Abstract
The β-keto amphetamine (cathinone, β-KA) designer drugs such as mephedrone (4-methylmethcathinone, 4-MMC) show a large degree of structural similarity to amphetamines like methamphetamine (METH). However, little is currently known about whether these substances also share the potential neurotoxic properties of their non-keto amphetamine counterparts, or what mechanisms could be involved. Here, we evaluate the cytotoxicity of β-KAs in SH-SY5Y cells using lactate dehydrogenase (LDH) assays, assess the redox potential of a range of β-KAs and non-keto amphetamines using the sensitive redox indicator 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-1), and explore the effect of 4-MMC on the formation of protein adducts using ultra-high performance liquid chromatography/high-resolution time-of-flight mass spectrometry (UHPLC-HR-TOFMS) and on the mitochondrial respiratory chain using high-resolution respirometry. We show that treatment with β-KAs increases LDH release. Further, we demonstrate that even under physiological pH, β-KAs are effective and selective—as compared with their non-keto analogues—reductants in the presence of electron acceptors. Increased pH (range 7.6–8.0) greatly enhanced the reactivity up to sixfold. We found no evidence of protein adduct formation, suggesting the reactivity is due to direct electron transfer by the β-KAs. Finally, we show that 4-MMC and METH produce dissimilar effects on the respiratory chain. Our results indicate that β-KAs such as 4-MMC possess cytotoxic properties in vitro. Furthermore, in the presence of an electron-accepting redox partner, the ketone moiety of β-KAs is vital for pH-dependent redox reactivity. Further work is needed to establish the importance of β-KA redox properties and its potential toxicological importance in vivo.
Keywords: cathinones, β-keto amphetamine, methamphetamine, mephedrone, neurotoxicity, reducing agent, respiratory chain, protein adducts
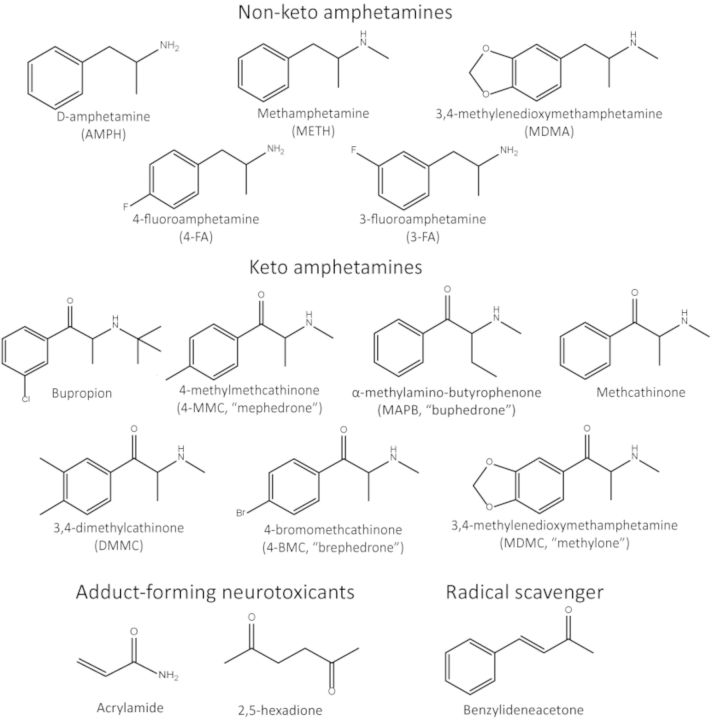
In the past years, there has been a large increase in the use of β-keto amphetamine (cathinone, β-KA) designer drugs such as mephedrone (4-methylmethcathinone, 4-MMC) (EMCDDA, 2012). Colloquially known as “bath salts,” a label initially used by internet vendors to circumvent laws banning their sale for human consumption, these substances are closely related to illicit amphetamines like methamphetamine (METH). In fact, 4-MMC is a close structural analogue of METH, differing only by the addition of the ketone group on the side chain and a methyl group on the phenyl ring (see Fig. 1). More than 30 β-KAs have been officially reported between 2005 and 2011, with likely many more unreported varieties in circulation on the market. Subsequent bans in the United States and the European Union have done little to curb their popularity, and 4-MMC remains among the most used drugs together with cannabis, ecstasy (3,4-methylenedioxymethamphetamine, MDMA), and cocaine in certain regions (EMCDDA, 2012).
FIG. 1.
Chemical structures of the compounds used and mentioned in this paper: non-keto amphetamines (top), β-KAs (middle), and the adduct forming neurotoxicants acrylamide and 2,5-hexadione (Lopachin and Decaprio 2005) and the radical scavenger benzylideneacetone (Dinkova-Kostova et al., 2007) (bottom).
The emergence of large numbers of novel β-KAs poses a challenge in terms of devising an evidence-based public health policy and harm reduction approach as it is infeasible to study in depth the toxicity of each individual compound. Because much is already known about amphetamine toxicity, a more viable approach is to study the biological effects of the most important structural distinction between keto and non-keto amphetamines: the ketone moiety on the side chain β-carbon. METH and MDMA have been extensively studied and the available evidence suggests that several factors, including oxidative stress, converge to produce a neurotoxic effect primarily affecting the brain's dopaminergic (DA) and serotonergic (5-HT) systems that may be associated with alterations in cognitive and neuropsychiatric functions, such as decreased memory performance and increased anxiety, both in humans and animal models (reviewed in Gouzoulis-Mayfrank and Daumann, 2009; Yamamoto et al., 2010).
Keto and non-keto amphetamines are pharmacologically similar in that they both increase the release and inhibit the reuptake of the monoamines DA, 5-HT, and norepinephrine (NE) (Hadlock et al., 2011; Lopez-Arnau et al., 2012). In terms of their toxicity profile, however, there appear to be distinct differences. Although 4-MMC produces a large increase in DA and 5-HT release following acute administration (Kehr et al., 2011; Wright et al., 2012), it does not appear to produce, even after high-dose binge treatments, any long-term (more than 1 week after the final treatment) decreases in DA or 5-HT levels, or any other biochemical gauges of toxicity (Angoa-Perez et al., 2013; den Hollander et al., 2013; Motbey et al., 2012), although 4-MMC does increase the toxicity of METH when the two drugs are administered together and causes decreased performance in rodent tests of memory functioning (den Hollander et al., 2013; Motbey et al., 2012). This warrants a closer investigation into the cellular and molecular mechanisms responsible for their differing toxicity. The fact that it has been hard to show any biochemical evidence of toxicity of 4-MMC leads us to investigate specific hypotheses regarding the effects of this drug on measures which may be related to toxicity.
We first tested and confirmed the ability of the β-KAs 4-MMC and methylone (3,4-methylonedioxymethcathinone, MDMC) to produce cytotoxicity in the SH-SY5Y neuroblastoma cell line and investigate a possible mechanism. Next, using the sensitive redox indicator 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-1) (Berridge and Tan, 1998; Peskin and Winterbourn, 2000; Tan and Berridge, 2000), we describe a difference in redox activity between β-KAs and non-keto amphetamines which we demonstrate to be dependent on the presence of the ketone moiety on the amphetamine molecule, suggesting that β-KAs may interfere with the cellular redox buffering system as has been observed with other redox-active, toxic compounds (Savory et al., 1999; Smith et al., 1997). It is also known that non-keto amphetamines can inhibit the mitochondrial electron transport chain (Brown et al., 2005; Burrows et al., 2000). Using mitochondrial respirometry, we show that 4-MMC and METH both reduce mitochondrial respiration, but produce a divergent effect at the level of complex II of the electron transport chain. Finally, it has been suggested that other carbonyl-containing compounds such as acrylamide and 2,5-hexadione may produce protein adducts due to their ability to form amino acid adducts with amino acids in neurofilament or synaptic vesicle proteins (Lopachin and Decaprio, 2005). For this reason, we investigated the ability of 4-MMC to induce adduct formation of amino acids using ultra-high performance liquid chromatography/high-resolution time-of-flight mass spectrometry (UHPLC-HR-TOFMS), but found no evidence of adduct formation.
MATERIALS AND METHODS
Drugs and reagents
All β-KAs were reference standard grade and supplied by the Department of Forensic Medicine, Hjelt Institute, University of Helsinki; MDMA was acquired from THC Pharm GmbH (Frankfurt, Germany); METH and other reagents were acquired from Sigma-Aldrich (St. Louis, MO) and were all of analytical quality.
Animals
Male C57BL/6J aged 8 weeks at arrival were obtained through Nova-Scanbur (Sollentuna, Sweden) and housed in 6 per cage with food pellets (Harlan BV., Horst, the Netherlands) and tap water available ad libitum at standard housing conditions (12-h light–dark cycle, lights on at 06:00 h; temperature, 20–23°C; relative humidity, 50–60%; and aspen chip beddings). All animal experiments were approved by the Laboratory Animal Committee of the Southern Finland Provincial Government.
Cell culture
SH-SY5Y neuroblastoma cells between passages 6 and 20 were seeded on 96-well plates at a density of ± 5000 cells/100 μl Dulbecco's Modified Eagle Medium (DMEM/F12) containing 10% Fetal Bovine Serum and antimycin-A and kept at 37°C/5% CO2. The cytotoxicity and cell proliferation assays were performed in medium with the same composition. After seeding, cells were grown for 4 days prior to drug treatments.
Cytotoxicity assay
The amount of lactate dehydrogenase (LDH) released into the growth medium 48 h after the start of the drug treatments was measured using the Cytotoxicity Detection Kit (Roche) according to the manufacturer's instructions. When drug treatments were combined with the DA transporter (DAT) blocker GBR12909, the cells were pre-incubated for 1 h with GBR12909 prior to application of the other drugs. The ability of this assay to measure substance-induced alterations in LDH release was assessed by applying similar 48-h treatments with 5mM nicotinamide adenine dinucleotide (NAD+) or 2mM hydrogen peroxide (H2O2) as positive and negative controls. Results are presented as percentages of the LDH released by untreated control cells.
Redox sensitivity/cell proliferation/WST-1 assay
After the 48-h drug treatments the medium was replaced by a medium containing WST-1 and the intermediate electron acceptor 1-methoxy-5-methyl-phenazinium methyl sulfate (mPMS) (Cell Proliferation Reagent, Roche). Following 60-min incubation with water-soluble WST-1, the resulting formazan dye production was quantitated in a spectrophotometer at 440 nm. The ability of this assay for measuring cellular viability/pro-liferation was validated by measuring the response to similar 48-h treatments with 5mM nicotinamide adenine dinucleotide (NAD+) and 2mM hydrogen peroxide (H2O2) as positive and negative controls. Results are presented as percentages of the reactivity produced by untreated control cells.
Analysis of reaction products
Aqueous 4-MMC, MDMC, and METH solutions (100 μl; 0.2mM; and 2mM) were incubated in phosphate-buffered saline (PBS) and NaHCO3 with and without the WST-1 reagent at 37°C for 85 min. A similar set of analyses were performed with the presence of DMEM/F12 amino acids at concentrations similar to those in the medium. An ultra-high performance liquid chromatography/high-resolution time-of-flight mass spectrometry (UHPLC-HR-TOFMS) method was used for the analysis of the incubated sample (1 μl). The chromatographic and mass spectrometric conditions were similar to those described previously (Sundstrom et al., 2013).
Mitochondrial respiration measurements using Seahorse XFe96
Microplates, Base Medium, and the XFe96 Analyzer were all obtained from Seahorse Bioscience (MA). Cells were seeded on 96-well XF Cell Culture Microplates at a density of ± 10.000 cells/80 μl well (previously determined to provide optimal sensitivity in this assay) and kept under the same condition as described above. One hour prior to the assay the DMEM/F12 medium was replaced with XF Base Medium with added glucose (10mM), pyruvic acid (10mM), and L-glutamine (1mM) and kept in a CO2-free incubator until analysis. Analysis of oxygen consumption rate (OCR) was performed in a Seahorse XFe96 analyzer according to the manufacturer's instructions. OCR was measured prior to, during, and after injections with the ATP synthase inhibitor oligomycin, the uncoupler carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), and finally a combined injection of the complex I+II inhibitors antimycin A + rotenone to yield final concentrations in the wells of 1μM, 0.5μM, and 1μM+1μM, respectively. Basal respiration was calculated by subtracting non-mitochondrial respiration (OCR following injection with antimycin A + rotenone) from the baseline respiration prior to drug injection. Maximum respiration was calculated by subtracting non-mitochondrial respiration from the maximum respiration measured following FCCP injection.
High-resolution respirometry with Oxygraph-2k
Mitochondrial respiration was measured using the Oxygraph-2k respirometer (Oroboros Instruments GmbH, Innsbruck, Austria). The Oxygraph-2k has not been employed previously to measure amphetamine-induced changes in mitochondrial respiration. For this reason, we opted to keep the tissue type similar to previous studies (Brown et al., 2005; Burrows et al., 2000) and employ mice brain tissue instead of SH-SY5Y cells in this experiment. Animals were sacrificed by decapitation following CO2 anesthesia. Samples were prepared according to instructions provided by the manufacturer of the Oxygraph-2k respirometer. Briefly, brains were dissected and 8 mg of frontal cortical tissue was immediately placed in shredder tubes containing 500 μl ice cold MiRO6 respiration buffer (0.5mM EGTA, 3mM MgCl2·6H20, 60mM K-lactobionate, 20mM taurine, 10mM KH2PO4, 20mM HEPES, 110mM sucrose, 1 g/l BSA, and 280 u/ml catalase) and shredded in a SG3 shredder (Pressure Biosciences Inc., MA) for 10 and 5 s at setting 1 and 2, respectively. The contents of the tube were dissolved in 4.5 ml of respiration buffer and ±2.5 ml was transferred to each of the two chambers of an Oxygraph-2k instrument. Water, METH, or 4-MMC (final concentration 20mM) was added to the chamber and allowed to incubate for 20 min prior to commencement of a substrate-uncoupler-inhibitor titration protocol (Pesta and Gnaiger, 2012). In our adaption of this protocol, the initially added substrates were L-malic acid (2mM), pyruvic acid (5mM), L-glutamic acid (10mM), ADP+Mg2+ (1mM), and succinate (10mM). Subsequently, FCCP was added, followed by the inhibitors rotenone (0.2μM), malonic acid (5μM), and antimycin A (2.5μM). Oxygen Flux Control Ratios (FCRs) for the respiratory states ROUTINE (baseline respiration without the presence of exogenous substrates), LEAK (resting state non-phosphorylating respiration after addition of L-malic acid, pyruvic acid, and L-glutamic acid), OXPHOS (ADP-activated phosphorylation through complex I), Complex I+II (after activation of complex II by succinate), and Complex II (after inhibition of complex I by rotenone) were determined according to methods described previously (Gnaiger, 2009; Pesta and Gnaiger, 2012).
Statistics
LDH, WST-1, and OCR data were analyzed using one-way or two-way ANOVAs followed by Dunnett's or Fisher's least significant difference post-hoc tests to compare the various drugs and concentrations against untreated control cells or each other. Mitochondrial FCRs based on the Oxygraph-2k data were determined in Datlab v. 5 (Oroboros Instruments GmbH, Innsbruck, Austria) and analyzed in a similar manner. All data were analyzed in SPSS v. 21 (IBM, Armonk, NY) or Prism v. 5.01 (GraphPad Software, Inc., CA).
RESULTS
WST-1 and LDH Validation Assays
The WST-1 and LDH assays were validated for their ability to detect substance-induced alterations in cellular proliferation/viability and LDH release by using H2O2 and NAD+ as positive and negative controls. For the WST-1 assay, a one-way ANOVA revealed a significant effect of these controls on WST-1 reactivity (F2 14 = 319.9, p < 0.0001). Post-hoc testing revealed that H2O2 significantly decreased (71% of control, p < 0.001) whereas NAD+ significantly increased (144% of control, p < 0.001) the measured WST-1 reactivity. A one-way ANOVA of the data from these controls for the LDH assay also revealed a significant effect (F2 17 = 7815, p < 0.0001), and post-hoc testing showed that H2O2 significantly increased (810% of control, p < 0.001) whereas NAD+ significantly decreased (71% of control, p < 0.01) cellular LDH release, indicating that both WST-1 and LDH assays properly measure substance-induced changes in cellular proliferation/viability and LDH release, respectively.
Effects on LDH Release, WST-1 Reactivity, and Morphology
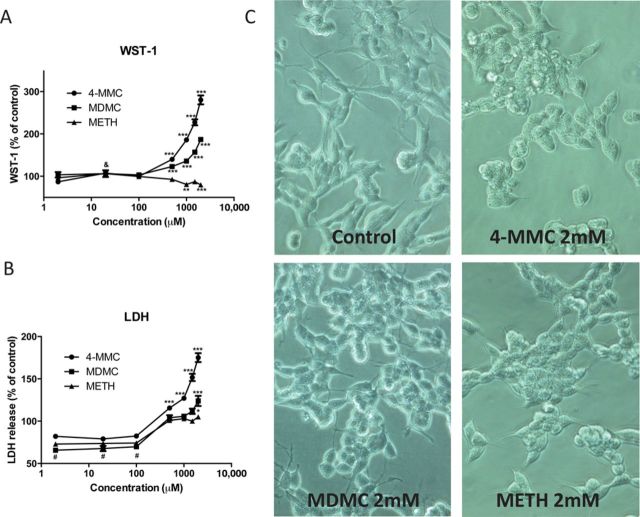
The effects of a 48-h treatment with varying doses (2μ–2mM) of 4-MMC, MDMC, and METH on WST-1 reactivity are shown in Figure 2A and the effects on LDH release are shown in Figure 2B. Morphology of untreated control cells and drug-treated cells (2mM) is shown in Figure 2C.
FIG. 2.
(A) SH-SY5Y cells were treated for 48 h with the β-keto amphetamine 4-MMC or MDMC or the non-keto amphetamine METH. METH (1 and 2mM) produced a decrease in WST-1 reactivity; indicative of a decrease is cell viability. The β-keto amphetamines, on the other hand, produced large increases in WST-1 reactivity. (B) SH-SY5Y cells were treated for 48 h with the β-keto amphetamine 4-MMC or MDMC or the non-keto amphetamine METH. All drugs increased the amount of LDH released into the medium, indicative of cytotoxic damage. The LDH release induced by 4-MMC and MDMC was larger than that in the METH treated cells. (C) Morphology of SH-SY5Y cells treated with 4-MMC, MDMC, and METH (all 2mM) as well as untreated control cells at 40× magnification. The large increase in WST-1 reactivity observed with the β-keto amphetamines did not correspond to the data obtained from the LDH assay or the morphology of the cells, suggesting a different mechanism to be responsible for the increased WST-1 reactivity. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control; &p < 0.01 MDMC compared with control; #p < 0.001 all conditions compared with control. Significance only marked if the measured value was higher or lower than 100% ± SD of untreated control cells. N ≥ 5 per group.
A two-way ANOVA of the WST-1 data revealed a significant effect of drug (F2 84 = 394.1, p < 0.0001), concentration (F6 84 = 141.1, p < 0.0001), and drug*concentration interaction (F12 84 = 75.88, p < 0.0001). Post-hoc testing following separate one-way ANOVA's revealed significant increases in WST-1 reactivity of both 4-MMC and MDMC at all concentrations above 500μM and for MDMC also at 20μM, whereas significant decreases were observed for METH at concentrations of 1 and 2mM (Fig. 2A).
A two-way ANOVA of the LDH data also revealed a significant effect of drug (F2 84 = 133.6, p < 0.0001), concentration (F6 84 = 176.0, p < 0.0001), and a drug*concentration interaction (F12 84 = 14.86, p < 0.0001). Post-hoc testing following separate one-way ANOVA's revealed a significant increase in LDH release due to 4-MMC at all concentrations above 500μM whereas MDMC and METH increased LDH release significantly only at 2mM. Notably, all drugs decreased LDH released at concentrations between 2 and 100μM (Fig. 2B).
METH, as expected, decreased WST-1 reactivity whereas increasing LDH release at the highest dose. The β-keto amphetamines 4-MMC and MDMC, on the other hand, produced an increase in both WST-1 reactivity and LDH release. Furthermore, when observed under a light microscope (Fig. 2C), there was no evidence of increased proliferation, suggesting the increase in WST-1 reactivity was due to another factor.
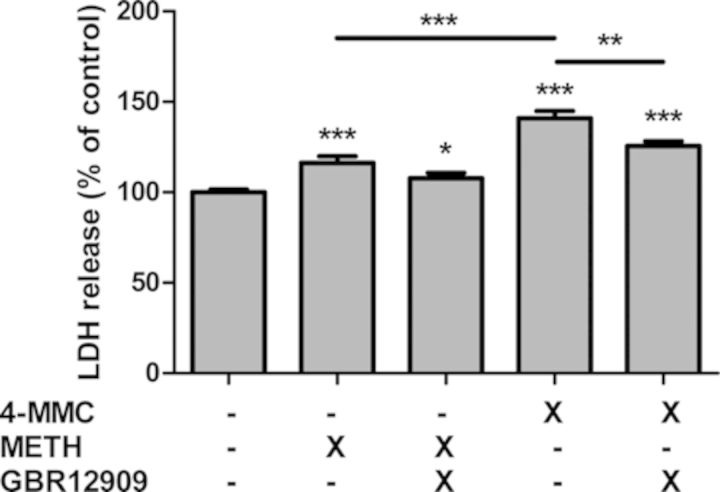
Next, we assessed the effect of the DAT blocker GBR12909 on LDH release induced by METH and 4-MMC (Fig. 3). A significant treatment effect was found (F4 45 = 40.43, p < 0.0001). Post-hoc testing revealed significantly increased LDH release following both METH (p < 0.05) and 4-MMC (p < 0.001). 4-MMC produced a larger increase than METH (p < 0.001). Finally, co-treatment with 1μM GBR12909 significantly decreased LDH release due to 4-MMC (p < 0.01) but not METH.
FIG. 3.
SH-SY5Y cells were treated for 48 h with the β-keto amphetamine 4-MMC or METH (2mM) alone, or in combination with the DAT blocker GBR12909 (1μM). Cells co-treated with GBR12909 were also pre-incubated with GBR12909 for 1 h prior to administration of 4-MMC or METH. *p < 0.05, **p < 0.01, ***p < 0.001. N ≥ 6 per group.
β-KA Redox Reactivity Measurements Using WST-1
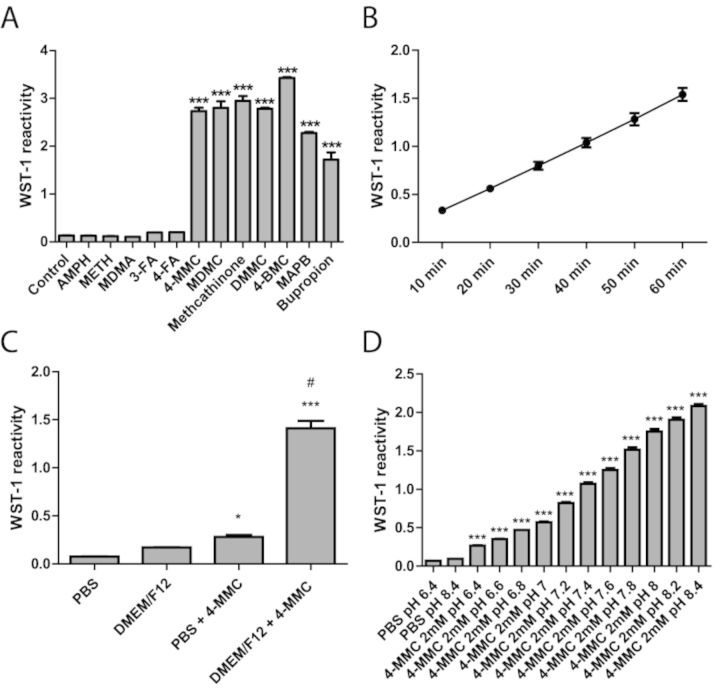
The following experiments were performing without the presence of SH-SY5Y cells. In the absence of cells, the WST-1 reagent is a sensitive redox-indicator based on the reduction of WST-1 to formazan via the intermediate electron acceptor mPMS. To determine whether the observed WST-1 reactivity was due to the ketone-group present in the β-KA, we investigated the effect of the non-keto amphetamines D-amphetamine, METH, MDMA (3,4-methylenedioxymethamphetamine, “ecstasy”), 3-FA (3-fluoroamphetamine) and 4-FA (4-fluoroamphetamine) and the β-KAs 4-MMC, MDMC, methcathinone, DMMC (3,4-dimethylcathinone), 4-BMC (4-bromomethylcathinone, “brephedrone”), MAPB (α-methylamino-butyrophenone, “buphedrone”), and bupropion on WST-1 reactivity following a 1-h incubation (Fig. 4A). A significant drug effect emerged (F12 72 = 741.0, p < 0.0001) which was due to an increase in reactivity with all the β-KAs but none of the non-keto amphetamines (p < 0.001). The reaction kinetics (10–60 min) for the β-KA 4-MMC with WST-1 is shown in Figure 4B and demonstrates a rapid increase in WST-1 reactivity over the course of the experiment. Similar results were obtained when using another tetrazolium redox-indicator 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, data not shown).
FIG. 4.
(A) The effect of the non-keto amphetamines D-amphetamine, METH, MDMA (3,4-methylenedioxymethamphetamine, “ecstasy”), 3-FA (3-fluoroamphetamine) and 4-FA (4-fluoroamphetamine) and the β-KAs 4-MMC, MDMC, methcathinone, DMMC (3,4-dimethylcathinone), 4-BMC (4-bromomethylcathinone, “brephedrone”), MAPB (α-methylamino-butyrophenone, “buphedrone”), and bupropion on WST-1 reactivity in culture medium without cells present. ***p < 0.001 compared with control; #p <0.001 compared with all other conditions. N=3–4 per group. (B) Reaction kinetics (10–60 min) for the reaction between 4-MMC (2mM) and WST-1 in culture medium. N=8 per group, bars indicate 95% confidence interval. (C) Effect of DMEM/F12 growth medium on WST-1 reactivity. 4-MMC (2mM) increased reactivity fourfold in PBS. However, with medium present the increase was significantly larger. *p < 0.05 ***p < 0.001 compared with PBS, #p < 0.001 compared with PBS + 4-MMC. (D) The effect of pH levels on 4-MMC (2mM) induced WST-1 reactivity in PBS. (A–D) *p < 0.05, ***p < 0.001 compared with control. N ≥ 3 per group.
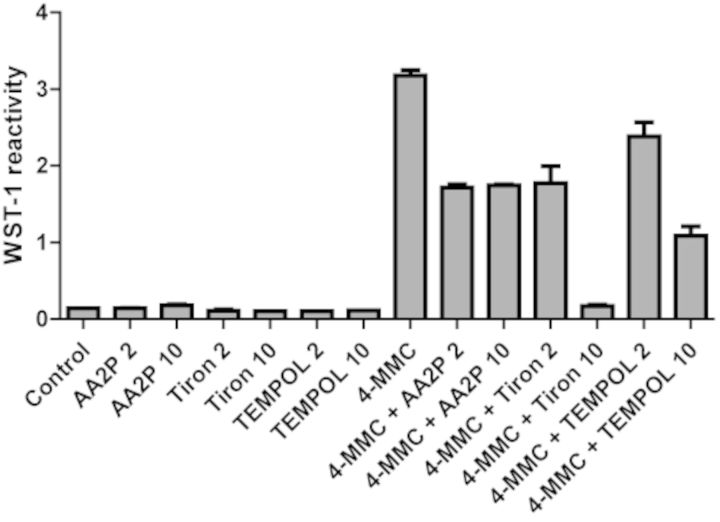
To determine the effect of the DMEM/F12 culture medium on WST-1 reactivity, we compared the effects in medium and PBS (F3,11 = 237.7, p < 0.0001). In PBS alone, 4-MMC produced a significant, fourfold increase in WST-1 reactivity. However, in culture medium, the reactivity was significantly higher with an eightfold increase in reactivity (Fig. 4C). Next, we determined the effect of pH levels, as adjusted by NaHCO3, on the 4-MMC-induced WST-1 reactivity. The reactivity was significantly affected by pH (F12, 40 = 1460, p < 0.0001) as the reactivity strongly increased with raising pH levels in the physiological range (Fig. 4D). We further demonstrated that the 4-MMC induced WST-1 reactivity was reduced by the antioxidant L-Ascorbic acid 2-phosphate (AA2P), the superoxide scavenger TEMPOL and the superoxide dismutase mimetic Tiron (Fig. 5).
FIG. 5.
The effect of the antioxidant L-Ascorbic acid 2-phosphate (AA2P) as well as TEMPOL and Tiron (2 and 10mM) on 4-MMC (2mM) induced WST-1 reactivity in culture medium. N ≥ 2 per group.
We proceeded to determine on which components of the cell culture medium the 4-MMC-induced WST reactivity was dependent. Starting with serum and antimycin-A in PBS, a significant treatment effect (F7, 23 = 64.07, p < 0.0001) was found only for 4-MMC, indicating that neither serum nor antimycin-A affected the reactivity. Similarly, no effect was found for glucose (F3, 26 = 474.0, p < 0.0001) or DMEM/F12 vitamins (F3, 31 = 1158, p < 0.0001) as the tests only revealed an effect of 4-MMC, but no inhibition by either glucose or vitamins. The DMEM/F12 metals, on the other hand, did have an effect on reactivity (F9, 71 = 789.1, p < 0.0001). Zinc, as well as a mix of zinc, iron, and copper caused a slight but significant drop in the 4-MMC induced WST-1 reactivity (a 17% and 27% reduction, respectively). Finally, addition of a DMEM/F12 amino acids mixture attenuated WST-1 reactivity with 4-MMC (F3, 31 = 1304, p < 0.0001).
Detection of Reaction Products
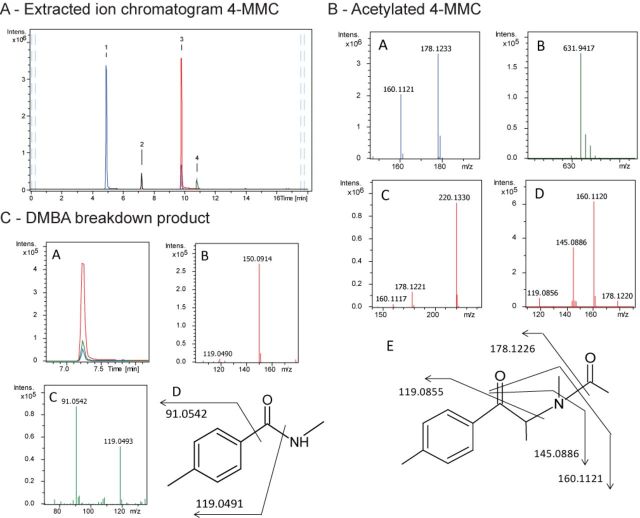
The decrease in WST-1 reactivity observed in the addition of amino acids leads us to investigate the possibility of β-KAs initiating the formation of protein adducts. We investigated the reaction products of 4-MMC, MDMC, and METH both in the absence and presence of DMEM/F12 amino acids. A representative figure of the extracted ion chromatograms for 4-MMC in the absence of amino acids after 85-min reaction time is shown in Figure 6A. In the reaction with 4-MMC and WST-1, we detected one peak with molecular formula C13H18NO2 which corresponds to acetylated 4-MMC (N-Ac-4-MMC). The product ions observed in both MS and broad-band collision induced dissociation (bbCID) MS/MS spectra support the structure of N-Ac-4-MMC (Fig. 6B). We also observed the presence of N,4-dimethylbenzamide (DMBA) in the 4-MMC sample (Fig. 6C). Similarly, in the MDMC sample we observed a product corresponding to acetylated MDMC (N-Ac-4-MDMC) and the product methylenedioxy-N-methylbenzamide (MDMBA) (data not shown). Both 4-MMC and MDMC samples revealed traces of reduced WST-1 reagent, whereas no such traces were observed in the METH samples. Finally, the samples containing amino acids did not indicate the presence of any drug–protein adducts in any of the conditions.
FIG. 6.
A: Extracted ion chromatograms of 4-MMC (1) and WST-1 in PBS + NaCHO3 in the absence of amino acids. Two unknown peaks corresponding to acetylated 4-MMC (3) and N,4-dimethylbenzamide (DMBA) (2) as well as a peak for reduced WST-1 (4) were detected. B: MS full range acquisition (A, B, C) and bbCID MS/MS full range acquisition (D) of 4-MMC (A), reduced WST-1 (B), and acetylated 4-MMC (C, D). Structure of acetylated 4-MMC indicating fragmentation sites (E). C: Extracted ion chromatograms of DMBA and two of its bbCID fragments in the absence of amino acids (A), MS full range (B), and bbCID MS/MS full range acquisition (C) of N,4-dimethylbenzamide (DMBA). Structure of DMBA indicating fragmentation sites.
Mitochondrial Respiration
Basal respiration
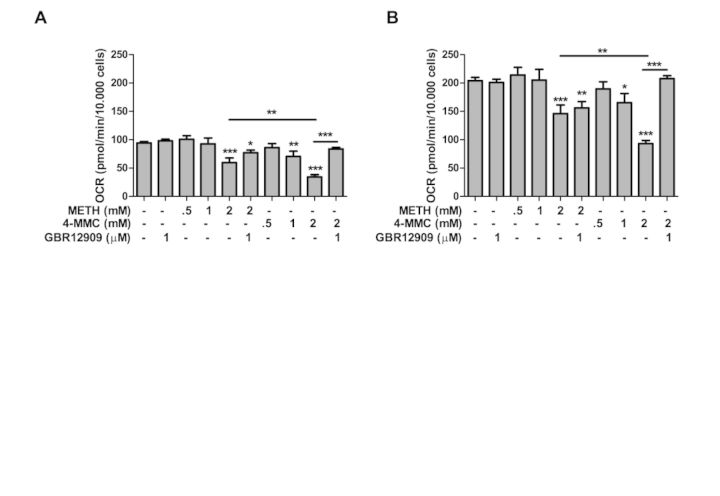
The effect of treatment with METH and 4-MMC alone or in combination with GBR12909 is shown in Figure 7A. An ANOVA revealed a significant effect of treatment on basal respiration (F9 83 = 11.50, p < 0.001). METH reduced basal respiration at a concentration of 2mM (p < 0.001) whereas 4-MMC reduced respiration at concentrations of both 1mM (p < 0.01) and 2mM (p < 0.001). 4-MMC produced a significantly larger decrease in respiration than METH at 2mM (p < 0.01). Furthermore, GBR1290 significantly attenuated the decrease in respiration due to 4-MMC (p < 0.001) but not METH.
FIG. 7.
The effect of treatment with METH or 4-MMC, either alone or in combination with GBR12909, on (A) basal respiration and (B) maximum respiration. The assay was performed in a Seahorse Bioscience XFe96 analyzer. Basal and maximum mitochondrial respiration/OCR was calculated by subtracting baseline OCR and FCCP-induced maximum OCR from the non-mitochondrial OCR after injection with antimycin A + rotenone. *p < 0.05, **p < 0.01, ***p < 0.001. N ≥ 5 per group.
Maximum respiration
As shown in Figure 7B, treatment with METH and 4-MMC with or without GBR12909 also produced a significant effect on FCCP-induced maximum respiration (F9 83 = 9.56, p < 0.001). METH reduced maximum respiration at a concentration of 2mM (p < 0.001) whereas 4-MMC reduced maximum respiration at concentrations of 1mM (p < 0.05) and 2mM (p < 0.001). Maximum respiration was also more strongly reduced by 4-MMC, compared with METH at 2mM (p < 0.01). GBR12909 blocked the 4-MMC-induced decrease in maximum respiration (p < 0.001) but had no effect on METH-induced decrease in respiration rate.
Complex I and II Assay
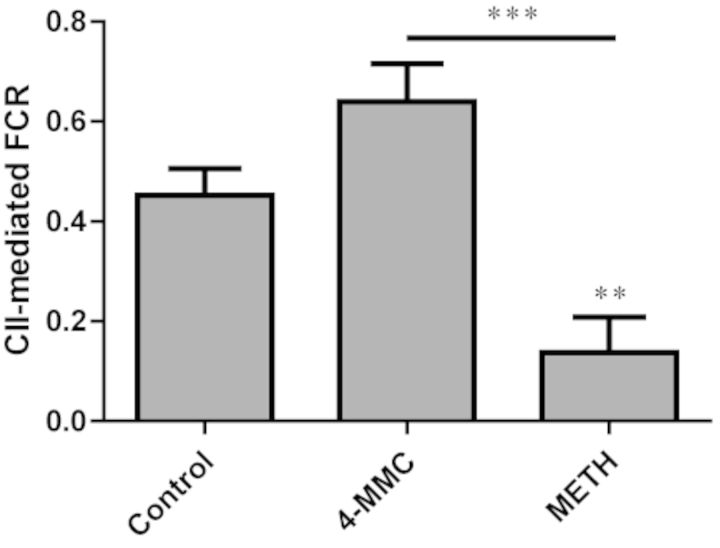
Analysis of the FCR in the ROUTINE, LEAK, OXPHOS (complex I), CI+CII, and CII states yielded a significant effect only for CII (F2, 9 = 14.69, p < 0.05) (Fig. 8). A post-hoc test revealed this was due to a significant (p < 0.01) decrease in the complex II-mediated FCR following incubation with METH, compared with control. This effect was not seen following incubation with 4-MMC. In fact, 4-MMC appeared to produce an increase in complex II-dependent FCR. Although this increase was not statistically significant compared with control (p = 0.08), the difference between 4-MMC and METH was significant (p < 0.001).
FIG. 8.
A 20-min incubation with METH (20mM) but not 4-MMC (20mM) produced a significant decrease in the complex II-mediated FCR. **p < 0.01, ***p < 0.001. N ≥ 4 per group.
DISCUSSION
Here we show cytotoxic effects of the 4-MMC at concentrations of 500μM and higher. MDMC and METH were cytotoxic only at 2mM. However, all drugs decreased LDH release between 2 and 100μM. Moreover, we demonstrate that treatment with GBR12909, a competitive inhibitor of DAT, suppresses the toxicity of 4-MMC. Further, we demonstrate redox electron donor reactivity of β-KAs and show a correlation of increased pH with the redox activity and formation of an N-acetylated form of 4-MMC. We found no evidence of protein adduct formation by β-KAs. Both 4-MMC and METH reduce basal and maximum mitochondrial respiration, but 4-MMC has different effect than METH at the level of mitochondrial complex II.
The experiments in the SH-SY5Y cell line demonstrate that incubation with 4-MMC and MDMC produces cytotoxicity at high concentrations, whereas at lower concentrations we noted small but significant decreases in LDH release. The METH-induced change in cytotoxicity and viability were in a similar range as other studies employing SH-SY5Y cells but much lower than those observed in primary cultures (Ali et al., 2009; Cai and Cadet, 2008; Pendyala et al., 2012; Wu et al., 2007). The SH-SY5Y cells, in its undifferentiated form, display a primarily NE neuron-like phenotype (Xie et al., 2010). Mechanisms such as the oxidation of DA and the subsequent generation toxic free radicals, thought to play an important role in METH toxicity (Yamamoto et al., 2010), may thus not be appropriately modelled in this cell line. Furthermore, the presence of serum also attenuates the toxic response, but is required to maintain viability even of untreated control cells for the duration of the experiment. Nevertheless, at exposure to concentrations between 1 and 2mM of METH the cells show increased reactive oxygen species (ROS) production and mitochondrial damage, mimicking the effects of exposure to binge-like METH treatments in vivo (Wang et al., 2008; Wu et al., 2007) and indicating that, at least at higher concentrations, SH-SY5Y cells model mechanism relevant to toxicity in vivo. Furthermore, we demonstrate that cytotoxicity is reduced by the DAT inhibitor GBR12909, suggesting that DA-related mechanisms are at least partially responsible for the cytotoxic response in this cell line.
Subsequent attempts to measure cell viability using the WST-1 reagent produced a false-positive reaction. False-positive reactions in tetrazolium-based cell viability assays have been reported previously for several compounds, including anti-oxidants (Bernhard et al., 2003; Chakrabarti et al., 2000; Funk et al., 2007; Natarajan et al., 2000). In this case, the false-positive reaction led to the discovery that β-KAs such as 4-MMC are reductants in the presence of an electron acceptor. It is difficult to predict the biological relevance of these reducing properties. Oxidative stress plays a key role in mediating the neurotoxic effects of amphetamines (Yamamoto et al., 2010) and one could hypothesize that β-KAs may interfere with cellular redox buffering systems. Other compounds, such as redox-active iron, have been linked to oxidative stress involved in neurodegenerative processes (Savory et al., 1999; Smith et al., 1997). Furthermore, it would explain why 4-MMC increases the toxicity of METH on striatal DA neurons (Angoa-Perez et al., 2013) despite not being toxic on its own. Importantly, however, recent studies have reported no evidence of 4-MMC toxicity in vivo (Angoa-Perez et al., 2012; den Hollander et al., 2013; Motbey et al., 2012). Several factors such as differences in metabolism or blood-brain-barrier permeability could contribute to this. Furthermore, compounds such as bis(benzylidene)acetone that, like the neurotoxic acrylamide, contain an α,β-unsaturated ketone group were shown to be effective radical scavenging compounds (Dinkova-Kostova et al., 2007), demonstrating that the toxic and protective effects of these types of compounds are closely intertwined, something which is also supported by our results showing decreased cytotoxicity at lower concentrations.
Mitochondria have been identified as the primary source of ROS and free radicals during amphetamine-induced stress (Brown and Yamamoto, 2003). This is interesting as we demonstrated that the reductive potential of 4-MMC is much higher at an increased pH corresponding to the alkaline pH of mitochondria (Llopis et al., 1998). In our measurements this increase may be due to the extent of ionization of 4-MMC which, with a pKa of 8.69 (Santali et al., 2011), is a weak base. The redox potential of β-KAs suggests that they may interact at the level of the electron transport chain, which is already a known target for amphetamines. METH-induced complex II inhibition has been reported previously (Brown et al., 2005) and is confirmed here as we also observed a decrease in complex II-dependent FCR following exposure to METH. We show that both 4-MMC and METH reduce mitochondrial-dependent respiration and that the reduction in respiration due to 4-MMC is larger than METH and blocked by the DAT inhibitor GBR12909. Furthermore, 4-MMC distinguishes itself from METH in that it does not produce a decrease in complex II-mediated FCR. If anything, there appeared to be an increase in complex II-mediated FCR, although this was not statistically significant compared with the control group. The divergent effects of METH and 4-MMC on mitochondrial respiration are interesting considering the importance of mitochondria in amphetamine toxicity. A more detailed investigation of the differing effects of β-KAs and non-keto amphetamines on the electron transport chain is clearly warranted.
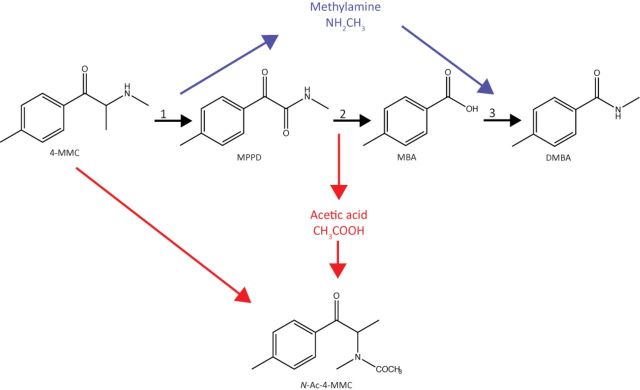
Our observation that the presence of amino acids quenched the 4-MMC-induced reactivity leads us to investigate the possibility that 4-MMC might form adducts with certain amino acids. Adduct formation by carbonyl-containing compounds has been described previously (Lopachin and Decaprio, 2005). We did, however, not find any evidence of protein adduct formation with neither 4-MMC nor MDMC, confirming that the WST-1 reactivity is indeed due to reduction of the WST-1 reagent by the KAs. We did observe the presence of N-acetyl-4-MMC and N-acetyl-MDMC in the 4-MMC and MDMC samples, respectively. This is an intriguing observation because acetylation of 4-MMC was recently suggested to happen under alkaline pH (Tsujikawa et al., 2012). Our results now imply that formation of such acetylated product from 4-MMC and MDMC can proceed under biological pH in the presence of an electron acceptor. Based on Tsujikawa and co-workers’ results on 4-MMC reaction kinetics under alkaline pH leading to formation of N-acetyl-4-MMC and N-acetyl-MDMC, we propose that the oxidative deamination of 4-MMC or MDMC followed by oxidative cleavage releases acetic acid that then acetylates 4-MMC or MDMC (Fig. 9). The identification of N-acetylated β-keto amphetamines may be interesting both from a toxicological and forensic point of view, considering the differing properties of N-acetylated amphetamines compared with their non-acetylated counterparts in vivo (Borges et al., 2001, 2002; Marvola, 1976). However, the presence of N-acetyl-4-MMC and N-acetyl-MDMC has not yet been reported in vivo. Further studies will have to elucidate whether formation of these compounds does occur in vivo, as well as its potential role in metabolism and toxicity of these compounds.
FIG. 9.
Schematic drawing of the mechanism by which acetylated 4-MMC, N-Ac-4-MMC, is produced: 1—oxidative deamination of 4-MMC produces 1-(4-methylphenyl)-1,2-propanedione (MPPD) and methylamine, 2—oxidative cleavage of MPPD produces 4-methylbenzoic acid (MBA) as well as acetic acid, 3—amidation of MBA produces N,4-dimethylbenzamide (DMBA). The acetic acid produced in reaction 2 reacts with 4-MMC to produce N-Ac-4-MMC (Tsujikawa et al., 2012). A similar reactivity was observed for MDMC (see the text).
One limitation of this study is that we only tested the interaction with two tetrazolium reagents. However, they are all based on the reduction of tetrazolium salts to formazans. Another limitation to the pH dependence of 4-MMC reactivity is that the redox reaction of tetrazolium reagents is pH dependent (Plumb et al., 1989). Finally, although the drug concentrations employed (1–2mM) here correspond to those normally used to investigate amphetamine-type drugs in this cell line (Chen et al., 2013; Wang et al., 2008; Wu et al., 2007), they are much higher than the low micromolar concentrations normally achieved by recreational users of these substances (Logan et al., 1998). The current experimental setup intended to compare β-KAs and non-keto amphetamines on certain variables does not permit translation of the results to conditions relevant for human recreational users. Hence, it is important to note that the in vitro results obtained in this study do not demonstrate that β-KAs are toxic in vivo. The results from these experiments should instead be viewed as a way of gaining a better understanding of mechanisms which may be involved in the in vivo effects of METH, 4-MMC, or their interactions.
It is important to continue to identify novel trends on the recreational stimulant drug market and to subject the potential toxicity of novel compounds to scientific scrutiny in order to produce an effective and evidence-based public health policy and harm reduction approach for these substances. Currently, the trend primarily consists of β-KAs such as mephedrone and methylone (EMCDDA, 2012). The biological and toxicological relevance of the redox reactivity difference, as observed here, between β-KAs and non-keto amphetamines should be investigated further in the future, particularly in light of the fact that drugs such as MDMA are now beginning to be used in clinical trials for treatment of anxiety disorders (Mithoefer et al., 2011, 2013). The fact that the subjective effects of β-KAs are similar to MDMA (Carhart-Harris et al., 2011) suggests that they may have similar applications if they are shown to have a comparable or more favorable safety profile.
CONCLUSION
The redox electron donor reactivity in the presence of the tetrazolium-based dyes means these dyes cannot be used for studying the effects of β-KAs on cellular viability and proliferation. Our results show that 4-MMC differs from METH in terms of cytotoxicity, redox reactivity, and effect on the electron transport chain. Future work is needed to elucidate the effects of β-KAs on redox systems, mitochondrial respiration, and its toxicological importance in vivo.
FUNDING
Alkoholitutkimussäätiö.
REFERENCES
- Ali S., Jiang H., Rongzhu L., Milatovic D., Aschner M. Methamphetamine dysregulates redox status in primary rat astrocyte and mesencephalic neuronal cultures. Am. J. Neuroprot. Neuroregen. 2009;1:52–59. doi: 10.1166/ajnn.2009.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Perez M., Kane M. J., Briggs D. I., Francescutti D. M., Sykes C. E., Shah M. M., Thomas D. M., Kuhn D. M. Mephedrone does not damage dopamine nerve endings of the striatum, but enhances the neurotoxicity of methamphetamine, amphetamine, and MDMA. J. Neurochem. 2013;125:102–110. doi: 10.1111/jnc.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Perez M., Kane M. J., Francescutti D. M., Sykes K. E., Shah M. M., Mohammed A. M., Thomas D. M., Kuhn D. M. Mephedrone, an abused psychoactive component of ‘bath salts’ and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. J. Neurochem. 2012;120:1097–1107. doi: 10.1111/j.1471-4159.2011.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard D., Schwaiger W., Crazzolara R., Tinhofer I., Kofler R., Csordas A. Enhanced MTT-reducing activity under growth inhibition by resveratrol in CEM-C7H2 lymphocytic leukemia cells. Cancer Lett. 2003;195:193–199. doi: 10.1016/s0304-3835(03)00157-5. [DOI] [PubMed] [Google Scholar]
- Berridge M. V., Tan A. S. Trans-plasma membrane electron transport: A cellular assay for NADH- and NADPH-oxidase based on extracellular, superoxide-mediated reduction of the sulfonated tetrazolium salt WST-1. Protoplasma. 1998;205:74–82. [Google Scholar]
- Borges C. R., Martin S. D., Meyer L. J., Wilkins D. G., Rollins D. E. Influx and efflux of amphetamine and N-acetylamphetamine in keratinocytes, pigmented melanocytes, and nonpigmented melanocytes. J. Pharm. Sci. 2002;91:1523–1535. doi: 10.1002/jps.10144. [DOI] [PubMed] [Google Scholar]
- Borges C. R., Wilkins D. G., Rollins D. E. Amphetamine and N-acetylamphetamine incorporation into hair: An investigation of the potential role of drug basicity in hair color bias. J. Anal. Toxicol. 2001;25:221–227. doi: 10.1093/jat/25.4.221. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Yamamoto B. K. Effects of amphetamines on mitochondrial function: role of free radicals and oxidative stress. Pharmacol Ther. 2003;99:45–53. doi: 10.1016/s0163-7258(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Quinton M. S., Yamamoto B. K. Methamphetamine-induced inhibition of mitochondrial complex II: Roles of glutamate and peroxynitrite. J. Neurochem. 2005;95:429–436. doi: 10.1111/j.1471-4159.2005.03379.x. [DOI] [PubMed] [Google Scholar]
- Burrows K. B., Gudelsky G., Yamamoto B. K. Rapid and transient inhibition of mitochondrial function following methamphetamine or 3,4-methylenedioxymethamphetamine administration. Eur. J. Pharmacol. 2000;398:11–18. doi: 10.1016/s0014-2999(00)00264-8. [DOI] [PubMed] [Google Scholar]
- Cai N. S., Cadet J. L. The combination of methamphetamine and of the HIV protein, Tat, induces death of the human neuroblastoma cell line, SH-SY5Y. Synapse. 2008;62:551–552. doi: 10.1002/syn.20512. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris R. L., King L. A., Nutt D. J. A web-based survey on mephedrone. Drug Alcohol Depend. 2011;118:19–22. doi: 10.1016/j.drugalcdep.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R., Kundu S., Kumar S., Chakrabarti R. Vitamin A as an enzyme that catalyzes the reduction of MTT to formazan by vitamin C. J. Cell. Biochem. 2000;80:133–138. doi: 10.1002/1097-4644(20010101)80:1<133::aid-jcb120>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Chen L., Huang E., Wang H., Qiu P., Liu C. RNA interference targeting alpha-synuclein attenuates methamphetamine-induced neurotoxicity in SH-SY5Y cells. Brain Res. 2013;1521:59–67. doi: 10.1016/j.brainres.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T., Cheah J., Samouilov A., Zweier J. L., Bozak R. E., Hicks R. J., Talalay P. Phenolic Michael reaction acceptors: Combined direct and indirect antioxidant defenses against electrophiles and oxidants. Med. Chem. 2007;3:261–268. doi: 10.2174/157340607780620680. [DOI] [PubMed] [Google Scholar]
- EMCDDA. Annual Report. 2012. Available at: http://www.emcdda.europa.eu/publications/annual-report/2012. Accessed January 15, ;2014. [Google Scholar]
- Funk D., Schrenk H. H., Frei E. Serum albumin leads to false-positive results in the XTT and the MTT assay. Biotechniques. 2007;43:178. doi: 10.2144/000112528. 180–182. [DOI] [PubMed] [Google Scholar]
- Gnaiger E. Capacity of oxidative phosphorylation in human skeletal muscle: New perspectives of mitochondrial physiology. Int. J. Biochem. Cell. Biol. 2009;41:1837–1845. doi: 10.1016/j.biocel.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E., Daumann J. Neurotoxicity of drugs of abuse—The case of methylenedioxyamphetamines (MDMA, ecstasy), and amphetamines. Dialogues Clin. Neurosci. 2009;11:305–317. doi: 10.31887/DCNS.2009.11.3/egmayfrank. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock G. C., Webb K. M., McFadden L. M., Chu P. W., Ellis J. D., Allen S. C., Andrenyak D. M., Vieira-Brock P. L., German C. L., Conrad K. M., et al. 4-Methylmethcathinone (mephedrone): Neuropharmacological effects of a designer stimulant of abuse. J. Pharmacol. Exp. Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander B., Rozov S., Linden A. M., Uusi-Oukari M., Ojanpera I., Korpi E. R. Long-term cognitive and neurochemical effects of “bath salt” designer drugs methylone and mephedrone. Pharmacol. Biochem. Behav. 2013;103:501–509. doi: 10.1016/j.pbb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Kehr J., Ichinose F., Yoshitake S., Goiny M., Sievertsson T., Nyberg F., Yoshitake T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br. J. Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llopis J., McCaffery J. M., Miyawaki A., Farquhar M. G., Tsien R. Y. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl Acad. Sci. U.S.A. 1998;95:6803–6808. doi: 10.1073/pnas.95.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan B. K., Fligner C. L., Haddix T. Cause and manner of death in fatalities involving methamphetamine. J. Forensic Sci. 1998;43:28–34. [PubMed] [Google Scholar]
- Lopachin R. M., Decaprio A. P. Protein adduct formation as a molecular mechanism in neurotoxicity. Toxicol. Sci. 2005;86:214–225. doi: 10.1093/toxsci/kfi197. [DOI] [PubMed] [Google Scholar]
- Lopez-Arnau R., Martinez-Clemente J., Pubill D., Escubedo E., Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: Butylone, mephedrone and methylone. Br. J. Pharmacol. 2012;167:407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvola M. Effect of acetylated derivatives of some sympathomimetic amines on the acute toxicity, locomotor activity and barbiturate anaesthesia time in mice. Acta Pharmacol. Toxicol. (Copenh.) 1976;38:474–489. doi: 10.1111/j.1600-0773.1976.tb03143.x. [DOI] [PubMed] [Google Scholar]
- Mithoefer M. C., Wagner M. T., Mithoefer A. T., Jerome L., Doblin R. The safety and efficacy of {+/-}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: The first randomized controlled pilot study. J. Psychopharmacol. 2011;25:439–452. doi: 10.1177/0269881110378371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer M. C., Wagner M. T., Mithoefer A. T., Jerome L., Martin S. F., Yazar-Klosinski B., Michel Y., Brewerton T. D., Doblin R. Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: A prospective long-term follow-up study. J. Psychopharmacol. 2013;27:28–39. doi: 10.1177/0269881112456611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motbey C. P., Karanges E., Li K. M., Wilkinson S., Winstock A. R., Ramsay J., Hicks C., Kendig M. D., Wyatt N., Callaghan P. D., et al. Mephedrone in adolescent rats: Residual memory impairment and acute but not lasting 5-HT depletion. PLoS One. 2012:e45473. doi: 10.1371/journal.pone.0045473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan M., Mohan S., Martinez B. R., Meltz M. L., Herman T. S. Antioxidant compounds interfere with the 3. Cancer Detect. Prev. 2000;24:405–414. [PubMed] [Google Scholar]
- Pendyala G., Ninemire C., Fox H. S. Protective role for the disulfide isomerase PDIA3 in methamphetamine neurotoxicity. PLoS One. 2012;7:e38909. doi: 10.1371/journal.pone.0038909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskin A. V., Winterbourn C. C. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1) Clin. Chim. Acta. 2000;293:157–166. doi: 10.1016/s0009-8981(99)00246-6. [DOI] [PubMed] [Google Scholar]
- Pesta D., Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012;810:25–58. doi: 10.1007/978-1-61779-382-0_3. [DOI] [PubMed] [Google Scholar]
- Plumb J. A., Milroy R., Kaye S. B. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989;15:4435–4440. [PubMed] [Google Scholar]
- Santali E. Y., Cadogan A. K., Daeid N. N., Savage K. A., Sutcliffe O. B. Synthesis, full chemical characterisation and development of validated methods for the quantification of (+/-)-4’-methylmethcathinone (mephedrone): A new “legal high”. J. Pharm. Biomed. Anal. 2011;56:246–255. doi: 10.1016/j.jpba.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Savory J., Rao J. K., Huang Y., Letada P. R., Herman M. M. Age-related hippocampal changes in Bcl-2:Bax ratio, oxidative stress, redox-active iron and apoptosis associated with aluminum-induced neurodegeneration: Increased susceptibility with aging. Neurotoxicology. 1999;20:805–817. [PubMed] [Google Scholar]
- Smith M. A., Harris P. L., Sayre L. M., Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl Acad. Sci. U.S.A. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom M., Pelander A., Angerer V., Hutter M., Kneisel S., Ojanpera I. A high-sensitivity ultra-high performance liquid chromatography/high-resolution time-of-flight mass spectrometry (UHPLC-HR-TOFMS) method for screening synthetic cannabinoids and other drugs of abuse in urine. Anal. Bioanal. Chem. 2013;405:8463–8474. doi: 10.1007/s00216-013-7272-8. [DOI] [PubMed] [Google Scholar]
- Tan A. S., Berridge M. V. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: A simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J. Immunol. Methods. 2000;238:59–68. doi: 10.1016/s0022-1759(00)00156-3. [DOI] [PubMed] [Google Scholar]
- Tsujikawa K., Mikuma T., Kuwayama K., Miyaguchi H., Kanamori T., Iwata Y. T., Inoue H. Degradation pathways of 4-methylmethcathinone in alkaline solution and stability of methcathinone analogs in various pH solutions. Forensic Sci. Int. 2012;220:103–110. doi: 10.1016/j.forsciint.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Wang S. F., Yen J. C., Yin P. H., Chi C. W., Lee H. C. Involvement of oxidative stress-activated JNK signaling in the methamphetamine-induced cell death of human SH-SY5Y cells. Toxicology. 2008;246:234–241. doi: 10.1016/j.tox.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Wright M. J. Jr., Angrish D., Aarde S. M., Barlow D. J., Buczynski M. W., Creehan K. M., Vandewater S. A., Parsons L. H., Parsons L. H., Houseknecht K. L., et al. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PLoS One. 2012;7:e44652. doi: 10.1371/journal.pone.0044652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. W., Ping Y. H., Yen J. C., Chang C. Y., Wang S. F., Yeh C. L., Chi C. W., Lee H. C. Enhanced oxidative stress and aberrant mitochondrial biogenesis in human neuroblastoma SH-SY5Y cells during methamphetamine induced apoptosis. Toxicol. Appl. Pharmacol. 2007;220:243–251. doi: 10.1016/j.taap.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Xie H. R., Hu L. S., Li G. Y. SH-SY5Y human neuroblastoma cell line: In vitro cell model of dopaminergic neurons in Parkinson's disease. Chin. Med. J. (Engl.) 2010;123:1086–1092. [PubMed] [Google Scholar]
- Yamamoto B. K., Moszczynska A., Gudelsky G. A. Amphetamine toxicities: Classical and emerging mechanisms. Ann. N.Y. Acad. Sci. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]