Abstract
The chicken egg genotoxicity assay (CEGA), which utilizes the liver of an intact and aseptic embryo-fetal test organism, was evaluated using four activation-dependent DNA-reactive carcinogens and four structurally related less potent carcinogens or non-carcinogens. In the assay, three daily doses of test substances were administered to eggs containing 9–11-day-old fetuses and the fetal livers were assessed for two endpoints, DNA breaks using the alkaline single cell gel electrophoresis (comet) assay and DNA adducts using the 32P-nucleotide postlabeling (NPL) assay. The effects of four carcinogens of different structures requiring distinct pathways of bioactivation, i.e., 2-acetylaminofluorene (AAF), aflatoxin B1 (AFB1), benzo[a]pyrene (B[a]P), and diethylnitrosamine (DEN), were compared with structurally related non-carcinogens fluorene (FLU) and benzo[e]pyrene (B[e]P) or weak carcinogens, aflatoxin B2 (AFB2) and N-nitrosodiethanolamine (NDELA). The four carcinogens all produced DNA breaks at microgram or low milligram total doses, whereas less potent carcinogens and non-carcinogens yielded borderline or negative results, respectively, at higher doses. AAF and B[a]P produced DNA adducts, whereas none was found with the related comparators FLU or B[e]P, consistent with comet results. DEN and NDELA were also negative for adducts, as expected in the case of DEN for an alkylating agent in the standard NPL assay. Also, AFB1 and AFB2 were negative in NPL, as expected, due to the nature of ring opened aflatoxin adducts, which are resistant to enzymatic digestion. Thus, the CEGA, using comet and NPL, is capable of detection of the genotoxicity of diverse DNA-reactive carcinogens, while not yielding false positives for non-carcinogens.
Avian eggs represent a unique model for toxicity testing which is intermediate between in vitro and in vivo models. Several testing approaches have been reported. The In Ovo Carcinogenicity Assessing (IOCA) assay, which has a turkey egg fetal liver as the target tissue, has shown responsiveness to carcinogenic chemicals (Enzmann et al., 1992, 2013; Williams et al., 2011a). The IOCA provides an aseptic intact developing organism, which is independent of maternal influences. In its current validated protocol, the assay involves a single dose followed by 24 days of incubation (Enzmann et al., 2013). The results obtained with several chemicals are supported by the demonstration that the turkey fetal liver expresses xenobiotic biotransformation enzymes that mediate DNA adduct formation (Perrone et al., 2004).
The Chicken Egg Genotoxicity Assay (CEGA), which is the subject of this report, is being developed as a rapid and mechanistic assay in the liver of chicken embryo-fetuses. The CEGA uses eggs of the white leghorn chicken and two endpoints for genotoxicity, the alkaline single cell gel (comet) assay which detects DNA breaks, as demonstrated in other systems and the 32P-nucleotide postlabeling (NPL) assay, which detects DNA adducts, also as demonstrated in other systems, including the turkey fetal liver (Perrone et al., 2004). Fetal chicken egg livers were shown to be similar in susceptibility to aflatoxin B1 (AFB1) induction of DNA breaks using the comet assay compared with that of the turkey egg fetal liver (Williams et al., 2011b). Xenobiotic biotransformation activity is expressed by the chicken embryo-fetal liver (Hamilton et al., 1983; Heinrich-Hirsh et al., 1990; Lorr and Bloom, 1987; Rifkind et al., 1979; Strittmatter and Umberger, 1969), like turkey (Perrone et al., 2004). Chicken eggs have also been used to monitor micronucleus induction in erythroid cells formed in the yolk sac (Wolf and Luepke, 1997).
The chicken embryo is highly susceptible to chemical toxicity up to 4 days of incubation (Clegg, 1964). On day 4, the liver and xenobiotic metabolism begins to develop (Hamilton et al., 1983). The basal activity of hepatic aryl hydrocarbon hydroxylase at day 10 and beyond is similar to that of adult chickens (Hamilton et al., 1983). Also, in chicken fetuses, there are no sex differences in hepatic activities of mixed function oxidases (Rifkind et al., 1979). In the protocol reported here, dosing of chicken eggs of undetermined sex was started on day 8 or 9 of incubation, and involved three daily injections until termination at day 11, at which point the liver is fully formed. The central nervous system does not form until after day 11 and hence exposures produce no pain to the embryo/fetus.
The compounds chosen for study were four activation-dependent DNA-reactive carcinogens (Fig. 1), which have been discussed by Williams et al. (2008), of different structural classes, and related weak or non-carcinogens, as follows: 2-acetylaminofluorene (AAF), a synthetic aromatic amine and fluorene (FLU), the latter lacking the critical N-acetyl group in the heterocyclic ring system; AFB1, a substituted coumarin mycotoxin containing a dihydrofurofuran moiety and aflatoxin B2 (AFB2), a 8,9-dihydro derivative of AFB1 lacking the critical unsaturated double bond; benzo[a]pyrene (B[a]P), a naturally occurring polycyclic aromatic hydrocarbon and benzo[e]pyrene (B[e]P), an isomer of B[a]P that cannot form the critical dihydrodiol epoxide; and diethylnitrosamine (DEN), a synthetic symmetric dialkyl N-nitroso compound and N-nitrosodiethanolamine (NDELA), an analog of DEN that has hydroxyl groups on the ethyl side chains at the α-position where DEN is oxidized.
FIG. 1.
Tested carcinogens (left column) and structurally related weak or non-carcinogens (right column).
We report here that, in the comet assay, the four carcinogens yielded clear evidence of DNA breaks, whereas structurally related weak carcinogens or non-carcinogens yielded borderline or negative results, respectively. In the NPL assay, AAF and B[a]P also produced adducts, whereas FLU and B[e]P did not, consistent with comet results. DEN did not yield positive NPL results due to the fact that the type of adduct (alkylation) formed is not detected by the standard NPL assay. AFB1 and AFB2 were also negative in the NPL assay, reflecting the fact that the initial 8,9-dihydro-8-(N7-guanyl)-9-hydroxyaflatoxin B1 adduct depurinates or ring opens and hence would not be detected by NPL.
Thus, the CEGA using livers of early stage chicken embryo-fetuses exhibited high sensitivity and specificity for carcinogens and non-carcinogens.
MATERIALS AND METHODS
White leghorn chicken (Gallus gallus) eggs (SPF Premium, fertile) of undetermined sex were purchased from Charles River (North Franklin, CT). Deionized water (DW) was prepared from a Picopure System (Hydro Services and Supplies, Garfield, NJ), which has an online resistance (10 MOhm) monitor. Solutol HS15 (Kolliphor HS15) was a gift from BASF SE (Ludwigshafen, Germany) and was prepared as a 20% aqueous solution (HS15).
Test substances
The structures of test substances are shown in Figure 1. The range of doses total is given in Table 1.
TABLE 1. Doses Tested.
| Chemical | Dose range testeda |
|---|---|
| 2-acetylaminofluorene | 0.02–0.6 mg/egg |
| Fluorene | 0.17–1.36 mg/egg |
| Aflatoxin B1 | 0.1–6.4 μg/egg |
| Aflatoxin B2 | 1.6–6.4 μg/egg |
| Benzo[a]pyrene | 15–500 μg/egg |
| Benzo[e]pyrene | 250–500 μg/egg |
| Diethylnitrosamine | 0.125–4 mg/egg |
| Nitrosodiethanolamine | 0.5–4 mg/egg |
aAdministered in three daily injections.
AAF (CAS: 53-96-3, mw 223.29, >95% pure as reported by the supplier) was obtained from Aldrich (Milwaukee, WI). AAF was initially dissolved in hot (80°C) HS15 and then DW was added to prepare the AAF 20% HS15 stock solution of the highest concentration used. At that point, AAF stayed in solution. Serial dilutions were made from the stock solution (4 mg/ml, with HS15 to obtain 0.007 to 0.2 mg/50 μl resulting in total doses of 0.02–0.6 mg/egg administered in three daily injections.
AFB1 (CAS: 1162-65-8, mw 312.27, 99% pure as reported by the supplier) was obtained from Sigma-Aldrich (St Louis, MO). It was dissolved in 20% HS15. Serial dilutions were made from the stock solution, 0.213 mg/ml, resulting in total doses of 0.1–6.4 μg/egg administered in three daily injections.
AFB2 (CAS: 7220-81-7, mw 314.29, 99.5% pure as reported by the supplier) was obtained from Sigma-Aldrich. It was dissolved in 20% HS15. Serial dilutions were made from the stock solution, 0.213 mg/ml, to yield total doses of 1.6–6.4 μg/egg administered in three daily injections.
B[a]P (CAS: 50-32-8, mw 252.31, 97% pure as reported by the supplier) was obtained from Sigma-Aldrich. B[a]P (20 mg) was dissolved in 1.2-g hot (80°C) HS15 and then 4.8-ml DW was added to obtain a 20% solution. At that point, some micro-crystals formed to yield a rather homogenous solution that passed through a 27G (0.4 mm) syringe needle used to inject the eggs. Serial dilutions were made from this stock solution, 3.34 mg/ml, resulting in total doses ranging from 15 to 500 μg/egg administered in three daily injections. Prior to injection, the solutions were vortexed to assure homogeneity.
B[e]P (CAS: 192-97-2 mw 252.31, 99.2% pure as reported by the supplier) was obtained from Sigma-Aldrich. B[e]P (20 mg) was dissolved in (1.2 g) hot (80°C) HS15 and then 4.8-ml DW was added to obtain a 20% solution. Serial dilutions were made from this stock solution, 3.34 mg/ml, resulting in total doses ranging from 250 to 500 μg/egg administered in three daily injections. Prior to injection, the solutions were vortexed to assure homogeneity.
DEN (CAS: 55-18-5, mw 102.14, its purity conforms to structure by nmr as reported by the supplier) was obtained from Sigma-Aldrich. It was pre-weighed in an Isopac. DW was added to yield a concentration of 26.7 mg/ml (1.33 mg/50 μl). Serial dilutions were made from this stock solution resulting in total doses of 0.125–4.0 mg/egg in three daily injections.
FLU (CAS: 86-73-7, mw 166.22, 99.7% pure as reported by the supplier) was obtained from Sigma-Aldrich. It was dissolved in hot (80°C) HS15 and then DW was added to obtain a 20% solution. Serial dilutions were made from this stock solution, 9.09 mg/ml, resulting in total doses ranging from 0.17 to 1.36 mg/egg administered in three daily injections. Prior to injection, the solutions were vortexed to assure homogeneity.
N-nitrosodiethanolamine (NDELA) (CAS: 1116-54-7, mw 134.13, its purity conforms to structure by nmr as reported by the supplier) was obtained from Sigma-Aldrich. It was dissolved in DW. Serial dilutions were made from the stock solution (26.7 mg/ml) to yield total doses of 0.5–4 mg/egg administered in three daily injections.
Dosing of eggs
Upon arrival, all eggs were numbered on the shell with a pencil and weighed. Prior to incubation, the eggs were stored at room temperature (usually 1 day after arrival, but always less than 3 days). Viability was assessed by transillumination, and those eggs which were not developed were eliminated prior to assigning viable eggs to test groups. Viable eggs were randomly divided into control and dosing groups (Table 1), and were placed in a GQF Manufacturing Company No. 1611 automatic egg turners (Murray McMurray Hatchery Inc., Webster City, IA), which were placed in GQF Manufacturing Company Hova Bator Model 2362N styrofoam incubators (Murray McMurray Hatchery Inc., Webster City, IA), and maintained at 37°C ± 0.5°C, and 60% ± 5% relative humidity. Control eggs were placed in separate incubators from dosed eggs to avoid any possible airborne cross contamination. The day on which incubation commenced was designated as Day 0. The injection site was marked with a 1–2-cm cross above the blunt end of the egg (over the air sac) with a pencil, sterilized using alcohol swabs, and pierced with sterilized 10-cm long FST scissors (Fine Science Tools, Foster City, CA).
The dose ranges selected were based on available toxicity data, usually in rodents or solubility limits. All injections were made into the air sac. The repeated dose regimen consisted of three injections either on days 8–10 or on days 9–11. Eggs were terminated on day 11. In the 9–11-day protocol, termination was 3 h after the third dose. For each dosing group, the amount given per egg represents the sum total of all three injections. Dose groups that were used for the measurement of DNA damage exhibited 70% or greater viability. At termination, the egg shells were opened at the blunt end with 15-cm long FST scissors, and the allanto-chorionic membrane was retracted to allow access to the entire anterior (visceral) aspect of the chicken fetus via the yolk sac. The fetuses were removed and decapitated. Fetal weights, including the head, were recorded after the removal of the surrounding excess yolk. The abdominal cavity was opened and the liver was removed and weighed.
Liver samples (three per compound/group and assay) were processed immediately for the comet assay and the remainder was frozen at −80°C for a subsequent NPL assay.
Alkaline single cell gel electrophoresis (comet) assay
The standard comet assay was performed according to Tice et al. (2000), as previously reported (Williams et al., 2011b). Livers of the fetuses were rinsed with and then placed in cold Hank's balanced salt solution (HBSS; Gibco, Grand Island, NY) on ice. To prepare cells for comet analysis, about 200 mg of tissue was placed in 1 ml of cold HBSS containing 20-mM EDTA and 10% dimethylsufoxide (DMSO), minced into fine pieces, and pieces allowed to settle and aliquots of the cell suspensions used.
Comet slides were prepared with 1.5% agarose coating and dried. Cell suspensions (∼100 k cells in 10 μl) were mixed with 75-μl low-melting point agarose in PBS, pH 7.4, and applied to the slides, which were covered with coverslips and kept on ice for 3–5 min to solidify the low-melting point agarose. The following steps were performed under dim light to prevent ultraviolet-induced DNA damage. After the removal of the coverslips, slides were covered with 90-μl low-melting point agarose and the coverslips replaced for a further 10 min of cooling before the glass coverslips were removed from the slides and they were immersed in cold (4°C) lysing solution pH 10.0 (2.5-M NaCl, 100-mM EDTA, 10-mM Tris, 1% Triton X100, 10% DMSO; the last two compounds being added immediately before use). Slides were kept at 4°C for 1.5 h. After lysis, the slides were placed on a horizontal electrophoresis box. The electrophoresis unit was filled with freshly prepared alkaline buffer (300-mM NaOH, 1-mM EDTA, pH 13), until slides were completely covered with buffer. After incubation of the slides for 30 min at room temperature in alkaline buffer, to allow DNA unwinding and DNA breakage at alkali-labile sites, DNA electrophoresis was performed at 20 V for 30 min. After electrophoresis, the slides were covered with neutralization buffer (0.4-M Tris-HCl, pH 7.5) for 5 min. This step was repeated twice. Slides were dried with ethanol and, finally, 50-μl ethidium bromide (5 μg/ml) was added onto each slide. The slides were then covered with a coverslip and kept for 5 min in the dark for DNA staining. DNA migration was analyzed by fluorescence microscopy using a Nikon OPTIPHOT microscope. The percentages of DNA-in-the-tail were determined using the Comet Score software v 1.5 (TriTek Corp, Sumerduck, VA), counting >100 cells. The obtained mean comet value from each of the dose groups was then compared with the corresponding negative (vehicle) control group, and a ratio value was obtained, which reflected the fold increase over the control value. Presently, within a limited data set of comet values, a working ratio value threshold for positive response was found to be >1.7-fold increase of the high dose percent DNA in the comet tail over the ratio value of the vehicle control.
Nucleotide 32P-postlabeling (NPL) assay for DNA adducts
DNA was isolated from the frozen liver samples using QIAGEN (Valencia, CA) G100 columns following the manufacturer's protocol (Qiagen, 2001). The isolated DNA had appropriate 260/280- and 260/230-nm absorbance ratios (generally greater than 1.8 and 2.0, respectively), indicating that purity was adequate. DNA was then processed for adducts analysis by the NPL method of Randerath (1981), as previously described (Jeffrey et al., 2002; Montandon and Williams, 1994).
Isolated DNA (10 μg) was enzymatically digested into 2′-deoxyribonucleoside 3′-monophosphates using micrococcal nuclease and spleen phosphodiesterase. The digest was then enriched for DNA-modified bases using both nuclease P1 digestion (NP1) (Reddy and and Randerath, 1986), which provides for better sensitivity, except for DNA adducts that are sensitive to this treatment, and OASIS HLB (HLB) column enrichment (Gupta, 1985). DNA bases were then labeled using 100 μCi γ-32P adenosine triphosphate (∼6000 Ci/mmole; Perkin-Elmer, Waltham, MA). The labeled modified bases were then resolved using two- or three-directional thin-layer chromatography (TLC) systems. Suitable solvent systems for the development of the TLC plates were established for bulky adducts (not for ethylated ones) and documented. The radioactivity on the TLC plates was detected using a Molecular Dynamics Storm 860 system with exposure times of 2 h to overnight (GE Health Care Life Sciences, Edison, NJ). DNA adducts in 108 of normal nucleotides (nts) were ascertained, and a mean value per group was calculated. The faint spots seen with some control samples were considered to represent intrinsically present modification in the DNA, as observed in other species (Randerath et al., 1986, 1993). These spots were subtracted and the remaining DNA-adduct spots were counted.
Statistical analyses
Statistical analysis was performed using SigmaStat for Windows version 3.11.0 (Systat Software Inc., Chicago, IL), with the most appropriate statistical analysis for the data sets, which included the Holm-Sidak pairwise multiple comparison procedures, the Kruskal-Walis one-way ANOVA on ranks, and multiple comparisons (Systat Software Manual, 2004).
RESULTS
Viability of eggs was greater than 88% in all of the control groups (Figs. 3a, 6a, 7a, and 10a). Among test compound groups, survival was reduced by the high total doses of 3.2-μg AFB1 (survival, 75%) and 500-μg B[a]P (85%). Surviving eggs in these groups were included for analysis. Results for the comet and NPL assays are summarized in Table 2.
FIG. 3.
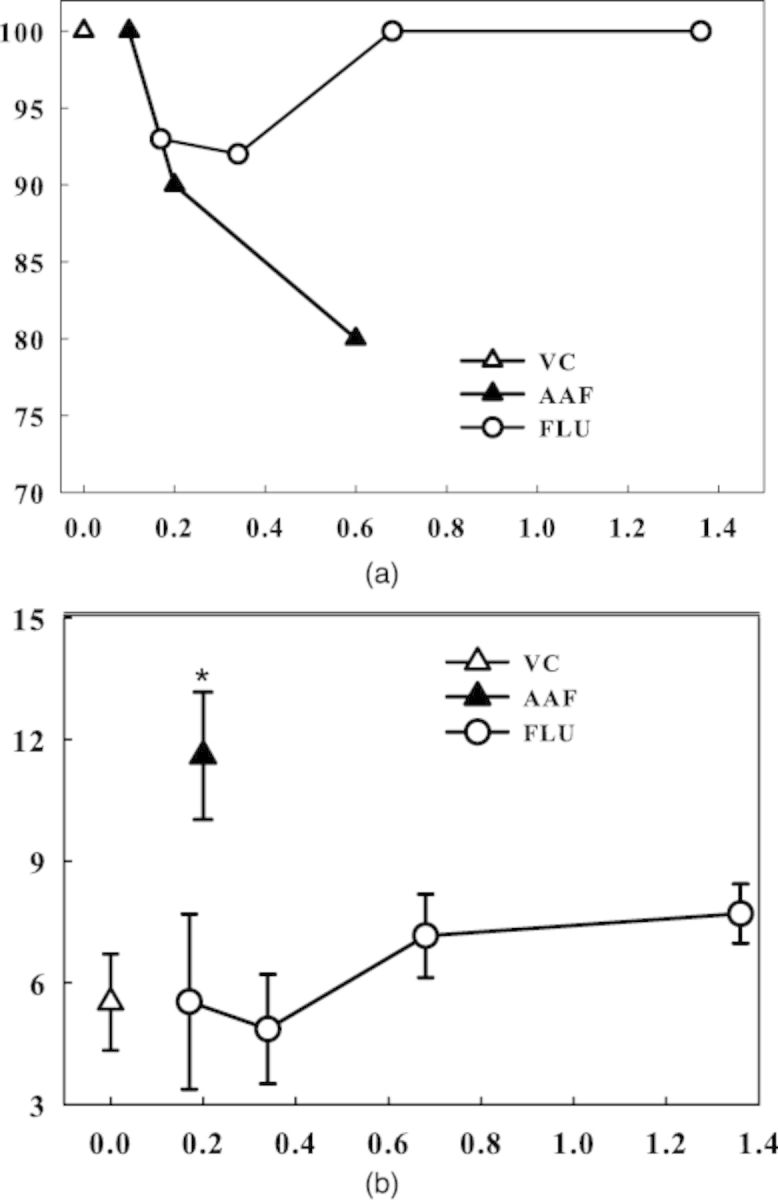
(a) AAF, 2-acetylaminofluorene; FLU, fluorene; VC, vehicle (HS15) control; The fetuses were dosed 48h, 24h, and 3h before termination. (b) AAF, 2-acetylaminofluorene; FLU, fluorene; VC, vehicle (HS15) control; dosed 48h, 24h and 3h before termination; *, the comet value ratio was >1.7-fold higher compared with the VC value denoting a positive assay outcome, as well as being statistically significant (at p < 0.05); for FLU the comet value ratio was below 1.5-fold denoting a negative outcome.
FIG. 6.

(a) AFB1, aflatoxin B1; AFB2, aflatoxin B2; VC, vehicle (HS15) control; The fetuses were dosed 48h, 24h and 3h before termination. (b) AFB1, aflatoxin B1; AFB2, aflatoxin B2; VC, vehicle (HS15) control; the comet value ratio for AFB1 was >1.7-fold for the 0.4- or 0.8-μg/egg doses denoting a positive outcome, whereas the ratio for AFB2 at the 1.6-μg/egg dose level was <1.6-fold denoting a negative outcome, the 3.2- or 6.4-μg/egg doses had a value of >1.7-fold denoting a positive outcome; dosed 48 h-24 h-3 h before termination; *, these dose levels were also statistically significant (at p < 0.05).
FIG. 7.

(a) B[a]P, benzo[a]pyrene; B[e]P, benzo[e]pyrene; VC, vehicle (HS15) control; The fetuses were dosed 48h, 24h and 3h before termination. (b) B[a]P, benzo[a]pyrene; B[e]P, benzo[e]pyrene; VC, vehicle (HS15) control; the comet value ratio for B[a]P was >1.7-fold for the 125- or 250-μg/egg doses denoting a positive outcome, whereas the ratio value for B[e]P at 250 or 500 μg/egg was below 1.4-fold denoting a negative outcome; dosed 48 h-24 h-3 h before termination; *, these dose levels were also statistically significant (at p < 0.05).
FIG. 10.

(a) DEN, diethylnitrosamine; NDELA, N-nitrosodiethanolamine; VC, vehicle (deionized water) control; the fetuses were dosed 48h, 24h and 3h before termination. (b) DEN, diethylnitrosamine; NDELA, N-nitrosodiethanolamine; VC, vehicle (deionized water) control; the comet value ratio for DEN was >1.7-fold for the 0.5- or 1-mg/egg doses denoting a positive outcome, whereas the ratio for NDELA at 1, 2, or 4 mg/egg was below 1.4-fold denoting a negative outcome; dosed 48h, 24h, 3h before termination; *, this dose level was also statistically significant (at p <0.5).
TABLE 2. Results for Compounds Tested in the CEGAa.
| CEGA | |||
|---|---|---|---|
| Compound (lowest positive dose/egg) | Comet b | NPL c | |
| NP1 (enrichment) | HLB (enrichment) | ||
| 2-acetylaminofluorene (0.1 mg/egg) | + | + | + |
| Fluorene (none) | − | − | − |
| Aflatoxin B1 (0.4 μg/egg) | + | − | − |
| Aflatoxin B2 (3.2 μg/egg) | ± | − | − |
| Benzo[a]pyrene (125 μg/egg) | + | + | + |
| Benzo[e]pyrene (none) | − | − | − |
| Diethylnitrosamine (0.5 mg/egg) | + | − | − |
| Nitrosodiethanolamine (none) | − | − | − |
aEggs were injected three times at 48, 24, and 3 h before termination on day 11 of incubation.
bPositive 1.7-fold control value with dose response.
cPositive at more than one dose; +, positive; –, negative; ±, weakly positive.
2-AAF and FLU
In an initial experiment, AAF produced no toxicity at up to 0.6-mg/egg total dose. It was weakly positive in the fetal liver comet assay using an 8–10-day-dosing regimen in which the comet assay was undertaken 24 h after the last dosing (Fig. 2). In an effort to improve results, we evaluated a regimen of injections on days 9–11 with termination 3 h after the last injection to capture any DNA damage at approximate maximum substance/metabolite exposure. Under these conditions, AAF produced strong positive comet results starting at the total dose of 0.1 mg/egg (Fig. 2). Based on this finding, in the remainder of experiments, the 9–11-day-dosing regimen was used.
FIG. 2.
AAF, 2-acetylaminofluorene; comparator (positive control), the liver was obtained 3 or 24 h after the last dosing. *, the comet value ratio was >1.7-fold higher compared with the vehicle control value denoting a positive assay outcome; the 3-h time seems to be the optimal one with respect to DNA damage at the time of analysis.
FLU, the ring system analog of AAF, showed no toxicity up to the total dose of 1.4 mg/egg (Fig. 3a). It was negative in the comet assay at up to the total dose of 1.4 mg/egg (Fig. 3b), whereas AAF at 0.2 mg/egg was again positive (Fig. 3b).
Using the day 8–10-injection regimen, AAF was clearly positive in the NPL assay for DNA adducts, forming two adducts, using NP1 or HLB enrichment in a dose-response manner (Fig. 4), particularly when using the HLB enrichment. In contrast, FLU at a total dose of 1.36 mg was negative (Fig. 5).
FIG. 4.
Two major DNA adducts in egg livers indicated by arrows had a value of 1.8, 3.4, 8.2, and 14.9 in 108 of normal nucleotides, for the 0.02 mg, 0.07 mg, 0.2 mg or 0.6 mg/egg doses, respectively.
FIG. 5.
AAF, 2-acetylaminofluorene; dosed 48 h-24 h-3 h before termination; *, with AAF, at 0.2 mg/egg, the DNA adducts were 9.7 in 108 of normal nucleotides, using the HLB enrichment, and were statistically (p < 0.05) and biologically significant compared with the vehicle control value.
Aflatoxins B1 and B2
In the 9–11-day-dosing protocol, with AFB1, viability at the highest (3.2 μg/egg) total dose was 75%, whereas with AFB2 at the highest (6.4 μg/egg) total dose it was 100% (Fig. 6a). AFB1 produced positive comet results starting at the total dose of 0.4 μg/egg and increasing to 2-fold background at 3.2 μg/egg (data not shown). In a comparative test with AFB2, AFB1 was more potent in the comet assay than AFB2, although the latter gave a weak response of about 2-fold the background at the total dose of 6.4 μg/egg (Fig. 6b).
AFB1 and AFB2 were negative in the NPL assay using either enrichment procedures (data not shown), reflecting the fact that the initial 8,9-dihydro-8-(N7-guanyl)-9-hydroxyaflatoxin B1 adduct depurinates or ring opens and hence would not be detected by NPL.
B[a]P and B[e]P
B[a]P reduced survival starting at the total dose of 100 μg/egg, whereas B[e]P had no effect at up to 500 μg/egg (Fig. 7a). In the comet assay, B[a]P produced weak, but definitely positive results with the three highest doses starting at the total dose of 125 μg/egg (data not shown). In a comparative experiment with B[e]P, B[a]P was confirmed to produce positive responses at the total doses of 125 and 250 μg/egg, whereas B[e]P at total doses of 250 and 500 μg/egg was negative (Fig. 8b).
FIG. 8.
NPL analysis obtained with NP1 enrichment of untreated, vehicle (HS15) control, B[a]P dosed eggs (the fetuses were dosed 48h, 24h, and 3h before termination). Adducts were resolved in the second and third directions of chromatography and are indicated by arrows. Other spots present in VC represent indigenous DNA adducts.
In the NPL analysis with NP1 enrichment, a single B[a]P DNA adduct was detected (Fig. 8), reaching a level of 38.7 in 108 nts at the total dose of 250 μg/egg (Fig. 9). B[e]P yielded no detectable adducts (Fig. 9).
FIG. 9.
B[a]P, benzo[a]pyrene; the fetuses were dosed 48h, 24h, and 3h before termination. The DNA adduct had a value of 38.7 in 108 of normal nucleotides, using the NP1 enrichment; *, this dose level was statistically (at p < 0.05) and biologically significant compared with the vehicle control value; B[e]P at 250- and 500-μg/egg doses denotes a negative outcome.
DEN and N-nitrosodiethanolamine
DEN produced slight toxicity at the total dose of 4 mg/egg (highest dose) (Fig. 10a). DEN also produced clear positive comet results starting at the total dose of 0.25 mg/egg (data not shown). When compared with NDELA, DEN produced positive comet responses at the total doses of 0.5 and 1.0 mg/egg, whereas NDELA was negative at up to 4 mg/egg (Fig. 10b).
DEN was negative in the NPL assay (data not shown), as expected for an agent producing DNA alkylation, and NDELA was also negative.
DISCUSSION
In the CEGA, all four DNA-reactive carcinogens, produced breaks in chicken embryo-fetal liver DNA in the comet assay, whereas non-carcinogens or weak carcinogens were negative, as summarized in Table 2. These results are in line with published carcinogenicity and other genotoxicity data as shown in Table 3. The assay was undertaken 3 h after the last dosing to capture DNA damage at approximate highest substance/metabolite exposure, whereas the two prior dosings would allow accumulation of DNA adducts and possibly any poorly repaired adducts. As stated in the methods, within the present data set of comet values, a working threshold for positive response was found to be about 1.7-fold increase of the high dose percent DNA in the comet tail over the value of the vehicle control. In the NPL assay, the faint spots seen with some control samples were considered to represent intrinsically present modifications in the DNA, as observed in other species (Randerath et al., 1986, 1993). These spots were subtracted and the remaining DNA-adduct spots were counted. Adducts were detected at levels of 5 in 109 nts and above.
TABLE 3. Results of Compounds Tested in the CEGAa Compared with Other Data from the Literature.
| CEGA | Literature | ||||
|---|---|---|---|---|---|
| Compound | Comet | NPL | Rodent carcinogenicity | Ames assay | DNA adducts |
| 2-acetylaminofluorene | + | + | + | + (+S9) | + |
| Fluorene | − | − | − | − | ND |
| Aflatoxin B1 | + | − | + | + (+S9) | + |
| Aflatoxin B2 | ± | − | + | + | + |
| Benzo[a]pyrene | + | + | + | + | + |
| Benzo[e]pyrene | − | − | − | − | − |
| Diethylnitrosamine | + | − | + | + | + |
| Nitrosodiethanolamine | − | − | + | + | + |
aEggs were injected three times at 48, 24, and 3 h before termination on day 11 of incubation; ±, weak activity; ND, no data.
In both comet and NPL assays, the results obtained with unsexed fetuses generally showed small variations, which is consistent with the absence of sex differences in xenobiotic biotransformation activities in avian embryo-fetuses (Rifkind et al., 1979; Hamilton et al., 1983). In this report, viability was greater than 70% in all dosed groups, hence results potentially stemming from cytotoxicity (Hartmann et al., 2003) were reduced.
The results in the CEGA are consistent with previous reports in the turkey fetal liver in which AFB1 was reported to produce DNA breaks using the comet assay (Williams et al., 2011a), and in the turkey fetal liver In Ovo Carcinogenicity Assay, in which DEN produced preneoplastic-like liver lesions and also malformations (Williams et al., 2011b), and the tobacco-specific 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) produced preneoplastic-like lesions (Enzmann et al., 2014).
In the current tests in the comet assay, AFB1 and B[a]P were more potent than AAF or DEN, producing effects with microgram doses compared with low milligram doses with the latter. In fact, on a molar basis, AFB1 was about 2000 times more potent than DEN. AFB2 yielded weak positive comet results, which is in accord with its weak carcinogenicity, through mechanisms as yet undetermined, although conversion to AFB1 and activation to the reactive oxide has been described (Swensen et al., 1977). NDELA yielded negative comet results, which is consistent with its major adducts being a O6 guanine and, therefore, not a DNA alkaline labile site (Loeppky et al., 2002; Singh and Farmer, 2006). The positive comet results with AAF correspond to the report of Wolf et al. (2008) that AAF induced micronuclei in the hen's egg-micronucleus test. These latter results, together with the present, imply that fetal chicken egg tissues express the bioactivation activities for diverse activation-dependent carcinogens. We have previously reported that the turkey fetal liver expresses enzymatic activities suggesting the presence of CYP1A and 2E (Perrone et al., 2004). It was subsequently reported that the turkey liver expresses cytochromes P450 system (CYPs) involved in AFB1 bioactivation (Rawal and Coulombe, 2011).
The results with the weak carcinogens AFB2 and NDELA are of particular note. AFB2 lacks the critical 9,10 unsaturated double bond where AFB1 is epoxidized (IARC, 2002). Swensen et al. (1977) reported that AFB2 could be converted to AFB1 and activated to the reactive oxide. Roebuck et al. (1978) reported that 2–8% of AFB2 was converted by a post mitochondrial fraction of duck liver to AFB1, whereas none was formed by rat, mouse, or human fractions. Also, AFB2 was reported to elicit DNA repair synthesis in cultured rat hepatocytes (Williams et al., 1989). With regard to NDELA, its ethyl groups, which are present in DEN, have terminal hydroxyl groups, which could interfere with the activating oxidation reaction, leading to only a weak response. Furthermore, the negative comet supports the report of Wolf et al. (2003) that NDELA did not induce micronuclei in the hen's egg-micronucleus test.
In the NPL assay for DNA adducts, AAF and B[a]P were positive. Both have been reported in other systems to produce adducts, including in the fetal turkey liver (Perrone et al., 2004). The major adduct formed with AAF is N-7 guanine and with B[a]P, N-2 guanine (Godschalk et al., 1997; Guengerich et al., 1999; Guptaet al., 1982) of which the N-7 guanine is an alkaline labile site that can be easily detected in the comet assay. B[e]P and FLU were negative for adduct formation. DEN was also negative as would be expected for an alkylating agent in the standard NPL assay (Singh et al., 1997; Wilson et al., 1988). AFB1 and AFB2 were also negative. The pattern of adducts formed by AFB1 is complex, as shown by other methodologies. The major adduct formed is at N-7 guanine (Croy and Wogan, 1981; Lin et al., 1977). This adduct can convert to the ring opened foramidopyrimide adduct (Hertzog et al., 1982). These adducts do not undergo enzymatic digestion to mononucleotide adducts and are not detected in standard NPL analysis (Randerath et al., 1985).
In conclusion, in the CEGA assay, prototype DNA-reactive carcinogens yielded positive comet results that were supported by NPL results, where appropriate. This assay, therefore, has the potential for detection of activation-dependent DNA-reactive carcinogens requiring diverse bioactivation pathways. These findings support the utility of chicken eggs for genotoxicity testing as also demonstrated by Wolf et al. (2008) who described induction of erythroid micronuclei in chicken eggs by several genotoxic agents. Thus, the CEGA, using comet and NPL, is capable of detection of the genotoxicity of diverse DNA-reactive carcinogens, while not yielding false positives for non-carcinogens/weak carcinogens. Based on the present results testing in the comet assay first is recommended and the NPL can be used to support equivocal or negative data, but the NPL alone has limitations based on structure which could lead to false negatives.
Acknowledgments
This paper is dedicated to our deceased colleague Alan Jeffrey who was essential in the development of the CEGA model. We thank Sharon Brana for preparing the manuscript.
REFERENCES
- Clegg D. J. The hen egg in toxicity and teratogenicity studies. Food Cosmet. Toxicol. 1964;2:717–727. [PubMed] [Google Scholar]
- Croy R. G., Wogan G. N. Temporal patterns of covalent DNA adducts in rat liver after single and multiple doses of aflatoxin B1. Cancer Res. 1981;41:197–203. [PubMed] [Google Scholar]
- Enzmann H., Brunnemann K., Iatropoulos M. J., Shpyleva S., Lukyanova N., Todor I., Moore M., Spicher K., Chekhun V., Tsuda H., et al. Interlaborabory comparison of turkey in ovo carcinogenicity assessment (IOCA) of hepatocarcinogens. Exp. Toxicol. Pathol. 2013;65:729–735. doi: 10.1016/j.etp.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Enzmann H. G., Brunnemann K. D., Kaestner B., Iatropoulos M. J., Williams G. M. Dose-dependent induction of preneoplastic lesions by the tobacco-specific nitrosamine carcinogen NNK in the in ovo carcinogenicity assessmenet (IOCA) assay. Exp. Toxicol. Pathol. 2014;66:35–40. doi: 10.1016/j.etp.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Enzmann H., Kaliner G., Watta-Gebert B., Löser E. Foci of altered hepatocytes induced in embryonal turkey liver. Carcinogenesis. 1992;13:943–946. doi: 10.1093/carcin/13.6.943. [DOI] [PubMed] [Google Scholar]
- Godschalk R. W., Vermeer I. T., Kriek E., Floot B., Schilderman P. A., Moonen E. J., Kleinjans J. C., van Schooten F. J. Comparison of 32P-postlabeling and HPLC-FD analysis of DNA adducts in rats acutely exposed to benzo a pyrene. Chem. Biol. Interact. 1997;104:41–54. doi: 10.1016/s0009-2797(97)03765-4. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Mundkowski R. G., Voehler M., Kadlubar F. F. Formation and reactions of N7-aminoguanosine and derivatives. Chem. Res. Toxicol. 1999;12:906–916. doi: 10.1021/tx990094u. [DOI] [PubMed] [Google Scholar]
- Gupta R. C. Enhanced sensitivity of 32P-Postlabeling analysis of aromatic carcinogen: DNA adducts. Cancer Res. 1985;45:5656–5662. [PubMed] [Google Scholar]
- Gupta R. C., Reddy M. V., Randerath K. 32P-postlabeling analysis of non-radioactive carcinogen-DNA adducts. Carcinogenesis. 1982;3:1081–1092. doi: 10.1093/carcin/3.9.1081. [DOI] [PubMed] [Google Scholar]
- Hamilton J. W., Denison M. S., Bloom S. E. Development of basal and induced aryl hydrocarbon (benzo[a]pyrene) hydroxylase activity in the chicken embryo in ovo. Proc. Natl. Acad. Sci. U.S.A. 1983;80:3372–3376. doi: 10.1073/pnas.80.11.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A., Agurell E., Beevers C., Brendler-Schwaab S., Burlinson B., Clay P., Collins A., Smith A., Speit G., Thybaud V., et al. Recommendations for conducting the in vivo alkaline comet assay. Mutagenesis. 2003;18:45–51. doi: 10.1093/mutage/18.1.45. [DOI] [PubMed] [Google Scholar]
- Heinrich-Hirsh B., Hofmann D., Webb J., Neubert D. Activity of aldrinepoxidase, 7-ethoxycoumarin-O-deethylase and 7-ethoxyresorufin-O-deethylase during the development of chick embryos in ovo. Arch. Toxicol. 1990;64:127–134. doi: 10.1007/BF01974398. [DOI] [PubMed] [Google Scholar]
- Hertzog P. J., Smith J. R., Garner R. C. Characterization of the imidazole ring-opened forms of trans-8,9-dihyro-8,9-dihydro-8-(7-guanyl)9-hydroxy aflatoxin B1. Carcinogenesis. 1982;3:723–725. doi: 10.1093/carcin/3.6.723. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic ARisks to Humans, Volume 72, Some Traditional Herbal Medicines, Some Mycotoxins. Lyon, France: Naphthalene and Styrene; Aflatoxin B1 and Aflatoxin B2; pp. 171–300. [Google Scholar]
- Jeffrey A. M., Luo F. Q., Amin S., Krzeminski J., Zech K., Williams G. M. Lack of DNA binding in the rat nasal mucosa and other tissues of the nasal toxicants roflumilast, a phosphodiesterase 4 inhibitor, and a metabolite, 4-amino-3,5-dichloropyridine in contrast to the nasal carcinogen 2,6-dimethylaniline. Drug Chem. Toxicol. 2002;25:93–107. doi: 10.1081/dct-100108475. [DOI] [PubMed] [Google Scholar]
- Lin J. K., Miller J. A., Miller E. C. 2,3-Dihydro-2-(guan-7-yl)-3-hydroxy-aflatoxin B1, a major acid hydrolysis product of aflatoxin B1-DNA or -ribosomal RNA adducts formed in hepatic microsome-mediated reactions and in rat liver in vivo. Cancer Res. 1977;37:4430–4438. [PubMed] [Google Scholar]
- Loeppky R. N., Ye Q., Goelzer P., Chen Y. DNA adducts from N-nitrosodiethanolamine and related ß-oxidized nitrosamines in vivo: 32P-postlabeling methods for glyoxal- and O6-hydroxy-ethyldeoxy-guanosine adducts. Chem. Res. Toxicol. 2002;15:470–482. doi: 10.1021/tx0101393. [DOI] [PubMed] [Google Scholar]
- Lorr N. A., Bloom S. E. Ontogeny of the chicken cytochrome P-450 enzyme system. Expression and development of responsiveness to phenobarbital induction. Biochem. Pharmacol. 1987;36:3059–3067. doi: 10.1016/0006-2952(87)90224-3. [DOI] [PubMed] [Google Scholar]
- Montandon F., Williams G. M. Comparison of DNA reactivity of the polyphenylethylene hormonal agents diethylstilbestrol, tamoxifen and toremifene in rat and hamster liver. Arch. Toxicol. 1994;68:272–275. doi: 10.1007/s002040050068. [DOI] [PubMed] [Google Scholar]
- Perrone C. E., Ahr H. J., Duan J. D., Jeffrey A. M., Schmidt U., Williams G. M., Enzmann H. G. Embryonic turkey liver: Activities of biotransformation enzymes and activation of DNA-reactive carcinogens. Arch. Toxicol. 2004;78:589–598. doi: 10.1007/s00204-004-0580-1. [DOI] [PubMed] [Google Scholar]
- Qiagen. QIAGEN Genomic DNA Handbook. Valencia, CA, USA: Qiagen, Inc.; 2001. [Google Scholar]
- Randerath K., Li D., Moorthy B., Randerath E. I-compounds–endogenous DNA markers of nutritional status, ageing, tumour promotion and carcinogenesis. IARC Sci. Publ. 1993;124:157–165. [PubMed] [Google Scholar]
- Randerath K. I., Randerath E., Agrawal H. P., Gupta R. C., Schurdak M. E., Reddy M. V. Postlabeling methods for carcinogen-DNA adduct analysis. Environ. Health Perspect. 1985;62:57–65. doi: 10.1289/ehp.856257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K., Reddy M. V., Disher R. M. Age- and tissue-related DNA modifications in untreated rats: Detection by 32P-postlabeling assay and possible significance for spontaneous tumor induction and aging. Carcinogenesis. 1986;7:1615–1617. doi: 10.1093/carcin/7.9.1615. [DOI] [PubMed] [Google Scholar]
- Randerath K., Reddy M. V., Gupta R.C. 32P-labeling test for DNA damage. Proc. Natl. Acad. Sci. U.S.A. 1981;78:6126–6129. doi: 10.1073/pnas.78.10.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal S., Coulombe, Jr Metabolism of aflatoxin B1 in turkey liver microsomes: The relative roles of cytochromes P450 1A5 and 3A37. Toxicol. Appl. Pharmacol. 2011;254:349–354. doi: 10.1016/j.taap.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Reddy M. V., Randerath K. Nuclease P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 1986;7:1543–1551. doi: 10.1093/carcin/7.9.1543. [DOI] [PubMed] [Google Scholar]
- Rifkind A. B., Troeger M., Petschke T. Equality of the rates of mixed function oxidation in livers of male and female chick embryos. Biochem. Pharmacol. 1979;28:1681–1683. doi: 10.1016/0006-2952(79)90184-9. [DOI] [PubMed] [Google Scholar]
- Roebuck B. D., Siegel W. G., Wogan G. N. In vitro metabolism of aflatoxin B2 by animal and human liver. Cancer Res. 1978;38:999–1002. [PubMed] [Google Scholar]
- Singh R., Farmer P. B. Liquid chromatography-electrospray ionization-mass spectrometry: The future of DNA adduct detection. Carcinogenesis. 2006;27:178–196. doi: 10.1093/carcin/bgi260. [DOI] [PubMed] [Google Scholar]
- Singh R., Sweetman G. M. A., Farmer P. B., Shuker D. E. G., Rich K. J. Detection and characterization of two major ethylated deoxyguanosine adducts by high performance liquid chromatography, electrospray mass spectrometry, and 32P-postlabeling. Development of an approach for detection of phosphotriesters. Chem. Res. Toxicol. 1997;10:70–77. doi: 10.1021/tx960135b. [DOI] [PubMed] [Google Scholar]
- Strittmatter C. F., Umberger F. T. Oxidative enzyme components of avian liver microsomes: Changes during embryonic development and the effects of phenobarbital administration. Biochim. Biophys. Acta. 1969;180:18–27. doi: 10.1016/0005-2728(69)90189-3. [DOI] [PubMed] [Google Scholar]
- Swensen D. H., Lin J-K., Miller E. C., Miller J. A. Aflatoxin B1–2,3-oxide as a probable intermediate in the covalent binding of aflatoxins B1 and B2 to rat liver DNA and ribosomal RNA in vivo. Cancer Res. 1977;37:172–181. [PubMed] [Google Scholar]
- Systat Software Manual. Chicago, IL: Systat Software Inc.; 2004. [Google Scholar]
- Tice R. R., Agurell E., Anderson D., Burlinson B., Hartmann A., Kobayahsi H., Miyamae Y., Rojas E., Ryu J. C., Sasaki Y. F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000;35:206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Brunnemann K. D., Iatropoulos M. J., Smart D. J., Enzmann H. G. Production of liver preneoplasia and gallbladder agenesis in turkey fetuses administered diethylnitrosamine. Arch. Toxicol. 2011a;85:681–687. doi: 10.1007/s00204-010-0603-z. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Deschl U., Williams G. M. DNA damage in fetal liver cells of turkey and chicken eggs dosed with aflatoxin B1. Arch. Toxicol. 2011b;85:1167–1172. doi: 10.1007/s00204-011-0653-x. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Iatropoulos M. J., Enzmann H. G. Principles of testing for carcinogenic activity. In: Hayes A. Wallace., editor. Principles and Methods of Toxicology. 5th edition. Philadelphia, PA: Taylor and Francis; 2008. pp. 1265–1316. [Google Scholar]
- Williams G. M., Mori H., McQueen C. A. Structure-activity relationships in the rat hepatocyte DNA-repair test for 300 chemicals. Mutat. Res. 1989;221:263–286. doi: 10.1016/0165-1110(89)90039-0. [DOI] [PubMed] [Google Scholar]
- Wilson V. L., Basu A. K., Essigmann J. M., Smith R. A., Harris C. C. O6-Alkyldeoxyguanosine detection by 32P-postlabeling and nucleotide chromatographic analysis. Cancer Res. 1988;48:2156–2161. [PubMed] [Google Scholar]
- Wolf T., Luepke N.-P. Formation of micronuclei in incubated hen's eggs as a measure of genotoxicity. Mutat. Res. 1997;394:163–175. doi: 10.1016/s1383-5718(97)00136-8. [DOI] [PubMed] [Google Scholar]
- Wolf T., Niehaus-Rolf C., Banduhn N., Eschrich D., Scheel J., Luepke N. P. The hen's egg test for micronucleus induction (HET-MN): Novel analyses with a series of well characterized substances support the further evaluation of the test system. Mutat. Res. 2008;650:150–164. doi: 10.1016/j.mrgentox.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Wolf T., Niehaus-Rolf C., Leupke N. P. Investigating genotoxic and hematotoxic effects of N-nitrosodimethylamine, N-nitrosodiethylamine and N-nitrosodiethanolamine in the hen's egg-micronucleus test (HET-MN) Food Chem. Toxicol. 2003;41:561–573. doi: 10.1016/s0278-6915(02)00281-8. [DOI] [PubMed] [Google Scholar]








