Abstract
Ischemia/reperfusion (I/R)-induced acute kidney injury (AKI) is evoked by diverse pathophysiological conditions and/or surgical procedures. Here, we evaluated the nephropreventive effect of sulfotransferase (SULT) inhibitors, quercetin, and resveratrol, which hamper hepatic indoxyl sulfate (IS) production. I/R of the kidney caused severe renal injury with marked accumulation of serum and renal IS and urinary excretion of kidney injury molecule-1. Oral administration of AST-120 resulted in a significant restoration of kidney injury, suggesting that uremic toxins, which can be suppressed or adsorbed by AST-120 in the intestine, contribute to the progression or development of I/R-induced AKI. Oral administration of resveratrol or quercetin, SULT inhibitors, suppressed IS accumulation, accompanied by significant amelioration of renal dysfunction. The expression of nuclear factor E2-related factor 2 (Nrf2) in the renal nuclear fractions was markedly elevated by renal I/R, but suppressed by treatment with SULT inhibitors. IS is primarily taken up by HK-2 cells derived from human proximal tubular cells via organic anion transporters, which then evokes activation of Nrf2, most likely due to intracellular oxidative stress. Renal basolateral organic anion transporters OAT1 and OAT3, which mediate renal tubular uptake of IS in basolateral membrane, were markedly downregulated by renal I/R, but restored by SULT inhibitors. Our results suggest that renal accumulation of IS in ischemic AKI induces oxidative stress and downregulation of organic anion transporters resulting in kidney damage, which could be restored to some extent by inhibiting hepatic SULT activity as a nephropreventive target.
Keywords: acute kidney injury, sulfotransferase, indoxyl sulfate, Nrf2, phytochemical polyphenols
ABBREVIATIONS
- AKI
acute kidney injury
- ARE/EpRE
antioxidant/electrophile responsive element
- BUN
blood urea nitrogen
- CKD
chronic kidney disease
- COX
cyclooxygenase
- CYP
cytochrome P450
- ERK
extracellular signal-regulated kinase
- GLC
glutamate cysteine ligase
- GSH
glutathione
- GST
GSH S-transferase
- HO-1
heme oxygenase-1
- HPLC
high-performance liquid chromatography
- I/R
ischemia/reperfusion
- IS
indoxyl sulfate
- JNK
c-Jun N-terminal kinase
- Keap1
Kelch-like ECH-associated protein 1
- MDA
malondialdehyde
- MRP
multidrug resistance-associated protein
- NADPH
nicotinamide adenine dinucleotide phosphate
- NQO1
NADPH quinone oxidoreductase 1
- Nrf2
nuclear factor E2-related factor 2
- OAT
organic anion transporter
- PBS
phosphate-buffered saline
- PGE2
prostaglandin E2
- ROS
reactive oxygen species
- SCr
serum creatinine
- SDS
sodium dodecyl sulfate
- SDS-PAGE
SDS-polyacrylamide gel electrophoresis
- SULT
sulfotransferase
- TBARS
thiobarbituric acid-reactive substances
Ischemic acute kidney injury (AKI) is a syndrome characterized by sudden deterioration of renal function from several hours to a few days causing derangement of homeostatic maintenance of the body's fluids and electrolytes (Thadhani et al., 1996). This condition can result from disturbances to the circulatory system, a living-donor renal transplantation or pharmacological therapy using nephrotoxic drugs (Thadhani et al., 1996). Despite technological improvements in dialysis treatment and renal replacement therapy (RRT), AKI-related pathologic mortality remains high (∼50%) (Thadhani et al., 1996; Ympa et al., 2005). Moreover, many cases with AKI, develop end-stage renal diseases and are forced undergo dialysis (Ympa et al., 2005). Given there is no effective treatment other than dialysis or RRT, early diagnosis and therapeutic management of AKI is particularly important (Kaushal and Shah, 2014). AKI is associated with subsequent development or progression of chronic kidney disease (CKD) (Belayev and Palevsky, 2013).
Indoxyl sulfate (IS) is actively excreted in the urine under normal kidney function, but is one of a representative group of sulfate-conjugated metabolites that accumulate in the circulatory system during renal dysfunction (Neirynck et al., 2013; Vanholder et al., 2003). Serum IS levels rise markedly in patients undergoing dialysis treatment, with maximum serum concentrations of ∼940μM (Vanholder et al., 2003). Several reports show that IS may induce oxidative stress in the kidney and cardiovascular system (Fujii et al., 2011; Niwa, 2010, 2011). For example, IS is one of the most powerful inducers of free radicals among various low-molecular weight uremic toxins (Niwa, 2010, 2011). Indeed, cytotoxicity evoked by IS can be explained by dysregulated oxygen metabolism and induction of oxidative stress in renal proximal tubular cells (Chiang et al., 2012).
IS is predominantly produced in the liver. Dietary protein-derived tryptophan is first metabolized to indole by tryptophanase of intestinal bacteria such as Escherichia coli (Niwa, 2010, 2011). Following intestinal absorption, indole is hydroxylated to indoxyl by cytochrome P450 (CYP) 2E1 or CYP2A6, and subsequently conjugated to IS by sulfotransferase (SULT) 1A1 in the liver (Banoglu and King, 2002; Banoglu et al., 2001). IS produced in the liver enters the blood circulatory system and is efficiently taken up by renal proximal tubular cells via basolateral membrane-localized organic anion transporters, OAT1 and OAT3, before being excreted into the urine via unidentified apical membrane-localized transporters (Enomoto et al., 2002). Given that IS accumulates in the body during renal dysfunction as well as inducing oxidative stress, IS is reported to be a risk factor responsible for renal disease progression (Miyazaki et al., 1997; Niwa and Ise, 1994). However, the removal of IS is difficult by dialysis because of its high rate of protein-binding, chiefly to serum albumin (∼95%) (Niwa, 2013). Clinically, restriction of protein intake in the diet and/or administration of oral adsorptive charcoal beaded medicine, AST-120 (Kremezin), can reduce the accumulation of uremic toxins (Fujii et al., 2011; Neirynck et al., 2013; Niwa, 2010; Niwa and Ise, 1994; Ueda et al., 2008). Indeed, the therapeutic efficacy of AST-120 has been demonstrated by the efficient removal of uremic toxins in patients with CKD (Niwa, 2011; Schulman et al., 2006). However, medication adherence is a major issue for this treatment because patients need to take 6 g of fine grain or 20 capsules of AST-120 every day. As such new approaches and/or strategies to efficiently reduce or remove systemic accumulation of IS is recognized as an urgent issue in patients with kidney disease and uremia.
In experimental rats with cisplatin-induced AKI, oral administration of AST-120 suppressed serum and renal accumulation of IS in association with an improvement of AKI (Iwata et al., 2007; Morisaki et al., 2008). It was suggested that low-molecular weight uremic toxins, which can be removed by AST-120 treatment, contribute to progression of kidney diseases in not only CKD but also the development or derangement of AKI. In addition, it became clear that phytochemical polyphenol compounds such as resveratrol and quercetin, which show potent inhibitory effect against SULT1A1, significantly inhibited hepatic production of IS (Kusumoto et al., 2011). A nephropreventive effect of these phytochemicals was observed in cisplatin-induced AKI in association with decreased accumulation of IS in the serum and tissues of kidney and liver (Kusumoto et al., 2011). On the other hand, it was also reported that renal injury is reduced by the administration of bardoxolone methyl, which activates Nrf2 (nuclear factor-E2-related factor 2), the transcription factor of the oxidative stress defense enzymes in renal ischemia-induced AKI model rats (Wu et al., 2010). Phytochemical polyphenols including resveratrol and quercetin are known to activate Nrf2, i.e., translocation into the nucleus (Kumar et al., 2011; Palsamy and Subramanian, 2011). Sulforaphane, which is found in broccoli, reduces cisplatin-induced AKI by modifying the complex of Nrf2/Keap-1 (Kelch-like ECH-associated protein 1) to promote nuclear translocation of Nrf2, thereby enhancing antioxidant defense molecules by binding to antioxidant/electrophile responsive element (ARE/EpRE) (Guerrero-Beltrán et al., 2010).
In the present study, we aimed to clarify the involvement of IS in the development and progression of ischemic AKI. Specifically, we examined and compared the nephropreventive effects of AST-120, SULT-inhibiting phytochemicals, and sulforaphane by using model rats with ischemia-reperfusion (I/R)-evoked AKI.
MATERIALS AND METHODS
Drugs and chemicals
IS, quercetin, resveratrol, and thiobarbituric acid (TBA) were obtained from Sigma-Aldrich (St Louis, MO). Indole, carboxymethyl cellulose (CMC), methanol and nitric acid (HNO3) were obtained from Wako Pure Chemical Industries, Ltd (Osaka, Japan). Sulforaphane was obtained from Funakoshi Co., Ltd (Tokyo, Japan). AST-120 was kindly donated by Daiichi Sankyo Co., Ltd (Tokyo, Japan). Probenecid was obtained from Wako Pure Chemical Industries, Ltd. All other chemicals used were of analytical grade and commercially available.
Animal experiments
All procedures for animal experiments were approved by Kumamoto University ethical committee concerning animal experiments, and animals were treated in accordance with the Guidelines of the United States National Institutes of Health regarding the care and use of animals for experimental procedures, and the Guidelines of Kumamoto University for the care and use of laboratory animals. Male Sprague Dawley (SD) rats at 6 weeks of age were housed in a standard animal maintenance facility at a constant temperature (22°C ± 2°C) and humidity (50–70 %) and a 12/12 h light/dark cycle for about a week before the day of the experiment, with food and water available ad libitum. Rats were anesthetized using sodium pentobarbital (50 mg/kg intraperitoneally), and placed on a heating plate (39°C) to maintain a constant temperature. All surgery was conducted under anesthesia with pentobarbital, and all efforts were made to minimize animal suffering. The kidneys of male SD rats at 6 weeks of age were exposed via midline abdominal incisions. Renal I/R was induced by vascular clamps (AS ONE, Osaka, Japan) over both pedicles for 30 min, followed by release of the clamps. Sham animals (control) underwent anesthesia, laparotomy, and renal pedicle dissection only. Rats were divided into six different groups as follows: sham-operated rats (control rats), rats with I/R treatment, AST-120-administered rats with I/R treatment, resveratrol-administered rats with I/R treatment, quercetin-administered rats with I/R treatment, and sulforaphane-administered rats with I/R treatment. AST-120 (2.5 g/kg), resveratrol (5 mg/kg), quercetin (50 mg/kg), or sulforaphane (5 mg/kg) were orally administered to rats 24 and 1 h before and 24 h after renal I/R treatment. In the postadministration experiment, these test compounds were administered to rats 3, 6, and 24 h after renal I/R treatment. All administrations and sacrifices were performed under surgical anesthesia using diethyl ether. Blood was collected 48 h after I/R treatment from the abdominal aorta and centrifuged at 3000 × g for 10 min to obtain the serum sample. Methanol (100 μl) was added to serum (50 μl) and the mixture was centrifuged at 9000 × g for 10 min at 4°C. The obtained supernatant (50 μl) was diluted with HPLC mobile phase solution (300 μl) and centrifuged at 9000 × g for 10 min at 4°C. The supernatant was used for HPLC determination of IS concentration. Kidney was harvested 48 h after IR treatment and homogenized in phosphate-buffered saline (pH 7.4) using a Polytron PT 3000 (Kinematica AG, Lucerne, Switzerland). After centrifugation at 9000 × g for 10 min at 4°C, the obtained supernatant was used for HPLC assay of IS concentration. Levels of serum creatinine (SCr) (enzymatic method), blood urea nitrogen (BUN) (uricase ultraviolet (UV) method) were then measured.
HPLC determination of IS concentration
The HPLC system consisted of a Shimadzu LC-10ADVP pump and a Shimadzu RF-10AXL fluorescence spectrophotometer. A column of LiChrospher 100 RP-18 (Merck KGaA, Darmstadt, Germany) was used as the stationary phase and the mobile phase consisted of acetate buffer (0.2M, pH 4.5). The flow rate was 1.0 ml/min at a column temperature of 40°C. The presence of IS in the eluate was monitored by means of a fluorescence detector (excitation 280 nm, emission 375 nm). Samples from the in vitro screening assay were analyzed by HPLC using a gradient as described later.
Measurement of urine kidney injury molecule (Kim)-1
Urine samples were collected periodically from rats in metabolic cages 3–48 h after I/R treatment. Kim-1 concentration in urine was determined by using a Tim-1/Kim-1/HAVCR Immunoassay kit (R&D Systems, Inc., MN) (Kusumoto et al., 2011).
Western blot analysis
Western blot analysis for organic anion transporters, OAT1 and OAT3, was performed according to a previous report (Matsuzaki et al., 2007) with some modifications. Kidneys were homogenized in an ice-cold homogenization buffer consisting of 230mM sucrose, 5mM Tris (hydroxymethyl) aminomethane hydrochloride (Tris-HCl) (pH 7.5), 2 mM ethylenediaminetetraacetic acid, 0.1 mM phenylmethanesulfonyl fluoride, 1 μg/ml leupeptin and 1 μg/ml pepstatin A. After measuring of protein content using a bicinchoninic acid (BCA) protein assay reagent (Thermo Fisher Scientific, Rockford, IL), each sample was mixed in loading buffer (2% (wt/vol) sodium dodecyl sulfate (SDS), 125 mM Tris-HCl, 20% (vol/vol) glycerol and 5% (vol/vol) 2-mercaptoethanol) and heated at 95°C for 2 min. The samples were subjected to sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) electrophoresis using a 7.5% gel and transferred onto a polyvinylidene difluoride (PVDF) membrane (Immobilon-P) (Millipore, Bedford, MA) by semidry electroblotting. The membrane was blocked overnight at 4°C with 2% (vol/vol) ECL Advance Blocking Agent (GE Healthcare, Little Chalfont, UK) in 50mM Tris-buffered saline (pH 7.6) containing 0.3% (vol/vol) Tween 20, and then incubated for 1 h at room temperature with a primary antibody specific for rat OAT1 or OAT3 (gift from Dr Inui Kyoto University Hospital). Western blot analysis for Nrf2 was performed by using nuclear fractions of kidneys prepared with NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific). The nuclear fractions were mixed with loading buffer (2% (wt/vol) SDS, 125mM Tris-HCl, 20% (vol/vol) glycerol, and 5% (vol/vol) 2-mercaptoethanol) and heated at 95°C for 2 min. Samples were subjected to SDS-PAGE and transferred onto a PVDF membrane using the same protocol as described above. The blots were incubated with a primary antibody specific for Nrf2 (Santa Cruz Biotechnology, Inc., TX) and then washed with Tris-buffered saline containing 0.3% (vol/vol) Tween 20 before incubation with the secondary antibody (horseradish peroxidase-linked antirabbit immunoglobulin F(ab)2 or horseradish peroxidase-linked antimouse immunoglobulin F(ab)2 (GE Healthcare) for 1 h at room temperature. Immunoblots were visualized with an ECL system (ECL Advance Western Blotting Detection Kit; GE Healthcare).
Determination of Nrf2 activation in HK-2 cells
Human kidney (HK)-2 cells derived from human proximal tubular epithelial cells were obtained from ATCC (Manassas, VA). HK-2 cell were cultured in a keratinocyte serum-free medium (GIBCO BRL, Palo Alto, CA) containing 0.05 mg/ml bovine pituitary extract (GIBCO BRL) and 5 ng/ml human recombinant epidermal growth factor (GIBCO BRL) in an incubator (5% CO2–95% air) at 37°C. HK-2 cells were grown on plastic dishes (4-cm diameter) at a cell density of 5 × 105 cells in a 5% CO2 incubator for 2 days. IS with or without probenecid dissolved in the culture medium was added to HK-2 cells and the cells were incubated for 24 h in a 5% CO2 incubator. After incubation, HK-2 cells were washed twice with Dulbecco's modified phosphate-buffered saline (PBS, pH 7.4), and then harvested and lyzed in a lysis buffer (150 mM NaCl, 50 mM Tris-HCl, 1% Triton X-100, 1 μg/ml leupeptin, and 1 μg/ml pepstatin A, pH 8.0). After sonication at 4°C for 15 min, the protein extract was mixed with the loading buffer described above and heated at 100°C for 2 min. The samples were used for Western blot analysis for Nrf2 expression as described above.
Measurement of lipid peroxidation in kidney
The malondialdehyde (MDA) level, a measure of lipid peroxidation, was assayed as thiobarbituric acid-reactive substances (TBARS) in kidneys. Kidneys of I/R-treated rats or control rats were homogenized in ice-cold PBS buffer, and supernatants of homogenates centrifuged at 1500 × g for 5 min at 4°C were mixed with 1% (vol/vol) phosphoric acid and 0.6% (wt/vol) TBA. The mixtures were heated at 100°C for 1 h, and centrifuged at 3000 × g for 10 min after adding n-butanol. The absorbance of the n-butanol phase at 540 nm was measured using a UV/Visible spectrophotometer for calculation of TBARS (Ultrospec 2000; GE Healthcare). The standard used for this assay was 1,1,3,3-tetraethoxypropane. Protein content of each sample was quantified by BCA protein assay.
Statistical analysis
Data were analyzed statistically by analysis of variance, followed by Scheffé's multiple comparison test. A p-value of <0.05 was considered statistically significant. All data were represented the mean ± SD.
RESULTS
Effects of AST-120, SULT Inhibitors, and Sulforaphane on Renal Function and IS Concentration in Serum and Kidney of Rats Treated with Renal I/R
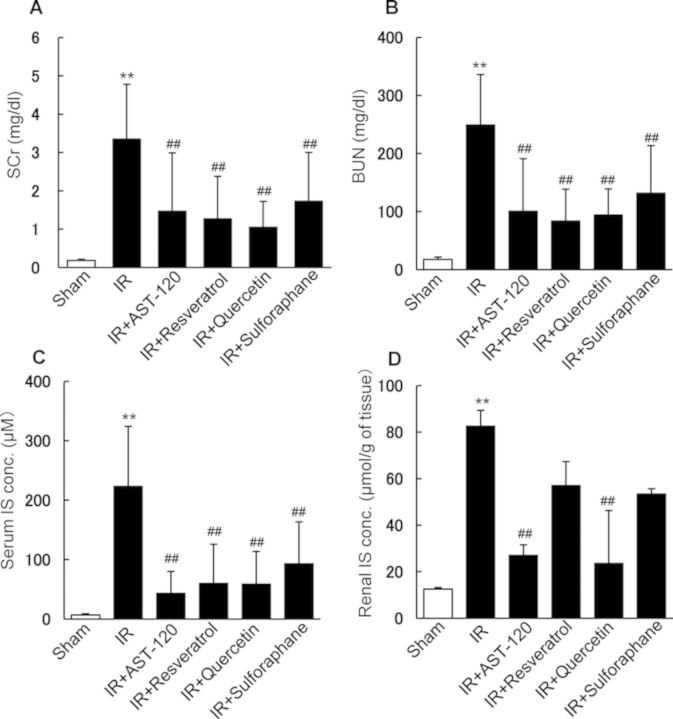
In our previous study (Kusumoto et al., 2011), we reported that phytochemical polyphenols such as quercetin, curcumin, and resveratrol, potent inhibitors of SULT, showed strong inhibitory effects on IS production with the apparent inhibition constant (Ki) values of 1.0, 1.8, and 1.5 μM, respectively, by using rat liver S9 fraction. By contrast, sulforaphane showed no inhibitory effect on IS production in the fraction (unpublished data). Thus, the effects of administration of the polyphenols, AST-120, and sulforaphane on renal function and IS accumulation in rats treated with renal I/R were examined. Renal I/R treatment evoked a marked elevation of SCr and BUN, indicating the onset of severe AKI (Figs. 1A and B). Oral administration of AST-120 resulted in a significant decrease in both SCr and BUN, suggesting that uremic toxins, which can be suppressed or adsorbed by AST-120 in the intestine, contribute to the progression or development of I/R-induced AKI. Oral administration of resveratrol, quercetin, and sulforaphane also showed a significant decrease in both SCr and BUN in I/R-treated rats, suggesting that these compounds have nephropreventing effects (Figs. 1A and B). Serum and renal IS concentrations were markedly elevated in rats treated with renal I/R compared with those of control rats (Fig. 1). Oral administration of AST-120, resveratrol, quercetin, or sulforaphane significantly suppressed serum IS accumulation (Fig. 1C). Administration of AST-120 and quercetin significantly suppressed IS accumulation in the ischemic kidney (Fig. 1D). Resveratrol and sulforaphane showed a tendency to suppress renal IS accumulation, but the effects were not significant (Fig. 1D). These observations suggested that SULT inhibitors suppress the hepatic production of IS in rats, resulting in the decreased accumulation of IS in serum and kidney, which may influence the onset of ischemic AKI. By contrast, sulforaphane, which has no inhibitory potency for hepatic IS production, also showed the preventive effect on ischemic AKI. However, the observed effect of sulforaphane might depend on other mechanisms including the activation of Nrf2 rather than the inhibition of IS production in ischemic kidney.
FIG. 1.
Effects of AST-120, SULT inhibitors, and sulforaphane on renal function and IS concentration in rats treated with renal I/R. AST-120, SULT inhibitors, and sulforaphane were orally administered to rats 24 and 1 h before and 24 h after renal I/R. After I/R treatment, SCr (A), BUN (B), serum concentration of IS (C), and renal concentration of IS (D) were determined. Each column represents the mean ± SD for 8–16 rats in each group. **p < 0.01 versus sham (control); ##p < 0.01 versus I/R.
Effect of AST-120, SULT Inhibitors, and Sulforaphane on Kim-1 Excretion in Urine
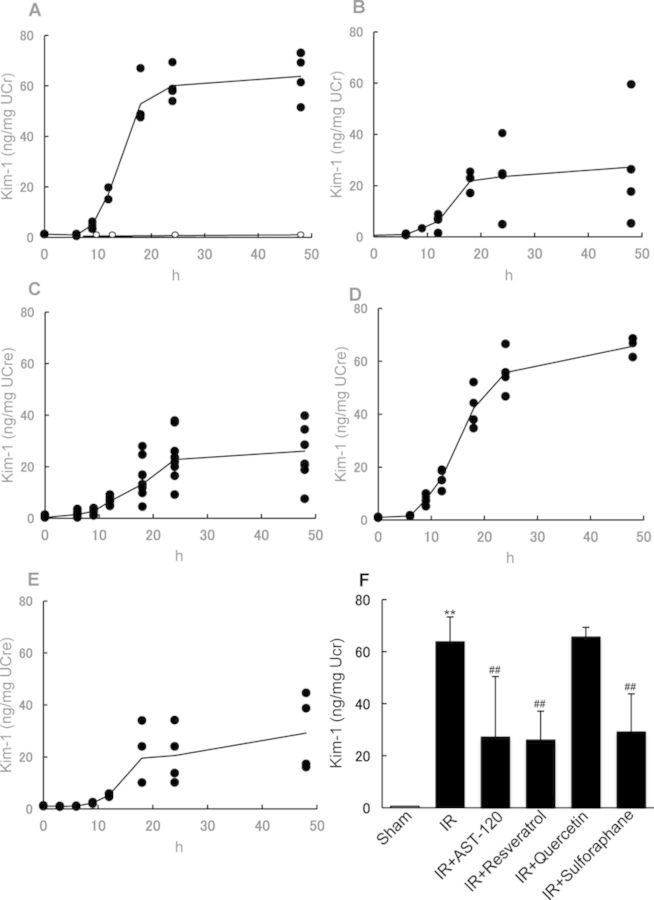
In order to clarify whether I/R-induced AKI was related to renal tubular cell damage, the preventive effect of AST-120, SULT inhibitors, or sulforaphane on urinary excretion of renal proximal tubule-specific biomarker Kim-1 was explored. Urinary Kim-1 excretion was markedly elevated in rats with renal I/R compared with that in control rats (Fig. 2A). Oral administration of AST-120, resveratrol, and sulforaphane significantly decreased urinary excretion of Kim-1 (Figs. 2B, C, E, and F). These observations suggested that the IS accumulation in the ischemic kidney could contribute to the progression or development of renal proximal tubule injury, and the compounds that suppress IS production and/or accumulation may help prevent renal tubular damage. To our surprise, quercetin showed no protective effect on urinary Kim-1 excretion (Fig. 2D), despite displaying a significant nephropreventive effect as observed by the restored SCr and BUN levels in renal ischemic rats.
FIG. 2.
Effect of AST-120, SULT inhibitors, and sulforaphane on Kim-1 excretion in urine. Urinary Kim-1 excretion was determined in rats with (A, solid circle) or without renal I/R treatment (A, open circle). AST-120 (B), resveratrol (C), quercetin (D), and sulforaphane (E) were orally administered to rats 24 and 1 h before and 24 h after renal I/R. Urine samples were collected periodically after I/R treatment. Urinary excretion of Kim-1 in each group at 48 h is depicted (F). Each line (A–E) represents the mean value of urinary Kim-1 excretion. Each column (F) represents the mean ± SD for 4–6 rats in each group. **p < 0.01 versus sham (control); ##p < 0.01 versus I/R.
Effect of Postoral Administration of AST-120 and SULT Inhibitors on Renal Function and IS Concentration in Serum and Kidney of Rats after Renal I/R Treatment
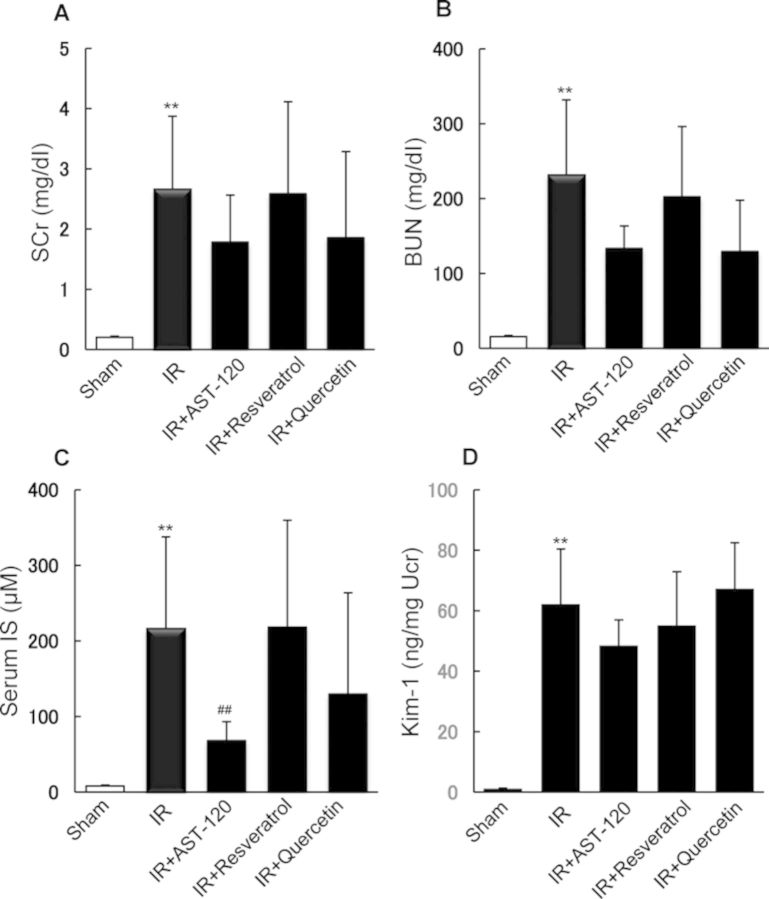
We also investigated the effect of commencing oral administration of the compounds 3, 6, and 24 h after renal I/R treatment. Postadministration of AST-120, but not resveratrol or quercetin, resulted in a significant decrease in serum IS level (Fig. 3C). However, AST-120 had no significant effect on the level of SCr or BUN (Figs. 3A and B). The increased excretion of urinary Kim-1 in I/R rats was unaffected by the postadministration of these test compounds (Fig. 3D). Our observations suggest the administration of a compound that suppresses IS accumulation prior to I/R of the kidney might be required in order to prevent the onset of ischemic AKI.
FIG. 3.
Effect of postoral administration of AST-120 and SULT inhibitors on renal function and IS concentration in serum and kidney of rats after renal I/R treatment. AST-120 and SULT inhibitors were orally administered to rats 3, 6, and 24 h after renal I/R. After I/R treatment, SCr (A), BUN (B), serum concentration of IS (C), and urinary excretion of Kim-1 (D) were determined. Each column represents the mean ± SD for 4–6 rats in each group. **p < 0.01 versus sham (control); ##p < 0.01 versus I/R.
Effect of AST-120, SULT Inhibitors, and Sulforaphane on Nuclear Nrf2 Expression in Kidney of Rats Treated with Renal I/R
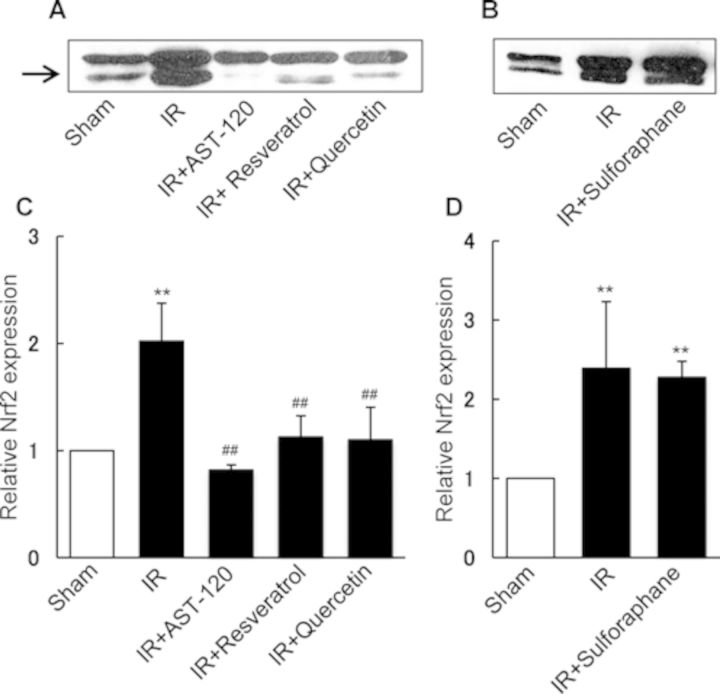
Nrf2 was reported to protect the kidney against oxidative stress by playing a pivotal role in the cooperative induction of genes that encode antioxidant and detoxifying enzymes (Saito, 2013). Next, we examined the effect of compounds suppressing IS accumulation on renal activation of Nrf2 after I/R treatment. As shown in Figure 4, renal I/R caused a marked increase in Nrf2 expression in the nuclear fraction of the kidney. However, oral administration of AST-120, resveratrol, and quercetin showed a significant suppressive effect on the Nrf2 activation (Figs. 4A and C). Administration of sulforaphane, a well-known potent activator of Nrf2, resulted in a significant increase in Nrf2 expression in the ischemic kidney compared with that of control rat kidney, which was comparable with the Nrf2 expression as observed for ischemic kidney (Figs. 4B and D).
FIG. 4.
Effect of AST-120, SULT inhibitors, and sulforaphane on nuclear Nrf2 expression in the kidney of rats treated with renal I/R. AST-120, resveratrol, quercetin, and sulforaphane were orally administered to rats 24 and 1 h before and 24 h after renal I/R. Renal tissue samples for three rats in each group were collected 48 h after I/R treatment, and used for immunoblotting of Nrf2. Figures A and B show the representative blots of Nrf2 (57 kDa). The band density of Nrf2 was determined densitometrically and the relative density ratio to control (sham) without I/R treatment are represented as columns (C and D) with the mean ± SD for three rats in each group. **p < 0.01 versus sham (control); ##p < 0.01 versus I/R.
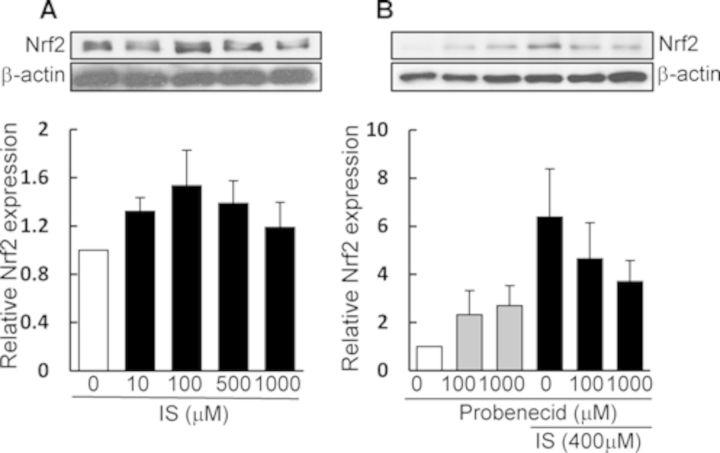
Effect of IS on Nrf2 Activation in HK-2 Cells
To investigate whether IS has a stimulating effect on Nrf2 activation in renal cells, an in vitro experiment was performed by using HK-2 cells. As depicted in Figure 5A, IS treatment caused an upregulation of Nrf2 protein expression at concentrations of <100 μM, but the expression decreased at higher concentrations (from 500 to 1000 μM). In addition, the IS (400 μM)-induced upregulation of Nrf2 was suppressed by the presence of probenecid, a potent inhibitor of organic anion transporters (Fig. 5B). These data suggested that IS is primarily taken up by HK-2 cells via organic anion transporters, which then evokes activation of Nrf2 (most likely due to induction of intracellular oxidative stress).
FIG. 5.
Effect of IS treatment on Nrf2 activation in HK-2 cells. HK-2 cells were treated with IS in the absence (A) or presence (B) of probenecid for 24 h. After incubation, cell lysates were prepared and used for immunoblotting (A and B). The band density of Nrf2 (57 kDa) was determined densitometrically and corrected with respect to the band density of β-actin. Each column shows the relative ratio against the Nrf2 band under IS-free conditions and represents the mean ± SD for four samples (A) and the mean for two samples (B).
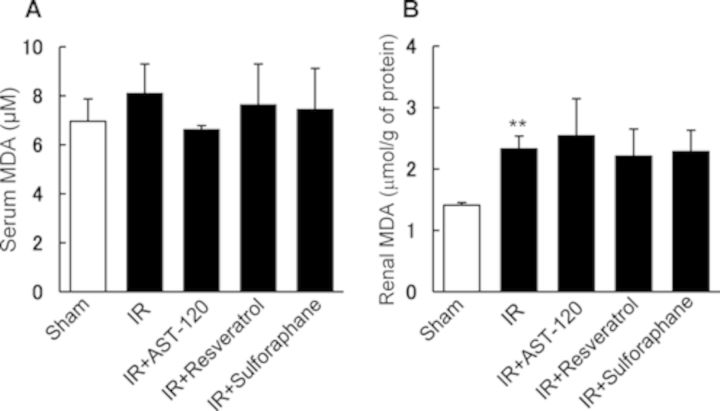
Effect of AST-120, SULT Inhibitors, and Sulforaphane on Serum and Renal MDA Levels in Rats Treated with Renal I/R
Figure 6 depicts lipid peroxidation in serum and kidney of rats with renal I/R treatment. There was no significant elevation of serum MDA levels among rats treated with or without administration of AST-120, resveratrol, and sulforaphane (Fig. 6A). By contrast, renal I/R caused a significant increase in the level of MDA in the kidney compared with control rat kidney, but the compounds examined showed no effect on MDA levels in the I/R kidney (Fig. 6B).
FIG. 6.
Effect of AST-120, SULT inhibitors, and sulforaphane on serum and renal MDA levels in rats treated with renal I/R. AST-120, resveratrol, and sulforaphane were orally administered to rats 24 and 1 h before and 24 h after renal I/R. Renal tissue samples were collected 48 h after I/R treatment, and used for determination of MDA levels in serum (A) and kidneys (B). Each column represents the mean ± SD for four rats in each group. **p < 0.01 versus sham (control).
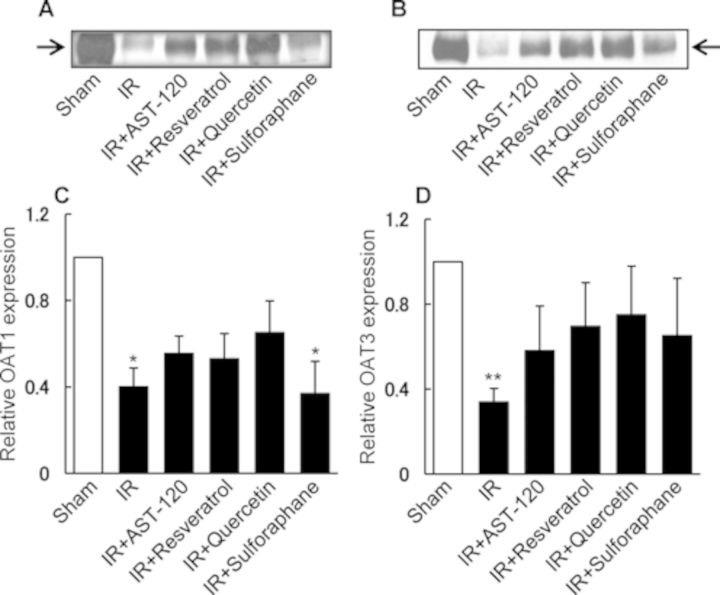
Effect of AST-120, SULT Inhibitors, and Sulforaphane on Renal Expression of OAT1 and OAT3 in Rats Treated with I/R
Next, we examined renal I/R and oral administration of compounds on renal protein expression of organic anion transporters, OAT1 and OAT3, which mediate basolateral influx of anionic uremic toxins including IS and p-cresyl sulfate (Enomoto et al., 2002; Saito, 2010). In a previous report, we observed a marked downregulation of these transporters after I/R of the rat kidney (Matsuzaki et al., 2007). As shown in Figure 7, treatment with renal I/R resulted in a marked downregulation in the expression of both OAT1 and OAT3 (Figs. 7A and B). By contrast, oral administration of AST-120, resveratrol, or quercetin reestablished the normal levels of expression for both these transporters (Fig. 7). Sulforaphane reestablished the expression of OAT3, but not OAT1 (Figs. 7C and D).
FIG. 7.
Effect of AST-120, SULT inhibitors, and sulforaphane on OAT1 and OAT3 expressions in the kidney of rats treated with I/R. AST-120, resveratrol, and sulforaphane were orally administered to rats 24 and 1 h before and 24 h after renal I/R. Renal tissue samples were collected 48 h after I/R treatment, and used for immunoblotting of OAT1 and OAT3. Figures A and B show the representative blots of OAT1 (77 kDa) or OAT3 (72 kDa). The band density of OAT1 and OAT3 were determined densitometrically and the relative density ratio to control (sham) is represented as columns (C and D) with the mean ± SD for three rats in each group. *p < 0.05 versus sham (control); **p < 0.01 versus sham (control).
DISCUSSION
The putative uremic toxin, IS, is thought to play a toxicopharmacological role in the development and/or progression of renal injury. Herein, we demonstrate the nephropreventive effects of AST-120, SULT inhibitors, and sulforaphane by using ischemic AKI model rats. Previously, it was reported that IS enhanced reactive oxygen species (ROS) production and induced cytotoxicity in endothelial cells (Adelibieke et al., 2012; Niwa, 2010, 2011; Niwa and Shimizu, 2012), glomerular mesangial cells (Gelasco and Raymond, 2006; Niwa, 2011; Niwa and Ise, 1994), renal tubular cells (Niwa, 2011; Shimizu et al., 2013), and osteoblasts (Nii-Kono et al., 2007). Dou et al. (2007) found that IS increased NADPH oxidase activity and decreased glutathione levels in endothelial cells. Gelasco et al. (2006) found that IS induced the production of intracellular ROS in mesangial cells, and the ROS generation was partially inhibited by an inhibitor of NADPH oxidase. Shimizu et al. (2012) reported that IS upregulates renal expression of monocyte chemotactic protein-1 through production of ROS, and activation of NF-κB, p53, ERK, and JNK in proximal tubular cells. Furthermore, IS appeared to reduce superoxide scavenging activity in the kidneys of normal and uremic rats (Owada et al., 2008). Thus, the toxic properties of IS may be induced by stimulating the production of ROS and disturbing the antioxidant system in not only the kidney but also several other tissues.
Previously, we reported that endogenous IS levels were markedly elevated in ischemic rats compared with that of control rats from 6 h after renal I/R (Matsuzaki et al., 2007). Furthermore, we have found a significant correlation between the level of BUN or SCr with the concentration of serum IS in both control and I/R rats, suggesting that IS could be an endogenous functional biomarker for evaluating renal dysfunction and renal tubular secretion of organic anions (Matsuzaki et al., 2007). A previous report demonstrated that SULT activity mediating conjugation of indoxyl to IS in the liver could be the major inhibition target for IS production rather than CYPs activities in oxidation of indole to indoxyl (Kusumoto et al., 2011). In addition, administration of AST-120 and SULT inhibitors, including phytochemical polyphenols, to cisplatin-induced AKI model rats reduced renal tissue damage and dysfunction, suggesting IS contribute to the development or progression of kidney injury (Kusumoto et al., 2011). In the present study, oral administration of AST-120 or phytochemical polyphenols with a potent SULT inhibitory effect appeared to improve I/R-induced AKI in association with the suppression of serum and renal accumulations of IS. Taken together, our results confirm that IS plays a toxicopharmacological role in the development and progression of I/R-induced renal dysfunction, thereby constituting a potential target for therapeutic management. After intestinal absorption, indole is distributed in the liver via portal vein, and then hydroxylated by CYP2A6 and/or CYP2E1 to indoxyl. Indoxyl is predominantly conjugated with sulfate by SULT. Under the suppression or inhibition of SULT, indoxyl is conjugated with glucuronic acid by hepatic UDP-glucuronyltransferase to indoxyl glucuronide as an alternative pathway for indoxyl metabolism. We have observed using rat liver S9 fraction that indoxyl glucuronide formation was elevated when IS production was blocked by phytochemicals including resveratrol and quercetin (unpublished data).
Renal I/R evoked a marked elevation in the level of both SCr and BUN, which was significantly suppressed by the administration of AST-120 or SULT inhibitors, suggesting that serum accumulation of IS might be associated with decreased glomerular function in I/R-induced AKI. In addition, we observed that postadministration of AST-120 or SULT inhibitors after I/R treatment had no preventive effect on I/R-induced renal damage, as judged by the levels of SCr and BUN, despite the fact that AST-120 decreased the serum level of IS (Fig. 3). Therefore, suppressing serum IS accumulation prior to the onset of I/R-induced renal injury (i.e., preconditioned inhibition of IS production or accumulation) appears to be an efficient way to prevent glomerular dysfunction in AKI. Considering these findings, we suppose preconditioned treatments with SULT inhibitor could be efficient and applied under clinical settings of renal ischemic conditions such as partial nephrectomy in tumor surgery, renal transplantation surgery, and treatments with nephrotoxicity-evoking drugs.
The marked increase in urinary Kim-1 excretion suggested that I/R treatment caused severe damage to renal proximal tubular cells; Kim-1 is known to be a highly sensitive biomarker for proximal tubular injury (Bonventre and Yang, 2010). Notably, oral administration of AST-120 resulted in a significant decrease in urinary Kim-1 excretion in I/R rats in association with decreased IS accumulation in the kidney, suggesting that IS is also involved in ischemic renal tubular cell damage. Furthermore, resveratrol and sulforaphane also showed a potent preventive effect on urinary Kim-1 excretion. The administration dose (i.e., 5 mg/kg for three times) of resveratrol was relatively low when compared with the doses exhibiting its antioxidant effects in rat kidney: e.g., 30 mg/kg for 12 weeks in diabetic nephropathy rats (Wu et al., 2012) or 10 mg/kg for 7 weeks in cholestasis rats with renal oxidative stress (Ara et al., 2005). However, in the present experimental conditions, antioxidant action of resveratrol in the preventive effect on renal tubular cells could not be excluded. In contrast, quercetin had no significant preventive effect on urinary Kim-1 excretion in ischemic rats, despite the fact that quercetin decreased I/R-induced renal injury and suppressed renal accumulation of IS. Quercetin may have different properties for the Kim-1 response of renal tubular cells against I/R-induced AKI. The difference between resveratrol and quercetin in terms of their preventive effect on Kim-1 excretion will be addressed in future experiments.
We found that the Nrf2 expression in renal nuclear fractions was elevated by renal I/R treatment, and was depressed by administration of AST-120 or SULT inhibitors, but not of sulforaphane. These results imply that oxidative stress was generated by accumulated IS in renal tissues after I/R treatment, thereby promoting nuclear translocation of cytoplasmic Nrf2 to induce antioxidant and detoxifying molecules including NQO1, GST, GLC, HO-1, and MRPs through binding to ARE/EpRE (Saito, 2013). The observation that incubation of HK-2 cells with IS activated Nrf2 expression, which was suppressed by probenecid, supports the idea of IS uptake via OAT1 or OAT3 inducing oxidative stress. Phytochemical polyphenols, including resveratrol and quercetin, are reported to enhance nuclear translocation of Nrf2 (Kumar et al., 2011; Palsamy and Subramanian, 2011), thereby reducing oxidative stress in various types of mammalian cells. In the present study, both resveratrol and quercetin apparently showed no stimulatory effects on the Nrf2 activation in the kidney of I/R-treated rats (Fig. 4), suggesting that oral administration of resveratrol and quercetin at a dose of 5 and 50 mg/kg, respectively, had no effects on the Nrf2 activation, but decreased oxidative stress by reducing IS accumulation in the kidney. We speculate that renal I/R-induced Nrf2 activation could be primarily due to oxidative stress evoked by I/R treatment of the kidney. Notably, the activation of Nrf2 was markedly suppressed by oral administration of AST-120 (Figs. 4A and 4C), which had no direct suppressive effect on oxidative stress, but blocked IS accumulation in the kidney (Fig. 1D) by adsorbing indole in the intestine. In contrast, oral administration of resveratrol or quercetin suppressed the I/R-induced activation of Nrf2 (Fig. 4A and C) in association with the decrease in renal IS accumulations (Fig. 1D). In a previous report, oral resveratrol administration (5 mg/kg) for 30 days had no effect on both mRNA and protein levels of Nrf2 in the kidney of diabetic model rats (Palsamy and Subramanian, 2011). To our knowledge, there is no report or finding indicating the suppressive effect of resveratrol on nuclear Nrf2 activation. Taking these results and previous findings into consideration, we suggest that renal accumulation of IS could play a key role in the production of oxidative stress in the kidney of ischemic AKI rats, thereby activating Nrf2. Administration of resveratrol or quercetin in AKI rats may suppress secondarily renal accumulation of IS by inhibiting hepatic SULT, thereby preventing ROS generation and Nrf2 activation. The suppressive effect of AST-120 on Nrf2 activation can be interpreted by suppression of renal IS accumulation through intestinal adsorption of indole followed by decreased hepatic IS production. It is suggested that indoxyl sulfate plays a pivotal role in the generation of oxidative stress in renal cells. The level of MDA in I/R-treated kidney was significantly elevated compared with control rat kidney, but was not suppressed by administration of AST-120, resveratrol nor sulforaphane (Fig. 6). This increase in MDA could be evoked primarily by ROS induced after I/R treatment of the kidney. Therefore, I/R-induced lipid peroxidation of the injured kidney could not be blocked by the pharmacological treatment that suppresses accumulation of IS in the kidney. After renal I/R treatment, continuous suppression of IS levels by the administration of AST-120 or SULT inhibitor may prevent further production of ROS in the injured kidney.
Sulforaphane had no additive stimulatory effect on the nuclear Nrf2 expression in the kidney of I/R rats, compared with that in I/R-treated rats without sulforaphane administration (Fig. 4B). As sulforaphane has no inhibitory effect on hepatic IS production (data not shown), and showed no significant suppressive effect on renal IS accumulation (Fig. 1D), the result of sulforaphane effect on the Nrf2 activation might be interpreted by I/R-induced oxidative stress and likely IS accumulation rather than its direct modification of Nrf2/Keap-1. Histological studies suggested that pretreatment with sulforaphane prevented morphological damage to the rat kidney following I/R, but resulted in slight swelling of the tubular epithelium and a slight loss of brush border (Yoon et al., 2008). In HK-2 cells, the cytoplasmic suppressing protein, Keap1 is bound directly to Nrf2 under normal quiescent conditions, thereby repressing its nuclear translocation (Yoon et al., 2008). However, exposure of HK-2 cells to sulforaphane for 12 h decreased the cytoplasmic level of Keap1 protein and increased the expression level of nuclear Nrf2. Sulforaphane effectively reduces renal dysfunction or injury caused by I/R treatment of the kidney. The nephroprotective mechanism of sulforaphane is considered to be mediated by preconditioning of the kidney by activation of Nrf2 and the resultant induction of phase 2 enzymes such as heme oxygenase-1, NADPH: quinone oxidoreductase 1, GSH reductase, and GSH peroxidase (Yoon et al., 2008).
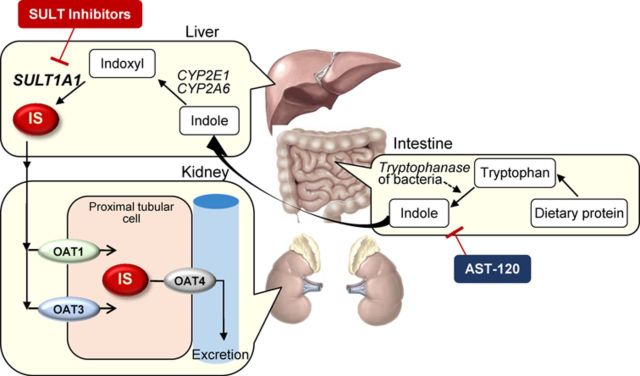
OAT1 and OAT3 are expressed in the basolateral membranes of proximal tubule epithelial cells in the kidney, mediating efficient IS uptake from the blood into renal cells (Fig. 8) (Saito, 2010, 2013). Our previous report suggested that the level of both OAT1 and OAT3 at the mRNA and protein levels were markedly reduced in I/R-treated kidney (Matsuzaki et al., 2007). We speculated that the downregulation of renal OAT1 and OAT3 could be responsible for the increase in serum IS level of renal I/R-treated rats (Matsuzaki et al., 2007). The report suggested that (1) I/R of rat kidney induces the serum accumulation of IS, which was caused by the downregulation of renal OAT1 and OAT3, accompanied by decreased transport activities, and (2) intake of cobalt chloride, that acts as a stabilizer of hypoxia-inducible factor-1, ameliorated the progression of renal dysfunction, but it had no significant preventive effect on the downregulation of OAT1 and OAT3, but showed partial restoration of OAT3 function. Pathophysiological role of IS in regulation of these transporters under ischemic AKI was not examined. In the present study, both OAT1 and OAT3 expressions were markedly and significantly downregulated by the renal I/R treatment (Fig. 7). The downregulation of both transporters was partially prevented in I/R-treated rats administered with AST-120 or SULT inhibitor (resveratrol or quercetin) with no significant difference compared with that in control (sham) rats, suggesting that AST-120 and SULT inhibitors have a partial preventive effect on the downregulation of both OAT1 and OAT3. We suggest that the effect of AST-120 and SULT inhibitors on the partial prevention/restoration of these OAT transporters may be related to the decreased accumulation of IS, probably by reducing IS-associated oxidative stress in renal tubular cells. The prevention of downregulation or restoration of the expression of both OAT1 and OAT3 could play a key role in urinary excretion of anionic uremic toxins to decrease accumulation of these toxins in serum and renal tissues, which may well be associated with the nephropreventive properties of these compounds. However, sulforaphane also prevented the downregulation of OAT3, but not OAT1, suggesting that OAT3 responds to the Nrf2-dependent antioxidant process in renal tubular cells.
FIG. 8.
Schematic diagram showing the production and excretion of IS. Tryptophan derived from dietary protein is metabolized to indole by tryptophanase of intestinal bacteria such as Escherichia coli. AST-120 adsorbs indole generated in intestine. Following intestinal absorption, indole is hydroxylated to indoxyl by CYP 2E1 and/or CYP2A6, and subsequently conjugated to IS by SULT1A1 in the liver. IS produced in the liver enters the circulatory system, followed by influx into renal proximal tubular cells via OAT1 and OAT3 localized in basolateral membrane, and is then excreted into the urine via OAT4 localized in brush-border/apical membrane. SULT inhibitors impede hepatic SULT activity and thereby reduce IS production.
OAT1 and OAT3 transporters are reported to be very sensitive to renal ischemic condition, i.e., the downregulation by cyclooxygenase (COX)-mediated elevation of prostaglandin E2 (PGE2) (Shneider et al., 2007, 2009). The treatments with AST-120 or SULT inhibitor could decrease the I/R-induced oxidative stress, as suggested by suppression of Nrf2 activation, by decreasing IS accumulation in the kidney, thereby partially preventing oxidative stress-inducible COX-mediated PGE2 production and subsequent downregulation of OAT1 and OAT3. Partial prevention or restoration of both transporters implies enhancement of IS uptake by renal tubular cells at basolateral membranes. However, the present findings that renal accumulation of IS was decreased in association with the partial restoration of OAT1 and OAT3 suggest that “renal net tubular secretion of IS” might be recovered. Among the organic anion transporters localized in the apical/brush-border membrane of renal proximal tubules, human OAT4 was shown to mediate bidirectional transport of IS (Enomoto et al., 2003), suggesting one of the candidate transporter involved in apical efflux/secretion of IS. In terms of the rate-limiting step/transport for tubular secretion of IS, there is no definite reports indicating quantitative kinetical comparison of the renal basolateral and apical/brush-border membrane transport of IS. We speculate that apical membrane efflux transporter(s), possibly OAT4, might be restored as well as basolateral OAT1 and OAT3 by treatment with AST-120 or SULT inhibitors, because renal accumulation of IS was decreased by these agents in ischemic AKI rats. OAT3 may be much more sensitive to oxidative stress or ROS than OAT1. The downregulation of these transporters could be one of the defense systems to prevent renal tubular accumulation of endogenous anionic toxic metabolites or toxins, which evoke cellular injury by generating oxidative stress or ROS. IS has been suggested to aggravate not only CKD-induced cardiac fibrosis (Lekawanvijit et al., 2012a,b) but aortic calcification in predialysis CKD patients (Goto et al., 2013). Thus, therapeutic medication strategy for reduction of serum IS may be useful to improve cardiorenal syndrome as well as progression of kidney disease.
In summary, in the systemic production and excretion processes of IS, we propose that hepatic SULT could serve as a potential nephropreventing target for development and/or progression of ischemic AKI and renal tubular damage by suppressing SULT-mediated production of IS and its subsequent accumulation in serum and kidneys (Fig. 8).
FUNDING
Japan Society for the Promotion of Science (JSPS) KAKENHI (25293040 to H.S.); Adaptable & Seamless Technology Transfer Program through Target-driven R&D (A-STEP) (AS242Z02505Q to H.S.); Japan Science and Technology Agency (JST) Accelerating Utilization of University IP Program (FY2013-300-148007 to H.S.).
REFERENCES
- Adelibieke Y., Shimizu H., Muteliefu G., Bolati D., Niwa T. Indoxyl sulfate induces endothelial cell senescence by increasing reactive oxygen species production and p53 activity. J. Ren. Nutr. 2012;22:86–89. doi: 10.1053/j.jrn.2011.10.027. [DOI] [PubMed] [Google Scholar]
- Ara C., Karabulut A. B., Kirimlioglu H., Coban S., Ugras M., Kirimliglu V., Yilmaz S. Protective effect of resveratrol against renal oxidative stress in cholestasis. Ren. Fail. 2005;27:435–440. [PubMed] [Google Scholar]
- Banoglu E., King R. S. Sulfation of indoxyl by human and rat aryl (phenol) sulfotransferases to form indoxyl sulfate. Eur. J. Drug Metab. Pharmacokinet. 2002;27:135–140. doi: 10.1007/BF03190428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banoglu E., Jha G. G., King R. S. Hepatic microsomal metabolism of indole to indoxyl, a precursor of indoxyl sulfate. Eur. J. Drug Metab. Pharmacokinet. 2001;26:235–240. doi: 10.1007/BF03226377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belayev L. Y., Palevsky P. M. The link between acute kidney injury and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2013;23:149–154. doi: 10.1097/01.mnh.0000441051.36783.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre J. V., Yang L. Kidney injury molecule-1. Curr. Opin. Crit. Care. 2010;16:556–561. doi: 10.1097/MCC.0b013e32834008d3. [DOI] [PubMed] [Google Scholar]
- Chiang C. K., Tanaka T., Nangaku M. Dysregulated oxygen metabolism of the kidney by uremic toxins: Review. J. Ren. Nutr. 2012;22:77–80. doi: 10.1053/j.jrn.2011.10.028. [DOI] [PubMed] [Google Scholar]
- Dou L., Jourde-Chiche N., Faure V., Cerini C., Berland Y., Dignat-George, Brunet P. F. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J. Thromb. Haemost. 2007;5:1302–1328. doi: 10.1111/j.1538-7836.2007.02540.x. [DOI] [PubMed] [Google Scholar]
- Enomoto A., Takeda M., Tojo A., Sekine T., Cha S. H., Khamdang S., Takayama F., Aoyama I., Nakamura S., Endou H., Niwa T. Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J. Am. Soc. Nephrol. 2002;13:1711–1720. doi: 10.1097/01.asn.0000022017.96399.b2. [DOI] [PubMed] [Google Scholar]
- Enomoto A., Takeda M., Taki K., Takayama F., Noshiro R., Niwa T., Endou H. Interactions of human organic anion as well as cation transporters with indoxyl sulfate. Eur. J. Pharmacol. 2003;466:13–20. doi: 10.1016/s0014-2999(03)01530-9. [DOI] [PubMed] [Google Scholar]
- Fujii H., Nakai K., Fukagawa M. Role of oxidative stress and indoxyl sulfate in progression of cardiovascular disease in chronic kidney disease. Ther. Apher. Dial. 2011;15:125–128. doi: 10.1111/j.1744-9987.2010.00883.x. [DOI] [PubMed] [Google Scholar]
- Gelasco A. K., Raymond J. R. Indoxyl sulfate induces complex redox alterations in mesangial cells. Am. J. Physiol. Renal Physiol. 2006;290:F1551–F1558. doi: 10.1152/ajprenal.00281.2004. [DOI] [PubMed] [Google Scholar]
- Goto S., Kitamura K., Kono K., Nakai K., Fujii H., Nishi S. Association between AST-120 and abdominal aortic calcification in predialysis patients with chronic kidney disease. Clin. Exp. Nephrol. 2013;17:365–371. doi: 10.1007/s10157-012-0717-0. [DOI] [PubMed] [Google Scholar]
- Guerrero-Beltrán C. E., Calderón-Oliver M., Tapia E., Medina-Campos O. N., Sánchez-González D. J., Martínez-Martínez C. M., Ortiz-Vega K. M., Franco M., Pedraza-Chaverri J. Sulforaphane protects against cisplatin-induced nephrotoxicity. Toxicol. Lett. 2010;192:278–285. doi: 10.1016/j.toxlet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Iwata K., Watanabe H., Morisaki T., Matsuzaki T., Ohmura T., Hamada A., Saito H. Involvement of indoxyl sulfate in renal and central nervous system toxicities during cisplatin-induced acute renal failure. Pharm. Res. 2007;24:662–671. doi: 10.1007/s11095-006-9183-2. [DOI] [PubMed] [Google Scholar]
- Kaushal G. P., Shah S. V. Challenges and advances in the treatment of AKI. J. Am. Soc. Nephrol. 2014;25:877–883. doi: 10.1681/ASN.2013070780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Singh C. K., Lavoie H. A., Dipette D. J., Singh U. S. Resveratrol restores Nrf2 level and prevents ethanol-induced toxic effects in the cerebellum of a rodent model of fetal alcohol spectrum disorders. Mol. Pharmacol. 2011;80:446–457. doi: 10.1124/mol.111.071126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumoto M., Kamobayashi H., Sato D., Komori M., Yoshimura M., Hamada A., Saito H. Alleviation of cisplatin-induced acute kidney injury using phytochemical polyphenols is accompanied by reduced accumulation of indoxyl sulfate in rats. Clin. Exp. Nephrol. 2011;15:820–830. doi: 10.1007/s10157-011-0524-z. [DOI] [PubMed] [Google Scholar]
- Lekawanvijit S., Kompa A. R., Wang B. H., Kelly D. J., Krum H. Cardiorenal syndrome: The emerging role of protein-bound uremic toxins. Circ. Res. 2012a;111:1470–1483. doi: 10.1161/CIRCRESAHA.112.278457. [DOI] [PubMed] [Google Scholar]
- Lekawanvijit S., Kompa A. R., Manabe M., Wang B. H., Langham R. G., Nishijima F., Kelly D,J., Krum H. Chronic kidney disease-induced cardiac fibrosis is ameliorated by reducing circulating levels of a non-dialysable uremic toxin, indoxyl sulfate. PLoS One. 2012b;7:e41281. doi: 10.1371/journal.pone.0041281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki T., Watanabe H., Yoshitome K., Morisaki T., Hamada A., Nonoguchi H., Kohda Y., Tomita K., Inui K., Saito H. Downregulation of organic anion transporters in rat kidney under ischemia/reperfusion-induced acute renal failure. Kidney Int. 2007;71:539–547. doi: 10.1038/sj.ki.5002104. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Ise M., Hirata M., Endo K., Ito Y., Seo H., Niwa T. Indoxyl sulfate stimulates renal synthesis of transforming growth factor-beta 1 and progression of renal failure. Kidney Int. 1997;63:S211–S214. [PubMed] [Google Scholar]
- Morisaki T., Matsuzaki T., Yokoo K., Kusumoto M., Iwata K., Hamada A., Saito H. Regulation of renal organic ion transporters in cisplatin-induced acute kidney injury and uremia in rats. Pharm. Res. 2008;25:2526–2533. doi: 10.1007/s11095-008-9668-2. [DOI] [PubMed] [Google Scholar]
- Neirynck N., Vanholder R., Schepers E., Eloot S., Pletinck A., Glorieux G. An update on uremic toxins. Int. Urol. Nephrol. 2013;45:139–150. doi: 10.1007/s11255-012-0258-1. [DOI] [PubMed] [Google Scholar]
- Nii-Kono T., Iwasaki Y., Uchida M., Fujieda A., Hosokawa A., Motojima M., Yamato H., Kurokawa K., Fukagawa M. Indoxyl sulfate induces skeletal resistance to parathyroid hormone in cultured osteoblastic cells. Kidney Int. 2007;71:738–743. doi: 10.1038/sj.ki.5002097. [DOI] [PubMed] [Google Scholar]
- Niwa T. Indoxyl sulfate is a nephro-vascular toxin. J. Ren. Nutr. 2010;20:S2–S6. doi: 10.1053/j.jrn.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Niwa T. Role of indoxyl sulfate in the progression of chronic kidney disease and cardiovascular disease: Experimental and clinical effects of oral sorbent AST-120. Ther. Apher. Dial. 2011;15:120–124. doi: 10.1111/j.1744-9987.2010.00882.x. [DOI] [PubMed] [Google Scholar]
- Niwa T. Removal of protein-bound uraemic toxins by haemodialysis. Blood Purif. 2013;35:20–25. doi: 10.1159/000350843. [DOI] [PubMed] [Google Scholar]
- Niwa T., Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J. Lab. Clin. Med. 1994;124:96–104. [PubMed] [Google Scholar]
- Niwa T., Shimizu H. Indoxyl sulfate induces nephrovascular senescence. J. Ren. Nutr. 2012;22:102–106. doi: 10.1053/j.jrn.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Owada S., Goto S., Bannai K., Hayashi H., Nishijima F., Niwa T. Indoxyl sulfate reduces superoxide scavenging activity in the kidneys of normal and uremic rats. Am. J. Nephrol. 2008;28:446–454. doi: 10.1159/000112823. [DOI] [PubMed] [Google Scholar]
- Palsamy P., Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim. Biophys. Acta. 2011;1812:719–731. doi: 10.1016/j.bbadis.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Saito H. Pathophysiological regulation of renal SLC22A organic ion transporters in acute kidney injury: Pharmacological and toxicological implications. Pharmacol. Ther. 2010;125:79–91. doi: 10.1016/j.pharmthera.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Saito H. Toxico-pharmacological perspective of the Nrf2-Keap1 defense system against oxidative stress in kidney diseases. Biochem. Pharmacol. 2013;85:865–872. doi: 10.1016/j.bcp.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Shneider R., Sauvant C., Betz B., Otremba M., Fischer D., Holzinger H., Wanner C., Galle J., Gekle M. Downregulation of organic anion transporters OAT1 and OAT3 correlates with impaired secretion of para-aminohippurate after ischemic acute renal failure in rats. Am. J. Physiol. Renal Physiol. 2007;292:F1599–F1605. doi: 10.1152/ajprenal.00473.2006. [DOI] [PubMed] [Google Scholar]
- Shneider R., Meusel M., Renke S., Baue C., Holzinger H., Roeder M., Wanner C., Gekle M., Sauvant C. Low-dose indomethacin after ischemic acute kidney injury prevents downregulation of Oat1/3 and improves renal outcome. Am. J. Physiol. Renal Physiol. 2009;297:F1614–F1621. doi: 10.1152/ajprenal.00268.2009. [DOI] [PubMed] [Google Scholar]
- Schulman G., Agarwal R., Acharya M., Berl T., Blumenthal S., Kopyt N. A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of AST-120 (Kremezin) in patients with moderate to severe CKD. Am. J. Kidney Dis. 2006;47:565–577. doi: 10.1053/j.ajkd.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Bolati D., Higashiyama Y., Nishijima F., Shimizu K., Niwa T. Indoxyl sulfate upregulates renal expression of MCP-1 via production of ROS and activation of NF-κB, p53, ERK, and JNK in proximal tubular cells. Life Sci. 2012;90:525–530. doi: 10.1016/j.lfs.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Yisireyili M., Higashiyama Y., Nishijima F., Niwa T. Indoxyl sulfate upregulates renal expression of ICAM-1 via production of ROS and activation of NF-κB and p53 in proximal tubular cells. Life Sci. 2013;92:143–148. doi: 10.1016/j.lfs.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Thadhani R., Pascual M., Bonventre J. V. Acute renal failure. N. Engl. J. Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- Ueda H., Shibahara N., Takagi S., Inoue T., Katsuoka Y. AST-120 treatment in pre-dialysis period affects the prognosis in patients on hemodialysis. Ren. Fail. 2008;30:856–860. doi: 10.1080/08860220802356531. [DOI] [PubMed] [Google Scholar]
- Vanholder R., De Smet R., Glorieux G., Argilés A., Baurmeister U., Clark W., Cohen G., De Deyn P. P., Deppisch R., et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- Wu Q. Q., Wang Y., Senitko M., Meyer C., Wigley W. C., Ferguson D. A., Grossman E., Chen J., Zhoum X,J., Hartono J., et al. Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARγ, and HO-1. Am. J. Physiol. Renal Physiol. 2010;300:F1180–F1192. doi: 10.1152/ajprenal.00353.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Zhang Y., Ma X., Zhang N., Qin G. The effect of resveratrol on FoxO1 expression in kidneys of diabetic nephropathy rats. Mol. Biol. Rep. 2012;39:9085–9093. doi: 10.1007/s11033-012-1780-z. [DOI] [PubMed] [Google Scholar]
- Ympa Y. P., Sakr Y., Reinhart K., Vincent J. L. Has mortality from acute renal failure decreased? A systematic review of the literature. Am. J. Med. 2005;118:827–832. doi: 10.1016/j.amjmed.2005.01.069. [DOI] [PubMed] [Google Scholar]
- Yoon H. Y., Kang N. I., Lee H. K., Jang K. Y., Park J. W., Park B. H. Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem. Pharmacol. 2008;75:2214–2223. doi: 10.1016/j.bcp.2008.02.029. [DOI] [PubMed] [Google Scholar]