Abstract
Lipopolysaccharides (LPS) through Toll-like receptor 2 (TLR2) and Toll-like receptor 4 (TLR4) activation induce systemic inflammation where oxidative damage plays a key role in multiple organ failure. Because of the neutralization of LPS toxicity by sialic acid (SA), we determined its effect and mechanisms on repurified LPS (rLPS)-evoked acute renal failure. We assessed the effect of intravenous SA (10 mg/kg body weight) on rLPS-induced renal injury in female Wistar rats by evaluating blood and kidney reactive oxygen species (ROS) responses, renal and systemic hemodynamics, renal function, histopathology, and molecular mechanisms. SA can interact with rLPS through a high binding affinity. rLPS dose- and time-dependently reduced arterial blood pressure, renal microcirculation and blood flow, and increased vascular resistance in the rats. rLPS enhanced monocyte/macrophage (ED-1) infiltration and ROS production and impaired kidneys by triggering p-IRE1α/p-JNK/CHOP/GRP78/ATF4-mediated endoplasmic reticulum (ER) stress, Bax/PARP-mediated apoptosis, Beclin-1/Atg5-Atg12/LC3-II-mediated autophagy, and caspase 1/IL-1β-mediated pyroptosis in the kidneys. SA treatment at 30 min, but not 60 min after rLPS stimulation, gp91 siRNA and protein kinase C-α (PKC) inhibitor efficiently rescued rLPS-induced acute renal failure via inhibition of TLR4/PKC/NADPH oxidase gp91-mediated ER stress, apoptosis, autophagy and pyroptosis in renal proximal tubular cells, and rat kidneys. In response to rLPS or IFNγ, the enhanced Atg5, FADD, LC3-II, and PARP expression can be inhibited by Atg5 siRNA. Albumin (10 mg/kg body weight) did not rescue rLPS-induced injury. In conclusion, early treatment (within 30 min) of SA attenuates rLPS-induced renal failure via the reduction in LPS toxicity and subsequently inhibiting rLPS-activated TLR4/PKC/gp91/ER stress/apoptosis/autophagy/pyroptosis signaling.
Keywords: lipopolysaccharide, sialic acid, reactive oxygen species, apoptosis, autophagy, pyroptosis, toll-like receptors
Sepsis, a major medical problem often leading to multiple organ failure, is one of the main causes of death in critical care medicine. The development of early sepsis into septic shock involves inflammatory cytokines and reactive oxygen species (ROS) production, which may be caused by the migration of leukocytes, lymphocytes, and platelets to the infected areas and increase endothelial damage, microvascular permeability, platelet aggregation, local blood flow reduction, and ischemia/reperfusion injury consequently leading to multiple organ failure (Bhattacharyya et al., 2004; Ho et al., 2009). Bacterial endotoxin, lipopolysaccharides (LPS) of Gram-negative bacteria, has been widely applied to the animals for the induction of systemic inflammation and hypotension (Ho et al., 2009; Yang et al., 2007). Acute renal failure is a common complication in septic patients (Angus et al., 2001; Hotchkiss and Karl, 2003). Exacerbated ROS production leads to acute renal failure through ROS-evoked abnormal signal transduction, inflammatory monocyte/macrophage (ED-1) infiltration, cellular dysfunction, and death in the several kinds of renal cells (Chung et al., 2012; Chien et al., 2001; Zimmerman, 1995). Three types of programmed cell death, apoptosis, autophagy, and pyroptosis, have been identified for their specific characteristics (Fink and Cookson, 2005). Autophagy is a programmed process that might promote or prevent cell death through the formation of autophagosome. Pyroptosis is an inherently proinflammatory pathway to cell death mediated by the activation of caspase 1, a protease that also activates the inflammatory cytokines, IL-1β, and features cell lysis and release of inflammatory cellular contents (Fink and Cookson, 2007). Pyroptosis occurs most frequently upon infection with intracellular pathogens (Fink and Cookson, 2007). Pyroptosis occurs independently of other proapoptotic caspases because pyroptosis is accompanied by plasma membrane rupture, water influx, cellular swelling, osmotic lysis, and the release of proinflammatory cytokines IL-1β and IL-18 other cellular contents (Orrenius et al., 2011).
ROS generation from the NADPH (nicotinamide adenine dinucleotide phosphate) oxidase or mitochondria may induce apoptosis, autophagy, or pyroptosis, via execution by caspases, lysosomal proteases, or endonucleases (Chung et al., 2012; Sumimoto et al., 2005; Taylor et al., 1995; Yang et al., 2008, 2013). The increased ROS trigger apoptosis by activating Bax expression/caspase 3 activity/poly-(ADP-ribose)-polymerase (PARP) fragments, enhance autophagy by activating Beclin-1/Atg5-Atg12/LC3-II pathway and/or evoke pyroptosis via the activation of caspase 1/IL-1β signaling (Chung et al., 2012; Yang et al., 2008, 2013). Recent studies suggest a critical cytoprotective or detrimental role for autophagy in toxin-mediated and ischemia/reperfusion-induced acute kidney injury (Kimura et al., 2011). The inhibition of apoptosis/autophagy (Oberholzer et al., 2001) or induction of autophagy in sepsis (Howell et al., 2013; Jiang et al., 2012; Kimura et al., 2011, Takahashi et al., 2012) is a new target for therapeutic exploration. Cumulated evidence showed that there is a cross talk between autophagy and apoptosis via Bcl-2 family (Fink and Cookson, 2005; Orrenius et al., 2011). Atg5 promotes autophagy and also enhances susceptibility toward apoptotic stimuli (Yousefi et al., 2006). Because both apoptosis and autophagy are simultaneously occurring in LPS-induced sepsis (Ho et al., 2009), a temporal study for determining if autophagy occurs first and then switches to apoptosis has never been explored. In response to IFNγ (TLR4 signaling), Atg5 interacts with FADD, an adaptor protein involved in death receptor-mediated apoptosis, to trigger autophagic cell death (Codogno and Meijer, 2006; Zhande et al., 2007). The role of Atg5 in the interaction between LPS-induced autophagy and apoptosis in the kidney has not clearly demonstrated. On the other hand, endoplasmic reticulum (ER) plays a central role in protein synthesis and folding and excessive protein synthesis and accumulation of unfolded proteins in the ER lumen, resulting in ER stress. ER stress triggered via GRP78/Bip-mediated the phopshorylation of inositol-requiring enzyme 1α (IRE1α) and activated the proapoptotic C/EBP homologues protein (CHOP), Jun N-terminal kinase (JNK), ATF4, and caspase 12 to subsequently promote apoptotic cell death (Hetz, 2012). ROS-evoked ER stress signaling may involve apoptosis or autophagy contributing to LPS-induced acute renal failure.
Several therapeutic strategies for treating sepsis are aimed at eradicating bacteria by antibiotics, by blocking the actions of cytokines, and most importantly at neutralizing LPS (Heine et al., 2001). A few antibodies and peptides reactive to LPS are able to efficiently block its proinflammatory activity (Gallay et al., 1994; Hamann et al., 2002; Levy and Elsbach, 2001). Although Polymyxin B with potent endotoxin-neutralizing properties has been applied to treat sepsis, the toxic antibiotic is associated with neurotoxic and nephrotoxic adverse effects (Danner et al., 1989). N-Acetylneuraminic acid, the most common sialic acid (SA), is found as the terminal position of cell surface glycoproteins and glycolipids (Varki, 1997) and is participated in E-selectin binding (Varki, 1997). SA affects the adherence of influenza virus particles to their target cells (Schauer and Kamerling, 1997). Previously, the use of exogenous SA can bind to lipid A, LPS and Helicobacter pylori, neutralize the toxicity, and subsequently ameliorate LPS-induced hepatic failure (Ho et al., 2009). However, LPS containing different components like endotoxin and endotoxin protein may evoke differential activation of Toll-like receptor 2 (TLR2) and Toll-like receptor 4 (TLR4) to facilitate the elimination of invading microorganisms, produce severe sepsis and inflammation (Bosshart and Heinzelmann, 2007; Takeuchi and Akira, 2010), and autophagy/pyroptosis formation (Bortoluci and Medzhitov, 2010). It has been argued that the commercial LPS prepared by a single phenol extraction may contain some endotoxin proteins and conserved bacterial structures called pathogen-associated molecular patterns (PAMPs) with possible TLR2 agonist activity (Hirschfeld et al., 2000). Therefore, to eliminate the possible interference from contaminant endotoxin proteins, the SA effect and mechanism on a repurified LPS (rLPS)-induced septic shock was required to be determined.
In the present study, we have rLPS to remove endotoxin proteins and to clearly delineate the interaction between SA and rLPS and a specific activation to TLR4 by rLPS. We explored whether SA's neutralizing effect improves acute renal injury via the inhibition of TLR4/PKC/NADPH oxidase/ROS-mediated ER stress, autophagy, apoptosis, and pyroptosis signaling simultaneously for the first time. We examined whether ROS overproduction-induced Atg5 switches autophagy to apoptosis in rLPS-induced kidney tissues. Also, we determined whether Atg5 can interact with FADD to trigger autophagic cell death under the stimulation of rLPS and interferonγ (IFNγ, a specific activation of TLR4 activation). We investigated whether the new insight of Atg5-FADD interaction between autophagy and apoptosis in the septic kidneys under in vitro and in vivo conditions. We also evaluated the timing effect and therapeutic mechanisms of SA administration in rLPS-induced acute multiple organ failure and systemic inflammation. These findings will provide an important clue for clinic application in future.
MATERIALS AND METHODS
Repurification of LPS
For removing endotoxin proteins and RAMPs, the commercial LPS were further purified as described previously (Hirschfeld et al., 2000). Briefly, 50 mg of LPS (Escherichia coli 0127:B8, Sigma-Aldrich, St Louis, MO) were suspended in 10 ml of endotoxin-free water containing 0.2% triethylamine (TEA). The sample was split into two 5 ml aliquots, and one aliquot was stored at 4°C without further manipulation (“unextracted LPS”). For phenol re-extracted LPS, deoxycholate was added to the remaining aliquot to a final concentration of 0.5% and followed by the addition of 5 ml of water-saturated phenol. The top aqueous layer was transferred to a new tube, and the phenol phase was re-extracted with 5 ml of 0.2% TEA/0.5% deoxycholate. The aqueous phases were pooled and re-extracted with 10 ml of water-saturated phenol. The pooled aqueous phases were adjusted to 75% ethanol and 30mM sodium acetate and precipitated at −20°C for 1 h. The precipitates after centrifuge at 4°C for 10 min at 10,000 × g, wash in 10 ml of cold 100% ethanol, and air-dry were resuspended in 5 ml of 0.2% TEA. One hundred percent recovery was assumed for the purified LPS, which was regarded as “rLPS”.
Biacore T200 for determining SA and LPS interaction
We had analyzed the binding affinity between unpurified LPS and SA by a BIAcoreX previously (Ho et al., 2009). We further explored the binding effect of SA on rLPS, which may contain different concentrations of highly bioactive contaminants like endotoxin protein and RAMPs (Hirschfeld et al., 2000), which may affect the binding effect between SA and rLPS. We prepared SA at the concentration of 1 mg/ml and rLPS standards. We determined the biomolecular interactions between SA and rLPS by using Biacore T200 optical biosensors and research-grade CM5 biosensor (GE Healthcare, Piscataway, NJ). Standard EDC/NHS coupling was used to covalently immobilize the therapeutic SA to CM5 sensor chips by amine coupling. CM5 chips were activated with EDC/NHS for 7 min with excess activated carboxyl groups blocked with ethanolamine for 7 min following immobilization of SA (diluted to 20 μg/ml in 10mM sodium acetate, pH 4.0). The absolute concentration of carboxyl groups contained per unit volume in the dextran surface layer is proprietary information (personal communication, GE Healthcare). We used amine coupling kit to fix SA on the surface channel of CM5 chip (Fc = 2). The procedures were implicated in Figure 1A. The channel Fc = 1 was used as reference value. rLPS (1 μg/ml) was injected into the surface channels for data analysis. Once the SA layer was stable, the rLPS solution (100 μg/ml) in distilled water was injected over the sensor chip surface and the subsequent dimensional, refractive index, and deposited mass changes were recorded. The Biacore sensorgrams were processed as described for the Biacore kinetic measurements using Biacore T200 software. Both the KD and ka values for binding analysis were calculated from the experimental curve with the BIAcore software package.
FIG. 1.

The procedures for measurement of kinetic interaction between SA and rLPS. (A) The procedures for immobilization of SA on an amine coupling kit activated CM5 chip. (B) The original tracing of SA interacts with rLPS at the level of RU = 165 during 100–210 s. (C) Representative sensograms of kinetic experiments between the increased rLPS concentration and immobilized SA. Using the single-cycle kinetics approach, samples are injected one after the other in the same cycle with no intervening regeneration steps. Here, a dilution series of rLPS was prepared at concentrations of 1, 2, 3, and 4 μg/ml and sequentially injected over a sensor surface prepared with SA (MW = 309.27) immobilized at 165 RU. Red line contrasts the measured data from the simulated fits (black line).
Surgery and animal preparation
Female Wistar rats (220–250 g) were purchased from BioLASCO Taiwan Co. Ltd (Taipei) and housed at the Experimental Animal Center, National Taiwan Normal University, at a constant temperature and with a consistent light cycle (light from 0700 h to 1800 h). All the surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee of the National Taiwan Normal University and were in accordance with the guidelines of the National Science Council of Republic of China (NSC 1997). All efforts were made to minimize animal suffering and the number of animals used throughout the experiment.
On the experimental day, the rats were anesthetized with subcutaneous urethane (1.2 g/kg). The body temperature was kept at 36.5°C–37.0°C by an infrared light and was monitored with a rectal thermometer. PE-50 catheters were placed in the left carotid artery for measurements of heart rate and arterial blood pressure (ABP) by using an ADI system (PowerLab/16S, ADI Instruments, Pty Ltd, Castle Hill, Australia) with a transducer (P23 1D, Gould-Statham, Quincy), and in the left femoral vein for administration of tested drugs or anesthetics when needed.
In this study, we used rLPS intravenously injected to the rats through the femoral vein to induce endotoxemia and renal injury. Total 97 rats were divided into following groups: normal group was given saline (n = 8); rLPS group was given different dose of rLPS (10, 25, and 50 mg/kg, iv, n = 8 each); rLPS + SA rats were challenged with rLPS (10, 25, and 50 mg/kg, iv, n = 8 each) and SA treatment (10 mg/kg, iv) 30 or 60 min after rLPS treatment; LPS group with unextracted LPS (50 mg/kg, iv, n = 6); LPS + SA rats were challenged with unextracted LPS and SA treatment (10 mg/kg, iv, n = 6) 30 min after LPS. Given that LPS binds to many molecules (e.g., albumin), it is possible that early administration of large doses (e.g., 10 mg/kg) of albumin other than SA would be equally effective in terms of inhibiting rLPS-induced organ damage in vivo. Therefore, we explored the effect of albumin (10 mg/kg body weight, iv) at 30 min post-rLPS treatment on rLPS-induced systemic inflammation in five rats. We determined the ROS level in the kidney, blood, and several biochemical parameters including blood urea nitrogen (BUN) and creatinine using commercially available analytical kits (Sigma, St Louis, MO). In some rats (n = 5 each), NADPH oxidase inhibitor diphenylene iodonium (DPI from Sigma, 3 mg/kg, ip), TLR4 inhibitor TAK-242 (resatorvid from Takeda Pharmaceutical Company Limited, Osaka, Japan, 3 mg/kg iv) or protein kinase C-α inhibitor α-tocopherol (90 mg/kg body weight, Sigma) were administered in the rLPS treated rats for determining TLR4/PKC/NADPH oxidase/ROS signaling.
At the end of each experiment, the animals were sacrificed with an intravenous potassium chloride injection (0.1 mg/ml). The kidneys was immediately removed and divided into two parts. One part was stored in 10% neutral buffered formalin for immunocytochemic assay, and another was frozen in liquid nitrogen and stored at –70°C for protein isolation or other assay.
Renal microcirculation measurement
The SA effect on LPS-induced acute renal failure was examined with a full-field laser perfusion imager and a transonic recording system. A full-field laser perfusion imager (MoorFLPI, Moor Instruments Ltd, Devon, UK) was used to continuously record microcirculatory blood flow intensity in the tested kidney. The amount of blood cells moved within the region of interest (ROI) is processed to produce a 16-color coded image that correlates with the value of renal blood flow. The ROI in blue is recognized as lower flow, whereas that in red is identified as higher flow. The microcirculatory blood flow intensity of each ROI was displayed as perfusion unit. The images were real-time analyzed by the MoorFLPI software version 3.0 (Moor Instruments Ltd). In some rats, we also measured the microcirculatory blood flow intensity in heart, lung, liver, kidney, and intestine simultaneously and examined their responses to LPS challenge and/or sialic acid treatment. We also measured total renal blood flow by placing a flow probe (Probe no. 0.1VBB517, Transonic Systems, Inc., Ithaca, NY) around the left renal artery by a transonic recording system (Transonic Systems, Inc.). We calculated the mean renal microvascular resistance by using the following formula:
 |
Endotoxin assay
The endotoxin level in plasma samples was assayed by using the chromogenic Limulus amebocyte lysate endpoint assay kit from Cambrex (Walkersville, MD) according to the manufacturer's instruction. The pyrogen-free water supplied with the kit was utilized to reconstitute both the endotoxin standard (E. coli O111:B4) and plasma samples. Serial dilutions of plasma samples were prepared to bring their concentrations into the range of the standard curve (from 0.1 to 1 EU/ml). Absorbance values of blank and endotoxin activity values of standards and samples were found acceptable according to directions of CambrexR for this kit.
In vivo and in vitro chemiluminescence recording of ROS activity
The ROS response to rLPS toxicity was directly evaluated from the kidney by intrarenal arterial infusion of a superoxide anion probe, 2-methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazo-[1,2-a]-pyrazin-3-one-hydrochloride (MCLA) (0.2 mg/ml/h, TCI-Ace, Tokyo Kasei Kogyo Co. Ltd, Tokyo, Japan) using a Chemiluminescence Analyzing System (CLA-ID3, Tohoku Electronic Industrial Co., LTD., Sendai, Japan) (Chien et al., 2001). The real-time displayed chemiluminescence signal was recognized as in vivo kidney ROS level and normalized to the kidney weight. The measurement of O2−• amount in blood and proximal tubular cells was performed by a lucigenin-amplified method (Chien et al., 2001) and normalized to the milligrams of total cellular proteins.
Apoptosis and autophagy assay of proximal tubular cells
Proximal tubular cell death was assayed in triplicate using trypan blue exclusion assay (>300 cells), terminal deoxynucleotidyl transferase-mediated nick-end labeling method and autophagic vacuoles were labeled in triplicate with 0.05 mmol/l monodansylcadaverin (MDC, Chien et al., 2001; Yang et al., 2008). After labeling, the cells were washed four times with PBS and immediately fixed with 4% paraformaldehyde and observed under a fluorescence microscope (Leica model DMRD, Wetzlar, Germany).
In situ demonstration of oxidative stress, autophagy, apoptosis, and pyroptosis formation
We performed Beclin-1-related autophagy, terminal deoxynucleotidyl transferase-mediated nick-end labeling (TUNEL) apoptosis method (Chung et al., 2012), and caspase 1-mediated pyroptosis to investigate the presence and extent of three types of programmed cell death in rLPS-induced renal injury. The renal sections (5 μm) were prepared, deparaffinized, and stained by the hematoxylin & eosin (H&E), Beclin-1 staining, TUNEL-avidin-biotin-complex methods, and caspase 1 stains. For renal macrophage (ED-1) staining as an inflammatory marker, these sections were incubated overnight at 4°C with a mouse antirat antibody to ED-1 (CD68, 1:200, Serotec, Sydney, NSW, Australia). A biotinylated secondary antibody (Dako, Botany, NSW, Australia) was then applied followed by streptavidin conjugated to HRP (Dako). The chromogen used was Dako Liquid diaminobenzene (DAB). Twenty high-power (×400) fields were randomly selected for each section, and the value of each oxidative stress was analyzed using a Sonix Image Setup (Sonix Technology Co., Ltd) containing image analyzing software Carl Zeiss AxioVision Rel.4.8.2 (Future Optics & Tech. Co. Ltd, Hangzhou, China).
Transfections
Small interfering RNAs (siRNA) for targeting NADPH oxidase gp91 and Atg5 were purchased from Santa Cruz Biotechnology (CA), and a universal control siRNA was obtained from Qiagen. The transfection of siRNA was performed in isolated primary renal proximal tubule cells (Chien et al., 2001) with TransMessenger transfection reagent (Qiagen) according to the manufacturer's instructions. One microliter of siRNA (10 μmol/l) was used for each well of cells (48-well plate). After transfection, cells were maintained in normal culture medium for another 4 h before 4 h of rLPS treatment (10 μg/ml) or IFNγ (1000 units/ml, Sigma).
Measurement of PKC activity
NADPH oxidase-dependent ROS play significant roles in the pathophysiology of sepsis. Previous study reported LPS/TLR4 activation induced greater PKC-induced NADPH oxidase-dependent ROS generation in the proximal tubules and the damaged kidney (Kong et al., 2010). Therefore, we determined PKC activity in response to LPS stimulation. Proximal tubule cells and tissue homogenates were lysed in cell lysis buffer (Cell Signaling Technology, Inc., Danvers, MA). PKC activity was measured using Cyclex protein kinase C assay kit (CycLex, Nagano, Japan). Aliquots of lysates were used for protein estimation.
Apoptosis, autophagy, pyroptosis, and ER stress-related proteins expression by Western blotting
The expression levels of TLR2, TLR4, gp91, apoptosis-related proteins including Bcl-2, Bax, caspase 3, PARP, and autophagy-related proteins Beclin-1, Atg5, Atg5-Atg12 and LC3-II, and ER stress signaling-related marker glucose-regulated protein (GRP78), JNK and p-JNK, ATF4, and CHOP were analyzed by Western blotting in proximal tubular cells and kidney tissues from rLPS rats with or without specific treatments. Western blotting method has been described elsewhere (Chung et al., 2012). Antibodies raised against ATF4 (Abcam Inc., Cambridge, MA), truncated Atg5 (ab77580, 23 kDa, Abcam Inc.), Atg5/Atg12 (Gene Tex, Alton Parkway, Irvine, CA), Bax (Chemicon), Bcl-2 (Transduction, Bluegrass-Lexington, KY), Beclin-1 (Cell Signaling Technology, Inc.), the activation fragments (32 kDa of proenzyme and 17 kDa of cleaved product) of caspase 3 (CPP32/Yama/Apopain, Upstate Biotechnology, Lake Placid, NY), C/EBP-homologus protein (CHOP, L63F7, Cell Signaling Technology, Inc.), caspase 12 (Santa Cruz Biotechnology), Fas-associated protein with death domain (FADD, Bioss Inc., West Cummings Park, MA), gp91phox (NOX2, Abcam Inc.), GRP78/Bip (Santa Cruz Biotechnology), IRE1a (Abcam Inc.), p-IRE1a (Abcam), JNK (Proteintech Group Inc., Manchester, UK), p-JNK (Santa Cruz Biotechnology), LC3-II (Cell Signaling Technology, Inc.), TLR2 (rabbit anti-TLR2 polyclonal antibody, Bioss, Inc., Woburn, MA), TLR4 (rabbit antirat TLR4 antibody, Santa Cruz Biotechnology), PARP (Cell Signaling Technology, Inc.), and β-actin (Sigma, St Louis, MI) were used. The density of the band with the appropriate molecular mass was determined semiquantitatively by densitometry using an image analyzing system (Alpha Innotech, San Leandro, CA).
Statistical analysis
All values were expressed as mean ± standard error mean (SEM). Differences within groups were evaluated by paired t-test. One-way analysis of variance was used for establishing differences among groups. Intergroup comparisons were made by Duncan's multiple-range test. Differences were regarded as significant if p < 0.05 was attained.
RESULTS
SA Can Interact with rLPS by High Structural Binding Affinity
Biacore surface techniques can be used to determine binding kinetics and affinity (Drake et al., 2012). As shown in Figure 1B, we found that SA can interact with rLPS (endotoxins) through a high binding affinity. Our data showed that SA (molecular weight = 309.27) can bind to rLPS at the responses of △RU = 165. After termination of rLPS injection (time = 225 s), SA still combined with rLPS and did not detach from rLPS indicating very high binding affinity between SA and rLPS. The interaction between increased rLPS concentration and immobilized SA were displayed in Figure 1C. Using the single-cycle kinetics approach, the samples are injected one after the other in the same cycle with no intervening regeneration steps. An increased rLPS concentration was prepared at concentrations of 1, 2, 3, and 4 μg/ml and sequentially injected over a sensor surface prepared with SA immobilized at 165 RU. The single-cycle kinetics simplifies analyses involving targets that are difficult to regenerate and reduces assay development time. The kinetics of rLPS and SA binding are calculated as follows: the binding affinity constant (KD) = 2.13 × 10−11 (M), association rate (ka) = 12689.19 (1/M s), and dissociation rate (kd) = 2.7 × 10−7 (1/s).
SA Treatment Attenuates rLPS-Induced Reduction in Systemic and Renal Hemodynamics
As shown in Figure 2A, rLPS markedly decreased renal microvascular blood flow after 2 h of rLPS stimulation and the reduction was still maintained until 6 h. SA treatment at 30 min post-LPS efficiently restored LPS-induced reduction in renal microvascular blood flow. LPS also significantly (p < 0.05) reduced the level of arterial blood pressure after 2 h of LPS (Fig. 2B), renal blood flow after 4 h of LPS (Fig. 2C) and increased renal vascular resistance after 4 h of LPS (Fig. 2D) in the rats compared with respective controls. SA treatment significantly improved the alteration in the arterial blood pressure, renal blood flow, and vascular resistance. However, 10 mg/kg of albumin did not improve the reduction in arterial blood pressure and renal blood flow and the increases in renal vascular resistance. Our data also found that the hemodynamic parameters were similar between LPS and rLPS treated groups.
FIG. 2.

Effect of SA on 50 mg of LPS or rLPS-induced changes of arterial blood pressure, renal blood flow, and vascular resistance in the rats. (A) Typical laser speckle imaging perfusions are displayed on a 16-level color palette in four rats receiving saline (control), rLPS treatment, rLPS plus SA treatment (LPS + SA), or rLPS plus albumin treatment (LPS + Alb), respectively. The mean changes of mean arterial blood pressure (B), total renal blood flow (C), and renal vascular resistance (D) was continuously recorded during 6 h of rLPS treatment. *p < 0.05 versus the matched time-course point of the control group without rLPS and SA treatment. #p < 0.05 versus the matched time-course point of the rLPS group.
SA Reduces rLPS-Induced Oxidative Stress and Improves Renal Dysfunction
As shown in Figure 3, we evaluated the SA effect on rLPS-induced oxidative stress in the kidney and blood and renal dysfunction in the rats. rLPS dose-dependently and significantly (p < 0.05) increased the level of kidney ROS in vivo (Fig. 3A), blood ROS in vitro (Fig. 3B), plasma BUN (Fig. 3C), and creatinine (Fig. 3D) after 6 h of rLPS treatment when compared with respective control values. Thirty minutes after LPS treatment, SA significantly decreased the level of kidney ROS, blood ROS, plasma BUN, and creatinine indicating the improvement or attenuation of rLPS-induced acute renal injury. We also found that ROS response and the level of renal dysfunction were similar between LPS and rLPS treated groups.
FIG. 3.
Effect of SA on LPS or rLPS-induced oxidative stress in the kidney and blood and renal dysfunction in the rats. The level of kidney ROS (A), blood ROS (B), BUN (C), and creatinine (D) was significantly and dose-dependently elevated after 6 h of rLPS (10, 25, and 50 mg) treatment. Thirty minutes after LPS or rLPS treatment, SA at 10 mg significantly decreased the oxidative stress markers and ameliorated renal dysfunction. All the parameters were similar between 50 mg of rLPS or LPS treatment. *p < 0.05 versus the control group without rLPS and SA treatment. #p < 0.05 versus respective dosage of rLPS or LPS treatment.
SA Reduces Necrosis, Inflammation, Autophagy, and Apoptosis in the rLPS Kidneys
rLPS treatment significantly increased tubular pathologic scores by H&E stain (Fig. 4B), ED-1 positive stains (Fig. 4E), Beclin-1 (Fig. 4H), TUNEL (Fig. 4K), and caspase 1 stains (Fig. 4N) compared with sham control (Figs. 4A, 4D, 4G, 4J, and 4M). The degree of LPS-enhanced necrosis, ED-1, Beclin-1 TUNEL, and caspase 1 stains are markedly depressed by SA treatment, 30 min post-rLPS (Figs. 4C, 4F, 4I, 4L, and 4O). All statistical data are shown in Figures 4P–T.
FIG. 4.

Effect of SA treatment on rLPS-induced renal injury. rLPS induced marked histologic changes (B), ED-1 infiltration (E), Beclin-1-autophagy (H), TUNEL-apoptosis formation (K), and caspase 1-pyroptosis (N) in the damaged kidneys when compared with respective control sections (A, D, G, J, and M). Intravenous SA at 10 mg significantly improved rLPS-induced pathologic parameters (C, F, I, L, and O). These markers of oxidative injury are indicated by green arrows. The scale bar is 50 μm. The statistic data is shown in P–T. *p < 0.05 versus the control group without rLPS and SA treatment. #p < 0.05 versus rLPS treatment.
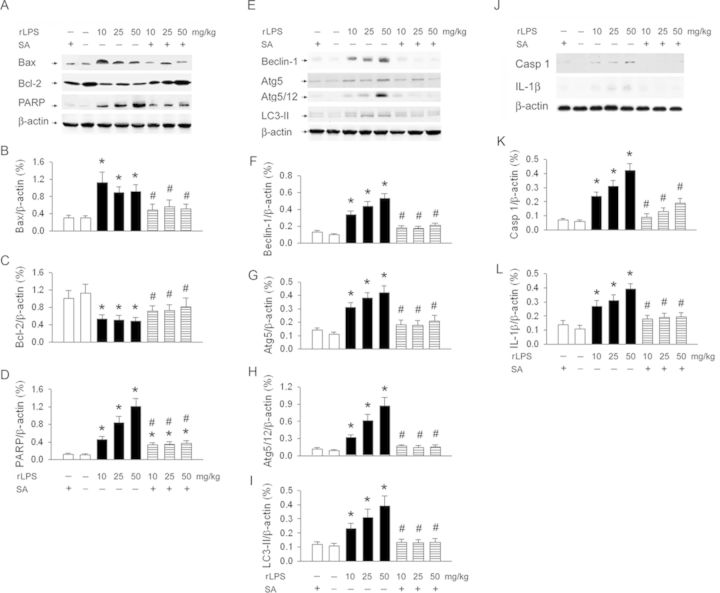
SA Depresses rLPS-Enhanced Renal Apoptosis-, Autophagy-, and Pyroptosis-Related Proteins Expression
Original data of apoptosis-related Bax, Bcl-2, and PARP are shown in Figure 5A. rLPS dose-dependently increased Bax (Fig. 5B) and PARP (Fig. 5D) expression and depressed Bcl-2 expression (Fig. 5C) in the kidneys. SA treatment significantly restored rLPS-depressed Bcl-2 expression and decreased rLPS-enhanced Bax and PARP expression in the kidneys. Typical graph of autophagy-related Beclin-1, truncated Atg5, Atg5/12, and LC3-II proteins expression was demonstrated in Figure 5E. rLPS dose-dependently increased the renal Beclin-1 (Fig. 5F), truncated Atg5 (Fig. 5G), Atg5/Atg12 (Fig. 5H), and LC3-II expression (Fig. 5I) expression. SA treatment at 30 min post-rLPS significantly decreased the enhanced renal Beclin-1, truncated Atg5, Atg5–Atg12, and LC3-II expression. Figure 5J demonstrated original data of pyroptosis-related proteins caspase 1 and IL-1β expression in the kidneys. rLPS significantly enhanced renal caspase 1 (Fig. 5K) and IL-1 IL-1β expression (Fig. 5L). SA treatment at 30 min post-rLPS significantly decreased the enhanced caspase 1 and IL-1β expression. According to our data, SA treatment in the control rats did not significantly affect the renal apoptosis-, autophagy-, and pyroptosis-related proteins expression.
FIG. 5.
Effect of SA treatment on rLPS induced apoptosis-, autophagy-, and pyroptosis-related proteins expression in the rat kidneys. Original data of apoptosis-related Bax, Bcl-2, and PARP and autophagy-related Beclin-1, Atg5/12, and LC3-II proteins was demonstrated in (A), (E), and (J), respectively. The statistic data was indicated in (B–D), (F–I), and (K–L), respectively. *p < 0.05 versus the control group without rLPS and SA treatment. #p < 0.05 versus rLPS treatment.
SA and gp91 siRNA Depresses rLPS-Enhanced Renal Proximal Tubular Apoptosis-, Autophagy-, and Pyroptosis-Related Proteins Expression
In vitro study with proximal tubular cells, the use of siRNA gp91, PKC-α inhibitor, and SA treatment was applied to determine the TLR4/PKC/NADPH oxidase gp91 signaling on ROS amount, cell death and autophagy-, apoptosis-, and pyroptosis-related proteins expression. (Fig. 6). Original data of TLR4, gp91, apoptosis-related PARP, autophagy-related Beclin-1, pyroptosis-related caspase 1, and IL-1β proteins in the renal proximal tubules are shown in Figure 6A. rLPS increased the TLR4, gp91, PARP, Beclin-1, caspase 1, and IL-1β proteins expression in the kidneys (Figs. 6B–G). SA significantly decreased rLPS enhanced O2−• production (Fig. 6H) and downregulated TLR4, percentage of cell death (Fig. 6I), and MDC positive autophagy (Fig. 6J) in the kidneys. gp91 siRNA treatment significantly decreased O2−• production, the percentage of cell death and MDC positive autophagy, renal gp91, PARP, Beclin-1, caspase 1, and IL-1β proteins expression, except TLR4. PKC-α inhibitor displayed a similar pattern like gp91 siRNA treatment. To explore the temporal effect of rLPS on autophagy and apoptosis, we determined Atg5, LC3-II, and PARP during 6 h of rLPS treatment. Enhanced truncated Atg5 appeared at 1 h, LC3-II at 2 h and PARP at 4 h after rLPS treatment (Fig. 6K). Autophogy occurs first and then switches to apoptosis by the activation of truncated Atg5. rLPS and IFNγ treatment upregulated FADD, LC3-II, and PARP expressions, whereas the treatment of Atg5 siRNA significantly inhibited the FADD, LC3-II, and PARP upregulation (Fig. 6L).
FIG. 6.
Effect of gp91 siRNA or SA treatment on rLPS induced TLR4, gp91, apoptosis-, autophagy-, and pyroptosis-related proteins expression (A), O2−• production (H), percentage of cell death (I), and MDC positive autophagic cells (J) in the renal proximal tubular cells. Original data of TLR4, gp91, PARP, and autophagy-related Beclin-1 and pyroptosis-related caspase 1 (casp 1) and IL-1β protein expressions is demonstrated in (A). The statistic data are indicated in (B–G), respectively. The temporal response of Atg5, LC3-II, and PARP to rLPS is indicated in (K). The effect of siRNA Atg5 on rLPS- or IFNγ-stimulated LC3-II and PARP is demonstrated in (L). *p < 0.05 versus the control group without rLPS and SA treatment. #p < 0.05 versus respective rLPS treatment.
rLPS via TLR4/PKC/gp91 Mediated ER Stress, Autophagy, and Apoptosis in the Kidney
In vivo study with rats, we determined the TLR4 inhibitor, PKC inhibitor, and NADPH oxidase gp91 inhibitor on TLR4/PKC/gp91-mediated p-IRE1α/p-JNK/CHOP/GRP78/ATF4-mediated endoplasmic reticulum (ER) stress, caspase 3-mediated apoptosis, Atg5/FADD-mediated autophagy, and caspase 1-mediated pyroptosis in the LPS-treated kidneys (Fig. 7). rLPS through TLR4/PKC/gp91 activation promoted p-IRE1α/p-JNK/CHOP/GRP78/ATF4-mediated ER stress, caspase 3-mediated apoptosis, Atg5/FADD-mediated autophagy, and caspase 1-mediated pyroptosis in the rLPS-treated kidneys. The administration of TLR4 inhibitor, PKC inhibitor, and NADPH oxidase gp91 inhibitor significantly inhibited p-IRE1α/p-JNK/CHOP/GRP78/ATF4-mediated ER stress, caspase 3-mediated apoptosis, Atg5/FADD-mediated autophagy, and caspase 1-mediated pyroptosis in the rLPS-treated kidneys.
FIG. 7.
Effect of SA, NADPH oxidase inhibitor, PKC inhibitor, and TLR4 inhibitor on rLPS-induced ER stress, FADD, apoptosis-, autophagy-, and pyroptosis-related proteins expression in the kidneys. Original data and statistic data of p-IRE1a/IRE1a (A) p-JNK/JNK (B), ATF4 (C), CHOP (D), caspase 12 (E), GRP78 (F), caspase 1 (G), Atg5 (H), FADD (I), caspase 3 (J) expression are demonstrated, respectively. Con, control; S, sialic acid; L, repurified lipopolysaccharide; LS, repurified lipopolysaccharide and sialic acid treatment; LP, repurified lipopolysaccharide and PKC-α inhibitor; LD, repurified lipopolysaccharide plus NADPH oxidase inhibitor DPI; LT, repurified lipopolysaccharide plus TLR4 inhibitor. *p < 0.05 versus Con group. #p < 0.05 versus rLPS treatment.
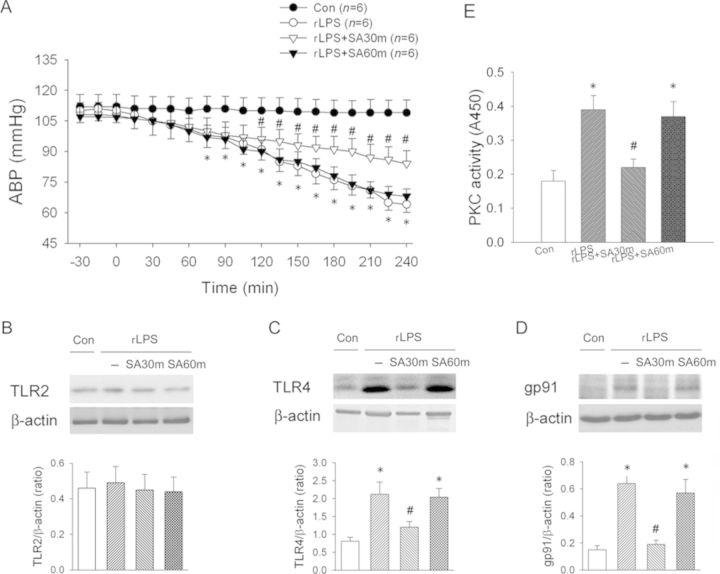
Thirty Minutes, not Sixty Minutes of SA Treatment Rescues rLPS-Induced Hypotension
As shown in Figure 8A, rLPS significantly depressed arterial blood pressure to 60 mmHg after 4 h of rLPS. SA administration at 30 min after rLPS treatment significantly attenuated the degree of hypotension. However, SA administration at 60 min after rLPS did not rescue the degree of hypotension. We found that rLPS significantly activated renal TLR4 (Fig. 8C) and NADPH oxidase gp91 (Fig. 8D) expression, and PKC activity (Fig. 8E), but did not affect TLR2 expression (Fig. 8B). SA administration at 30 min after rLPS treatment significantly depressed the enhanced TLR4 and NADPH oxidase gp91 expression and PKC activity, whereas SA administration at 60 min post-rLPS treatment did not reduce the enhanced TLR4 and gp91 expression and PKC activity.
FIG. 8.
Time effect of intravenous SA treatment on arterial blood pressure (A), TLR2 (B), TLR4 (C), NADPH oxidase gp91 subunit expression (D), and PKC activity (E) in the rLPS kidneys with or without SA treatment. Con, control rats without rLPS and SA treatment; rLPS, the rats with iv 50 mg/kg rLPS; rLPS + SA30m, 30 min after rLPS plus iv 10 mg SA; rLPS+SA60m, 60 min after rLPS plus iv 10 mg SA. *p < 0.05 versus the Con group without LPS and SA treatment. #p < 0.05 rLPS30m versus rLPS group.
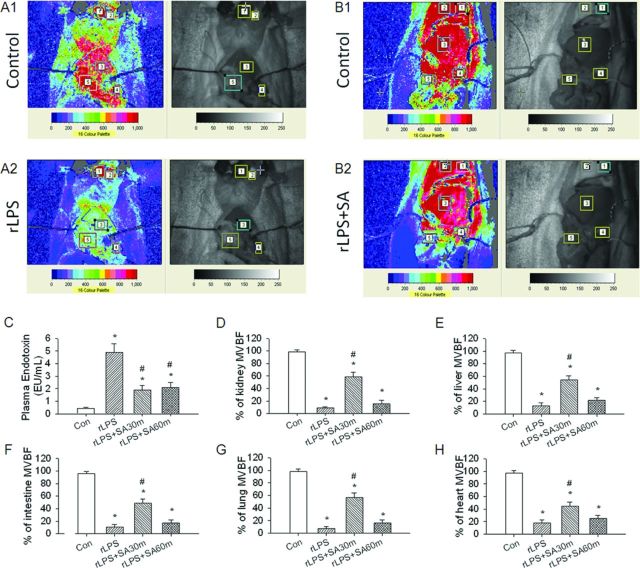
Thirty Minutes, not Sixty Minutes of SA Treatment Rescues rLPS-Induced Multiple Organ Failure
Figure 9 showed the effect of SA on rLPS-induced microvascular blood flow in multiple organs. When compared with the level before rLPS treatment (Fig. 9A-1), there were significant decreases in microvascular blood flow in the kidney, liver, small intestine, lung, and heart after 240 min of rLPS (Fig. 9A-2). The microvascular blood flow response of SA treatment after 30 min of rLPS on these five organs is indicated in Figure 9B-2 when compared with control value (Fig. 9B-1). The statistic data in Figure 9C showed that the treatment of SA at 30 or 60 min after rLPS significantly decreased rLPS-increased level of plasma endotoxin. Four hours of rLPS treatment significantly decreased the percentage of perfusion units in the kidney (Fig. 9D), liver (Fig. 9E), small intestine (Fig. 9F), lung (Fig. 9G), and heart (Fig. 9H). However, SA treatment at 30 not 60 min post-rLPS injury can delay the significant decrease of microvascular blood flow in these organs.
FIG. 9.
Systemic effect of SA on rLPS-induced microvascular blood flow in multiple organs. rLPS induced the reduction of microvascular blood flow in the kidney, liver, small intestine, lung, and heart. After 240 min of rLPS, the decreased percentage of microcirculation of four groups is indicated in (F). Con, control rats without rLPS and SA treatment; rLPS, the rats with iv 50 mg/kg rLPS; rLPS + SA30m, 30 min after rLPS plus iv 10 mg SA; rLPS + SA60m, 60 min after rLPS plus iv 10 mg SA. *p < 0.05 versus the Con group without rLPS and SA treatment. #p < 0.05 rLPS30m versus rLPS group.
DISCUSSION
In the present study, we further confirm that SA can interact with rLPS with a high binding affinity possibly through small molecular SA insertion or attachment to the macromolecular LPS as described previously (Ho et al., 2009). SA does not easily detach from LPS indicating their high binding affinity between SA and LPS. For determining the binding affinity, the structures in SA and LPS both contain rich negative charges, therefore, the high binding affinity between SA and LPS by Biacore T200 analysis may be possibly ascribed to small molecular SA insertion or attachment to the macromolecular LPS. Our previous report (Ho et al., 2009) and the present study demonstrate that a safely used SA by oral intake or intravenous administration did not evoke any neural, renal, and hepatic toxicity. We also show that intravenous SA administration within 30 min of lethal rLPS-induced sepsis can efficiently attenuate renal and systemic hypotension, oxidative stress, inflammation, apoptosis, autophagy, and pyroptosis in acute renal injury.
LPS from Gram-negative bacteria or Gram-positive bacteria would activate TLR2 and TLR4 signaling to induce endotoxemia and sepsis. TLR4 is well characterized as the receptor for LPS endotoxin, a cell wall component of Gram-negative bacteria, whereas TLR2 has been demonstrated to act as a receptor for components of LPS endotoxin protein and of Gram-positive bacteria such as staphylococcal and streptococcal exotoxins (superantigens), peptidoglycan, and lipoteichoic acid (Bannan et al., 1999; Schwandner et al., 1999). Activation of TLR2 and TLR4 by their ligands can lead to excess production of proinflammatory cytokines like tumor necrosis factor-α and interleukin-6 leading to sepsis. Synergistic interactions between TLR2 and TLR4 increased mortality in animal models of septic shock (Dalpke and Heeg, 2003) and possibly in human. Both TLR2 and TLR4 are important in the inflammatory response to bacterial infection because endotoxin proteins and LPS are present in the context of whole bacteria (Hirschfeld et al., 2000). rLPS could not actually be present in cases of sepsis in the clinic, however, rLPS/TLR4 signaling is one of the major pathways for induction of endotoxemia and sepsis. Therefore, it is important to delineate the contribution of individual (rLPS) or combination of microbial components (LPS) and their subsequent activation of various TLRs to the pathogenesis of inflammatory events.
Our data demonstrated that rLPS toxicity decreases arterial blood pressure, renal microcirculation, and renal blood flow and increases renal vascular resistance. These depressed hemodynamic parameters by rLPS could induce ischemia/hypotension-mediated oxidative stress in the kidneys, because our data found the increased renal ROS in vivo and blood ROS in vitro by a chemiluminescent amplification technique and increased renal NADPH oxidase gp91 expression. The renal oxidative stress in LPS-treated kidney was found in ED-1 macrophages or damaged renal cells located in the glomerular and tubulointerstitial area. Wang et al. (2003) reported that during endotoxemic acute renal failure, superoxide dismutase is decreased in the kidney, resulting in increased O2− and thus decreased vascular NO bioavailability with resultant renal vasoconstriction and a decrease in renal blood flow and glomerular filtration rate. Our data show that the destruction of renal structures including tubular cell death, erythrocyte leakage, and accumulation may possibly contribute to the acute renal failure. In this study, we also found that the major ROS source, NADPH oxidase gp91 was highly expressed via the TLR4 activation and PKC-α activity (Fig. 7). Using NADPH oxidase inhibitor DPI, siRNA gp91, SA, and PKC-α inhibitor α-tocopherol significantly decreased ROS formation and reduced three types of cell programmed death. Based on our data with pharmacologic inhibitors, rLPS via TLR4 activation to excite PKC-α activity and subsequently increased NADPH oxidase gp91 activity.
Increased ROS production from mitochondria or other intracellular compartments can induce three types of programmed cell death—apoptosis, autophagy, or pyroptosis—by activating caspases, lysosomal proteases, or endonucleases, respectively (Chung et al., 2012; Miao et al., 2011; Oberholzer et al., 2001). In this study, we demonstrated an increase in the expression of Beclin-1/Atg5/Atg12/LC3-II-autophagic pathways, Bax/caspase 3/PARP-apoptotic signaling, and caspase 1/IL-1β-pyroptotic signaling upon rLPS stimulation in vitro and in vivo. The increased autophagy, apoptosis, and pyroptosis associated with increased oxidative stress are also found in ischemia/reperfusion and unilateral ureteral obstruction kidney (Chung et al., 2012; Forbes et al., 2012). We also found that truncated Atg5 at 24 kDa seems to play a critical role in triggering autophagy and apoptosis formation in the rLPS treated kidneys (Fig. 6). Truncated Atg5 triggered autophagy induction earlier than apoptosis formation suggesting its role to switch autophagy to apoptosis (Codogno and Meijer, 2006; Zhande et al., 2007). Furthermore, the increased truncated Atg5 also activated FADD-induced autophagic cell death, because the treatment of Atg5 siRNA significantly ameliorated LC3-II and PARP expression. Recently, it has been reported that autophagy and inflammation are intimately linked (Howell et al., 2013). These events subsequently led to the acute renal failure. On the other hand, some findings indicate that a loss or reduction of renal autophagy is detrimental to the kidney in the experimental nephrotoxic, LPS, ischemia/reperfusion or inhibited autophagy by autophagy inhibitor 3-methyladenine or bafilomycin models (Howell et al., 2013; Jiang et al., 2012; Kimura et al., 2011, Takahashi et al., 2012). Hsiao et al. (2012) showed that the level of LC3-II elevated transiently at 3 h but declined at 9 h until 18 h after cecal ligation and puncture-induced sepsis. We suspect that the differential autophagy responses may be caused by differential degrees of injury, various types of stimulation or different time course of damage. However, further studies are required to be determined.
In our study, SA can efficiently attenuate rLPS-induced renal apoptosis, autophagy, and pyroptosis. How does SA affect rLPS-induced programmed cell death in the kidney? Azuma et al. (2007) reported that α2,6-linked SA via the action of enhancement of X-linked inhibitor of apoptosis protein directly or indirectly suppresses caspase 3 activation resulting in the inhibition of apoptosis. Helicobacter pylori, a Gram-negative bacterium capable of chronic colonization of the human stomach, infected the target organ via the structure of LPS or lipid A (Cullen et al., 2011). The use of SA showed a direct antiadhesion effect to H. pylori binding to gastric cells (Yang et al., 2008, 2013). In addition, in the H. pylori infected mice, SA can depress the increased oxidative stress, inducible nitric oxide synthase, NADPH oxidase gp91phox, CD68, caspase 1/IL-1β-mediated pyroptosis, and Bax/caspase 3/PARP-evoked apoptosis in the gastric mucosa (Yang et al., 2008, 2013).
Why SA treatment at 30 min not 60 min post-rLPS injury efficiently ameliorates rLPS-induced systemic inflammation? TLRs play an important role in the recognition of molecules derived from microbes and TLR4 plays a critical role in sepsis and inflammation (Takeuchi and Akira, 2010). Both TLR2 and TLR4 are important in the inflammatory response to Gram-negative bacterial infection because both endotoxin proteins and LPS are present in the context of whole bacteria (Cullen et al., 2011). In this study, the rLPS we used did not activate renal TLR2 expression suggesting the factors for activating TLR2 signaling may be removed in the extracted preparation. Activation of the TLR4 by rLPS increases production of proinflammatory cytokines and chemokines (Tsuboi et al., 2002) and specifically activates the NF-κB pathway which has been implicated in the regulation of multiple biological phenomena including apoptosis (Yu et al., 2007). Modest stimulation of TLR4 facilitates the elimination of invading microorganisms, whereas potent TLR4 stimulation produces severe reactions in the host, often leading to multiple organ failure and death (Bosshart and Heinzelmann, 2007). ROS are known to be critical in intracellular signaling, including the TLR signaling, and the scavenging of ROS or the inhibition of NADPH oxidase suppresses LPS-induced cytokine production (Kong et al., 2010). Kong et al. (2010) demonstrated that NADPH oxidase gp91 is the primary source, whereas mitochondria is the secondary source of ROS production that contributes to elevated ROS levels in macrophages after LPS stimulation. In vivo studies showed greater LPS-induced pulmonary inflammation and LPS shock and polymicrobial sepsis induced early and greater mortality was significantly reduced by ablation of NADPH oxidase gp91phox (Kong et al., 2010) implicating that NADPH oxidase-dependent ROS mediates TLR4 activation in sepsis. Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression (Shah et al., 2012). In our study, we found that 30 min, not 60 min of SA treatment after rLPS injury attenuates acute kidney injury and severe hypotension by the depressed renal TLR4 and gp91 expression. SA depressed the number of inflammatory ED-1-positive cells also suggesting its anti-inflammation on immune cells. However, the detailed mechanism needs to be determined. We suggest that early treatment of SA at a critical timing (<30 min) may neutralize LPS toxicity and prevent the activation of TLR4 and gp91 signaling for triggering inflammation and oxidative injury and subsequent apoptotic, autophagic and pyroptotic cell death. A delayed treatment of SA (>60 min) would damp the protective efficiency because of overt activation of TLR4 and gp91 by rLPS. We suggest that SA could be used for preventive strategy or at earlier therapeutic stage to counteract rLPS toxicity and TLR4 activation. Recently, SA has been reported to attenuate H. pylori-triggered epithelial caspase 1 activity and eradicate infection via its antiadhesion effect (Yang et al., 2013). All data suggest that SA may inhibit lipid A, endotoxin (rLPS), and Gram-negative bacteria-induced toxicity and infection.
Previous study has shown that Polymyxin B, a relatively toxic antibiotic, has potent endotoxin-neutralizing property that is used as an adjunctive therapy in Gram-negative sepsis by blocking an early step in the neutrophil priming process, possibly LPS attachment to or insertion into the neutrophil membrane (Danner et al., 1989). In clinical trial, blood purification techniques including hemoperfusion, plasma exchange, and hemofiltration with hemoperfusion using Polymyxin B hemoperfusion from Japan were associated with lower mortality in patients with sepsis (Zhou et al., 2013). However, Polymyxin B exhibits neuromuscular blocking, neurotoxic, or nephrotoxic effects, which hinder its application. We suggest that the use of SA may be applied to the dialyzer, intravenous injection, or oral intake for reducing sepsis mortality in future.
In summary, in our animal models, SA treatment can counteract rLPS-induced acute renal failure by its neutralization potential and reduce acute kidney injury via the antioxidant, anti-ER stress, antiapoptotic, antiautophagic, and antipyroptotic mechanisms. Early treatment of intravenous SA within 30 min may rescue rLPS-induced lethal septic shock possibly via the depression of rLPS toxicity-activated TLR4/PKC/NADPH oxidase gp91/ROS signaling and consequently p-IRE1α/p-JNK/CHOP/GRP78/ATF4-mediated endoplasmic reticulum (ER) stress, Bax/PARP-mediated apoptosis, Beclin-1/Atg5-Atg12/LC3-II-mediated autophagy, and caspase 1/IL-1β-mediated pyroptosis in the kidneys. However, the difficulty (impossibility) of determining endotoxemia within 30 min in the clinical setting requires further solution in future.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Science Council of the Republic of China (NSC 98-2320-B-002-043-MY3).
Supplementary Material
Footnotes
These authors contributed equally to this work.
REFERENCES
- Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Azuma Y., Higurashi K., Matsumoto K. Immobilized alpha2,6-linked sialic acid suppresses caspase-3 activation during anti-IgM antibody-induced apoptosis in Ramos cells. Biochim. Biophys. Acta. 2007;1770:279–285. doi: 10.1016/j.bbagen.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Bannan J., Visvanathan K., Zabriskie J. B. Structure and function of streptococcal and staphylococcal superantigens in septic shock. Infect. Dis. Clin. North Am. 1999;13:387–396. doi: 10.1016/s0891-5520(05)70081-7. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya J., Biswas S., Datta A. G. Mode of action of endotoxin: Role of free radicals and antioxidants. Curr. Med. Chem. 2004;11:359–368. doi: 10.2174/0929867043456098. [DOI] [PubMed] [Google Scholar]
- Bortoluci K. R., Medzhitov R. Control of infection by pyroptosis and autophagy: Role of TLR and NLR. Cell. Mol. Life Sci. 2010;67:1643–1651. doi: 10.1007/s00018-010-0335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshart H., Heinzelmann M. Targeting bacterial endotoxin: Two sides of a coin. Ann. N. Y. Acad. Sci. 2007;1096:1–17. doi: 10.1196/annals.1397.064. [DOI] [PubMed] [Google Scholar]
- Chien C. T., Lee P. H., Chen C. F., Ma M. C., Lai M. K., Hsu S. M. De novo demonstration and co-localization of free-radical production and apoptosis formation in rat kidney subjected to ischemia/reperfusion. J. Am. Soc. Nephrol. 2001;12:973–982. doi: 10.1681/ASN.V125973. [DOI] [PubMed] [Google Scholar]
- Chung S. D., Lai T. Y., Chien C. T., Yu H. J. Activating nrf-2 signaling depresses unilateral ureteral obstruction-evoked mitochondrial stress-related autophagy, apoptosis and pyroptosis in kidney. PLoS One. 2012;7:e47299. doi: 10.1371/journal.pone.0047299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codogno P., Meijer A. J. Atg5: More than an autophagy factor. Nat. Cell Biol. 2006;8:1045–1047. doi: 10.1038/ncb1006-1045. [DOI] [PubMed] [Google Scholar]
- Cullen T. W., Giles D. K., Wolf L. N., Ecobichon C., Boneca I. G., Trent M. S. Helicobacter pylori versus the host: Remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog. 2011;7:e1002454. doi: 10.1371/journal.ppat.1002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpke A. H., Heeg K. Synergistic and antagonistic interactions between LPS and superantigens. J. Endotoxin Res. 2003;9:51–54. doi: 10.1179/096805103125001342. [DOI] [PubMed] [Google Scholar]
- Danner R. L., Joiner K. A., Rubin M., Patterson W. H., Johnson N., Ayers K. M., Parrillo J. E. Purification, toxicity, and antiendotoxin activity of polymyxin B nonapeptide. Antimicrob. Agents Chemother. 1989;33:1428–1434. doi: 10.1128/aac.33.9.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake A. W., Tang M. L., Papalia G. A., Landes G., Haak-Frendscho M., Klakamp S. L. Biacore surface matrix effects on the binding kinetics and affinity of an antigen/antibody complex. Analyt. Biochem. 2012;429:58–69. doi: 10.1016/j.ab.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Fink S. L., Cookson B. T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink S. L., Cookson B. T. Pyroptosis and host cell death responses during Salmonella infection. Cell Microbiol. 2007;9:2562–2570. doi: 10.1111/j.1462-5822.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- Forbes M. S., Thornhill B. A., Minor J. J., Gordon K. A., Galarreta C. I., Chevalier R. L. Fight-or-flight: Murine unilateral ureteral obstruction causes extensive proximal tubular degeneration, collecting duct dilatation, and minimal fibrosis. Am. J. Physiol. Renal Physiol. 2012;303:F120–129. doi: 10.1152/ajprenal.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallay P., Heumann D., Le Roy D., Barras C., Glauser M. P. Mode of action of anti-lipopolysaccaride-binding protein antibodies for prevention of endotoxemic shock in mice. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7922–7926. doi: 10.1073/pnas.91.17.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann L., Stammec C., Ulmera A. J., Schumann R. R. Inhibition of LPS-induced activation of alveolar macrophages by high concentrations of LPS-binding protein. Biochem. Biophys. Res. Commun. 2002;295:553–560. doi: 10.1016/s0006-291x(02)00710-6. [DOI] [PubMed] [Google Scholar]
- Heine H., Rietschel E. T., Ulmer A. J. The biology of endotoxin. Mol. Biotechnol. 2001;19:279–296. doi: 10.1385/MB:19:3:279. [DOI] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M., Ma Y., Weis J. H., Vogel S. N., Weis J. J. Cutting edge: Repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- Ho C. H., Hsu S. P., Yang C. C., Lee Y. H., Chien C. T. Sialic acid reduces acute endotoxemia-induced liver dysfunction in the rat. Shock. 2009;32:228–235. doi: 10.1097/SHK.0b013e318197118e. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R. S., Karl I. E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Howell G. M., Gomez H., Collage R. D., Loughran P., Zhang X., Escobar D. A., Billiar T. R., Zuckerbraun B. S., Rosengart M. R. Augmenting autophagy to treat acute kidney injury during endotoxemia in mice. PLoS One. 2013;8:e69520. doi: 10.1371/journal.pone.0069520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao H. W., Tsai K. L., Wang L. F., Chen Y. H., Chiang P. C., Chuang S. M., Hsu C. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock. 2012;37:289–296. doi: 10.1097/SHK.0b013e318240b52a. [DOI] [PubMed] [Google Scholar]
- Jiang M., Wei Q., Dong G., Komatsu M., Su Y., Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271–1283. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Takabatake Y., Takahashi A., Kaimori J. Y., Matsui I., Namba T., Kitamura H., Niimura F., Matsusaka T., Soga T., et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J. Am. Soc. Nephrol. 2011;22:902–913. doi: 10.1681/ASN.2010070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X., Thimmulappa R., Kombairaju P., Biswal S. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J. Immunol. 2010;185:569–577. doi: 10.4049/jimmunol.0902315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O., Elsbach P. Bactericidal/permeability-increasing protein in host defense and its efficacy in the treatment of bacterial sepsis. Curr. Infect. Dis. Rep. 2001;3:407–412. doi: 10.1007/s11908-007-1007-y. [DOI] [PubMed] [Google Scholar]
- Miao E. A., Rajan J. V., Aderem A. Caspase-1-induced pyroptotic cell death. Immunol. Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberholzer C., Oberholzer A., Clare-Salzler M., Moldawer L. L. Apoptosis in sepsis: A new target for therapeutic exploration. FASEB J. 2001;15:879–892. doi: 10.1096/fj.00-058rev. [DOI] [PubMed] [Google Scholar]
- Orrenius S., Nicotera P., Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol. Sci. 2011;119:3–19. doi: 10.1093/toxsci/kfq268. [DOI] [PubMed] [Google Scholar]
- Schauer R., Kamerling J. P. Chemistry, biochemistry and biology of sialic acids. In: Montreuil J., Vliegenthart J. F. G., Schachter H., editors. Glycoproteins II. Amsterdam: Elsevier; 1997. [Google Scholar]
- Schwandner R., Dziarski R., Wesche H., Rothe M., Kirschning C. J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- Shah N., Dhar D., El Zahraa Mohammed F., Habtesion A., Davies N. A., Jover-Cobos M., Macnaughtan J., Sharma V., Olde Damink S. W., Mookerjee R. P., et al. Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression. J. Hepatol. 2012;56:1047–1053. doi: 10.1016/j.jhep.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Sumimoto H., Miyano K., Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem. Biophys. Res. Commun. 2005;338:677–686. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Kimura T., Takabatake Y., Namba T., Kaimori J., Kitamura H., Matsui I., Niimura F., Matsusaka T., Fujita N., Yoshimori T., Isaka Y., Rakugi H. Autophagy guards against cisplatin-induced acute kidney injury. Am. J. Pathol. 2012;180:517–525. doi: 10.1016/j.ajpath.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Ghio A. J., Piantadosi C. A. Reactive oxygen species produced by liver mitochondria of rats in sepsis. Arch. Biochem. Biophys. 1995;316:70–76. doi: 10.1006/abbi.1995.1011. [DOI] [PubMed] [Google Scholar]
- Tsuboi N., Yoshikai Y., Matsuo S., Kikuchi T., Iwami K., Nagai Y., Takeuchi O., Akira S., Matsuguchi T. Roles of toll-like receptors in C–C chemokine production by renal tubular epithelial cells. J. Immunol. 2002;169:2026–2033. doi: 10.4049/jimmunol.169.4.2026. [DOI] [PubMed] [Google Scholar]
- Yousefi S., Perozzo R., Schmid I., Ziemiecki A., Schaffner T., Scapozza L., Brunner T., Simon H. U. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat. Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- Varki A. Sialic acids as ligands in recognition phenomena. FASEB J. 1997;11:248–255. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- Wang W., Jittikanont S., Falk S. A., Li P., Feng L., Gengaro P. E., Poole B. D., Bowler R. P., Day B. J., Crapo J. D., et al. Interaction among nitric oxide, reactive oxygen species, and antioxidants during endotoxemia-related acute renal failure. Am. J. Physiol. Renal Physiol. 2003;284:F532–F537. doi: 10.1152/ajprenal.00323.2002. [DOI] [PubMed] [Google Scholar]
- Yang C. C., Ma M. C., Chien C. T., Wu M. S., Sun W. K., Chen C. F. Hypoxic preconditioning attenuates lipopolysaccharide-induced oxidative stress in rat kidneys. J. Physiol. 2007;582:407–419. doi: 10.1113/jphysiol.2006.122747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. C., Shun C. T., Chien C. T., Wang T. H. Effective prevention and treatment of Helicobacter pylori infection using a combination of catechins and sialic acid in AGS cells and BALB/c mice. J. Nutr. 2008;138:2084–2090. doi: 10.3945/jn.108.090985. [DOI] [PubMed] [Google Scholar]
- Yang J. C., Yang H. C., Shun C. T., Wang T. H., Chien C. T., Kao J. Y. Catechins and sialic acid attenuate Helicobacter pylori-triggered epithelial caspase-1 activity and eradicate Helicobacter pylori infection. Evid. Complement Alternat. Med. 2013;2013:248585. doi: 10.1155/2013/248585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Shao D., Liu J., Zhu J., Zhang Z., Xu J. Effects of ketamine on levels of cytokines, NF-Kappa B and TLRs in rat intestine during CLP-induced sepsis. Int. Immunopharmacol. 2007;7:1076–1082. doi: 10.1016/j.intimp.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Zhou F., Peng Z., Murugan R., Kellum J. A. Blood purification and mortality in sepsis: A meta-analysis of randomized trials. Crit. Care Med. 2013;41:2209–2220. doi: 10.1097/CCM.0b013e31828cf412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhande R., Dauphinee S. M., Thomas J. A., Yamamoto M., Akira S., Karsan A. FADD negatively regulates lipopolysaccharide signaling by impairing interleukin-1 receptor-associated kinase 1-MyD88 interaction. Mol. Cell. Biol. 2007;27:7394–7404. doi: 10.1128/MCB.00600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman J. J. Definition the role of oxyradicals in the pathogenesis of sepsis. Crit. Care Med. 1995;23:616–617. doi: 10.1097/00003246-199504000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.