2005). Primary cultures of mouse Leydig cells from CBA and C57BL mice were treated with MEHP (90 μM) for 48 h followed by media were collected for steroids measurement and the cells were incubated in fresh DMEM/F12 phenol-free media with WST-1 for 3 h. The level of formazan produced was measured with a filter for 450/10 nm using a Wallac1420 microplate spectrofluorimeter (Perkin Elmer). To prove that WST-1 was reduced into formazan by superoxide, the cells were preincubated with superoxide dismutase (SOD) (20 µg/ml), an enzyme that converts superoxide into hydrogen peroxide.
Cell proliferation assay
The influence of MEHP on proliferation of immature Leydig cells was assayed by CyQUANT Cell Proliferation Assay Kit (C7026, Invitrogen, Ltd, UK) according to the manufacturer’s protocol.
ATP assay
Cellular ATP levels in mouse Leydig cells were assessed using the Promega luminescent cell viability assay kit (Promega, Fisher Scientific) according to the manufactureŕs protocol. Briefly, mouse Leydig cells incubated with or without MEHP for 48 h were lysed with Promega Cell Titer-Glo substrate at room temperature on an orbital shaker for 2 min, followed by a 10-min standing incubation at room temperature enabling cell lysis. Samples were analyzed for overall luminescence using a Wallac1420 microplate spectrofluorometer (Perkin Elmer).
Statistical analysis
The differences between various values were analyzed for statistical significance by 1-way ANOVA followed by Dunnett t-test, using the SigmaPlot (v. 11) program (Systat Software, Inc, California). In the case of single comparisons of 2 groups t-test was used. P < .05 was considered to be statistically significant.
RESULTS
MEHP Stimulates Basal Steroidogenesis but Has No Effect on hCG-Activated Androgen Production by Mouse Leydig Cells From CBA/Lac and C57BL/6j Mice
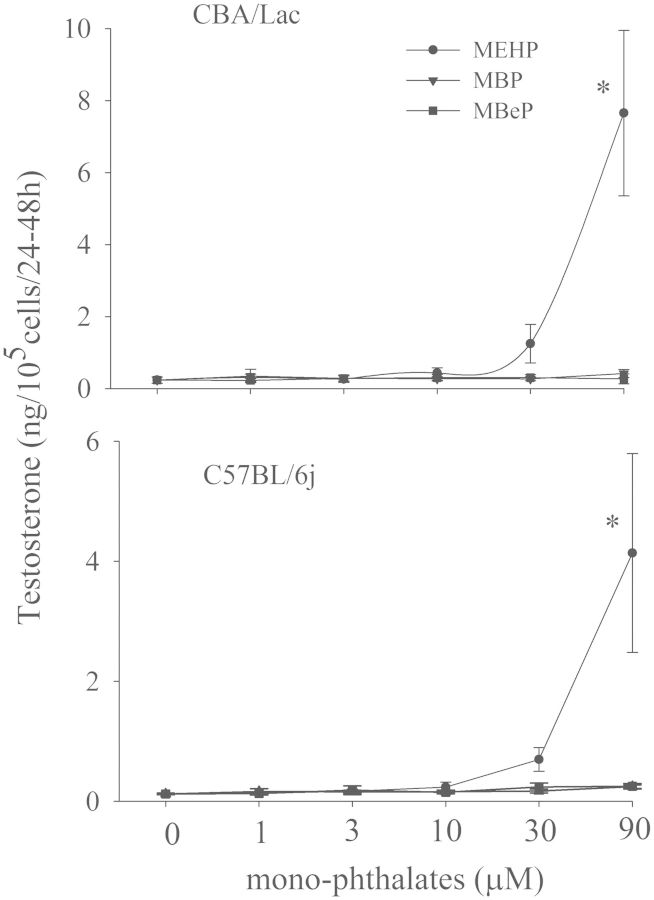
We first explored the effects of the monophthalates on basal and hCG-activated steroidogenesis in mouse Leydig cells with different androgen production potential. We found that only MEHP was able to significantly stimulate biosynthesis of testosterone in mouse Leydig cells from both genotypes at the highest concentration (90 µM) used (Fig. 1). In contrast to these basal effects, MEHP and other monophthalates investigated did not affect hCG-stimulated steroidogenesis in Leydig cells from both mouse genotypes (Supplementary Fig.S 1). It is important to note that the Leydig cells from C57BL/6j mice had attenuated (7-fold, P < .01) capacity to synthesize testosterone in response to hCG compared with the cells from CBA mice. Similarly, these cells from different mouse genotypes demonstrated significant difference in responsiveness to hCG (65- and 239-fold increase in comparison to basal secretion for C57BL/6j and CBA mice, respectively).
FIG. 1.
Effects of monophthalates on basal steroidogenesis in Leydig cells from CBA/Lac and C57BL/6j mice. Primary cultures of mouse Leydig cells were treated with increasing concentrations of monophthalates for 48 h followed by testosterone production in the medium was measured by RIA. The results are means ± SE for 4 independent Leydig cell preparations. *P < .05 compared with untreated control.
Monophthalates Have No Toxic Effects on Mouse Leydig Cell Function
Further, we investigated if MEHP, MBP, and MBeP can suppress the overall metabolism in mouse Leydig cells at the highest concentration used (90 µM). We observed that none of the monophthalates caused toxic effects on mouse Leydig cells from both genotypes. MEHP was the only monophthalate with potential to stimulate reduction of WST-1 into formazan (Figs. 2A and 2B), suggesting a perturbation in the redox state in the Leydig cells.
FIG. 2.
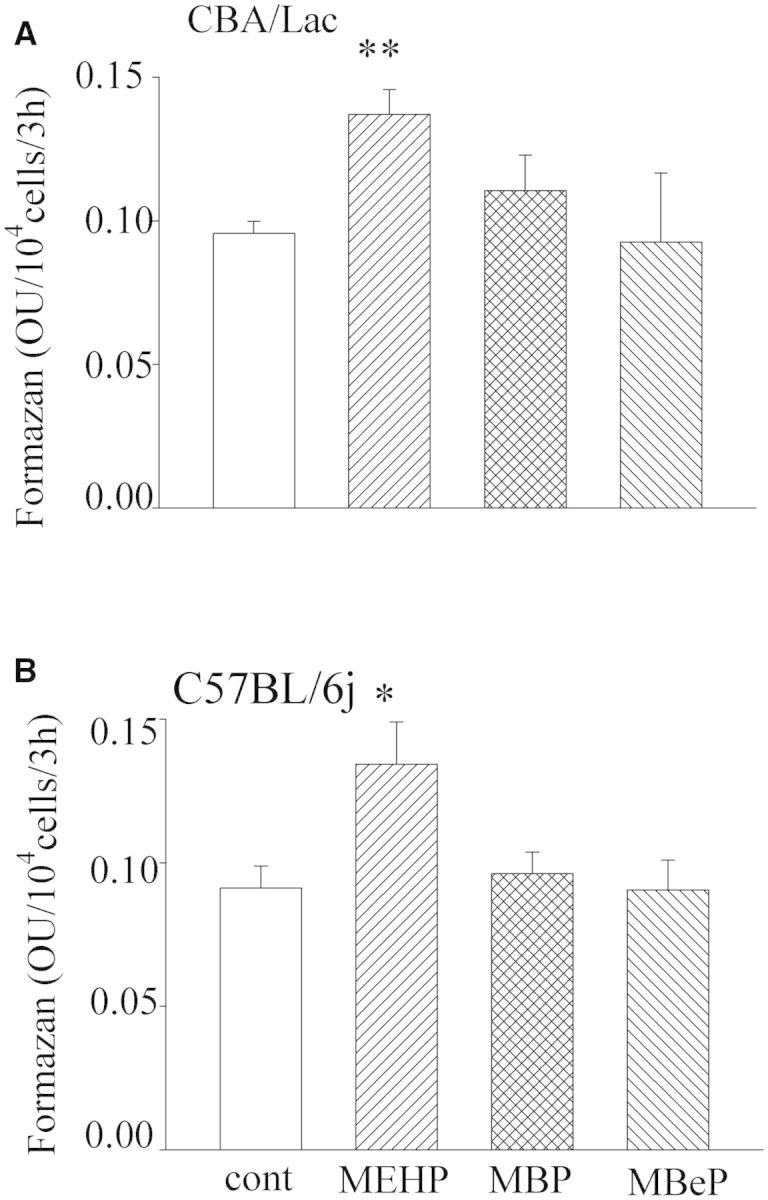
Effects of monophthalates on viability of mouse Leydig cells. Leydig cells were treated with monophthalates (90 µM) for 48 h. The reduction of WST-1 to formazan was then measured. The means ± SE for 4 independent Leydig cell preparations are shown. *P < .05, **P < .01 compared with untreated control.
MEHP Upregulates StAR Protein Expression in Leydig Cells From CBA/Lac and C57BL/6j Mice
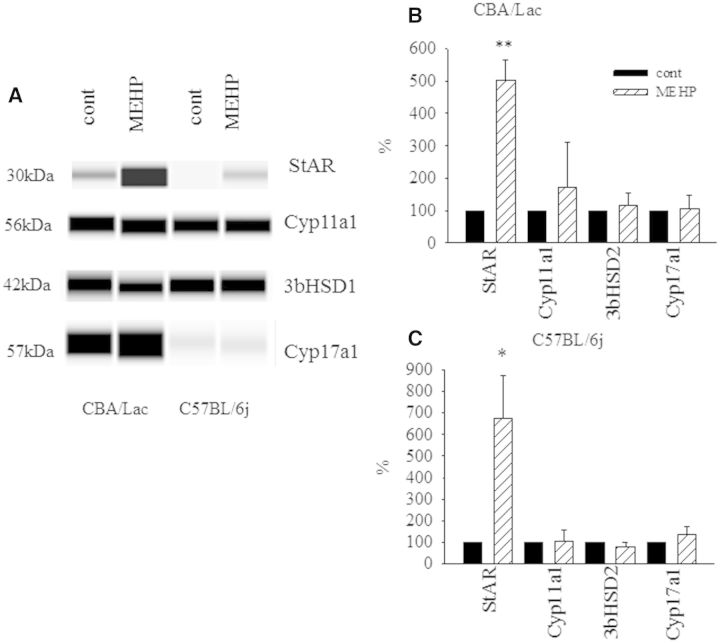
Because MEHP was the only monophthalate with stimulatory effects on mouse Leydig cell steroidogenesis, we further performed a comprehensive analysis of its effects on the expression of several steroidogenic genes in mouse Leydig cells with different androgen production potential. We found that MEHP had no significant influence on the expression of steroidogenic genes after treatment for 24 h (Supplementary Fig. S2) but prolongation of treatment with the monophthalate during 48 h markedly declined the levels of mRNA of downstream steroidogenic enzymes (data not shown). Prominent activation of steroidogenesis by MEHP with no clear upregulation of the steroidogenic gene expression in mouse Leydig cells brought us to explore steroidogenic enzyme expression at the protein level. As shown in Figure 3, MEHP significantly upregulated the expression of StAR protein in Leydig cells from C57BL/6j and CBA/Lac mice (6.5-and 5-fold compared with control, respectively), strongly suggesting that StAR protein, which is crucial for the transport of cholesterol into mitochondria (Stocco, 2001), underlies the stimulatory effect of MEHP on basal Leydig cell steroidogenesis. No significant effects of MEHP on the expression of other steroidogenic enzymes in both mouse Leydig cell genotypes were found. We also observed dramatic decrease in the basal levels of StAR and CYP17 expression in Leydig cells from C57BL/6j mice compared with CBA/Lac genotype (Fig. 3A), an observation that may explain the attenuated capacity of Leydig cells from C57BL/6j mice to synthesize testosterone.
FIG. 3.
Effect of mono-2-ethylhexyl phthalate (MEHP) on steroidogenic enzyme expression in mouse Leydig cells. Leydig cells were treated with MEHP (90 µM) for 48 h. Protein extracts were then analyzed by employing a Wes automated Western blotting system as described in “Materials and Methods”. Band density differences were expressed as percent of control values. Data from 3 to 4 independent experiments are presented. *P < .05, **P < .01 compared with untreated control.
MEHP Attenuates ATP Production by Mouse Leydig Cells With No Effect on Their Proliferation
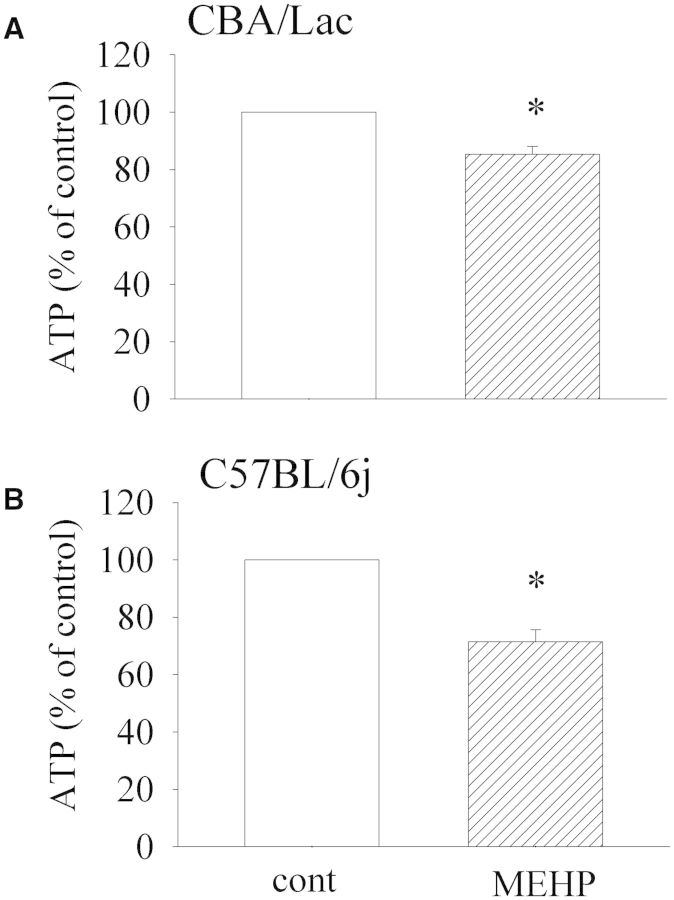
We further investigated the potential of MEHP to affect Leydig cell capacity to produce ATP and affect the proliferative activity. We found that MEHP had no marked effect on Leydig cell proliferation in both mouse genotypes (Supplementary Fig.S3) but significantly suppressed the total concentrations of ATP in these cells (Fig. 4), strongly suggesting that the decline in ATP levels in mouse Leydig cells was the result of suppressive effects of MEHP on mitochondrial function but was not due to a decreasing number of Leydig cells.
FIG. 4.
Effect of MEHP on ATP generation by mouse Leydig cells. Leydig cells from both mouse genotypes were treated with MEHP (90 µM) for 48 h. The levels of ATP were then measured as described in “Materials and Methods”. The means ± SE for 4 independent Leydig cell preparations are shown. *P < .05 compared with untreated control.
MEHP Activates Superoxide Production by Mouse Leydig Cells
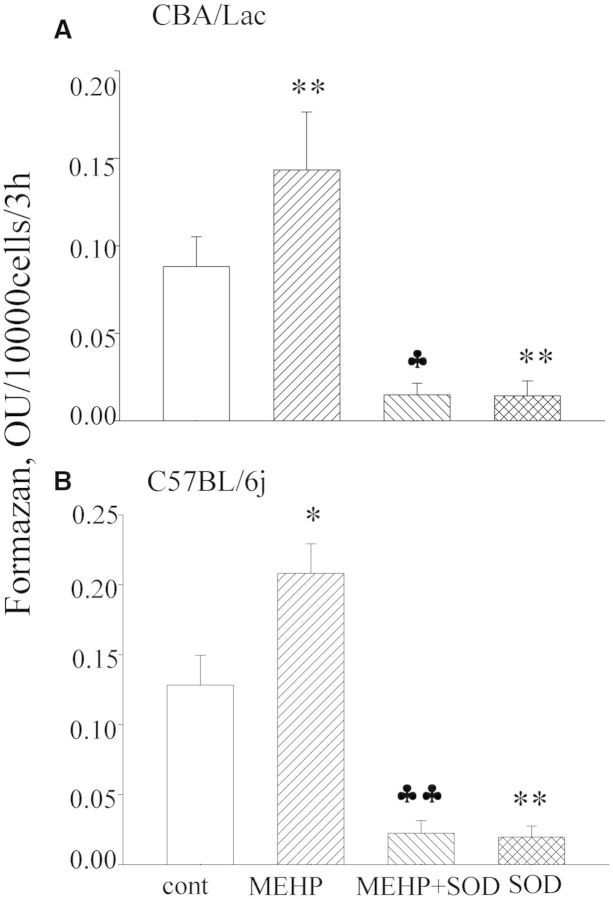
Attenuated ATP production by MEHP-treated mouse Leydig cells let us to suggest that this monophthalate may disturb the activity of the mitochondrial electron transport chain and increase production and secretion of reactive oxygen species (ROS) by defective mitochondria. Therefore, we further explored levels of extracellular ROS production by mouse Leydig cells. We observed that MEHP-treated mouse Leydig cells from both genotypes significantly (1.6-fold over control) increased reduction of WST-1 to formazan (Fig. 5), a process that reflected enhanced generation of superoxide on the cell surface (Berridge et al., 2005). The finding that cotreatment with SOD dramatically suppressed formation of formazan (Fig. 5) confirmed the superoxide selectivity of the method used and strongly supported the suggestion of an active generation of superoxide radicals by MEHP-treated mouse Leydig cells.
FIG. 5.
Superoxide production by mouse Leydig cells treated with MEHP. Leydig cells from both mouse genotypes were treated with MEHP (90 µM) for 48 h. Superoxide production was then estimated by monitoring reduction of WST-1 to formazan in the presence or absence of 20 µg/ml of superoxide dismutase (SOD). Data are expressed as means ± SE for 4 independent Leydig cell preparations. *P < .05, **P < .01 compared with control; ♣P < .05, ♣♣P < .01 compared with MEHP-treated cells.
DISCUSSION
This study has demonstrated that among 3 monophthalates investigated (ie, MEHP, MBP, and MBeP) only MEHP had clear biological effects on mouse Leydig cell function. Responsiveness of the steroidogenic machinery of immature Leydig cells from different mouse genotypes to MEHP was similar and not dependent on their androgen production potential. We observed that MEHP had no significant effect on hCG-activated androgen production but markedly stimulated basal steroidogenesis in immature Leydig cells from both mouse genotypes. Activation of basal steroidogenesis by MEHP was accompanied by significant upregulation of StAR protein expression in these steroidogenic cells. Further, we found that MEHP suppressed mitochondrial function by attenuating ATP production in mouse Leydig cells that was associated with increased production of extracellular superoxide, suggesting disturbance in the function of the mitochondrial electron transport chain. To the best of our knowledge, this finding represents the first example of suppressive effect of MEHP on the energetic state of the mitochondria that is accompanied by an increase in the production of extracellular ROS by developing mouse Leydig cells.
In this study, we observed that among 3 monophthalates tested only MEHP had potential to stimulate steroidogenesis in mouse Leydig cells. These differences in biological action of monophthalates may result from the length and extent of branching in the alkyl chains of monopthalates (Gray et al., 2000). The structural differences in the monophthalates could affect their metabolism as well as their ability to interfere with signaling pathways and transcription factors controlling steroidogenic gene expression.
Our observation that mouse Leydig cells with different steroidogenic potential had similar responsiveness to MEHP strongly suggest that the efficiency of the Leydig cells to produce androgens is not associated with their sensitivity to monophthalate. Further, in this context, one can suggest that signaling pathways and molecular events controlling StAR expression and initiated by MEHP were equally operative in both types of Leydig cells with low and high capacity to produce testosterone and caused the same cellular response.
The mechanism(s) by which MEHP can stimulate steroidogenesis in the Leydig cells is under debate. We reported recently that MEHP stimulated basal steroidogenesis associated with increased StAR protein expression in rat progenitor Leydig cells and immature granulosa cells (Svechnikova et al., 2011). Similarly, low MEHP concentration was shown to slightly stimulate androgen production by MA-10 Leydig cell line with a resulting gene profile similar to that obtained with hCG alone (Fan et al., 2010). Activation of steroidogenesis by MEHP was also demonstrated in mouse Leydig tumor cells (MLTC-1) (Forgacs et al., 2012; Gunnarsson et al., 2008), but this process was StAR independent and associated with an upregulation of the enzymes involved in cholesterol mobilization, suggesting that MEHP may increase the amount of cholesterol available for steroidogenesis (Gunnarsson et al., 2008). The mechanism(s) underlying activation of StAR expression by MEHP in mouse Leydig cells is yet unknown but several hypotheses can be suggested. First, MEHP can stimulate StAR expression via activation of the family of peroxisome proliferator-activated receptors, which was reported to upregulate StAR expression in MA-10 mouse Leydig tumor cells (Kowalewski et al., 2009). Second, increased expression of StAR in MEHP-treated Leydig cells can be induced by ROS, generated by affected mitochondria. This suggestion is supported by the observation that low levels of MEHP (20–200 µM) can elevate ROS production associated with increased StAR expression in primary cultures of rat Leydig cells (Zhao et al., 2012). Further, a recent study has demonstrated a link between an elevated ROS production induced by cAMP analogs and activation of phosphorylation of ERK1/2 in the Leydig cells (Tai and Ascoli, 2011), the MAPKs that upregulate the expression and phosphorylation of StAR in the immature Leydig cells (Martinelle et al., 2004). Thus, one can suggest that MEHP via the activation of the ROS-ERK1/2 signaling pathway could upregulate StAR expression and increase basal androgen production by mouse Leydig cells. Altogether, from the present data we conclude that low concentrations of MEHP are proandrogenic and stimulate basal steroidogenesis in primary cultures of rodent Leydig cells and their tumor cell lines in vitro (Chen et al., 2013; Ge et al., 2007; Gunnarsson et al., 2008; Svechnikova et al., 2011; Zhao et al., 2012).
We also observed that cotreatment of mouse Leydig cells with MEHP and hCG did not increase their capacity to produce testosterone compare with hCG alone. This finding could indicate the presence of mechanism(s) which precisely control the required production of testosterone by the Leydig cells. High levels of androgens released by the Leydig cells activated by MEHP and hCG could downregulate steroidogenic enzyme expression via a feedback mechanism described earlier for StAR (Houk et al., 2004) avoiding the overproduction of testosterone.
It is of importance to note that the concentration of MEHP (90 μM) employed in the present in vitro investigation is relevant to in vivo situations in humans. It has been reported that plasma concentrations of MEHP in infants exposed to exchange transfusions ranged from 2.4 to 15 µg per ml (9–54 µM) (Sjoberg et al., 1985). Further, in vitro data reported in this study are consistent with in vivo findings, showing that exposure to relatively low (ie, 10 and 100 mg/kg) doses of MEHP significantly upregulated the expression of StAR and Cyp17a1 in the testes of prepubertal rats (Lahousse et al., 2006). In line with this, it was demonstrated that exposure of prepubertal rats to low environmentally relevant DEHP levels for 28 days increased their Leydig cells capacity to produce testosterone and elevated the serum levels of the androgen. This was associated with advanced pubertal onset in those animals (Akingbemi et al., 2001; Ge et al., 2007). This observation together with our findings of the pro-androgenic effect of MEHP on developing mouse Leydig cells may indicate a potential of this monophthalate to promote an earlier start of puberty in boys due to increasing levels of circulating testosterone. Similarly, significantly elevated serum levels of phthalates (eg, DEHP, DBP, and MEHP) were found in girls in Puerto Rico with premature breast development, suggesting a possible association between exposure to plasticizers with known estrogenic and antiandrogenic activity and the cause of premature breast development in a human female population (Colon et al., 2000).
Further, we observed that MEHP suppressed ATP production in Leydig cells from both mouse genotypes. This was associated with elevated generation of superoxide at the cell surface, a process indicating mitochondrial dysfunction. The exact cellular events that lead to enhanced generation of extracellular superoxide by mouse Leydig cells are not yet known but one can hypothesize that due to structural alterations in the mitochondrial electron transport chain induced by MEHP, defective mitochondria have an attenuated capacity to oxidize reduced molecules (eg, NADH and FADH2). An excess of these molecules can be released from the mitochondria and transferred to the plasma membrane electron transport chain (PMETC), where the reducing molecules donate electrons and undergo reoxidation (Bedard and Krause, 2007; Morre et al., 2000). The transfer of electrons by the PMETC to oxygen could subsequently result to the generation of superoxide on the cell surface (Berridge et al., 2005). This suggested mechanism is supported by our findings showing that MEHP-treated mouse Leydig cells produce increased levels of superoxide and that addition of SOD completely prevents reduction of WST-1 to formazan. Increased production of highly reactive superoxide by MEHP affected Leydig cells may create oxidative stress in surrounding testicular cells (eg, Sertoli and germ cells) and their elimination via apoptosis in the testis. This mechanism can partly explain the testicular toxicity of MEHP described earlier (Awal et al., 2005). Our observation is also in line with a previous report showing that MEHP can stimulate the generation of ROS by MA-10 Leydig cell line (Fan et al., 2010) due to impairment of mitochondrial function. We also demonstrated that MEHP-treated mouse Leydig cells retained an activated steroidogenesis despite disturbed mitochondrial function. This finding agrees well with a previous study demonstrating that suppression of mitochondrial function by specific mitochondrial toxins increase basal testosterone production by rat Leydig cells (Midzak et al., 2007).
Altogether, our study demonstrated that the efficiency of mouse Leydig cells to produce androgens was not associated with their responsiveness to monophthalates. We demonstrated that of 3 monophthalates investigated (ie, MEHP, MBP, and MBeP) only MEHP affected mouse Leydig cell function. This monophthalate stimulated basal steroidogenesis associated with upregulation of StAR expression and disturbed mitochondrial function in Leydig cells from both mouse genotypes with low and high potential to produce testosterone. We suggest that this effect of MEHP observed in Leydig cells of rodent origin needs to be taken into consideration in the discussion of the causes behind the observed earlier start of puberty in boys and may highlight an influence of phthalates on the reproductive health of males.
FUNDING
This work was supported by the Swedish Research Council; the Finnish Academy; the Children’s Cancer Fund; Frimurare Barnhuset Foundation; Kronprinsessan Lovisas Foundation; the “Sällskapet Barnavård”; the “Stiftelsen Samariten”; the “Stiftelsen Olle Engkvist Byggmästare” and the “Stiftelsen Gunvor och Josef Anérs”.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
REFERENCES
- Akingbemi B. T., Youker R. T., Sottas C. M., Ge R., Katz E., Klinefelter G. R., Zirkin B. R., Hardy M. P. (2001). Modulation of rat Leydig cell steroidogenic function by di(2-ethylhexyl)phthalate. Biol. Reprod 65, 1252–1259. [DOI] [PubMed] [Google Scholar]
- Albert O., Jegou B. (2014). A critical assessment of the endocrine susceptibility of the human testis to phthalates from fetal life to adulthood. Hum. Reprod. Update 20, 231–249. [DOI] [PubMed] [Google Scholar]
- Albro P. W., Chapin R. E., Corbett J. T., Schroeder J., Phelps J. L.. (1989). Mono-2-ethylhexyl phthalate, a metabolite of di-(2-ethylhexyl) phthalate, causally linked to testicular atrophy in rats. Toxicol. Appl. Pharmacol 100, 193–200. [DOI] [PubMed] [Google Scholar]
- Awal M. A., Kurohmaru M., Andriana B. B., Kanai Y., Hayashi Y. (2005). Mono-(2-ethylhexyl) phthalate (MEHP) induces testicular alterations in male guinea pigs at prepubertal stage. Tissue Cell 37, 167–175. [DOI] [PubMed] [Google Scholar]
- Bedard K., Krause K. H. (2007). The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev 87, 245–313. [DOI] [PubMed] [Google Scholar]
- Berridge M. V., Herst P. M., Tan A. S. (2005). Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol. Annu. Rev 11, 127–152. [DOI] [PubMed] [Google Scholar]
- Chen X., Liu Y. N., Zhou Q. H., Leng L., Chang Y., Tang N. J. (2013). Effects of low concentrations of di-(2-ethylhexyl) and mono-(2-ethylhexyl) phthalate on steroidogenesis pathways and apoptosis in the murine leydig tumor cell line MLTC-1. Biomed. Environ. Sci 26, 986–989. [DOI] [PubMed] [Google Scholar]
- Clewell R. A., Campbell J. L., Ross S. M., Gaido K. W., Clewell H. J., III, Andersen M. E. (2010). Assessing the relevance of in vitro measures of phthalate inhibition of steroidogenesis for in vivo response. Toxicol. in vitro 24, 327–334. [DOI] [PubMed] [Google Scholar]
- Colon I., Caro D., Bourdony C. J., Rosario O. (2000). Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environ. Health Perspect 108, 895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dees J. H., Gazouli M., Papadopoulos V. (2001). Effect of mono-ethylhexyl phthalate on MA-10 Leydig tumor cells. Reprod. Toxicol 15, 171–187. [DOI] [PubMed] [Google Scholar]
- Fan J., Traore K., Li W., Amri H., Huang H., Wu C., Chen H., Zirkin B., Papadopoulos V. (2010). Molecular mechanisms mediating the effect of mono-(2-ethylhexyl) phthalate on hormone-stimulated steroidogenesis in MA-10 mouse tumor Leydig cells. Endocrinology 151, 3348–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgacs A. L., Ding Q., Jaremba R. G., Huhtaniemi I. T., Rahman N. A., Zacharewski T. R. (2012). BLTK1 murine Leydig cells: a novel steroidogenic model for evaluating the effects of reproductive and developmental toxicants. Toxicol. Sci 127, 391–402. [DOI] [PubMed] [Google Scholar]
- Ge R. S., Chen G. R., Dong Q., Akingbemi B., Sottas C. M., Santos M., Sealfon S. C., Bernard D. J., Hardy M. P. (2007). Biphasic effects of postnatal exposure to diethylhexylphthalate on the timing of puberty in male rats. J. Androl 28, 513–520. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Jr, Ostby J., Furr J., Price M., Veeramachaneni D. N., Parks L. (2000). Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci 58, 350–365. [DOI] [PubMed] [Google Scholar]
- Gunnarsson D., Leffler P., Ekwurtzel E., Martinsson G., Liu K., Selstam G. (2008). Mono-(2-ethylhexyl) phthalate stimulates basal steroidogenesis by a cAMP-independent mechanism in mouse gonadal cells of both sexes. Reproduction 135, 693–703. [DOI] [PubMed] [Google Scholar]
- Houk C. P., Pearson E. J., Martinelle N., Donahoe P. K., Teixeira J. (2004). Feedback inhibition of steroidogenic acute regulatory protein expression in vitro and in vivo by androgens. Endocrinology 145, 1269–1275. [DOI] [PubMed] [Google Scholar]
- Koch H. M., Preuss R., Angerer J. (2006). Di(2-ethylhexyl)phthalate (DEHP): Human metabolism and internal exposure– an update and latest results. Int. J. Androl 29, 155–165. [DOI] [PubMed] [Google Scholar]
- Kowalewski M. P., Dyson M. T., Manna P. R., Stocco D. M. (2009). Involvement of peroxisome proliferator-activated receptor gamma in gonadal steroidogenesis and steroidogenic acute regulatory protein expression. Reprod. Fertil. Dev 21, 909–922. [DOI] [PubMed] [Google Scholar]
- Lahousse S. A., Wallace D. G., Liu D., Gaido K. W., Johnson K. J. (2006). Testicular gene expression profiling following prepubertal rat mono-(2-ethylhexyl) phthalate exposure suggests a common initial genetic response at fetal and prepubertal ages. Toxicol. Sci 93, 369–381. [DOI] [PubMed] [Google Scholar]
- Martinelle N., Holst M., Soder O., Svechnikov K. (2004). Extracellular signal-regulated kinases are involved in the acute activation of steroidogenesis in immature rat Leydig cells by human chorionic gonadotropin. Endocrinology 145, 4629–4634. [DOI] [PubMed] [Google Scholar]
- Midzak A. S., Liu J., Zirkin B. R., Chen H. (2007). Effect of myxothiazol on Leydig cell steroidogenesis: Inhibition of luteinizing hormone-mediated testosterone synthesis but stimulation of basal steroidogenesis. Endocrinology 148, 2583–2590. [DOI] [PubMed] [Google Scholar]
- Morre D. M., Lenaz G., Morre D. J. (2000). Surface oxidase and oxidative stress propagation in aging. J. Exp. Biol 203, 1513–1521. [DOI] [PubMed] [Google Scholar]
- Savchuk I., Soder O., Svechnikov K. (2013). Mouse leydig cells with different androgen production potential are resistant to estrogenic stimuli but responsive to bisphenol a which attenuates testosterone metabolism. PLoS One 8, e71722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoberg P. O., Bondesson U. G., Sedin E. G., Gustafsson J. P. (1985). Exposure of newborn infants to plasticizers. Plasma levels of di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate during exchange transfusion. Transfusion 25, 424–428. [DOI] [PubMed] [Google Scholar]
- Stocco D. M. (2001). StAR protein and the regulation of steroid hormone biosynthesis. Annu. Rev. Physiol 63, 193–213. [DOI] [PubMed] [Google Scholar]
- Svechnikov K., Landreh L., Weisser J., Izzo G., Colon E., Svechnikova I., Soder O. (2010). Origin, development and regulation of human Leydig cells. Horm. Res. Paediatr 73, 93–101. [DOI] [PubMed] [Google Scholar]
- Svechnikov K., Svechnikova I., Soder O. (2008). Inhibitory effects of mono-ethylhexyl phthalate on steroidogenesis in immature and adult rat Leydig cells in vitro. Reprod. Toxicol 25, 485–490. [DOI] [PubMed] [Google Scholar]
- Svechnikov K. V., Sultana T., Söder O. (2001). Age-dependent stimulation of Leydig cell steroidogenesis by interleukin-1 isoforms. Mol. Cell Endocrinol 182, 193–201. [DOI] [PubMed] [Google Scholar]
- Svechnikova K., Svechnikova I., Soder O. (2011). Gender-specific adverse effects of mono-ethylhexyl phthalate on steroidogenesis in immature granulosa cells and rat leydig cell progenitors in vitro. Front. Endocrinol 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P., Ascoli M. (2011). Reactive oxygen species (ROS) play a critical role in the cAMP-induced activation of Ras and the phosphorylation of ERK1/2 in Leydig cells. Mol. Endocrinol 25, 885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Thomas M. J. (1984). Biological effects of di-(2-ethylhexyl) phthalate and other phthalic acid esters. Crit. Rev. Toxicol 13, 283–317. [DOI] [PubMed] [Google Scholar]
- Wormuth M., Scheringer M., Vollenweider M., Hungerbuhler K. (2006). What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal . 26, 803–824. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Ao H., Chen L., Sottas C. M., Ge R. S., Li L., Zhang Y. (2012). Mono-(2-ethylhexyl) phthalate affects the steroidogenesis in rat Leydig cells through provoking ROS perturbation. Toxicol. in vitro 26, 950–955. [DOI] [PubMed] [Google Scholar]
- Zhou L., Beattie M. C., Lin C. Y., Liu J., Traore K., Papadopoulos V., Zirkin B. R., Chen H. (2013). Oxidative stress and phthalate-induced down-regulation of steroidogenesis in MA-10 Leydig cells. Reprod. Toxicol 42, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.