Abstract
In the present study, a previously established integrated testing strategy (ITS) for in vitro estrogenicity testing was extended with additional in vitro assays in order to broaden its sensitivity to different modes of action resulting in apparent estrogenicity, i.e., other than estrogen receptor (ER) binding. To this end, an extra set of 10 estrogenic compounds with modes of action in part different from ER binding, were tested in the previously defined ITS, consisting of a yeast estrogen reporter gene assay, an U2OS ERα CALUX reporter gene assay and a cell-free coregulator binding assay. Two androgen reporter gene assays and the enhanced H295R steroidogenesis assay were added to that previous defined ITS. These assays had added value, as several estrogenic model compounds also elicited clear and potent antiandrogenic properties and in addition also showed effects on steroidogenesis that might potentiate their apparent estrogenic effects in vivo. Adding these assays, examining mechanisms of action for estrogenicity apart from ERα binding, gives a more complete and comprehensive assessment of the ability of test compounds to interfere with endocrine signaling. It was concluded that the extended ITS will go beyond in vivo estrogenicity testing by the uterotrophic assay, thereby contributing to the 3R-principles.
Keywords: estrogenicity, yeast bioassay, CALUX bioassay, coregulators, MARCoNI, H295R steroidogenesis assay, high-throughput in vitro methods
Testing chemicals for their endocrine-disrupting potential, including interference with estrogen receptor signaling, is an important factor to be taken into account when assessing the safety of chemicals. Presently, the standard test for disruption of normal estrogen function is the Allen and Doisy test, a 3-day uterotrophic assay in immature or ovariectomised rodents with uterus weight as the crucial read-out parameter (OECD, 2007; Owens and Ashby, 2002). Due to the high costs, ethical objections and labor intensiveness of the in vivo uterotrophic assay, the development of an in vitro battery for the prediction of in vivo estrogenicity has high priority. It was previously demonstrated that combination of a yeast or U2OS estrogen receptor α (ERα) reporter gene assay with a cell-free coregulator binding assay, would have the potential to form an adequate part of an integrated testing strategy (ITS) for in vitro estrogenicity testing in order to replace the in vivo uterotrophic assay (Wang et al., forthcoming; Wang et al., 2013). However, also the uterotrophic assay has its limits for estrogenicity testing, e.g., because it does not include estrogenicity due to effects on the hypothalamic-pituitary-gonadal (HPG) axis, male organs or steroidogenesis, because the assay uses either ovariectomized or immature rodents, i.e., female animals without endogenous estrogen production. Additional in vivo assays, e.g., the female and male pubertal assays (OPPTS 890.1450 and OPPTS 890.1500, respectively), are needed to achieve more comprehensive estrogenicity screening, and provide data on multiple mechanisms and organs. Indeed, the shortcoming of the uterotrophic assay has been demonstrated by the fact that several compounds, such as atrazine and vinclozolin, failed to show a uterotrophic effect, whereas eliciting (anti)estrogenic effects in other in vivo tests. The herbicide atrazine delayed puberty and sexual development in both male and female rodents (Laws et al., 2000a; Stoker et al., 2000). The fungicide vinclozolin has been shown to be an endocrine disruptor with antiandrogenic effects and vinclozolin-treated rat male off-spring display female-like anogenital distance at birth, retained nipples, cleft phallus with hypospadias, a blind vaginal pouch and small to absent sex accessory glands (Gray et al., 1994; Ostby et al., 1999). In addition, some compounds even have combined modes of action, e.g., several estrogenic compounds like 17α-ethinyl estradiol (EE2) and diethylstilbestrol (DES) are also antiandrogenic, and in this way might enhance the overall ‘estrogenic’ effect in vivo (Bovee et al., 2010; Gross-Sorokin et al., 2006; Toorians et al., 2010). It has been shown that EE2 is ∼25 times more potent compared with E2 in a zebrafish model (Cosnefroy et al., 2012), whereas in transcription activation assays based on subtypes of zebrafish ER, EE2 has about the same estrogenic potency as E2 (Van den Belt et al., 2004). EE2 has also been shown to be one of the main substances responsible for the widespread feminization of male fish in rivers, due to its combined estrogenic and antiandrogenic activities (Filby et al., 2007; Gross-Sorokin et al., 2006). The previously established ITS correlates well with the in vivo uterotrophic assay, but is expected to suffer from the same drawbacks as the uterotrophic assay, as the selected in vitro bioassays only measure effects directly mediated by ERα, and are not able to detect chemicals that elicit their estrogenic effects through indirect mechanisms, such as, alteration of hormone biosynthesis, hormone metabolism and transport, and mixed estrogenic/antiandrogenic effects. This emphasizes the need to extend the previously established in vitro ITS, by including androgen reporter gene assays (Bovee et al., 2010; van der Burg et al., 2010a) and the H295R steroidogenesis assay (Rijk et al., 2012). Such an extended ITS would have a clear advantage in that it would surpass the uterotrophic assay with respect to the multitude of mechanisms interfering with estrogen signaling pathways that can be detected. As such, the extended ITS would allow a further reduction or eventually even an elimination of the need for testing certain endocrine disrupting effects in animal models.
The aim of the present study was to demonstrate whether the extension of the previously established ITS for in vitro estrogenicity testing by adding two androgen reporter gene assays as well as the enhanced H295R steroidogenesis assay goes beyond an ultimate replacement of the in vivo uterotrophic assay, i.e., is able to detect estrogenic effects, antiandrogenic effects, and effects on steroidogenesis as observed in intact animals in the female and male pubertal assays.
Materials and Methods
Chemicals
Atrazine, bisphenol A (BPA), bisphenol B (BPB), bisphenol C1 (BPC1), bisphenol C2 (BPC2), butyl paraben, equilin, 17β-estradiol (E2), flutamide, mestranol, tamoxifen, vinclozolin, 4-dimethyl-aminopyridine, 2-methyl-6-nitrobenzoic anhydride, picolinic acid, triethylamine, tetrahydrofuran, Dulbecco's modified Eagle medium/Ham's F-12 nutrient mix (DMEM/F12), NaHCO3 and PBS were obtained from Sigma-Aldrich Chemie B.V. (Zwijndrecht, The Netherlands). Diethylstilbestrol monomethyl ether (DES-ME) was purchased from LGC Standards GmbH (Wesel, Germany). Ammonia, acetic acid, formic acid, and dimethyl sulfoxide (DMSO) were obtained from Merck (Darmstadt, Germany). Methanol, acetonitrile, acetone, and ethanol were from Biosolve (Valkenswaard, The Netherlands). Pregnenolone, 17α-OH-pregnenolone, progesterone, 17α-OH-progesterone, dehydroepiandrosterone (DHEA), androstenedione, testosterone (T), estrone, 11-deoxycorticosterone, corticosterone, 11-deoxycortisol, cortisol, and dihydrotestosterone (DHT) were obtained from Steraloids (Newport, RI). The deuterium-labeled internal steroid standards were from CDN isotopes (Point-Claire, Canada) and ITS+ premix and NuSerum from BD Biosciences (Bedford, MA). Chemicals to prepare the growth media for yeast were described previously (Bovee et al., 2009). Milli-Q water was obtained using a Purelab Ultra system from Elga (Bucks, UK).
CALUX bioassays
The ERα and AR agonist or antagonist potencies of a test compound were determined in the ERα and AR CALUX bioassays, respectively, as described previously (van der Burg et al., 2010a,b). In short, CALUX cells were plated in 96-well plates with phenol red-free DMEM/F12 (1:1) mixture supplemented with charcoal stripped Fetal Bovine Serum (csFBS). One day later, the medium was refreshed and cells were incubated with the compounds to be tested (final DMSO concentration: 0.1%). Each test compound at each concentration was tested in triplicate. After 24 h exposure, the medium was removed, cells were lysed in Triton lysis buffer, and luciferase activity was measured. To test for antagonism, CALUX cells were incubated with test compound in combination with a nonsaturating level of agonist (around the EC50 value of the reference compound), i.e., 10−11M E2 in the ERα CALUX bioassay, and 2 × 10−10M DHT in the AR CALUX bioassay.
Yeast estrogen and androgen bioassays
The estrogenic, antiestrogenic, androgenic, and antiandrogenic properties of the compounds were tested as described previously (Bovee et al., 2008, 2010). In short, cultures of the yeast estrogen or androgen biosensor were grown overnight at 30°C with vigorous orbital shaking. At the late log phase, the culture was diluted in selective MM/L medium till an OD value at 630 nm between 0.04 and 0.06 was reached. To expose the yeast cells, 200 μl aliquots of the diluted culture were pipetted into each well of a 96-well plate and 2 μl of a stock solution in DMSO was added when testing the agonistic properties of the compounds (final DMSO concentration: 1%). To test for antagonism, yeast cells were incubated with test compound in combination with a nonsaturating level of agonist (around the EC50 value of the reference compound), i.e., 10−9M E2 in the yeast estrogen bioassay, and 7 × 10−9M testosterone (T) in the yeast androgen bioassay. DMSO (negative control), and E2 or T (positive controls) were included in each experiment, and each sample concentration was assayed in triplicate. Exposure was performed for 24 h at 30°C with orbital shaking at 125 rpm. Fluorescence was measured at 0 and 24 h directly in a Synergy HT Multi-Detection Microplate Reader (BioTek Instruments Inc.) using excitation at 485 nm and emission at 530 nm. Normally, the cell density at 630 nm increases from 0.05 at 0 h to ∼0.9 at 24 h. If the OD at 24 h was <0.7, concentrations of the test compound were considered to cause cytotoxicity and the corresponding data were rejected for analysis.
Microarray assay for real-time coregulator-nuclear receptor interaction (MARCoNI)
Ligand-modulated interaction of ERα-LBD-His with coregulators was assessed using a PamChip plate (PamGene International B.V., 's-Hertogenbosch, The Netherlands) containing 96 identical peptide microarrays as described previously (Aarts et al., 2013; Houtman et al., 2012; Koppen et al., 2009). The peptide microarray was incubated with the test solution containing ERα-LBD-His in the absence or presence of ligand by pumping the sample up and down the three-dimensional metal oxide carrier. In short, assay mixtures were prepared on ice in a master 96-well plate and contained ERα-LBD-His (optimal assay concentration of a crude lysate containing ERα-LBD-His was empirically determined, and estimated to lie between 1 and 10 nM), 25 nM of an Alexa Fluor 488-conjugated polyhistidine antibody (penta-His Alexa Fluor 488 conjugate, Qiagen no. 35310), and ligand at the indicated concentration in reaction buffer (20mM Tris–HCl, pH 7.5, 500mM NaCl, 0.2% BSA, 0.05% Tween-20). All assays were performed in a fully automated microarray processing platform (PamStation96, PamGene International B.V.) at 20°C applying two incubation cycles per minute. For each compound, eight concentrations added from 50-fold concentrated stock solutions with fivefold serial dilutions in DMSO were tested in singular (final DMSO concentration: 2%). After removal of the unbound receptor by washing each array with 25 μl Tris-buffered saline, tiff images were obtained by the CCD camera which is part of the PamStation96 platform. The tested compounds were distributed over one plate run using 2% DMSO as the negative control and 50μM E2 as the positive control (each control measured in four technical replicates).
Enhanced H295R steroidogenesis assay
Human H295R adrenocarcinoma cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and were cultured according to the protocol described in the OECD test guideline 456. Cells were routinely grown at 37°C under 5% CO2 atmosphere in 75 cm2 culture flasks containing 12 ml DMEM/F12 culture medium supplemented with 1.2 g/l NaHCO3, 1% ITS+ premix, and 2.5% NuSerum. For subculturing, the H295R cells were washed three times with PBS, detached by trypsin/EDTA (0.25%/0.05% (vol/vol) in Hank's Balanced Salt Solution (HBSS)) and seeded in a 1:3 ratio. For testing, 1 ml cell suspension containing 2 × 105 to 3 × 105 cells was seeded in each well of the 24-well plate. After 24 h, the medium was refreshed and compounds dissolved in DMSO (1 μl) were added. Exposures were performed in triplicate and the final concentration of the solvent carrier DMSO was 0.1%. After 48 h of exposure, the medium was stored at −80°C for steroid hormone analysis. Effects on viability and cytotoxicity were evaluated by the live/dead viability/cytotoxicity kit (Molecular Probes, Eugene, OR) using the protocol described by the OECD (OECD TG 456). After washing the cells with PBS twice, a calcein and ethidium bromide solution was added to the cells. After 1 h, fluorescence was measured (excitation/emission at 530 nm/645 nm and excitation/emission at 485 nm/530 nm) using a Synergy HT multi-detection microplate reader (BioTek Instruments Inc.). Exposures showing a decrease in cell viability were excluded from hormone analysis.
For hormone analysis, 900 μl H295R medium aliquots were adjusted to 1 ml with 18 μl of deuterium labeled internal steroid standard mix and Milli-Q water. Similarly, a standard steroid curve was prepared by spiking 900 μl of supplemented DMEM-F12 medium with a mix of steroid standards, resulting in final concentrations of 10, 25, 50, 100, 250, 500, 1000, 2500, 5000, 10,000, and 25,000 pg/ml. Next, both standards and samples were subjected to solid-phase extraction (SPE) using OASIS HLB cartridges (Waters, 60 mg) in 96-wells format, previously conditioned with 1 ml methanol and 1 ml Milli-Q water. Washing was carried out with subsequently 1 ml Milli-Q water, 1 ml of methanol/water/acetic acid (55%:43%:2%, vol/vol/vol), 1 ml of methanol/water/25% ammonia (30%:62%:8%, vol/vol/vol), 1 ml Mill-Q water and 1 ml acetonitrile/water (35%/65%, vol/vol). The free steroids were eluted with 1 ml acetone and this eluate was evaporated to dryness at 45°C under nitrogen. The derivatizing reagent was prepared freshly before use by mixing 1 mg 4-dimethyl-aminopyridine, 5 mg 2-methyl-6-nitrobenzoic anhydride, and 3 mg picolinic acid in 1 ml tetrahydrofuran, after which 10 μl of triethylamine was added. Picolinoyl derivatization was achieved by incubating the dried sample extracts with 35 μl of derivatization reagent for 45 min at room temperature. The reaction was terminated by adding 50 μl of a 5% ammonia solution after which the samples were analyzed directly by ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) according to the method described by Rijk et al. (in preparation). Steroid hormone concentrations in the samples were calculated using the steroid standard reference line, constructed by plotting the peak area ratios versus the spiked concentration. For some samples, the estradiol levels were below the UPLC-MS/MS detection limit and these samples were further analyzed by an enzyme-linked immunosorbant assay according to the manufacturer's protocol (Oxford Biomedical Research, Oxford, MI).
Data analysis
For the CALUX bioassays, luciferase activity per well was measured as relative light units (RLU). Fold induction was calculated by dividing the mean value of light units from exposed and nonexposed (DMSO control) wells. For the yeast bioassays, the fluorescence signals were corrected for the signal obtained with the MM/L medium containing DMSO solvent only. The relative estrogenic potency (REP), defined as the ratio of the concentration of E2 needed to achieve 50% of maximal response (EC50) and the concentration of the test compounds required to achieve a similar effect multiplied by 100, was calculated from fitted dose-response curves (four parameter sigmoidal dose-response curve, Graphpad Prism software version 5.04). The REP value for E2 is, hereby, set at 100 for both the CALUX and the yeast ERα reporter gene assays. The relative transactivation activity (RTA) of each compound tested was calculated as the ratio of maximal luciferase or yEGFP reporter gene induction values of each compound and the maximal induction value of reference compound E2. The antiandrogenic activity was characterized by the IC50, i.e., the concentration that inhibited the response of DHT or T by 50%.
For the coregulator binding assay, image analysis was performed using BioNavigator software (PamGene International B.V.) as described previously (Aarts et al., 2013). In short, the boundaries of a spot are determined and the median fluorescent signal was quantified within the spot (signal) as well as in a defined area surrounding it (background). The signal-minus-background value was subsequently used as the quantitative parameter of binding. Ligand dose-response curve fitting and hierarchical clustering (Euclidean distance, average linkage) were performed using the drc and stats packages in R (version 2.12.0, www.r-project.org). A sigmoidal, 4-parameter Hill (logistic) model was fitted to the dose-response data and the goodness-of-fit parameter and EC50 values as calculated by the drc package were recorded.
Fold changes in steroids levels in the H295R steroidogenesis assay were calculated by comparing the mean steroid levels of the DMSO solvent control (SC) versus the mean steroid levels in medium of H295R cells exposed to the compound under investigation. A one-tailed Student's t-test was used to test for significance. For comparison of the presented in vitro data with estrogenicity in vivo, uterotrophic assay data were used that were derived from the Endocrine Disruptor Knowledge Base (EDKB), designed and produced by the National Center for Toxicological Research (NCTR, USA) (Ding et al., 2010).
Results
Estrogenic and Antiestrogenic Activities
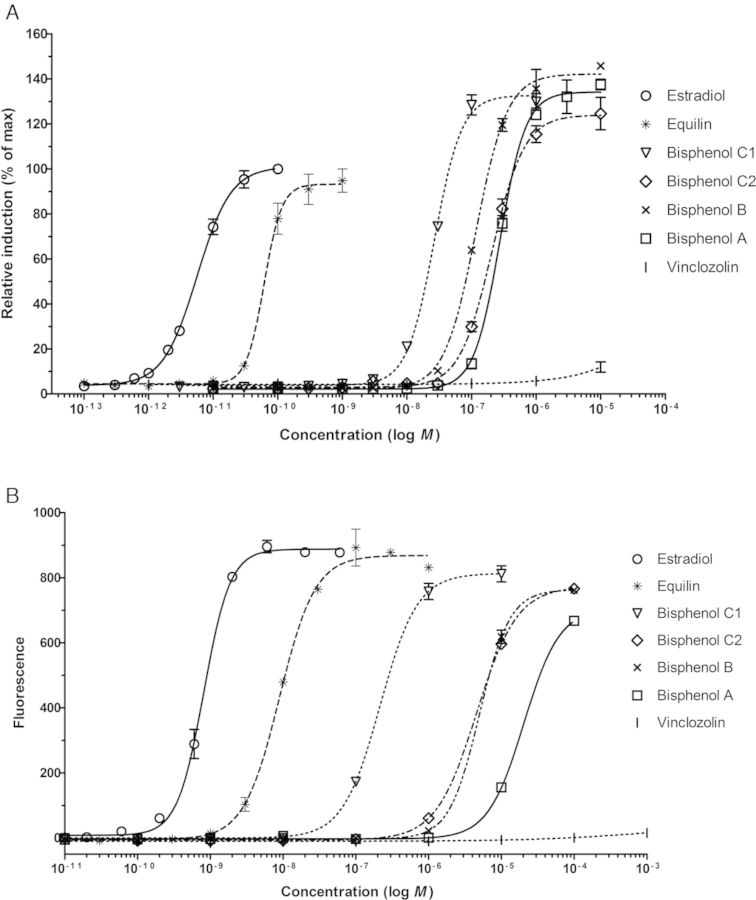
Ten compounds were selected, based on their ability to disrupt normal estrogen action by different modes of action, and tested in the U2OS ERα CALUX and the yeast estrogen bioassay for estrogenic and antiestrogenic activities. Figure 1 shows typical dose-response curves for several of the test compounds as obtained in these two assays. The EC50 and relative transcriptional activity (RTA) values of each compound were calculated from the fitted dose-response curve and are shown in Table 1. The reference compound E2 was the most potent ER agonist and showed an EC50 of 8.8 × 10−12 and 8.7 × 10−10M in the U2OS ERα CALUX and the yeast estrogen bioassay, respectively. Equilin, mestranol, and DES ME were ∼2–30 times less potent compared with E2 and the obtained RTA values were between 80 and 105% (E2 being 100%). All four phenolic compounds showed full agonistic responses in the two reporter gene assays (Fig. 1 and Table 1), with BPC1 being the most potent phenolic compound and BPA being the weakest in both assays. Butyl paraben was clearly positive in the yeast and U2OS cell based estrogen bioassays and induced a much higher maximal response (RTA = 256%) than E2 in the U2OS ERα CALUX assay. The fungicide vinclozolin was slightly active (RTA < 10%) in both reporter gene assays, but no EC50 value could be calculated as it only showed a response at the highest concentration tested. The herbicide atrazine was not active in these two reporter gene assays. All the compounds were tested in combination with E2 for potential antagonist activities, but no ER-antagonism was observed for any of the compounds tested (data not shown).
FIG. 1.
Dose-response curves of the indicated test compounds in the ERα CALUX (A) and yeast estrogen bioassay (B). The response is displayed as the mean ± SD of a triplicate measurement.
TABLE 1. Estrogenic Activities of the Test Compounds in the In Vivo Uterotrophic Bioassay, U2OS ERα CALUX Assay, Yeast Estrogen Bioassay, and ERα Coregulator Binding Assay.
| Compound | CAS no. | Uterotrophic assay log RPa | U2OS ERα CALUX bioassay | Yeast estrogen bioassay | ERα coregulator binding assay | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| EC50 (M) | log REP | RTA (%) | EC50 (M) | log REP | RTA (%) | Median EC50e/IC50f (M) | log RBP | |||
| Estradiol | 50-28-2 | 2.0 | 8.8 × 10−12 | 2.0 | 100 | 8.7 × 10−10 | 2.0 | 100 | 7.5 × 10−9e | 2.0 |
| Equilin | 474-86-2 | 1.7 | 6.5 × 10−11 | 1.1 | 95 | 8.7 × 10−09 | 1.0 | 105 | 3.4 × 10−8e | 1.3 |
| Mestranol | 72-33-3 | 2.0 | 3.2 × 10−10 | 0.4 | 84 | 7.9 × 10−09 | 1.0 | 88 | 5.1 × 10−7e | 0.2 |
| DES-ME | 7773-60-6 | 1.9 | 2.4 × 10−10 | 0.6 | 88 | 1.8 × 10−09 | 1.7 | 94 | 1.7 × 10−7e | 0.6 |
| Bisphenol A | 80-05-7 | −1.6 | 2.7 × 10−07 | −2.5 | 136 | 2.0 × 10−05 | −2.4 | 76 | — | −5.0 |
| Bisphenol B | 77-40-7 | +b | 1.2 × 10−07 | −2.1 | 144 | 5.0 × 10−06 | −1.8 | 87 | 3.2 × 10−6f | na |
| Bisphenol C1 | 14868-03-2 | NAc | 2.7 × 10−08 | −1.5 | 133 | 2.2 × 10−07 | −0.4 | 93 | 2.7 × 10−8f | na |
| Bisphenol C2 | 79-97-0 | NA | 2.1 × 10−07 | −2.4 | 125 | 4.5 × 10−06 | −1.6 | 87 | 7.4 × 10−4f | na |
| Butyl paraben | 94-26-8 | + | 2.9 × 10−06 | −3.5 | 256 | 5.1 × 10−06 | −1.8 | 86 | — | −5.0 |
| Atrazine | 1912-24-9 | −5.0d | —b | −5.0 | 1 | — | −5.0 | 1 | — | −5.0 |
| Vinclozolin | 50471-44-8 | −5.0 | — | −5.0 | 8 | — | −5.0 | 4 | — | −5.0 |
aMedian relative potency values based on the uterotrophic assay in mouse or rat, derived from EDKB (NCTR, USA) (Ding et al., 2010). Estradiol is used as a reference chemical and is defined to have a relative potency of 100 (log RP = 2.0).
b+ = positive; − = negative.
cNA = not available; na = not applicable.
dA cut-off value of −5.0 is listed for compounds showing no effect.
Coregulator-Nuclear Receptor Interaction
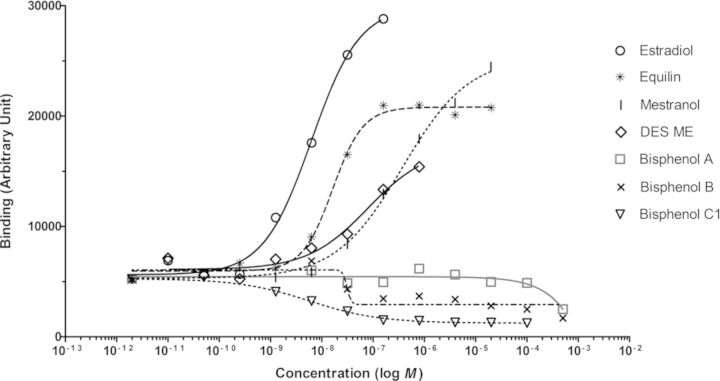
The 10 compounds were also tested in the coregulator binding assay to evaluate their capacity to modulate ERα-LBD binding to NR-coregulator motifs. As an example, Fig. 2 shows the induction of ERα-LBD-His binding to a peptide representing the NR-binding motif within amino acid 677–700 of the well-known nuclear receptor coactivator 1 (NCOA1_677_700) by several test compounds, i.e., single measurements of eight concentrations per compound on 1 of the 154 unique coregulator peptides on the array. The lowest concentration of E2 resulting in a detectable binding of ERα-LBD-His to NCOA1_677_700 was 1.3 × 10−9M, and a half maximal binding level (EC50) was reached at ∼6.4 × 10−9M. Equilin, mestranol and DES-ME also induced binding of ERα-LBD to NCOA1_677_700, whereas the bisphenol compounds displayed antagonist binding curves on NCOA1_677_700 when compared with the solvent control DMSO and the known ER-agonists (Fig. 2).
FIG. 2.
Binding of ERα-LBD-His to the coregulator binding motif peptide NCOA1_677_700 as function of the concentration of the indicated compounds measured in the ERα-coregulator binding assay.
Dose-response curves were generated for all 154 coregulator-derived NR-binding motifs for each compound in order to obtain an overall measure for the potency as an inducer of ERα-coregulator binding. These dose-response curves allowed to determine EC50 values for each compound on each coregulator peptide. The median EC50 value of each compound was determined over 48 coregulator peptides that showed a comparatively good fit of the applied dose-response model (goodness-of-fit of the four parameter Hill equation above 0.9) and are shown in Table 1. The selection of these coregulators was based on a previous study where the potency of compounds to induce binding of ERα-LBD-GST to these coregulators was demonstrated to correlate well (R2 ≥ 0.80) with their in vivo determined estrogenic potency in the uterotrophic assay (Wang et al., 2013). Equilin, mestranol, and DES-ME resulted in coregulator binding profiles similar to that of E2 and the calculated median EC50 values were in the nanomolar range. BPA showed a slightly bell-shaped dose-response binding profile on several coregulators, i.e., inducing weak binding to coactivators at low concentrations, but inhibitory effects (lower binding compared with the DMSO control) at high concentrations (Fig. 2). In contrast, BPB, BPC1, and BPC2 strongly inhibited binding of ERα-LBD to almost all coactivator peptides on the peptide microarray, however, no increased binding was observed for any of the compounds on the corepressors present on the peptide microarray (see Supplementary fig. 1 for BPC1 as an example). To provide an overall measure of the antagonistic binding potency for the bisphenols, the median IC50 values were calculated and are shown in Table 1. Vinclozolin showed no statistically significant altered binding signals compared with the DMSO solvent control. Butyl paraben was negative in the concentration range tested in a previous study using the ERα coregulator binding assay (20nM−400μM) (Aarts et al., 2013) and higher concentrations of butyl paraben as tested in the current study led to precipitation in the assay buffer and resulted in an overall negative binding profile. Atrazine was also tested in our previous study (negative) and the results for the coregulator binding assay were taken from that study. For each test compound and each of the 154 coregulator-nuclear receptor binding motifs represented by the peptide microarray, the modulation index (MI) was calculated as the log-transformed ratio of receptor binding at a saturating compound concentration over that in the absence of ligand. The MIs for the 154 binding motif peptides constitute an ERα-coregulator binding profile for each compound tested. Unsupervised hierarchical clustering of these MI values over the various compounds over all coregulator peptides on the array revealed that compounds with structural similarity tend to cluster together (Fig. 3).
FIG. 3.
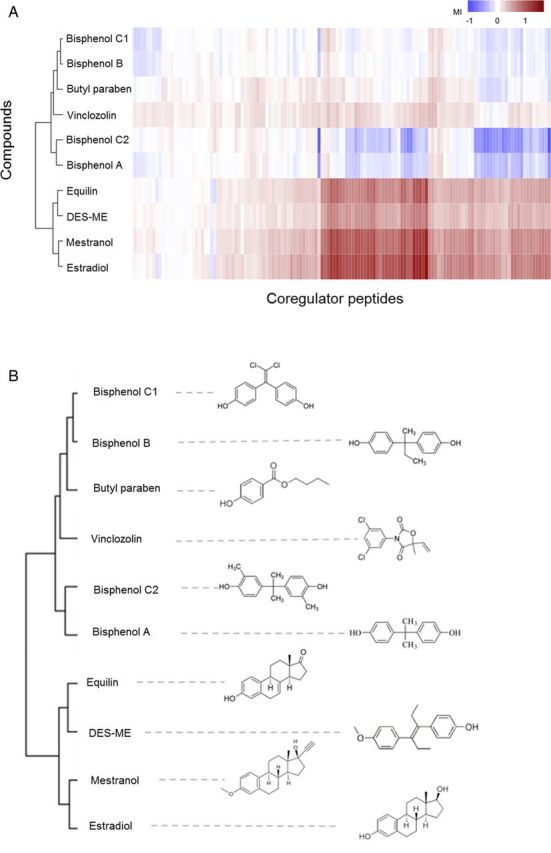
Heatmap of the unsupervised hierarchically clustered values for modulation of ERα-coregulator binding across compounds and coregulators shows structural similarity of compounds. (A) Two-dimensional clustering heatmap. (B) Clustering pattern of compounds.
Comparison with the In Vivo Uterotrophic Assay
In general, the U2OS ERα CALUX and the yeast estrogen bioassay showed 100% concordance with the uterotrophic assay, i.e., no misclassification on the eight compounds with known estrogenicity in vivo (Table 1). To compare the determined relative estrogenic potencies of the compounds with their uterotrophic potencies in vivo, the relative estrogenic potencies (REP) or relative binding potencies (RBPs) of these compounds were calculated on the basis of the obtained EC50 values (Table 1). The estrogenic potencies obtained from the yeast estrogen bioassay and the U2OS ERα CALUX assay showed very good correlations with the potency outcomes of the in vivo uterotrophic assay, i.e., in both cases R2 = 0.99 (p < 0.0001, n = 6). Because no in vivo estrogenic potency data were available for butyl paraben, BPB, BPC1, and BPC2, these four compounds were excluded from correlation analysis. In addition, the U2OS ERα CALUX assay and the yeast estrogen bioassay resulted in similar potency ranking of the 10 compounds and the correlation between the relative potency values as obtained from these two in vitro assays was excellent (R2 = 0.93, p < 0.0001, n = 10).
In the ERα coregulator binding assay, butyl paraben was classified as negative. BPA, BPB, BPC1, and BPC2 showed antagonistic binding profiles and can therefore not be compared with the agonistic effects as observed in the uterotrophic assay or in the reporter gene assays. This assay gave a lower correlation (R2 = 0.86, p < 0.0001, n = 6) and lower concordance (83%; 5/6) with the in vivo uterotrophic assay. However, the potency ranking of equilin, mestranol, and DES-ME in the ERα coregulator binding assay was the same as in the U2OS ERα CALUX assay.
Antiandrogenic Activities
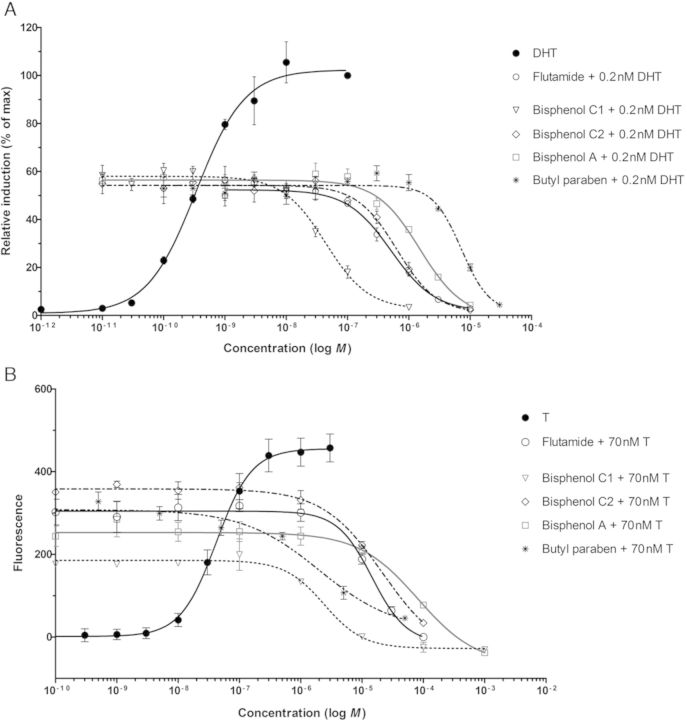
No androgen agonism was observed for any of the compounds tested (data not shown). The potential antiandrogenic activity of the test compounds was studied by coexposure of each compound with DHT or T in the AR CALUX assay or the yeast androgen bioassay, respectively. Figure 4 shows the antagonistic effects of several of the compounds tested. The potent androgen antagonist flutamide was used as positive control in both assays. Vinclozolin, BPC1, and BPC2 showed potent antiandrogenic activities in both reporter gene assays (Fig. 4 and Table 2). Although less potent, also BPA and butyl paraben inhibited the response induced by the potent androgens (DHT or T) to the baseline level. Atrazine, equilin, mestranol, and DES-ME only showed weak antiandrogenic activities at relative high concentrations. Due to the poor fitting of the applied dose-response model to the data, no IC50 value could be calculated for these four compounds.
FIG. 4.
Antagonistic effect of several compounds, including the reference antiandrogen flutamide, measured in the AR CALUX (A) and the yeast androgen bioassay (B). Signals are displayed as the mean ± SD of triplicate measurements.
TABLE 2. Antiandrogenic Activities of the Test Compounds in the U2OS AR CALUX and Yeast Androgen Bioassay.
| Compound | U2OS AR CALUX assay IC50 (M) | Yeast androgen bioassay IC50 (M) |
|---|---|---|
| Equilin | +a | + |
| Mestranol | + | + |
| DES-ME | + | + |
| Bisphenol A | 1.5 × 10−06 | 8.1 × 10−05 |
| Bisphenol B | 9.3 × 10−07 | −a |
| Bisphenol C1 | 4.3 × 10−08 | 2.4 × 10−06 |
| Bisphenol C2 | 6.3 × 10−07 | 2.2 × 10−05 |
| Butyl paraben | 7.1 × 10−06 | + |
| Atrazine | + | + |
| Vinclozolin | 4.8 × 10−08 | 1.9 × 10−06 |
| Flutamideb | 5.1 × 10−07 | 1.5 × 10−05 |
a+ = positive; − = negative.
bFlutamide was used as a positive control.
Modulation of Steroidogenesis
Effects on steroidogenesis were evaluated using the enhanced H295R steroidogenesis assay. The effects of seven of the model compounds were assessed by measuring levels of 13 steroids in medium of H295R cells, i.e., for each of these 13 steroid hormone intermediates a dose-response bar graph was constructed upon exposure to these seven compounds. Figure 5B, e.g., shows the BPA dose-dependent changes in these 13 hormone levels in medium of H295R cells being exposed for 48 h. Exposing H295R cells to increasing concentrations of BPA resulted in increased levels of pregnenolone, progesterone, and estradiol, whereas levels of 17α-OH-progesterone, 11-deoxycortisol, cortisol, androstenedione, and testosterone decreased dose-dependently. Levels of the other steroids were not significantly affected by BPA. Among the eight steroids modulated by BPA, androstenedione, and testosterone were the two most sensitive endpoints with a lowest observed effective concentration (LOEC) of 0.03 and 0.1μM BPA, respectively.
FIG. 5.
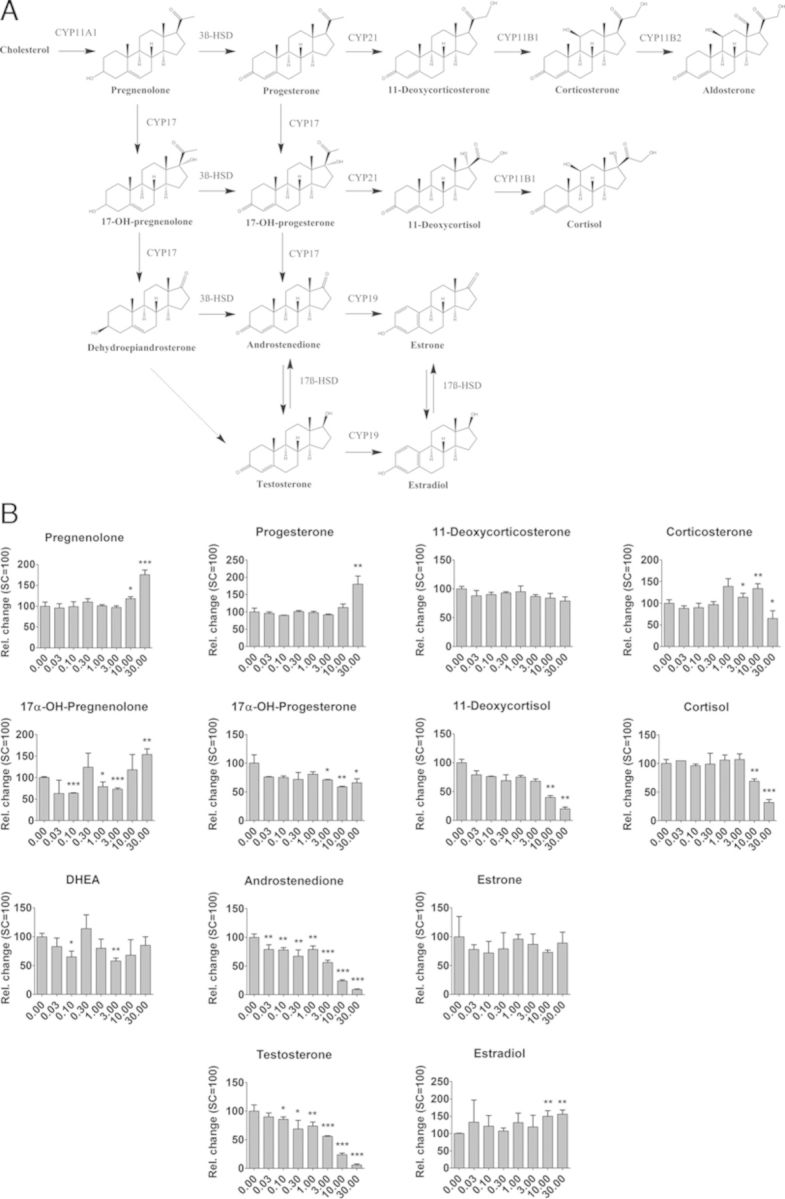
Enhanced H295R steroidogenesis assay. (A) Steroid biosynthesis pathway. (B) Changes in hormone levels in medium of H295R cells exposed to BPA. Changes in hormone levels are expressed relative to the DMSO solvent control (mean ± SD, n = 3). Statistical significance: *p < 0.05, **p < 0.01, and ***p < 0.001.
A summary of the results of all compounds tested in the H295R steroidogenesis assay is presented in Table 3, showing the LOEC together with the maximal fold change of induction or inhibition. The hormone profile caused by BPB was similar to that caused by BPA, showing a dose-dependent decrease in androgens (androstenedione and testosterone) and glucocorticoids (11-deoxycortisol and cortisol), and an increase in pregnenolone, progesterone, and less pronounced, in estrone. BPC1 resulted in a dose-dependent increase in levels of pregnenolone, progesterone, 17α-OH-progesterone, and estradiol with an LOEC of 10, 0.3, 0.3, and 10μM, respectively. With the exception of testosterone and estrone, levels of all other steroids were decreased by BPC1. BPC2 resulted in a dose-dependent decrease in the levels of most steroid hormones, whereas progesterone and estradiol were up-regulated, but only at the highest nontoxic test-concentration of BPC2 (10μM). The highest concentration of BPC2 (30μM) was cytotoxic for the H295R cells (data not shown). In general, it is observed that all four bisphenolic compounds show a dose-dependent decrease in androgens, whereas estrogen levels increased. Exposure of H295R cells to the fungicide vinclozolin resulted in a maximum 1.99-fold increase in estradiol (LOEC of 10μM), whereas levels of progesterone, 17α-OH-progesterone, androstenedione, testosterone, 11-deoxycorticosterone, and 11-deoxycortisol were down-regulated by vinclozolin. Exposure to butyl paraben resulted in a dose-dependent increase in levels of pregnenolone, progesterone, 17α-OH-progesterone, and estradiol, whereas levels of corticosterone, 11-deoxycortisol, androstenedione, and testosterone decreased. Atrazine increased the levels of eight steroids and resulted in a maximum 4.70-fold increase in the estradiol level (LOEC of 1μM).
TABLE 3. Effects on Steroid Hormone Production in Exposed H295R Cells.
| BPA | BPB | BPC1 | BPC2 | Vinclozolin | Butyl paraben | Atrazine | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOECa (μM) | Maxb change | LOEC (μM) | Max change | LOEC (μM) | Max change | LOEC (μM) | Max change | LOEC (μM) | Max change | LOEC (μM) | Max change | LOEC (μM) | Max change | |
| Pregnenolone | 10 | 1.76 | 10 | 1.97 | 10 | 1.70 | 3 | 0.38 | NE | — | 30 | 2.46 | 10 | 1.40 |
| 17α-OH-pregnenolone | Eq.c | — | NE | — | 0.1 | 0.25 | 0.3 | 0.17 | NE | — | NE | — | NE | — |
| Progesterone | 30 | 1.80 | 3 | 2.41 | 0.3 | 6.35 | 10 | 1.30 | 3 | 0.66 | 10 | 3.72 | 1 | 2.01 |
| 17α-OH-progesterone | 3 | 0.71 | 1 | 1.69 | 0.3 | 5.98 | Eq. | — | 3 | 0.53 | 1 | 4.39 | 0.3 | 1.83 |
| DHEA | Eq. | — | 10 | 0.56 | 1 | 0.15 | Eq. | — | Eq. | — | NE | — | NE | — |
| Androstenedione | 0.03 | 0.09 | 3 | 0.19 | 10 | 0.13 | 3 | 0.09 | 10 | 0.72 | 30 | 0.23 | NE | — |
| Testosterone | 0.1 | 0.08 | 3 | 0.16 | Eq. | — | 0.1 | 0.10 | 3 | 0.70 | 3 | 0.23 | 10 | 1.19 |
| Estrone | NEd | — | 1 | 1.34 | NE | — | NE | — | NE | — | NE | — | NE | — |
| Estradiol | 10 | 1.56 | NE | — | 10 | 1.86 | 10 | 1.86 | 10 | 1.99 | 3e | 1.60e | 1e | 4.70e |
| 11-Deoxycorticosterone | NE | — | 30 | 0.82 | 3 | 0.15 | 1 | 0.39 | 3 | 0.49 | Eq. | — | 0.3 | 1.87 |
| 11-Deoxycortisol | 10 | 0.23 | 3 | 0.36 | 10 | 0.04 | 0.30 | 0.14 | 1 | 0.53 | 30 | 0.19 | 3 | 1.35 |
| Corticosterone | Eq. | — | Eq. | — | 10 | 0.32 | Eq. | — | NE | — | 30 | 0.20 | 10 | 1.34 |
| Cortisol | 10 | 0.32 | 10 | 0.29 | 1 | 0.04 | 3 | 0.20 | NE | — | NE | — | NE | — |
aLowest observed effective concentration (LOEC in μM), which is defined as the lowest concentration statistically significant different (p < 0.05) from the solvent control average.
bMaximum fold change (up or down) of the average response observed at any concentration significantly different from the average solvent control level.
cEquivocal results.
dNE: no effect observed at the highest noncytotoxic concentration tested.
eEstradiol levels were analyzed by enzyme-linked immunosorbant assay according to the manufacturer's protocol.
Discussion
Our previous studies showed that the yeast estrogen bioassay, the U2OS ERα CALUX reporter gene assay, and the ERα coregulator binding assay revealed good correlations with the in vivo uterotrophic assay (based on a set of 23 compounds, most of which were selected from the ICCVAM list of compounds defined for validation of in vitro tests for estrogenicity testing) (Wang et al., forthcoming; Wang et al., 2013). It was advised to include these assays in an ITS for estrogenicity testing and prioritization of chemicals, aiming at refinement, reduction, and ultimately replacement of current animal testing for (anti)estrogenic effects, as these three high-throughput in vitro assays were also shown to be reproducible, transferable, fast, and robust. The present study with 10 extra compounds with specific modes of action was set up in order to further validate the previously established ITS and to demonstrate the added value of including in vitro tests for AR-(ant)agonism and interference with steroidogenesis. Among these compounds, five of them (i.e., equilin, DES-ME, BPB, BPC1, and BPC2) have not been tested before in any of the three in vitro assays of this ITS and the other five compounds (i.e., BPA, mestranol, butyl paraben, vinclozolin, and atrazine) have been tested only in some of the assays of our previously proposed ITS or by other groups using the same assays. The present study shows that for the extra set of compounds, the U2OS ERα CALUX and yeast estrogen bioassays both revealed 100% concordance with the in vivo uterotrophic assay, i.e., no misclassification of any compound for which uterotrophic data are available. In addition, these two reporter gene assays resulted in similar potency ranking of the 10 compounds. However, superinduction was observed for butyl paraben in the U2OS ERα CALUX assay. It has previously been shown that superinduction in CALUX assays can be caused by stabilization of the firefly luciferase reporter enzyme by the test compound, thus increasing the bioluminescent signal as determined at the end of this assay (Sotoca et al., 2010). Butyl paraben did not result in superinduction in the green fluorescent protein-based yeast estrogen bioassay, where it induced a similar maximal response as E2. The yEGFP yeast-based bioassays do not suffer from the superinduction artifact. In the ERα coregulator binding assay, only butyl paraben was misclassified as negative. Overall, these results demonstrated that the previously established ITS enables accurate prediction of the estrogenic properties in vivo as observed in the uterotrophic assay and is informative regarding the mode of action of the test compound. However, quantitative discrepancies do exist between the results obtained in vitro and in vivo, as well as between the results obtained by the different in vitro assays. Mestranol and DES-ME were ∼20–300 times less potent than E2 in the ITS, whereas these two compounds showed an estrogenic potency similar to E2 in the in vivo uterotrophic assay. Differences in metabolism might provide an explanation for these discrepancies, as it is known that mestranol is demethylated in vivo into the more potent ER-agonist EE2 (Christin-Maitre, 2013; Schmider et al., 1997). The observed in vitro/in vivo discrepancy for mestranol could thus be due to the lack of metabolism in the in vitro systems used in the current ITS. A similar explanation might be valid for DES-ME, as this compound can be metabolised in vivo into the more potent ER-agonist DES (Bolt, 1994). Taken together, this strengthens the idea that the U2OS cell line on which the ERα CALUX is based is limited in its metabolic capacity, whereas the yeast based reporter gene assay and the cell-free coregulator binding assay are lacking mammalian steroid metabolism. Therefore, the combination of the ITS with biotransformation steps, e.g., metabolism leading to either deactivation or activation, might further improve its predictive capacity for estrogenic potency in vivo. In general, most mammalian cell lines are rather limited with regard to their metabolic capacities and should be combined with metabolic models using, e.g., S9 liver fractions or physiologically based kinetic modeling to describe in vivo toxicokinetics (Jacobs et al.,2013).
Numerous studies have shown that BPA exhibits (weak) estrogenic activity (Grignard et al., 2012; Kitamura et al., 2005; Matthews et al., 2000) and strong antiandrogenic activity in vitro (Lee et al., 2003; Sohoni and Sumpter, 1998; Xu et al., 2005). In our study, all three BPA analogues showed stronger estrogenic activities than BPA, and BPC1 was even 10–100 times more potent than BPA in the reporter gene assays. In addition to the estrogenic effects, unique coregulator binding characteristics were observed in the ERα coregulator binding assay for the bisphenols. BPA showed slight nonmonotonic dose-response curves on several coregulators, i.e., inducing coregulator binding at low concentrations and inhibiting binding to the same coregulator at high concentrations. However, whether these observations on the coregulator binding level provide an explanation for the low dose effect of BPA as reported in several in vitro and in vivo studies remains to be elucidated. Moreover, the coregulator binding profile obtained for BPC1 was essentially the same as that of the selective estrogen receptor modulator (SERM) tamoxifen reported in our previous study (Wang et al., 2013). Interestingly, no estrogen antagonism was observed for the bisphenols when tested in combination with E2 in the two reporter gene assays. However, a recent study of Delfosse et al. shows that both tamoxifen and BPC1 prevent NOCA1 binding to ERα LBD (Delfosse et al., 2012). As the coregulator binding assay uses ERα-LBD that contains the activation function 2 (AF-2; localized within the C-terminal of LBD), our study reveals that BPB, BPC1, and BPC2 act as general AF-2 antagonists, similar as tamoxifen. The estrogen reporter gene assays used in the present study stably express full-length ERα. This further suggests that the ERα activation function 1 (AF-1; localized within the N-terminal A/B domain) is needed for the estrogenic activities of the bisphenols. BPC1 and BPC2 also showed significant inhibitory effects on the transcriptional activity induced by DHT or T in the AR CALUX and the yeast androgen bioassay, respectively. The IC50 values of BPC1 and BPC2 were 2–30 times lower than that of BPA and were comparable with the potent AR antagonist flutamide, demonstrating the strong antiandrogenic activities of BPC1 and BPC2. Additionally, the phenolic compounds elicited strong disruptions on the hormone synthesis in the H295R cells, in general resulting in decreased levels of androgens and elevated levels of estrogens. Together these properties of the phenolic compounds, i.e., ER-agonist, AR-antagonist, and decreasing androgen levels and elevating estrogen levels in the H295R assay, possibly direct these compounds to be stronger “estrogens” in vivo than predicted by the in vitro ER-agonist properties alone. BPA was shown to be weakly estrogenic in several in vitro models (Grignard et al., 2012; Laws et al., 2000b; Schafer et al., 1999), and some studies suggest that BPA has also a greater in vivo potency than would be predicted based on the results obtained in vitro. Thus, the combined actions as determined in vitro in the present study might explain the in vivo potency of BPA more accurately. BPC1 is apparently the most potent bisphenol analogue that possesses estrogenic and antiandrogenic properties and at the same time affects steroidogenesis into the direction of decreased androgen levels and elevated estrogen levels. BPA and its analogues are widely used as raw material in the production of polycarbonate plastics and epoxy resins, and are found to contaminate a broad range of end products (Brotons et al., 1995; Krishnan et al., 1993; Olea et al., 1996; Vandenberg et al., 2007). Given the complex biological activities of BPC1 and the on-going debate on endocrine disrupting chemicals and BPA in particular, further in vivo testing of BPC1 in animal models should have high priority, as our data suggest that the use of BPA analogues might lead to a higher risk than BPA itself. Similar concerns were published by Grignard et al., showing that bisphenol S (BPS), used as a BPA substitute in the production of plastic baby bottles, has a comparable estrogenic potency as BPA in in vitro transcriptional activation assays (Grignard et al., 2012).
It has been shown that atrazine does not bind to the ER or AR (Blair et al., 2000; Fang et al., 2003) and, consistently, failed to induce ER- or AR-dependent transcription in several reporter gene assays (Kavlock and Dix, 2010; Kojima et al., 2004; Kolle et al., 2010; Vandenberg et al., 2007). Atrazine is also unable to stimulate estrogen-dependent MCF-7 cell proliferation in the E-screen (Connor et al., 1996; Fukamachi et al., 2004). These findings are in agreement with the outcomes of the present study, i.e., atrazine has no affinity for the ER and showed very weak antiandrogenic activities (only at the highest concentration tested) in the AR reporter gene assays. However, atrazine showed clear effects in the H295R steroidogenesis assay, resulting in elevated levels of estradiol and testosterone. Thus, the results from the current study indicate that further testing of atrazine in the in vivo uterotrophic assay is not needed, as atrazine did not show clear effects via the ER or AR and only affected the steroidogenesis. Therefore, atrazine should be tested in the male and female pubertal assays rather than the uterotrophic assay. Indeed, this was corroborated by the fact that atrazine failed to show estrogenic or androgenic activities in the uterotrophic assay or Hershberger assay (Yamasaki et al., 2000, 2004), respectively, but delayed puberty and sexual development in both male and female rodents in pubertal assays (Laws et al., 2000a; Stoker et al., 2000). The fungicide vinclozolin does not bind to the ER and was also unable to induce uterotrophic effects in vivo (Blair et al., 2000; Laws et al., 1996), but has been reported to have antiandrogenic effects in vitro and in vivo (Hellwig et al., 2000; Korner et al., 2004). In the present study, vinclozolin did not show any estrogenic effects, but elicited strong antiandrogenic activity. It also increased estrogen levels and decreased androgen levels in the H295R cells, suggesting that vinclozolin may induce aromatase activity.
In conclusion, the ITS consisting of the U2OS ERα CALUX, yeast estrogen bioassay and ERα coregulator binding assay enables an accurate prediction of the estrogenic effects in vivo and provides mechanistic insights. The extended ITS, including the enhanced H295R steroidogenesis assay and androgen reporter gene assays, was demonstrated to result in a better prediction than in vivo estrogenicity testing by the uterotrophic assay alone, because it can detect possible (anti)androgenic effects and effects on steroidogenesis. The latter was illustrated for atrazine and vinclozolin, whose endocrine disrupting activities were not detected by the in vivo uterotrophic assay. The extended ITS presented in this study may therefore allow easy high-throughput screening and prioritization of chemicals, thereby contributing to refinement, reduction, and to some extent even a replacement of current animal testing for (anti)estrogenic effects. However, the examples of mestranol and DES-ME showing differences between in vitro and in vivo potencies, indicate that the ITS has to be combined with additional types of in vitro assays, including physiologically based kinetic modeling of in vivo toxicokinetics in order to further improve its predictive capacity for in vivo estrogenicity.
Supplementary Data
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
Funding
The Netherlands Genomics Initiative and The Netherlands Toxicogenomics Centre (6162500134).
Supplementary Material
Acknowledgments
We thank Diana Melchers, PamGene International B.V., 's-Hertogenbosch, The Netherlands, for her technical support with the PamChip peptide microarray experiment.
References
- Aarts J. M. M. J. G., Wang S., Houtman R., van Beuningen R. M. G. J., Westerink W. M. A., Van De Waart B. J., Rietjens I. M. C. M., Bovee T. F. H. Robust array-based coregulator binding assay predicting ERα-agonist potency and generating binding profiles reflecting ligand structure. Chem. Res. Toxicol. 2013;26:336–346. doi: 10.1021/tx300463b. [DOI] [PubMed] [Google Scholar]
- Van den Belt K., Berckmans P., Vangenechten C., Verheyen R., Witters H. Comparative study on the in vitro/in vivo estrogenic potencies of 17β-estradiol, estrone, 17α-ethynylestradiol and nonylphenol. Aquat. Toxicol. 2004;66:183–195. doi: 10.1016/j.aquatox.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Blair R. M., Fang H., Branham W. S., Hass B. S., Dial S. L., Moland C. L., Tong W., Shi L., Perkins R., Sheehan D. M. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: Structural diversity of ligands. Toxicol. Sci. 2000;54:138–153. doi: 10.1093/toxsci/54.1.138. [DOI] [PubMed] [Google Scholar]
- Bolt H. M. Interactions between clinically used drugs and oral contraceptives. Environ. Health Perspect. 1994;102(Suppl. 9):35–38. doi: 10.1289/ehp.94102s935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovee T. F. H., Bor G., Becue I., Daamen F. E. J., van Duursen M. B. M., Lehmann S., Vollmer G., De Maria R., Fox J. E., Witters H., et al. Inter-laboratory comparison of a yeast bioassay for the determination of estrogenic activity in biological samples. Anal. Chim. Acta. 2009;637:265–272. doi: 10.1016/j.aca.2008.09.064. [DOI] [PubMed] [Google Scholar]
- Bovee T. F., Schoonen W. G., Hamers A. R., Bento M. J., Peijnenburg A. A. Screening of synthetic and plant-derived compounds for (anti) estrogenic and (anti) androgenic activities. Anal. Bioanal. Chem. 2008;390:1111–1119. doi: 10.1007/s00216-007-1772-3. [DOI] [PubMed] [Google Scholar]
- Bovee T. F., Thevis M., Hamers A. R., Peijnenburg A. A., Nielen M. W., Schoonen W. G. SERMs and SARMs: Detection of their activities with yeast based bioassays. J. Steroid Biochem. Mol. Biol. 2010;118:85–92. doi: 10.1016/j.jsbmb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Brotons J. A., Olea-Serrano M. F., Villalobos M., Pedraza V., Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ. Health Perspect. 1995;103:608–612. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Burg B., Winter R., Man H.-y., Vangenechten C., Berckmans P., Weimer M., Witters H., van der Linden S. Optimization and prevalidation of the in vitro AR CALUX method to test androgenic and antiandrogenic activity of compounds. Reprod. Toxicol. 2010a;30:18–24. doi: 10.1016/j.reprotox.2010.04.012. [DOI] [PubMed] [Google Scholar]
- van der Burg B., Winter R., Weimer M., Berckmans P., Suzuki G., Gijsbers L., Jonas A., van der Linden S., Witters H., Aarts J., et al. Optimization and prevalidation of the in vitro ERα CALUX method to test estrogenic and antiestrogenic activity of compounds. Reprod. Toxicol. 2010b;30:73–80. doi: 10.1016/j.reprotox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Christin-Maitre S. History of oral contraceptive drugs and their use worldwide. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:3–12. doi: 10.1016/j.beem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Connor K., Howell J., Chen I., Liu H., Berhane K., Sciarretta C., Safe S., Zacharewski T. Failure of chloro-S-triazine-derived compounds to induce estrogen receptor-mediated responses in vivo and in vitro. Fundam. Appl. Toxicol. 1996;30:93–101. [PubMed] [Google Scholar]
- Cosnefroy A., Brion F., Maillot-Maréchal E., Porcher J.-M., Pakdel F., Balaguer P., Aït-Aïssa S. Selective activation of zebrafish estrogen receptor subtypes by chemicals by using stable reporter gene assay developed in a zebrafish liver cell line. Toxicol. Sci. 2012;125:439–449. doi: 10.1093/toxsci/kfr297. [DOI] [PubMed] [Google Scholar]
- Delfosse V., Grimaldi M., Pons J.-L., Boulahtouf A., le Maire A., Cavailles V., Labesse G., Bourguet W., Balaguer P. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc. Natl. Acad. Sci. U.S.A. 2012;109:14930–14935. doi: 10.1073/pnas.1203574109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D., Xu L., Fang H., Hong H., Perkins R., Harris S., Bearden E., Shi L., Tong W. The EDKB: An established knowledge base for endocrine disrupting chemicals. BMC Bioinformatics. 2010;11(Suppl 6):S5. doi: 10.1186/1471-2105-11-S6-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H., Tong W., Branham W. S., Moland C. L., Dial S. L., Hong H., Xie Q., Perkins R., Owens W., Sheehan D. M. Study of 202 natural, synthetic, and environmental chemicals for binding to the androgen receptor. Chem. Res. Toxicol. 2003;16:1338–1358. doi: 10.1021/tx030011g. [DOI] [PubMed] [Google Scholar]
- Filby A. L., Thorpe K. L., Maack G., Tyler C. R. Gene expression profiles revealing the mechanisms of anti-androgen- and estrogen-induced feminization in fish. Aquat. Toxicol. 2007;81:219–231. doi: 10.1016/j.aquatox.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Fukamachi K., Han B. S., Kim C. K., Takasuka N., Matsuoka Y., Matsuda E., Yamasaki T., Tsuda H. Possible enhancing effects of atrazine and nonylphenol on 7,12-dimethylbenz[a]anthracene-induced mammary tumor development in human c-Ha-ras proto-oncogene transgenic rats. Cancer Sci. 2004;95:404–410. doi: 10.1111/j.1349-7006.2004.tb03223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L. E., Ostby J. S., Kelce W. R. Developmental effects of an environmental antiandrogen: The fungicide vinclozolin alters sex differentiation of the male rat. Toxicol. Appl. Pharmacol. 1994;129:46–52. doi: 10.1006/taap.1994.1227. [DOI] [PubMed] [Google Scholar]
- Grignard E., Lapenna S., Bremer S. Weak estrogenic transcriptional activities of Bisphenol A and Bisphenol S. Toxicol. In Vitro. 2012;26:727–731. doi: 10.1016/j.tiv.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Gross-Sorokin M. Y., Roast S. D., Brighty G. C. Assessment of feminization of male fish in English rivers by the Environment Agency of England and Wales. Environ. Health Perspect. 2006;114(Suppl. 1):147–151. doi: 10.1289/ehp.8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig J., van Ravenzwaay B., Mayer M., Gembardt C. Pre- and postnatal oral toxicity of vinclozolin in Wistar and Long-Evans rats. Reg. Toxicol. Pharmacol. 2000;32:42–50. doi: 10.1006/rtph.2000.1400. [DOI] [PubMed] [Google Scholar]
- Houtman R., de Leeuw R., Rondaij M., Melchers D., Verwoerd D., Ruijtenbeek R., Martens J. W. M., Neefjes J., Michalides R. Serine-305 phosphorylation modulates estrogen receptor alpha binding to a coregulator peptide array, with potential application in predicting responses to tamoxifen. Mol. Cancer Ther. 2012;11:805–816. doi: 10.1158/1535-7163.MCT-11-0855. [DOI] [PubMed] [Google Scholar]
- Jacobs M.N., Laws S.C., Willett K., Schmieder P., Odum J., Bovee T.F. In vitro metabolism and bioavailability tests for endocrine active substances: What is needed next for regulatory purposes? ALTEX. 2013;30:331–351. doi: 10.14573/altex.2013.3.331. [DOI] [PubMed] [Google Scholar]
- Kavlock R., Dix D. Computational toxicology as implemented by the U.S. EPA: Providing high throughput decision support tools for screening and assessing chemical exposure, hazard and risk. J. Toxicol. Environ. Health - Part B: Crit. Rev. 2010;13:197–217. doi: 10.1080/10937404.2010.483935. [DOI] [PubMed] [Google Scholar]
- Kitamura S., Suzuki T., Sanoh S., Kohta R., Jinno N., Sugihara K., Yoshihara S. i., Fujimoto N., Watanabe H., Ohta S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. 2005;84:249–259. doi: 10.1093/toxsci/kfi074. [DOI] [PubMed] [Google Scholar]
- Kojima H., Katsura E., Takeuchi S., Niiyama K., Kobayashi K. Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ. Health Perspect. 2004;112:524–531. doi: 10.1289/ehp.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolle S. N., Kamp H. G., Huener H. A., Knickel J., Verlohner A., Woitkowiak C., Landsiedel R., van Ravenzwaay B. In house validation of recombinant yeast estrogen and androgen receptor agonist and antagonist screening assays. Toxicol. In Vitro. 2010;24:2030–2040. doi: 10.1016/j.tiv.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Koppen A., Houtman R., Pijnenburg D., Jeninga E. H., Ruijtenbeek R., Kalkhoven E. Nuclear receptor-coregulator interaction profiling identifies TRIP3 as a novel peroxisome proliferator-activated receptor γ cofactor. Mol. Cell. Proteomics. 2009;8:2212–2226. doi: 10.1074/mcp.M900209-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner W., Vinggaard A. M., Terouanne B., Ma R., Wieloch C., Schlumpf M., Sultan C., Soto A. M. Interlaboratory comparison of four in vitro assays for assessing androgenic and antiandrogenic activity of environmental chemicals. Environ. Health Perspect. 2004;112:695–702. doi: 10.1289/ehp.112-1241964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A. V., Stathis P., Permuth S. F., Tokes L., Feldman D. Bisphenol-A: An estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- Laws S. C., Carey S. A., Ferrell J. M., Bodman G. J., Cooper R. L. Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol. Sci. 2000b;54:154–167. doi: 10.1093/toxsci/54.1.154. [DOI] [PubMed] [Google Scholar]
- Laws S. C., Carey S. A., Kelce W. R., Cooper R. L., Gray L. E., Jr Vinclozolin does not alter progesterone receptor (PR) function in vivo despite inhibition of PR binding by its metabolites in vitro. Toxicology. 1996;112:173–182. doi: 10.1016/0300-483x(96)03354-9. [DOI] [PubMed] [Google Scholar]
- Laws S. C., Ferrell J. M., Stoker T. E., Schmid J., Cooper R. L. The effects of atrazine on female Wistar rats: An evaluation of the protocol for assessing pubertal development and thyroid function. Toxicol. Sci. 2000a;58:366–376. doi: 10.1093/toxsci/58.2.366. [DOI] [PubMed] [Google Scholar]
- Lee H. J., Chattopadhyay S., Gong E.-Y., Ahn R. S., Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol. Sci. 2003;75:40–46. doi: 10.1093/toxsci/kfg150. [DOI] [PubMed] [Google Scholar]
- Matthews J. B., Twomey K., Zacharewski T. R. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors α and β. Chem. Res. Toxicol. 2000;14:149–157. doi: 10.1021/tx0001833. [DOI] [PubMed] [Google Scholar]
- OECD. Test no. 440: Uterotrophic Bioassay in Rodents. In OECD Guidelines for the Testing of Chemicals. 2007 Available at: http://www.oecd-ilibrary.org/environment/test-no-440-uterotrophic-bioassay-in-rodents_9789264067417-en. [Google Scholar]
- Olea N., Pulgar R., Pérez P., Olea-Serrano F., Rivas A., Novillo-Fertrell A., Pedraza V., Soto A. M., Sonnenschein C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ. Health Perspect. 1996;104:298–305. doi: 10.1289/ehp.96104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby J., Monosson E., Kelce W. R., Gray L. E. Environmental antiandrogens: Low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol. Ind. Health. 1999;15:48–64. doi: 10.1177/074823379901500106. [DOI] [PubMed] [Google Scholar]
- Owens J. W., Ashby J. Critical review and evaluation of the Uterotrophic bioassay for the identification of possible estrogen agonists and antagonists: In support of the validation of the OECD Uterotrophic protocols for the laboratory rodent. Crit. Rev. Toxicol. 2002;32:445–520. doi: 10.1080/20024091064291. [DOI] [PubMed] [Google Scholar]
- Rijk J. C. W., Peijnenburg A. A. C. M., Blokland M. H., Lommen A., Hoogenboom R. L. A. P., Bovee T. F. H. Screening for modulatory effects on steroidogenesis using the human H295R adrenocortical cell line: A metabolomics approach. Chem. Res. Toxicol. 2012;25:1720–1731. doi: 10.1021/tx3001779. [DOI] [PubMed] [Google Scholar]
- Schafer T. E., Lapp C. A., Hanes C. M., Lewis J. B., Wataha J. C., Schuster G. S. Estrogenicity of bisphenol A and bisphenol A dimethacrylate in vitro. J. Biomed. Mater. Res. 1999;45:192–197. doi: 10.1002/(sici)1097-4636(19990605)45:3<192::aid-jbm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Schmider J., Greenblatt D., von Moltke L., Karsov D., Vena R., Friedman H., Shader R. Biotransformation of mestranol to ethinyl estradiol in vitro: The role of cytochrome P-450 2C9 and metabolic inhibitors. J. Clin. Pharmacol. 1997;37:193–200. doi: 10.1002/j.1552-4604.1997.tb04781.x. [DOI] [PubMed] [Google Scholar]
- Sohoni P., Sumpter J. Several environmental oestrogens are also anti-androgens. J. Endocrinol. 1998;158:327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- Sotoca A. M., Bovee T. F. H., Brand W., Velikova N., Boeren S., Murk A. J., Vervoort J., Rietjens I. M. C. M. Superinduction of estrogen receptor mediated gene expression in luciferase based reporter gene assays is mediated by a post-transcriptional mechanism. J. Steroid Biochem. Mol. Biol. 2010;122:204–211. doi: 10.1016/j.jsbmb.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Stoker T. E., Laws S. C., Guidici D. L., Cooper R. L. The effect of atrazine on puberty in male Wistar rats: An evaluation in the protocol for the assessment of pubertal development and thyroid function. Toxicol. Sci. 2000;58:50–59. doi: 10.1093/toxsci/58.1.50. [DOI] [PubMed] [Google Scholar]
- Toorians A. W. F. T., Bovee T. F. H., De Rooy J., Stolker L. A. A. M., Hoogenboom R. L. A. P. Gynaecomastia linked to the intake of a herbal supplement fortified with diethylstilbestrol. Food Addit. Contam.: Part A. 2010;27:917–925. doi: 10.1080/19440041003660869. [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N., Hauser R., Marcus M., Olea N., Welshons W. V. Human exposure to bisphenol A (BPA) Reprod. Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Wang S., Aarts J. M. M. J. G., de Haan L. H. J., Argyriou D., Peijnenburg A. A. C. M., Rietjens I. M. C. M., Bovee T. F. H. Towards an integrated in vitro strategy for estrogenicity testing. J. Appl. Toxicol. doi: 10.1002/jat.2928. (forthcoming) [DOI] [PubMed] [Google Scholar]
- Wang S., Houtman R., Melchers D., Aarts J., Peijnenburg A., Van Beuningen R., Rietjens I., Bovee T. A 155-plex high-throughput in vitro coregulator binding assay for (anti-)estrogenicity testing evaluated with 23 reference compounds. ALTEX. 2013;30:145–157. doi: 10.14573/altex.2013.2.145. [DOI] [PubMed] [Google Scholar]
- Xu L.-C., Sun H., Chen J.-F., Bian Q., Qian J., Song L., Wang X.-R. Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology. 2005;216:197–203. doi: 10.1016/j.tox.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Yamasaki K., Sawaki M., Noda S., Muroi T., Maekawa A. Immature rat uterotrophic assay of diethylstilbestrol, ethynyl estradiol and atrazine. J. Toxicol. Pathol. 2000;13:145–149. [Google Scholar]
- Yamasaki K., Sawaki M., Noda S., Muroi T., Takakura S., Mitoma H., Sakamoto S., Nakai M., Yakabe Y. Comparison of the Hershberger assay and androgen receptor binding assay of twelve chemicals. Toxicology. 2004;195:177–186. doi: 10.1016/j.tox.2003.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.