Abstract
Humans are exposed to distinct structural classes of insecticides with different neurotoxic modes of action. Because calcium homeostasis is essential for proper neuronal function and development, we investigated the effects of insecticides from different classes (pyrethroid: (α-)cypermethrin; organophosphate: chlorpyrifos; organochlorine: endosulfan; neonicotinoid: imidacloprid) and mixtures thereof on the intracellular calcium concentration ([Ca2+]i). Effects of acute (20 min) exposure to (mixtures of) insecticides on basal and depolarization-evoked [Ca2+]i were studied in vitro with Fura-2-loaded PC12 cells and high resolution single-cell fluorescence microscopy. The data demonstrate that cypermethrin, α-cypermethrin, endosulfan, and chlorpyrifos concentration-dependently decreased depolarization-evoked [Ca2+]i, with 50% (IC50) at 78nM, 239nM, 250nM, and 899nM, respectively. Additionally, acute exposure to chlorpyrifos or endosulfan (10μM) induced a modest increase in basal [Ca2+]i, amounting to 68 ± 8nM and 53 ± 8nM, respectively. Imidacloprid did not disturb basal or depolarization-evoked [Ca2+]i at 10μM. Following exposure to binary mixtures, effects on depolarization-evoked [Ca2+]i were within the expected effect additivity range, whereas the effect of the tertiary mixture was less than this expected additivity effect range. These results demonstrate that different types of insecticides inhibit depolarization-evoked [Ca2+]i in PC12 cells by inhibiting voltage-gated calcium channels (VGCCs) in vitro at concentrations comparable with human occupational exposure levels. Moreover, the effective concentrations in this study are below those for earlier described modes of action. Because inhibition of VGCCs appears to be a common and potentially additive mode of action of several classes of insecticides, this target should be considered in neurotoxicity risk assessment studies.
Keywords: in vitro neurotoxicity, mixture toxicity, calcium homeostasis, voltage-gated calcium channels, PC12 cells, insecticides
Abbreviations
- [Ca2+]i
intracellular calcium concentration
- CI
confidence intervals
- EI
expected inhibition
- IC20
concentration that induces an inhibitory effect of 20%
- IC50
concentration that induces an inhibitory effect of 50%
- MOE
margin of exposure
- MRL
maximum residue level
- nAChR
nicotinic acetylcholine receptor
- NOEC
no-observed-effect concentration
- OP
organophosphate
- TR
treatment ratio
- VGCC
voltage-gated calcium channel
- VGSC
voltage-gated sodium channel
Insecticides are potent neurotoxicants, which are used in agriculture and households worldwide. In many cases, their neurotoxic mode of action is not species-specific, and the widespread use of these chemicals may thus pose a risk to human health (Mackenzie Ross et al., 2013). There are many types of insecticides, commonly classified according to their structure and mode of action.
Pyrethroids induce neuronal overexcitation by delaying the inactivation of voltage-gated sodium channels (VGSCs; Soderlund, 2012). They are rapidly metabolized in humans (e.g., Leng et al., 2006) and mammals (Wolansky and Harrill, 2008). Nevertheless, acute oral exposure to cypermethrin (and other pyrethroids) can affect behavior in mice and rats (Wolansky and Harrill, 2008) and was shown in vitro to have several targets, including VGSCs (Meacham et al., 2008), potassium channels (Yu-Tao et al., 2009), and ATPase (Kakko et al., 2004). Moreover, cypermethrin residues are detected in food at concentrations that exceed the maximum residue level (MRL; EFSA, 2013).
Organophosphates (OPs) are known as potent, irreversible inhibitors of acetylcholinesterase (AChE). Nonetheless, chlorpyrifos, an extensively studied OP, was demonstrated to have several additional effects in vivo at concentrations below those required for AChE inhibition (reviewed in Eaton et al., 2008). Moreover, chlorpyrifos induces several adverse effects in neural cells in vitro, such as inhibition of neurite outgrowth (reviewed in Eaton et al., 2008) and inhibition of voltage-gated calcium channels (VGCCs; Meijer et al., 2014). Chlorpyrifos is widely used and frequently exceeds the MRL (EFSA, 2013).
Organochlorine insecticides are divided into two main groups: DDT-type insecticides that are known to target VGSCs, and chlorinated alicyclic insecticides such as endosulfan that target GABA and glycine receptors (Coats, 1990). Endosulfan can still be found in human tissues as it is poorly metabolized and highly persistent in the environment (Mrema et al., 2013). Chronic and acute exposure to endosulfan can affect behavior in adult rats (Castillo et al., 2002; Silva and Beauvais, 2010). Additionally, in vitro research demonstrated that endosulfan exposure affects a number of neurotoxicological targets and endpoints, such as caspase-3, NFκB, formation of reactive oxygen species as well as GABAA and glycine-gated chlorine channels (Jia and Misra, 2007; Vale et al., 2003).
Neonicotinoid insecticides can induce neuronal overexcitation by targeting nicotinic acetylcholine receptors (nAChR; Matsuda et al., 2009). However, the human health risk associated with neonicotinoids is presumably low due to the high selectivity for insect nAChR compared with mammals (Tomizawa and Casida, 2005). Nevertheless, imidacloprid has been shown to induce neurobehavioral changes in young rats following developmental exposure (Abou-Donia et al., 2008). Moreover, some in vitro studies indicate that imidacloprid has additional targets in neuronal cells as it activates the extracellular signal-regulated kinase cascade (Tomizawa and Casida, 2002) and can cause a depolarization shift of the membrane potential (Bal et al., 2010).
Calcium homeostasis is an important endpoint in neurotoxicity studies because proper regulation of the intracellular calcium concentration ([Ca2+]i) is critical for normal neurotransmission and neural development (Leclerc et al., 2011). Notably, even small changes in [Ca2+]i can result in adverse effects (Toescu and Verkhratsky, 2007).
It has previously been shown in rat PC12 cells that inhibition of the depolarization-evoked increase in [Ca2+]i, which is mediated by VGCCs, is a common mode of action for several persistent environmental pollutants (Dingemans et al., 2010; Langeveld et al., 2012), the organochlorine insecticides lindane and dieldrin (Heusinkveld and Westerink, 2012), the OP insecticides chlorpyrifos and parathion (Meijer et al., 2014), and several conazole fungicides (Heusinkveld et al., 2013).
In addition to inhibition of VGCCs, insecticides disturb calcium homeostasis through other mechanisms. Lindane and chlorpyrifos induce an increase in [Ca2+]i by depolarization of the membrane and by release of calcium from intracellular calcium stores, respectively (Heusinkveld et al., 2010; Meijer et al., 2014). On the other hand, imidacloprid can induce calcium influx through activation of calcium permeable nAChR as shown in rat cerebellar granule cells (Kimura-Kuroda et al., 2012), whereas pyrethroids, including cypermethrin, can increase sodium influx, resulting in depolarization and subsequent calcium influx via VGCCs as shown in mouse neocortical neurons (Cao et al., 2011).
To investigate if disturbance of calcium homeostasis is a common mode of action for insecticides, we investigated the effects of low concentrations (1nM–10μM) of (α-)cypermethrin, chlorpyrifos, endosulfan, and imidacloprid on basal and depolarization-evoked [Ca2+]i in PC12 cells. PC12 cells are well characterized and commonly used for mechanistic neurotoxicological and neurophysiological studies (Westerink, 2013; Westerink and Ewing, 2008) and express L-, N-, and P/Q-type VGCCs (Dingemans et al., 2009; Heusinkveld et al., 2010). Because humans are simultaneously exposed to multiple insecticides (EFSA, 2013) we also investigated if the effects of mixtures of insecticides are additive.
MATERIALS AND METHODS
Chemicals
Fura-2 AM was obtained from Molecular Probes (Invitrogen, Breda, The Netherlands). Cypermethrin (purity 95.1%), α-cypermethrin (purity 99.7%), chlorpyrifos (purity 99.9%), endosulfan (α:β 2:1; purity 99.9%), imidacloprid (purity 99.9%), and all other chemicals were obtained from Sigma-Aldrich (Zwijndrecht, The Netherlands) unless otherwise noted. Saline solutions (containing in mM: 125 NaCl, 5.5 KCl, 2 CaCl2, 0.8 MgCl2, 10 HEPES, 24 glucose and 36.5 sucrose at pH 7.3, adjusted with NaOH) were prepared with deionized water (Milli-Q; resistivity >18 MΩ·cm). Stock solutions were prepared in DMSO and final solutions (solvent concentration 0.1% DMSO) were prepared daily.
Cell culture
Rat pheochromocytoma (PC12) cells (Greene and Tischler, 1976) were cultured for up to 10 passages in RPMI 1640 medium (Invitrogen) supplemented with 10% horse serum, 5% fetal bovine serum and 2% penicillin/streptomycin (ICN Biomedicals, Zoetermeer, The Netherlands) as described previously (Dingemans et al., 2010; Langeveld et al., 2012; Meijer et al., 2014). All cell culture material was coated with poly-l-lysine (50 μg/ml). Cells were grown in a humidified incubator at 37°C and 5% CO2. Medium was refreshed every 2–3 days. For single-cell fluorescent microscopy Ca2+ imaging experiments, PC12 cells were subcultured (at ∼75% confluency) in glass-bottom dishes (MatTek, Ashland, MA) as described previously (Dingemans et al., 2010; Langeveld et al., 2012; Meijer et al., 2014).
[Ca2+]i measurements
Changes in [Ca2+]i were measured with the Ca2+ sensitive fluorescent ratio dye Fura-2 AM as described previously (Dingemans et al., 2010; Langeveld et al., 2012; Meijer et al., 2014). Briefly, PC12 cells were loaded with 5μM Fura-2 AM for 20 min in saline, followed by 15 min de-esterification in saline at room temperature. Cells were placed on the stage of an Axiovert 35M inverted microscope (40× oil-immersion objective, numerical aperture 1.0; Zeiss, Göttingen, Germany) equipped with a TILL Photonics Polychrome IV (Xenon Short Arc lamp, 150W; TILL Photonics GmBH, Gräfelfing, Germany) and continuously superfused with saline using a Valvelink 8.2 (Automate Scientific, CA). Fluorescence, excited by 340 and 380nM wavelengths (F340 and F380), was collected every 3 s at 510nM with an Image SensiCam digital camera (TILL Photonics GmBH). All experiments were performed at room temperature. Every experiment consisted of a 5 min baseline recording and a subsequent depolarization of the cells by superfusing with 100 mM K+ saline (containing in mM: 5.5 NaCl, 100 KCl, 2 CaCl2, 0.8 MgCl2, 10 HEPES, 24 glucose and 36.5 sucrose at pH 7.3, adjusted with NaOH) for 21 s. Next, cells were allowed to recover in saline for 8 min prior to superfusion with saline containing 0.1% DMSO (vehicle control) or (mixtures of) insecticides (1nM–10μM) for 20 min to determine effects of insecticide exposure on basal [Ca2+]i. Following this 20 min exposure, cells were depolarized for a second time in the presence of DMSO or (mixtures of) insecticides (see Fig. 1A for example recording) to determine effects of insecticide exposure on depolarization-evoked [Ca2+]i. At the end of each recording, cells were permeabilized with 5μM ionomycin to determine the maximum ratio (Rmax) after which all Ca2+ was chelated with 17mM ethylenediamine tetraacetic acid to determine the minimum ratio (Rmin) to calculate [Ca2+]i (see below).
FIG. 1.
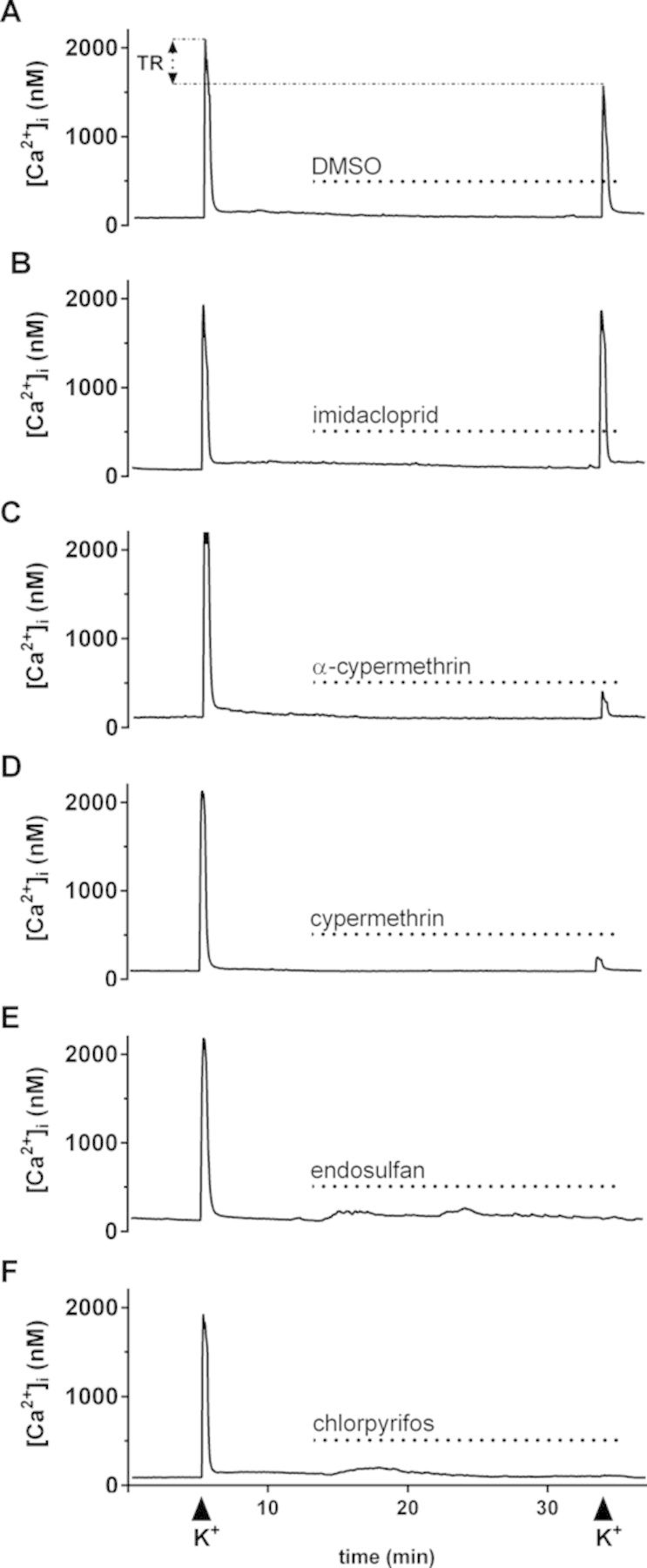
Representative recordings of [Ca2+]i from individual PC12 cells exposed to DMSO (A), 10μM imidacloprid (B), α-cypermethrin (C), cypermethrin (D), endosulfan (E), and chlorpyrifos (F). PC12 cells were exposed to saline containing test compound for 20 min as indicated by the dotted line. Before and following 20 min exposure of the cells, depolarization was evoked by high K+-containing saline as indicated by the arrowheads below the recordings. Changes in treatment ratio (TR = amplitude second depolarization/amplitude first depolarization * 100%), normalized to DMSO controls, were used as a measure for effects of insecticides on the depolarization-evoked increase in [Ca2+]i.
Individual insecticides were selected for the mixture experiments based on their potency to inhibit VGCCs and were combined at the (calculated) concentrations that induce an inhibition of the depolarization-evoked [Ca2+]i of 20% (IC20).
Data analysis and statistics
Data were processed with TILLVisION software (version 4.01) and further analyzed with a custom-made MS-Excel macro. A modified Grynkiewicz's equation [Ca2+]i = Kd* * (R − Rmin)/(Rmax − R), where Kd* is the dissociation constant of Fura-2 determined in the experimental setup, was used to calculate free cytosolic [Ca2+]i from background-corrected F340/F380 ratio values (Dingemans et al., 2010; Langeveld et al., 2012; Meijer et al., 2014).
Insecticide-induced effects on basal [Ca2+]i were expressed as the average increase in [Ca2+]i during the first 5 min of exposure of responding cells. Only cells that displayed an increase in [Ca2+]i during exposure ≥ basal + SD were regarded as responding cells and were selected for quantification of basal [Ca2+]i effects.
Effects on the depolarization-evoked increase in [Ca2+]i were expressed as a treatment ratio (TR) as described previously (Meijer et al., 2014; see Fig. 1A for illustration). Data are expressed as mean ± SEM (calculated from number of cells (n)), normalized to the control, unless otherwise noted. Cells that did not respond to the first depolarization (amplitude <3× basal) were excluded for further analysis. Additionally, cells that show effects outside the range of the average ± 2×SD are considered as outliers and were excluded for further analysis (∼15%). Per test condition ≥4 independent experiments (N) were performed to obtain ≥38 cells (n) after outlier exclusion. For the calculation of IC20s a non-linear regression curve with Hill-slope was fitted by use of GraphPad Prism software (v6, GraphPad Software, La Jolla, CA).
Cypermethrin, chlorpyrifos, and endosulfan were used at their IC20s in the mixture experiments to test if additivity applies. In view of the similar endpoint (i.e., inhibition of depolarization-evoked [Ca2+]i), concentration-addition could be assumed. However, this assumption cannot be confirmed because the actual underlying mechanism(s) of action is not known (i.e., different subtypes of VGCCs exist that have multiple molecular entities/binding sites that can be targeted by the individual chemicals) and the different chemicals could thus also target VGCCs via independent action. Therefore, from a theoretical point of view, effect-addition for these three insecticides cannot be excluded. Nevertheless, it has recently been suggested that in the case of effect-addition with different underlying mechanisms of action concentration-addition may also apply (Kortenkamp et al., 2012).
Effects of mixtures were considered additive if the measured effect was within the expected additivity effect range. This range was calculated from the expected inhibition (EI) of the mixtures in case of additivity (i.e., an EI of 40% for a binary IC20 mixture and an EI of 60% for a tertiary IC20 mixture) and the sum of the confidence intervals (CI) of the IC20s of the individual chemicals. Thus, the additivity effect range is 100−(EI1+EI2) ± (CI1+CI2). If effects were below or above this range, the effect of the mixture was considered less or more than additive, respectively.
Data of test conditions for which the TR approached 0% are by definition not normally distributed because the TR cannot be negative. However, data from controls and from conditions that did not exert a (near) maximal effect were normally distributed (Kolmogorov–Smirnov test and Shapiro-Wilk test) and continuous data were therefore compared with an unpaired t-test and concentration-response curves with a one-way ANOVA. Data were considered statistically significant if p < 0.05.
RESULTS
Effects of Insecticides on Basal [Ca2+]i
Basal [Ca2+]i in resting PC12 cells is low and stable (99 ± 1nM; n = 206, N = 22). Upon depolarization with high K+-containing saline, [Ca2+]i rapidly and transiently increased to 2.1 ± 0.1μM. During a subsequent 8 min recovery period, [Ca2+]i returned to near resting values and was unaffected by 20 min exposure to DMSO-containing saline (Fig. 1A). When cells were exposed to 10μM imidacloprid (Fig. 1B), α-cypermethrin (Fig. 1C) or cypermethrin (Fig. 1D), basal [Ca2+]i levels were unaffected. In contrast, in 40% of the cells exposed to 10μM endosulfan a small but sustained increase in [Ca2+]i was observed, which lasted for 20 min and amounted to 53 ± 8nM in the first 5 min (18 out of 45 cells (40%), N = 6; Fig. 1E). When cells were exposed to endosulfan in Ca2+-free medium, the increase in [Ca2+]i was no longer observed (data not shown). When cells were exposed to 10μM chlorpyrifos, 56% of the cells responded with a small but transient increase in [Ca2+]i, which sustained for ∼5 min and amounted to 68 ± 8nM (30 out of 54 cells (56%), N = 7; Fig. 1F). At concentrations ≤1μM, endosulfan and chlorpyrifos did not disturb basal [Ca2+]i (data not shown).
Effects of Insecticides on Depolarization-Evoked Increase in [Ca2+]i
The effects of insecticide exposure on the increase in depolarization-evoked [Ca2+]i, which is mediated by VGCCs, were investigated by depolarization of the cells for a second time with high K+-containing saline following the 20 min of exposure to saline containing DMSO (control) or insecticide. In control cells, the second depolarization-evoked increase in [Ca2+]i amounted to 1.5 ± 0.05μM (n = 206, N = 22), yielding a net TR of 69% (Fig. 1A). The TR of control cells was set to 100% and all data of insecticide-exposed cells were normalized to the control.
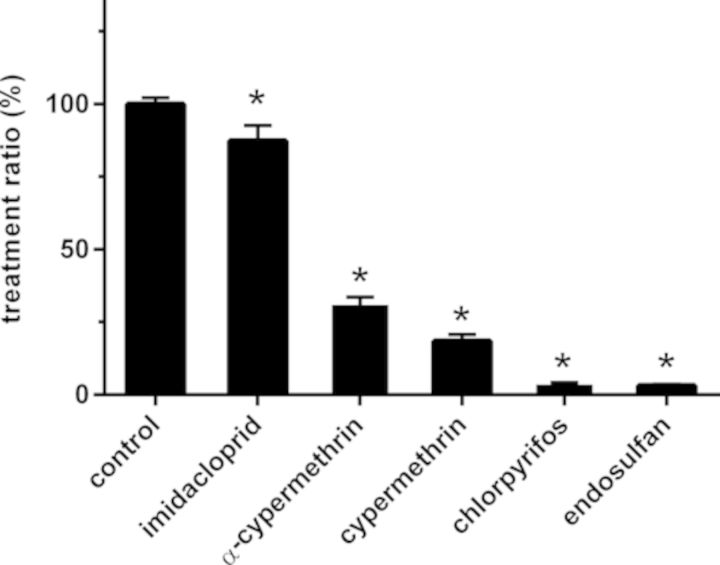
Effects on the depolarization-evoked increase in [Ca2+]i were initially tested at 10μM. All tested insecticides significantly decreased the TR with endosulfan yielding the largest inhibition, reducing the TR to 3 ± 0.5% (n = 45, N = 6; p < 0.001; Figs. 1E and 2). At 10μM, chlorpyrifos yielded a comparable decrease in TR compared with endosulfan (TR: 4 ± 1%; n = 54, N = 7; p < 0.001; Figs. 1F and 2; also see Meijer et al., 2014). At 10μM, α-cypermethrin and cypermethrin reduced the TR to 31 ± 3% (n = 57, N = 6; p < 0.001; Figs. 1C and 2) and 18 ± 2% (n = 50, N = 5; p < 0.001; Figs. 1D and 2), respectively. For concentration-response curves (Fig. 3), all insecticides were tested at lower concentrations, except for imidacloprid which had only a small effect at 10μM (TR: 87 ± 5%; n = 38, N = 4; p < 0.03; Figs. 1B and 2).
FIG. 2.
The inhibitory effects of insecticides on the depolarization-evoked increase in [Ca2+]i. At 10μM, α-cypermethrin, cypermethrin, chlorpyrifos, and endosulfan effectively inhibited the TR (depolarization-evoked increase in [Ca2+]i after exposure compared with depolarization-evoked increase in [Ca2+]i before exposure), whereas imidacloprid induced only a modest inhibition of the TR. Data represent mean ± SEM (n = 38–206, N = 4–22). * indicates a significant difference compared with control (p < 0.05).
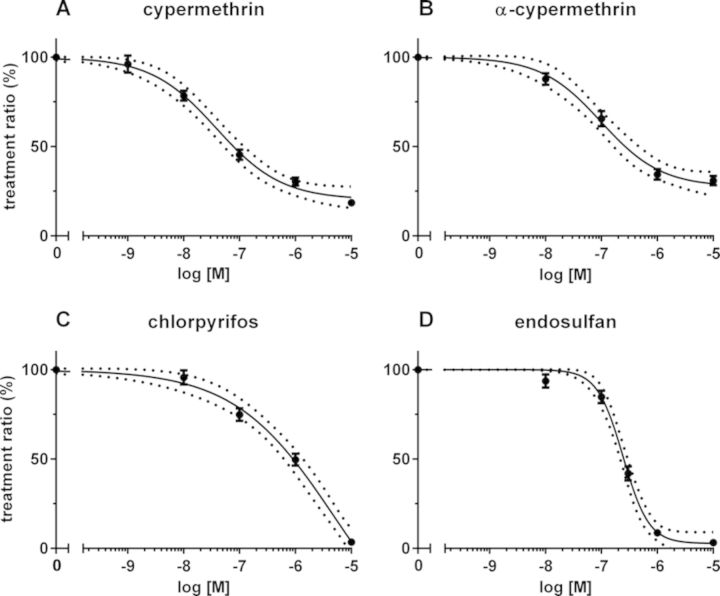
FIG. 3.
Concentration-response curves of inhibition of depolarization-evoked [Ca2+]i by the insecticides cypermethrin (A), α-cypermethrin (B), chlorpyrifos (C), and endosulfan (D). The selected insecticides concentration-dependently inhibited the TR, with cypermethrin being most potent. The effects were significantly different from control (p < 0.05) at ≥0.01μM (cypermethrin) and ≥0.1μM (α-cypermethrin, chlorpyrifos, and endosulfan). For calculated IC20s and IC50s, see Table 1. Data represent mean ± SEM (n = 38–206, N = 4–22). The dotted line indicates the 95% confidence interval of the fitted curve.
Compared with DMSO controls, cells exposed to cypermethrin for 20 min displayed a concentration-dependent inhibition of the TR (p < 0.001). This TR amounted to 78 ± 3% (n = 53, N = 6; p < 0.001) at 10nM, 45 ± 3% (n = 66, N = 5; p < 0.001) at 100nM, and 30 ± 2% (n = 63, N = 6; p < 0.001) at 1μM (Fig. 3A), resulting in IC20 and IC50 values of 9nM and 78nM, respectively. At 100nM, α-cypermethrin reduced the TR to 66 ± 4% (n = 65, N = 8; p < 0.001) and to 34% ± 3 (n = 60, N = 7; p < 0.001) at 1μM (Fig. 3B; IC20: 27nM, IC50: 239nM; Table 1). Notably, the effect of cypermethrin was significantly different from that of α-cypermethrin at ≥0.01μM. As also demonstrated previously (Meijer et al., 2014), chlorpyrifos concentration-dependently decreased the TR to 75 ± 4% (n = 52, N = 5; p < 0.001) at 100nM, and to 50 ± 3% (n = 59, N = 6; p < 0.001) at 1μM (Fig. 3C; IC20: 85nM, IC50: 899nM; Table 1). Endosulfan also concentration-dependently decreased the TR, amounting to 85 ± 4% (n = 59, N = 6; p < 0.001) at 100nM, to 42 ± 4% (n = 39, N = 7; p < 0.001) at 300nM, and to 9 ± 1% (n = 55, N = 7; p < 0.001) at 1μM (Fig. 3D; IC20: 118nM, IC50: 250nM; Table 1).
TABLE 1. Human Exposure Concentrations and (No) Effect Concentrations for Inhibition of VGCCs of Endosulfan, Cypermethrin and Chlorpyrifos (in μM) with the Estimated Margin of Exposurea.
| Insecticide | Human occupational exposure | (No) effect concentrations for VGCC inhibition | Margin of exposureb | |||
|---|---|---|---|---|---|---|
| Concentration | Estimated from | IC50 | IC20 | NOEC | ||
| α-cypermethrin | 0.0010–0.0037 | Internal daily dose from urinary metabolitec | 0.239 | 0.03 | 0.01 | 2.7–9.7 |
| Chlorpyrifos | 0.0071–0.1520 | Absorbed daily dose from urinary metabolited | 0.899 | 0.08 | 0.01 | 0.07–1.4 |
| Endosulfan | 1.3371 | Blood serume | 0.25 | 0.12 | 0.01 | 0.007 |
aRisk estimates presented here did not take into account possible detoxification or bioactivation pathways that may influence the risk assessment.
bMargin of exposure: NOEC/exposure concentration; NOEC, no-observed-effect concentration.
cSingleton et al., 2014.
dPhung et al., 2012.
eDalvie et al., 2009.
Effects of Mixtures of Insecticides on Depolarization-Evoked Increase in [Ca2+]i
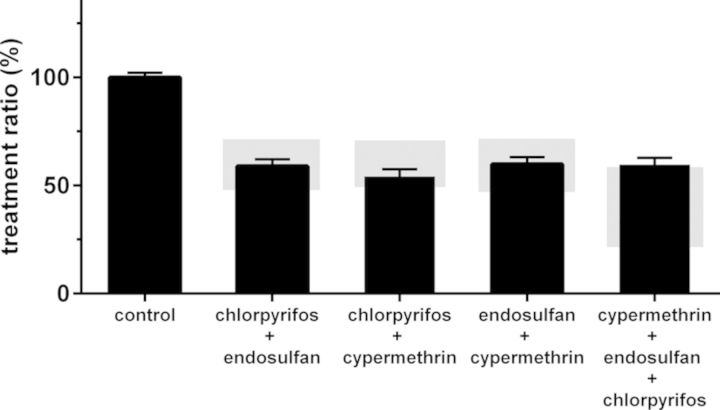
Insecticides with the highest potency (cypermethrin, chlorpyrifos, and endosulfan) were combined as binary mixtures for the investigation of additive effects on the depolarization-evoked [Ca2+]i. A mixture of chlorpyrifos (IC20) combined with endosulfan (IC20) resulted in a TR of 59 ± 3% (n = 48, N = 4), which was well within the expected additivity effect range (48–72%; Fig. 4). When chlorpyrifos (IC20) and cypermethrin (IC20) were combined, the TR amounted to 54 ± 3% (n = 47, N = 5), i.e., within the expected additivity effect range (49–71%; Fig. 4). Similarly, when endosulfan (IC20) was combined with cypermethrin (IC20) the TR amounted to 60 ± 3% (n = 49, N = 6), i.e., within the expected additivity effect range (48–72%; Fig. 4). However, when all three insecticides were combined, the TR amounted to 60 ± 3% (n = 46, N = 4), which was just above the expected additivity effect range (22–58%). Though the inhibitory effect of the tertiary mixture on TR is only slightly less than additive, these data indicate that mixture effects cannot completely be predicted based on additivity alone.
FIG. 4.
Effects of mixtures of insecticides on the depolarization-evoked increase in [Ca2+]i. Binary and tertiary mixtures of IC20s were investigated to determine if the inhibitory effects of mixtures on the TR are additive. All mixtures decreased the TR significantly compared with the control. The gray shaded areas indicate the expected additivity effect range, illustrating that the binary mixtures induced an additive inhibition compared with the single compounds and that the tertiary mixture induced a less than additive inhibition. Data represent mean ± SEM (n = 46–206, N = 4–22).
DISCUSSION
The present data demonstrate that the pyrethroids (α-)cypermethrin, the OP chlorpyrifos, and the organochlorine endosulfan at low concentrations acutely disturb calcium homeostasis through a concentration-dependent inhibition of VGCCs. Moreover, endosulfan and chlorpyrifos (and also its metabolite chlorpyrifos-oxon; Meijer et al., 2014) acutely increased basal calcium levels. The effects of insecticides on basal [Ca2+]i were observed only at relatively high concentrations (10μM), whereas the IC50s for inhibition of the depolarization-evoked [Ca2+]i were as low as 78nM, 239nM, 250nM, and 899nM for cypermethrin, α-cypermethrin, endosulfan, and chlorpyrifos, respectively. In this study, α-cypermethrin displayed a lower potency compared with cypermethrin although α-cypermethrin has the lowest acute toxicological reference value (ARfD; EFSA, 2013).
Effects of chlorpyrifos and endosulfan on VGCCs appeared not specific for a VGCC-subtype as complete inhibition of the TR was observed at 10μM (Fig. 3). On the other hand, a complete inhibition of VGCCs could not be reached for (α-)cypermethrin at concentrations up to 10μM, suggesting that inhibition of VGCC by pyrethroids could be VGCC-subtype specific or that higher concentrations are required to induce a complete block. Imidacloprid did not disturb basal calcium levels and inhibited the depolarization-evoked increase in [Ca2+]i only marginally. This was somewhat surprising as imidacloprid (≥1μM) was reported to induce an increase in basal [Ca2+]i in rat cerebellar granule cells. This effect was mediated by α7, α4β2, and α3β4 nAChRs (Kimura-Kuroda et al., 2012), of which at least α7 and α4β2 nAChRs are also expressed in PC12 cells (Mehrani and Golmanesh, 2008). However, the expression of functional (calcium permeable) nAChRs in PC12 cells is limited as acetylcholine-induced increases in [Ca2+]i are relatively small (amplitude ∼300nM; Hondebrink et al., 2012). Possibly, the lack of effect of imidacloprid on basal [Ca2+]i is due to differences in nAChR expression levels and/or functionality between PC12 cells and rat cerebellar granule cells.
Previously, several pesticides were demonstrated to potently inhibit VGCCs, including the organochlorine pesticides lindane and dieldrin (Heusinkveld and Westerink, 2012), several conazole fungicides (Heusinkveld et al., 2013), and the OP insecticides chlorpyrifos and parathion (Meijer et al., 2014). As VGCCs are also inhibited by endosulfan and (α-)cypermethrin (Figs. 1–3), inhibition of VGCCs appears to be a common mode of action for several distinct classes of pesticides. The neonicotinoid imidacloprid (Figs. 1–2) and the carbamate carbaryl (Meijer et al., 2014) did not exert this effect, which indicates that there are also (classes of) insecticides that do not target VGCCs.
For chemicals with a similar mode of action additivity is expected. Our mixture data demonstrated that insecticide mixtures induce additive effects (Fig. 4) comparable with those observed for the conazole fungicides (Heusinkveld et al., 2013) and organochlorine insecticides (Heusinkveld and Westerink, 2012). However, when cypermethrin, endosulfan, and chlorpyrifos were combined at their respective IC20s in a tertiary mixture, the effect was less than additive (Fig. 4). This is in line with other mixture studies showing that additivity does not always apply for mixtures with similar modes of action. Less than additive effects of binary OP mixtures on VGCC inhibition were observed (Meijer et al., 2014) and also the binary mixture of PCB53 and PCB95 (which both induce an increase in basal [Ca2+]i) did not induce additive effects on the basal increase in [Ca2+]i (Langeveld et al., 2012), indicating that effects of mixtures of chemicals with common modes of action cannot always be simply predicted by additivity.
Interestingly, effects of cypermethrin, endosulfan, and chlorpyrifos on VGCCs occur at lower concentrations compared with the presumed primary modes of action of pyrethroids, organochlorines, and OPs. For example, 10μM cypermethrin induced a small modification in VGSC currents in Xenopus oocytes (Meacham et al., 2008) and endosulfan inhibited GABA-R at 0.4μM (IC50; Vale et al., 2003) and 1μM (IC50; Huang and Casida, 1996) in cerebellar granule cells. These levels are higher than those required for inhibition of VGCCs (cypermethrin IC20: 9nM and endosulfan IC50: 0.25μM; Table 1). However, it should be noted that part of the differences in sensitivity may be explained by small differences in the ion channels and/or receptors expressed in the different experimental models and potential absence of (intracellular) feedback mechanisms in Xenopus oocytes. Previously described effects of chlorpyrifos in vitro generally occur at concentrations between ∼1 and 100μM (reviewed in Meijer et al., 2014), which are also higher compared with the IC50 for inhibition of VGCCs found in this study (chlorpyrifos IC50: 0.899μM; Table 1). The IC50 for inhibition of VGCCs for chlorpyrifos is somewhat higher in this study compared with Meijer et al. (2014), which is probably due to an improvement of the curve fit as a result of the inclusion of additional data points. Nonetheless, the IC50s are still in the same order of magnitude.
VGCCs are important regulators for neurotransmission and gene expression and as such they play an important role in neuronal development and function (Leclerc et al., 2011). In addition, altered expression and dysfunction of VGCCs are associated with neurological disorders, such as pain, epilepsy, migraine, and ataxia (Simms and Zamponi, 2014). Notably, some well-known neurobehavioral toxicants, such as OPs (Eaton et al., 2008), NDL-PCBs (Boix et al., 2011), and methylmercury (Bailey et al., 2013), also inhibit VGCCs. However, because these compounds have multiple effects, it is difficult to determine the contribution of inhibition of VGCC to the adverse health outcome. Nonetheless, the importance of calcium channels in neurobehavioral toxicity has been demonstrated for methylmercury because an L-type calcium channel blocker prevented or delayed the observed behavioral toxicity (Bailey et al., 2013). Yet considering the essential role of VGCCs it is not unlikely that compensatory mechanisms prevent the manifestation of severe health outcomes unless exposure is to high concentrations and/or chronic.
To estimate the relevance of the effects observed in the present study, we compared effective concentrations for the inhibition of VGGCs by insecticides with occupational human exposure levels. α-Cypermethrin was found in Egyptian agricultural workers at an estimated daily internal dose of 0.43–1.53 μg/kg/day (1.03–3.68nM; Singleton et al., 2014) amounting to a margin of exposure (MOE) of 2.7–9.7 (Table 1). In contrast with α-cypermethrin, occupational exposure levels of chlorpyrifos and endosulfan were higher than the effective concentrations for the inhibition of VGCCs found in this study. For chlorpyrifos, estimated absorbed daily doses for farmers were between 2.5 and 53.2 μg/kg/day (7.13–152nM; Phung et al., 2012). At these concentrations, chlorpyrifos inhibits VGCCs (IC20: 85nM; Table 1) and the MOE amounts only to 0.07–1.4 (Table 1). Endosulfan was also found in human tissues of agricultural workers. After endosulfan is sprayed, 544.1 μg/l (1.34μM) was found in blood serum, resulting in a MOE of only 0.007 (Table 1; Dalvie et al., 2009).
The calculated MOEs are thus insufficient to rule out effects on VGCCs in agricultural workers. However, it should be noted that for the calculation of MOEs it was assumed that the estimated absorbed daily doses and serum levels represent the insecticide concentration at the internal target sites, though the actual concentrations at the target cells in vivo could be different. Although more sophisticated physiologically-based pharmacokinetic (PBPK) data and/or actual measurements of the insecticide concentration at the target site are required to confirm that the MOEs are insufficient, our findings are of relevance for neurotoxicity risk assessment studies as it is also demonstrated here that additivity can occur when insecticides with common modes of action are combined.
In conclusion, the present study demonstrates that structurally diverse insecticides have inhibition of VGCCs as a common mode of action, resulting in the inhibition of the depolarization-evoked increase in [Ca2+]i. The tested insecticides exerted this effect at concentrations that are relevant for human (occupational) exposure situations. Our data further demonstrate that exposure to (binary) mixtures of insecticides can induce additive effects, illustrating the need to include mixture effects in human neurotoxicity risk assessment.
FUNDING
European Commission [DENAMIC project; FP7-ENV-2011-282957]; Faculty of Veterinary Medicine of Utrecht University.
Acknowledgments
The authors acknowledge the European Commission [DENAMIC project; grant number FP7-ENV-2011-282957] and the Faculty of Veterinary Medicine of Utrecht University for funding, and the members of the Neurotoxicology Research Group for helpful discussions.
REFERENCES
- Abou-Donia M., Goldstein L. B., Bullman S., Tu T., Khan W. A., Dechkovskaia A. M., Abdel-Rahman A. Imidacloprid induces neurobehavioral deficits and increases expression of glial fibrillary acidic protein in the motor cortex and hippocampus in offspring rats following in utero exposure. J. Toxicol. Environ. Health A. 2008;71:119–130. doi: 10.1080/15287390701613140. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Hutsell B. A., Newland M. C. Dietary nimodipine delays the onset of methylmercury neurotoxicity in mice. Neurotoxicology. 2013;37:108–117. doi: 10.1016/j.neuro.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal R., Erdogan S., Theophilidis G., Baydas G., Naziroglu M. Assessing the effects of the neonicotinoid insecticide imidacloprid in the cholinergic synapses of the stellate cells of the mouse cochlear nucleus using whole-cell patch-clamp recording. Neurotoxicology. 2010;31:113–120. doi: 10.1016/j.neuro.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Boix J., Cauli O., Leslie H., Felipo V. Differential long-term effects of developmental exposure to polychlorinated biphenyls 52, 138 or 180 on motor activity and neurotransmission. Gender dependence and mechanisms involved. Neurochem. Int. 2011;58:69–77. doi: 10.1016/j.neuint.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Cao Z., Shafer T., Murray T. Mechanisms of pyrethroid insecticide-induced stimulation of calcium influx in neocortical neurons. J. Pharmacol. Exp. Ther. 2011;336:197–205. doi: 10.1124/jpet.110.171850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo C. G., Montante M., Dufour L., Martínez M. L., Jiménez-Capdeville M. E. Behavioral effects of exposure to endosulfan and methyl parathion in adult rats. Neurotoxicol. Teratol. 2002;24:797–804. doi: 10.1016/s0892-0362(02)00268-4. [DOI] [PubMed] [Google Scholar]
- Coats J. R. Mechanisms of toxic action and structure-activity relationships for organochlorine and synthetic pyrethroid insecticides. Environ. Health Perspect. 1990;87:255–262. doi: 10.1289/ehp.9087255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvie M. A., Africa A., Solomons A., London L., Brouwer D., Kromhout H. Pesticide exposure and blood endosulfan levels after first season spray amongst farm workers in the western cape, south africa. J. Environ. Sci. Health B. 2009;44:271–277. doi: 10.1080/03601230902728351. [DOI] [PubMed] [Google Scholar]
- Dingemans M. M. L., van den Berg M., Bergman Å., Westerink R. H. S. Calcium-related processes involved in the inhibition of depolarization-evoked calcium increase by hydroxylated PBDEs in PC12 cells. Toxicol. Sci. 2010;114:302–309. doi: 10.1093/toxsci/kfp310. [DOI] [PubMed] [Google Scholar]
- Dingemans M. M. L., Heusinkveld H. J., de Groot A., Bergman Å., van den Berg M., Westerink R. H. S. Hexabromocyclododecane inhibits depolarization-induced increase in intracellular calcium levels and neurotransmitter release in PC12 cells. Toxicol. Sci. 2009;107:490–497. doi: 10.1093/toxsci/kfn249. [DOI] [PubMed] [Google Scholar]
- Eaton D. L., Daroff R. B., Autrup H., Bridges J., Buffler P., Costa L. G., Spencer P. S. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit. Rev. Toxicol. 2008;38:1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) The 2010 European Union Report on Pesticide Residues in Food. EFSA J. 2013. 2013;11:3130–3938. [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. U.S.A. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusinkveld H. J., Molendijk J., van den Berg M., Westerink R. H. S. Azole fungicides disturb intracellular Ca2+ in an additive manner in dopaminergic PC12 cells. Toxicol. Sci. 2013;134:374–381. doi: 10.1093/toxsci/kft119. [DOI] [PubMed] [Google Scholar]
- Heusinkveld H., Thomas G., Lamot I., van den Berg M., Kroese A. B. A., Westerink R. H. S. Dual actions of lindane (y-hexachlorocyclohexane) on calcium homeostasis and exocytosis in rat PC12 cells. Toxicol. Appl. Pharmacol. 2010;248:12–19. doi: 10.1016/j.taap.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Heusinkveld H. J., Westerink R. H. S. Organochlorine insecticides lindane and dieldrin and their binary mixture disturb calcium homeostasis in dopaminergic PC12 cells. Environ. Sci. Technol. 2012;46:1842–1848. doi: 10.1021/es203303r. [DOI] [PubMed] [Google Scholar]
- Hondebrink L., Meulenbelt J., Rietjens S., Meijer M., Westerink R.H.S. Methamphetamine, amphetamine, MDMA (‘ecstasy’), MDA and mCPP modulate electrical and cholinergic input in PC12 cells. Neurotoxicology. 2012;33:255–260. doi: 10.1016/j.neuro.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Huang J., Casida J. E. Characterization of [3H]ethynylbicycloorthobenzoate ([3H]EBOB) binding and the action of insecticides on the gamma-aminobutyric acid-gated chloride channel in cultured cerebellar granule neurons. J. Pharmacol. Exp. Ther. 1996;279:1191–1196. [PubMed] [Google Scholar]
- Jia Z, Misra H.P. Reactive oxygen species in in vitro pesticide-induced neuronal cell (SH-SY5Y) cytotoxicity: role of NFkappaB and caspase-3. Free Radic Biol Med. 2007;42:288–298. doi: 10.1016/j.freeradbiomed.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Kakko I., Toimela T., Tähti H. The toxicity of pyrethroid compounds in neural cell cultures studied with total ATP, mitochondrial enzyme activity and microscopic photographing. Environ. Toxicol. Pharmacol. 2004;15:95–102. doi: 10.1016/j.etap.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Kimura-Kuroda J., Komuta Y., Kuroda Y., Hayashi M., Kawano H. Nicotine-like effects of the neonicotinoid insecticides acetamiprid and imidacloprid on cerebellar neurons from neonatal rats. Plos One. 2012;7:1–11. doi: 10.1371/journal.pone.0032432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A., Evans R., Faust M., Kalberlah F., Scholze M., Schuhmacher-Wolz U. Investigation of the state of science on combined actions of chemicals in food through dissimilar modes of action and proposal for science-based approach for performing cumulative risk assessment. 2012 Scientific report submitted to EFSA (European Food Safety Authority) EN-232. [Google Scholar]
- Langeveld W. T., Meijer M., Westerink R. H. S. Differential effects of 20 non-dioxin-like PCBs on basal and depolarization-evoked intracellular calcium levels in PC12 cells. Toxicol. Sci. 2012;126:487–496. doi: 10.1093/toxsci/kfr346. [DOI] [PubMed] [Google Scholar]
- Leclerc C., Néant I., Moreau M. Early neural development in vertebrates is also a matter of calcium. Biochimie. 2011;93:2102–2111. doi: 10.1016/j.biochi.2011.06.032. [DOI] [PubMed] [Google Scholar]
- Leng G., Gries W., Selim S. Biomarker of pyrethrum exposure. Toxicol. Lett. 2006;162:195–201. doi: 10.1016/j.toxlet.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Mackenzie Ross S., McManus I. C., Harrison V., Mason O. Neurobehavioral problems following low-level exposure to organophosphate pesticides: A systematic and meta-analytic review. Crit. Rev. Toxicol. 2013;43:21–44. doi: 10.3109/10408444.2012.738645. [DOI] [PubMed] [Google Scholar]
- Matsuda K., Kanaoka S., Akamatsu M., Sattelle D. Diverse actions and target-site selectivity of neonicotinoids: Structural insights. Mol. Pharmacol. 2009;76:1–10. doi: 10.1124/mol.109.055186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham C. A., Brodfuehrer P. D., Watkins J. A., Shafer T. J. Developmentally-regulated sodium channel subunits are differentially sensitive to α-cyano containing pyrethroids. Toxicol. Appl. Pharmacol. 2008;231:273–281. doi: 10.1016/j.taap.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Mehrani H., Golmanesh L. Changes in mRNA and protein levels of nicotinic acetylcholine receptors in diazoxon exposed PC12 cells. Toxicol. In Vitro. 2008;22:1257–1263. doi: 10.1016/j.tiv.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Meijer M., Hamers T., Westerink R. H. S. Acute disturbance of calcium homeostasis in PC12 cells as a novel mechanism of action for (sub)micromolar concentrations of organophosphate insecticides. Neurotoxicology. 2014 doi: 10.1016/j.neuro.2014.01.008. Available at: http://dx.doi.org/10.1016/j.neuro.2014.01.008. Accessed June 17, 2014. [DOI] [PubMed] [Google Scholar]
- Mrema E., Rubino F., Brambilla G., Moretto A., Tsatsakis A., Colosio C. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology. 2013;307:74–88. doi: 10.1016/j.tox.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Phung D. T., Connell D., Miller G., Chu C. Probabilistic assessment of chlorpyrifos exposure to rice farmers in viet nam. J. Expo. Sci. Environ. Epidemiol. 2012;22:417–423. doi: 10.1038/jes.2012.32. [DOI] [PubMed] [Google Scholar]
- Silva M. H., Beauvais S. L. Human health risk assessment of endosulfan I: Toxicology and hazard identification. Regul. Toxicol. Pharmacol. 2010;56:4–17. doi: 10.1016/j.yrtph.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Simms B. A., Zamponi G. W. Neuronal voltage-gated calcium channels: Structure, function, and dysfunction. Neuron. 2014;82:24–45. doi: 10.1016/j.neuron.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Singleton S. T., Lein P. J., Farahat F. M., Farahat T., Bonner M. R., Knaak J. B., Olson J. R. Characterization of α-cypermethrin exposure in egyptian agricultural workers. Int. J. Hyg. Environ. Health. 2014;217:538–545. doi: 10.1016/j.ijheh.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund D. Molecular mechanisms of pyrethroid insecticide neurotoxicity: Recent advances. Arch. Toxicol. 2012;86:165–181. doi: 10.1007/s00204-011-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu E. C., Verkhratsky A. The importance of being subtle: Small changes in calcium homeostasis control cognitive decline in normal aging. Aging Cell. 2007;6:267–273. doi: 10.1111/j.1474-9726.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- Tomizawa M., Casida J. E. Desnitro-imidacloprid activates the extracellular signal-regulated kinase cascade via the nicotinic receptor and intracellular calcium mobilization in N1E-115 cells. Toxicol. Appl. Pharmacol. 2002;184:180–186. doi: 10.1006/taap.2002.9503. [DOI] [PubMed] [Google Scholar]
- Tomizawa M., Casida J. E. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 2005;45:247–268. doi: 10.1146/annurev.pharmtox.45.120403.095930. [DOI] [PubMed] [Google Scholar]
- Vale C., Fonfria E., Bujons Vilas J., Messeguer A., Rodriguez-Farré E., Sunol C. The organochlorine pesticides γ-hexachlorocyclohexane (lindane), α-endosulfan and dieldrin differentially interact with GABAA and glycine-gated chloride channels in primary cultures of cerebellar granule cells. Neuroscience. 2003;117:397–403. doi: 10.1016/s0306-4522(02)00875-8. [DOI] [PubMed] [Google Scholar]
- Westerink R. H. S. Do we really want to REACH out to in vitro? Neurotoxicology. 2013;39:169–172. doi: 10.1016/j.neuro.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Westerink R. H. S., Ewing A. G. The PC12 cell as model for neurosecretion. Acta Physiol. 2008;192:273–285. doi: 10.1111/j.1748-1716.2007.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolansky M. J., Harrill J. A. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: A critical review. Neurotoxicol. Teratol. 2008;30:55–78. doi: 10.1016/j.ntt.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Yu-Tao T., Zhao-Wei L., Yang Y., Zhuo Y., Tao Z. Effect of alpha-cypermethrin and theta-cypermethrin on delayed rectifier potassium currents in rat hippocampal neurons. Neurotoxicology. 2009;30:269–273. doi: 10.1016/j.neuro.2009.01.001. [DOI] [PubMed] [Google Scholar]