Abstract
Men are at risk of becoming completely infertile due to innumerable environmental chemicals and pollutants. These xenobiotics, hence, should be tested for their potential adverse effects on male fertility. However, the testing load, a monumental challenge for employing conventional animal models, compels the pursuit of alternative models. Towards this direction, we show here that Drosophila melanogaster, an invertebrate, with its well characterized/conserved male reproductive processes/proteome, recapitulates male reproductive toxicity phenotypes observed in mammals when exposed to a known reproductive toxicant, dibutyl phthalate (DBP). Analogous to mammals, exposure to DBP reduced fertility, sperm counts, seminal proteins, increased oxidative modification/damage in reproductive tract proteins and altered the activity of a hormone receptor (estrogen related receptor) in Drosophila males. In addition, we show here that DBP is metabolized to monobutyl phthalate (MBP) in exposed Drosophila males and that MBP is more toxic than DBP, as observed in higher organisms. These findings suggest Drosophila as a potential alternative to traditional animal models for the prescreening of chemicals for their reproductive adversities and also to gain mechanistic insights into chemical-mediated endocrine disruption and male infertility.
Keywords: Drosophila, male infertility, endocrine disruption, phthalate, estrogen related receptor
Increased incidence of infertility in men, in part, as a consequence of exposure to the environmental chemicals (xenobiotics), is of global concern. In the last two decades, sperm counts in men have reduced well below World Health Organization threshold of average concentration of 55 million/ml and significant alterations in semen quality parameters critical for male fertility have been reported (Carlsen et al., 1992; Rolland et al., 2012). Environmental chemicals hamper male fertility by adversely affecting the testicular signaling and reducing sperm counts as well as quality of the semen of exposed males. These xenobiotics inflict detrimental effects on male fertility through disruption of endocrine function and/or causing oxidative stress (Aitken et al., 2014; Knez, 2013). The magnitude of observed reduction in male fertility, accordingly, prompted the assessment of reproductive toxicity potential of various environmental chemicals.
Male reproductive toxicity assessment of xenobiotics primarily utilizes rodents or nonhuman primates, with a focus on human safety (Foster, 2006; Sharpe, 2001). Therefore, the assessment of innumerable chemicals on their ability to hamper male fertility would need a large number of these animals. However, the regulatory restrictions and ethical concerns along with limitations associated with using laboratory animals pose a greater challenge in taking in vivo reproductive toxicity assessment to high-throughput scale. In order to minimize these concerns and to promote the principle of three Rs (reduce, refine, and replace), European Centre for Validation of Alternate Methods (ECVAM) recommended the use of lower vertebrates and invertebrates as alternatives. Given the complexity of reproductive processes and their precise regulation through various feedback mechanisms, designing in vitro assays that can recapitulate the same is no trivial goal. Accordingly, existing in vitro male reproductive toxicity assays faithfully capture only certain aspects of male reproduction. In addition, the artificial nonphysiological conditions of the cultures, lack of homeostasis, impaired intracellular interactions, and dearth of defense mechanisms, which thrusts probably stronger impact on the precision of toxicity estimations (Hartung, 2007), exhort the pursuit of better substitutes. However, alternatives that facilitate the reproductive toxicity assessment in vivo are relatively scarce. In this context, Drosophila, with its well characterized development and reproduction, offers a potential alternative and a promising model for the male reproductive toxicity assessment. Interestingly, Drosophila is an OECD approved model for genotoxicity studies that quantifies DNA damage using sperm DNA (Siddique et al., 2005). However, despite the homology of genes associated with testicular development, spermatogenesis (Fischer et al., 2012; Mikhaylova et al., 2008), sperm maturation, and conservation of protein classes in seminal fluids from insects to mammals (Avila et al., 2011; LaFlamme et al., 2012), Drosophila remained under utilized for male reproductive toxicity assessment studies. A few studies have attempted to utilize Drosophila for reproductive toxicity assessment, but these have been mostly confined to the preliminary analysis of fertility. This is, in part, due to lack of predefined endpoints that can be attributed to male reproductive adversities in Drosophila. Therefore, in the present study, we carried out a systematic as well as comprehensive analysis of sperm as well as seminal fluid parameters at organismal, genetic, biochemical, and endocrine levels on male flies fed with a chemical to develop Drosophila melanogaster as an alternative model for the assessment of chemical-mediated male reproductive adversities.
We show here that Drosophila recapitulates male reproductive toxicity phenotypes observed in mammals when exposed to dibutyl phthalate (DBP), a chemical belonging to a group of phthalate esters and known to hamper male fertility (Foster, 2006). We observed significant reduction in male fertility, as a consequence of altered semen quality and endocrine disruption, in response to DBP. Exposure to DBP resulted in a significant decline in the expression as well as activity of a Drosophila hormone receptor, Estrogen-Related Receptor (dERR), a sole Drosophila ortholog of the vertebrate ERR nuclear receptor subclass. In addition, we detected monobutyl phthalate (MBP), a major metabolite of DBP in higher organisms, in Drosophila males exposed to DBP. Our data suggest that even in Drosophila, MBP is more toxic than DBP to male fertility. Our findings reflect the potential of Drosophila as an alternative for prescreening of innumerable environmental chemicals on their ability to hamper male fertility. This, together with its genetic tractability makes Drosophila an excellent model to gain insights into chemical-mediated reproductive toxicity and endocrine disruption.
MATERIALS AND METHODS
Fly strains
All experiments, except as stated, were carried out with wild-type flies (Oregon-R strain) of D. melanogaster. For sperm count analyses, flies carrying green fluorescent labeled sperm (w; P{Protamine B-EGFP}, Manier et al., 2010) were used. For protein analyses, males carrying ovulin-GFP (Lung and Wolfner, 1999) were used and w1118 of D. melanogaster were used as genetically matched controls for the same. For endocrine disruption studies, w1118; P{hs-GAL4-ERR.LBD} (Palanker et al., 2006) flies were employed in combination with UAS-lacZ (BL 3955) flies. All flies were reared under a 12:12 h light-dark cycle at 22 ± 1°C on standard Drosophila maize-sugar food medium supplemented with additional yeast in glass bottles/vials.
Exposure regimen
Oregon-R flies, reared on standard Drosophila medium supplemented with yeast, were transferred to glass bottles without food and kept on fasting for 2 h. Subsequently, flies were allowed to lay eggs on grape juice medium (comprising 3% agar-agar, 1.2% sucrose, 2% ethanol, 1% acetic acid, and 27.2% grape juice without any preservative) for 2 h. For chemical exposure, eggs (developmental exposure) or flies (adult exposure) were transferred to food vials with DBP (Sigma) or MBP (Sigma) at different concentrations or with Dimethyl sulfoxide (DMSO) (vehicular control for MBP experiments) or without test chemical (control). The concentrations of test chemical used ranged from 10μM–2M for DBP and 1μM–50mM for MBP depending upon the experiment. We added the test chemical to 5 ml of food, mixed well, poured into glass vials, and allowed the food to solidify. For developmental exposure, hundred eggs were placed in each vial and were allowed to develop to the adult stage at 22 ± 1°C. Altogether, we used three vials per batch (triplicates), with 100 eggs each, for further experimental setups. The unmated males from controls, DBP and MBP food vials were transferred to normal food, aged for 3 days and performed the experiments as described below. For adult exposure, unmated males from a similar setup without test chemical were isolated within 24 h of their emergence and were exposed to DBP through food as described above.
Determination of lethal concentration-50 (LC50)
To determine the LC50, i.e., the concentration at which 50% of eggs fail to reach the adulthood, for DBP or MBP exposure during development, the eggs were exposed to 10–500mM of DBP, or 1–50mM of MBP for the entire development span till the eclosion of the fly. To determine the LC50 concentration of DBP for the adult exposure, males were exposed to various concentrations of test chemical ranging from 10mM to 2M for 72 h. The LC50 was calculated through regression-based EPA probit analysis.
Quantitative estimation of DBP and MBP in exposed D. melanogaster flies
Flies (3 days old) were homogenized in 3 ml of Milli-Q water and the pH of the homogenate was adjusted to 6.0–6.5. β-Glucuronidase (Sigma) was added to the homogenate to release MBP from its glucuronide conjugate and the mixture was hydrolyzed at 37°C for 3 h. After the enzymatic hydrolysis, pH was readjusted to 4.0 using acetic acid. Extraction was performed using ethyl acetate, and the organic phase was separated by centrifugation at 6000 rpm. The processed extract (see supplementary methods for additional details) was used for GC-MS/MS analysis, on ThermoScientific Trace GC ultra gas chromatograph with a TriPlus auto sampler coupled to TSQ Quantum XLS triple quadrupole mass spectrometer (ThermoScientific) and equipped with a TG-5MS capillary column. Helium was used as the carrier gas. The oven temperature was programmed as follows: initial temperature was 100°C and then increased to 200°C at the rate of 15°C/min, maintained for 5 min and then further increased to 280°C at the rate of 10°C/min. Transfer line and ion source temperatures were set at 290 and 220°C, respectively. The quantity of DBP and MBP were estimated using selective reaction monitoring (SRM; see Supplementary table 1 for GC-MS/MS transitions).
Assessment of the reproductive performance of males in response to DBP and MBP
Males from developmental or adult exposure were allowed to mate with 3–5 days old wild-type Oregon-R virgin females. Males were removed immediately after mating. Females were allowed to lay eggs for 10 days ASM (after the start of mating), either with transfers to fresh control food vials every day (for assaying fecundity, fertility, and hatchability) or every 3 days (for assaying fertility alone). To determine fecundity, the number of eggs laid by females mated to control or exposed males were counted every 24 h for 10 days ASM (Kalb et al., 1993). Fertility was recorded as the number of progeny of these females. Hatchability was determined as the proportion of progeny eclosed/number of eggs laid. The differences in overall fertility, fecundity, and hatchability were statistically analyzed through one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison tests. For daywise comparisons, two-way analysis of variance and Bonferroni's post hoc test were used. All these assays were repeated more than three times with each group consisting of 20 replicates.
Sperm counts
Sperm counts were carried out as in Mueller et al. (2008), except that sperm were counted using transgenic flies, w; P{Protamine B-EGFP} containing EGFP (enhanced green fluorescent protein) labeled sperm and the exposure conditions were same as above. Initially, sperm in seminal vesicles and late spermatogenesis stages in testes of control and exposed males 3 days posteclosion were counted. Subsequently, males were subjected to brood mating (Gilchrist and Partridge, 1995). Briefly, 3-day-old control and exposed males were mated to females in three broods (each consisting of three virgin females) over 3 days. The first mating of every brood was observed, and the mated female was frozen, 2 h ASM. On the fourth day, an additional virgin female (10th mate) was provided, the mating was observed and the mated female was frozen at 2 h ASM. Males were allowed to recover for 48 h in isolation on fresh food. Subsequently, the reproductive tracts from males and mated females were dissected out in physiological saline (0.7% sodium chloride). Each tissue was transferred to a slide containing a droplet of physiological saline, covered with a cover glass, and sealed with nail polish. In males, number of sperm bundles in testes and mature sperm in seminal vesicles were counted twice under the fluorescence microscope (at a total magnification of ×600, with GFP filter; Leica DMLB, Germany). To determine the post-mating fate of sperm from exposed males, sperm in the sperm storage organs (seminal receptacle and paired spermathecae) of females mated to control or exposed males were counted in the first mate of the brood every day, for 3 days and in the 10th female (last female mated prior to keeping the males for recovery) at 2 h ASM. The number of sperm stored both in seminal receptacle and paired spermathecae were counted. The differences, if any, in sperm counts in the respective groups were analyzed statistically using Mann-Whitney U-test.
Analysis of transcripts of a few genes associated with Drosophila male reproduction
RNA extracted from 3 days old males collected from control and DBP food (10 flies/replicate, and 2 replicates/group) using RNazol RT (Molecular Research Centre) was reverse transcribed to cDNA (using Superscript III [Life Technologies]) according to the manufacturer's protocol. The transcript levels of 31 genes, including those encoding seminal fluid proteins, nuclear receptors (EcR, ERR), and other genes implicated in spermatogenesis and/or male fertility (including CG4760-fly homolog of vertebrate deleted in Azoospermia, DAZ; refer to Supplementary table 2 for details) were analyzed through semiquantitative polymerase chain reaction (PCR) employing gene-specific primers (Mueller et al., 2004). We used RPL32 (CG7939) as an internal control, for the quality and quantity of the template. The intensities of the amplified products from control and exposed organisms were assessed through densitometry using the volume analysis algorithm of Quantity One software (Bio-Rad). The differences in the intensities of amplified products between control and exposed groups were statistically analyzed using Student's t-test. The expression profiles of the candidate genes were validated by quantitative real time PCR (qPCR) with reverse transcribed cDNA corresponding to 150 ng/5 μl of the reaction volume and SYBR green (Life Technologies) as the reporter using Applied Biosystem detection system (see supplementary section for the details of conditions applied, and Supplementary table 3 for sequences of the real-time primers used). Differences in the fold change of transcript levels between groups were analyzed statistically by employing Student's t-test.
Confocal microscopy
Ovulin-GFP flies (that express ovulin-GFP in the male accessory glands under the control of ovulin promoter) were exposed to DBP as above. The accessory glands from control and developmentally exposed 3-day-old males (N = 10) were dissected out in physiological saline and mounted on a slide. The levels of ovulin were assessed using the intensity of GFP as a marker for the extent of expression of ovulin in response to developmental exposure to DBP under confocal microscope (Leica, Germany). The intensity of GFP fluorescence was quantified using ImageJ software (National Institute of Health, Bethesda, MD). The difference in the fluorescence intensity in accessory glands from control and exposed males was statistically analyzed using one-way ANOVA and Tuckey's post hoc test.
Sample preparation and Western blot analysis
Protein samples from the accessory glands of unmated control and exposed ovulin-GFP males and from the reproductive tracts of their mates, frozen 1 h ASM, were prepared independently and probed using anti-GFP antibodies (Cell Signaling) through Western blot analysis (Ravi Ram et al., 2005; see supplementary material for methodological details). The differences in the protein quantities in samples from control/exposed males and their mates were semiquantitatively assessed using volume analysis algorithm of Quantity One software (Bio-Rad), as described above.
Qualitative assay of oxidative modification of proteins
The oxidative modification of proteins in response to DBP exposure was assayed using OxyBlot Protein Oxidation detection kit (Millipore) following manufacturer's protocol. Two pairs of male reproductive tracts per batch were dissected and homogenized in 10 μl of lysis buffer (containing 20μM Tris (pH 6.8), 6% SDS, 2% β-mercaptoethanol). The glands were homogenized, and the homogenate was allowed to stand at 24 ± 1°C for 1 h. The carbonyl moieties, if any, introduced in the protein side chains by the reactive oxygen species (generated in response to exposure) were derivatized with 10 μl of 2,4-dinitro phenyl hydrazine (DNP) to form corresponding hydrazones. After 20 min, the derivatization reaction was stopped by adding 7.5 μl of neutralization reagent. Samples (20 μl of the supernatant) were then loaded on to 13% SDS-polyacrylamide gels and Western blotting was performed as mentioned above except that the blot was probed with DNP-specific anti-DNP antibody generated in rabbit (at 1:500 dilution) and peroxidase-Affinipure goat antirabit IgG (1:1000). The documentation was carried out and signal intensities were statistically analyzed as above.
X-gal staining
To analyze the ERR activity in response to DBP exposure, 3-day-old males (hs-GAL4-ERR.LBD; UAS-LacZ) derived from the cross between hs-GAL4-ERR.LBD and UAS-LacZ flies under control and exposure conditions, were placed at 37°C for 30 min, in separate glass vials. After the heat shock, flies were allowed to recover for an hour, transferred into the normal food vials, and subsequently were frozen after 1 h. Whole reproductive tracts from control and exposed males were dissected in physiological saline and these tissue were stained with X-gal following the protocol of Montell et al. (1992) with minor modifications (refer to supplementary material for details). We counted the number of X-gal spots (blue in color) and measured the intensities of these spots in the male reproductive tract using ImageJ software (National Institute of Health, Bethesda, MD).
RESULTS
DBP Is Metabolized to MBP in Exposed Drosophila Males
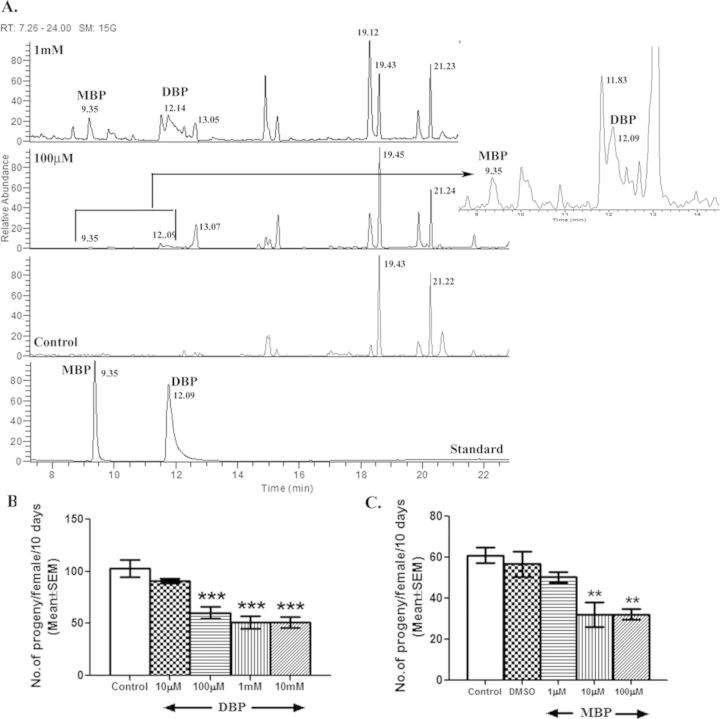
To determine the internalization of DBP and its metabolism in Drosophila, we estimated the levels of DBP and its major metabolite, MBP within control and exposed males through GC-MS/MS. The estimated levels of DBP were 0.30 ng/mg tissue and 0.83 ng/mg, whereas those of MBP were 63.375 ng/mg and 160 ng/mg in organisms developed from egg to adult stage (12–13 days) on 100μM or 1mM DBP, respectively, suggesting the hydrolysis of DBP into MBP. Expectedly, we did not detect a peak corresponding to DBP or MBP in control males (Fig. 1A). The concentration of DBP internalized, as observed, within the exposed fly are lower than exposure/doses generally used in mammalian studies, ranging from 100 to 500 mg/kg depending upon the route of exposure and phenotype assayed (Mylchreest et al., 1998).
FIG. 1.
Metabolism of DBP in Drosophila and the effect of DBP and MBP on Drosophila male fertility. To determine the chemical load in exposed organisms, the level of DBP within control and exposed males was estimated through GC-MS/MS. Panel (A) includes the total ion current chromatograms from GC-MS depicting MBP and DBP peaks (as labeled) in control males (control), flies exposed to 100μM or 1mM DBP and also with reference standard (Standard). Subsequently, fertility (the number of progeny produced over a period of 10 days) of females mated to males exposed to different concentrations of (B) DBP or (C) MBP throughout their development was analyzed. Developmental exposure of males to 100μM of DBP reduced (***p < 0.001) the fertility of their mates while MBP hampered fertility (**p < 0.01 when compared with vehicular control, DMSO) even at a concentration 10 times lower than that of DBP. All experiments were repeated three times (N = 15–20 males/mates per replicate/group).
Exposure to DBP or MBP Adversely Affects the Fertility of Drosophila Males
To determine the window of exposure, males were exposed to DBP during their entire development (egg to emergence of adult) or for 72 h after emergence of the adult and assayed for the fertility of their mates. We observed that the fertility (number of progeny produced/female/10 days) of females mated to males exposed to 10 or 50mM for 72 h was significantly reduced by 14.69 or 33.27%, respectively, when compared with that of control (see Supplementary fig. 1A). However, the fertility of females mated to males exposed to concentrations lower than 10mM (ranging from 0.1 to 1mM) of DBP did not significantly differ (p > 0.05) from their controls (see Supplementary fig. 1A), suggesting that LOAEL (lowest observed adverse effect level) for organismal/reproductive parameters during adult exposure to DBP in Drosophila is 10mM DBP [1/180 of LC50 concentration (1.815M) observed for 72 h exposure to DBP]. In contrast, we observed 49–52% reduction in the fertility of females mated to males exposed to 100μM, 1mM, or 10mM DBP during their development, when compared with that of control mates (p < 0.001; Fig. 1B). However, females mated to males exposed to 10μM of DBP during development had fertility comparable to their controls (p > 0.05), indicating the 100μM concentration to be the LOAEL for developmental exposure to DBP (which amounts to 1/620 of LC50 (62.03mM) observed for developmental exposure). Interestingly, flies were more vulnerable to MBP (with observed LC50 of 6.9mM) and LOAEL for developmental exposure to MBP was 10μM (p < 0.01; amounting to 1/690 of LC50 observed for MBP; Fig. 1C) as far as male fertility is concerned. In addition, we did not observe significant mortality or any gross morphological, reproductive tract abnormalities, in flies eclosed from DBP food, when compared with controls (see Supplementary fig. 2 for details). In view of the robust but adverse fertility consequence of developmental exposure to DBP, we focused on males exposed to DBP during their development for further determination of the Drosophila-based endpoints of male reproductive toxicity.
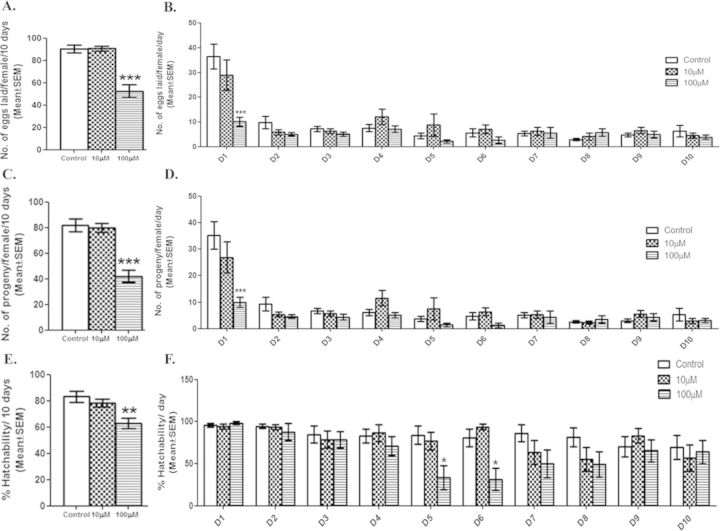
In Drosophila, females prior to mating lay a few unfertilized eggs. However, upon mating, females lay large number of fertilized eggs with percentage hatchability (proportion of eggs that reach the adult stage) in the range of 90–95%. This increase in egg-laying/production upto 24 h ASM is dependent upon SFPs whereas sustaining the same for a long-term (beyond 24 h) would require the presence of SFPs and sperm in the sperm storage organs (Ravi Ram and Wolfner, 2009). Therefore, significant reduction in the fertility can be consequence of (1) reduction in the number of fertilized eggs, (2) mortality at the embryonic level and/or during development, and (3) alterations in sperm in storage/SFPs. We initially tested for egg laying pattern and we observed that females mated to males exposed to 100μM DBP during development, laid significantly fewer eggs (p < 0.001) over a period of 10 days when compared with their controls (Fig. 2A). However, females mated to males exposed to 10μM DBP laid eggs at levels similar (p > 0.05) to their controls. Daywise analysis of the egg counts has revealed that 100μM DBP mates laid significantly fewer eggs within 24 h ASM (after start of mating; p < 0.001; Fig. 2B) when compared with control or 10μM DBP mates. In the remaining days, the number of eggs laid did not significantly differ within or between exposed/control mates (p > 0.05; Fig. 2B). A similar trend was observed in the overall (Fig. 2C) as well as daywise (Fig. 2D) fertility of exposed or control mates, over a period of 10 days. Interestingly, overall percentage hatchability of eggs laid by 100μM DBP mates, differed significantly from that of controls (p < 0.01; Fig. 2E). A daywise comparison revealed that the percentage hatchability of eggs laid by females mated to exposed males did not differ from that of controls on day 1 (24 h ASM), unlike as in the case of fecundity or fertility (Fig. 2F). In contrast, significantly fewer eggs from females mated to males exposed to 100μM DBP hatched on days 5 and 6, when compared with those from controls (p < 0.05; Fig. 2F). These results reflect that the observed reduction in the progeny production of 100μM DBP mates on day 1, is mainly due to the reduced number of eggs laid in the first 24 h ASM (p < 0.001; Fig. 2B). However, the overall decline in the fertility of DBP exposed mates is a consequence of reduced fecundity and hatchability.
FIG. 2.
Reduced reproductive performance of males exposed to DBP during development. The effect of DBP on the reproductive performance of exposed males was analyzed by mating control or exposed males to wild-type virgin females. We measured (A) the overall fecundity (number of eggs laid/ female over a period of 10 days) of females mated to control or exposed males (10 or 100μM DBP) and (B) daywise fecundity with transfer of mated females to fresh food every 24 h for 10 days (D1–D10). Subsequently, we scored these vials for the (C) overall fertility (number of progeny/female/10 days), and (D) daywise fertility of females mated to males exposed to 10 or 100μM DBP or their controls. We determined (E) overall percentage hatchability based on the proportion of eggs that reach adult stage and (F) at 24 h intervals for 10 days. The significant differences in fecundity, fertility and percentage hatchability between controls and exposed groups are denoted by *p < 0.05, **p < 0.01 or ***p < 0.001. All experiments were repeated three to four times (N = 15–20 males/mates per replicate/group).
Based on these findings, sperm counts and gene expression analyses have been performed at LOAEL concentration of DBP whereas biochemical analyses included 1mM DBP group in addition to 100μM DBP exposed males/mates (LOAEL group).
Altered Semen Quality in Males Exposed to DBP During Their Development
Seminal vesicles of males exposed to DBP during development contain significantly fewer sperm after successive matings
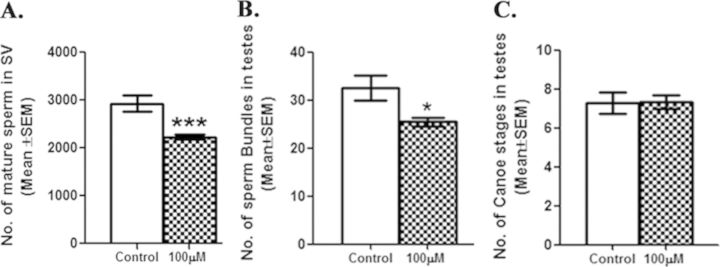
To determine the effect of DBP on spermatogenesis, number of mature sperm in the seminal vesicle, and late spermatogenesis stages such as sperm bundles and canoe stages in testes of control and exposed males were counted. Number of mature sperm and late spermatogenesis stages in unmated 3-day-old Protamine B-EGFP males exposed to 100μM DBP during development did not differ significantly from those in controls. However, after successive matings (potentially 10 matings), Protamine B-EGFP males, exposed to 100μM DBP during development, contained significantly fewer (p < 0.001) mature sperm in their seminal vesicles, when compared with those in controls (Fig. 3A). In addition, these males also had significantly fewer sperm bundles in their testes when compared with their controls (Fig. 3B). However, the number of canoe stages in these exposed males did not differ from those of controls (Fig. 3C).
FIG. 3.
Fewer mature sperm and late spermatogenesis stages in males developmentally exposed to DBP. To determine the effect of DBP on spermatogenesis, we counted the number of sperm in seminal vesicles and sperm bundles and canoe stages in testes of control and developmentally exposed w; P{Protamine B-EGFP} males after recurrent matings over 3 days followed by 48 h relaxation. The number of sperms in the seminal vesicle (A) and the number of sperm bundles in testes (B) of Protamine B-EGFP males exposed developmentally to 100μM DBP were significantly reduced (***p < 0.001; *p < 0.05) whereas the number of canoe stages (C) were similar to controls. All these experiments were carried out twice and sperm were counted in tissues twice with a repeatability index of 92% (N = 10–15/replicate/group).
Transcript levels of certain genes expressed in the reproductive tracts of males exposed to DBP are significantly reduced
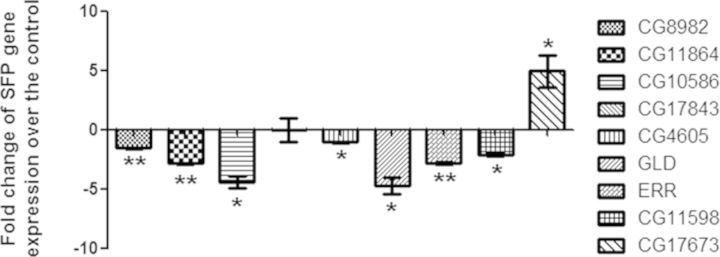
The expression profile of a few genes expressed in the male reproductive tract and/or encoding seminal fluid proteins was analyzed initially through semiquantitative PCR. Semiquantitative analysis of 31 genes in males exposed to 100μM DBP during development revealed that the transcript levels of six genes [CG8982 (ovulin, Acp26Aa), CG11864, CG11598, CG4605, glucose dehydrogenase (GLD), and estrogen related receptor (ERR)] were significantly lower (p < 0.05; Supplementary fig. 3) and transcript levels of CG17673 were significantly higher (p < 0.05; Supplementary fig. 3) in exposed males when compared with controls. However, the transcript levels of remaining 24 genes expressed in the male reproductive tract and/or encoding seminal proteins falling into varied conserved protein classes did not significantly differ from their controls (p > 0.05; Supplementary fig. 3).
Quantification of the transcript levels of CG8982, CG11864, CG4605, CG11598, GLD, CG17673, CG17843, ERR along with an additional candidate, seminase (CG10586), through qPCR (Fig. 4) confirmed the above observations. The expression of ovulin, CG11864, ERR, CG10586, CG11598, and GLD were down-regulated by 1.6 folds (p < 0.01), 2.9 folds (p < 0.01), 2.8 folds (p < 0.01), 4.5 folds (p < 0.05), 2.08 folds (p < 0.05), and 4.75 folds (p < 0.05), respectively, in 100μM DBP exposed males when compared with those in controls. Transcript levels of CG17843 did not significantly differ from controls in the qPCR whereas CG17673 was significantly up-regulated (p < 0.05; Fig. 4), as observed in the semiquantitative analysis.
FIG. 4.
Alteration of a few genes, expressed in the reproductive tract or encoding seminal proteins in males exposed to 100μM DBP during development. To validate the semiquantitative PCR data, we analyzed the transcript levels of candidate genes (Ovulin, CG11864, CG10586, CG4605, CG11598, CG17673, GLD, ERR, and CG17843) through real-time PCR. Ovulin, CG11864, CG10586, CG11598, GLD, and ERR were significantly down-regulated, and transcript levels of CG17673 were significantly higher when compared with control (*p < 0.05; **p < 0.01). The Δct values were determined through normalization against RPL32, which was used as an internal control for the quality of the template.
Ovulin protein levels are reduced in the accessory glands from ovulin-GFP transgenic males exposed to DBP
To determine if reduction in transcript levels is reflected at the protein level, transgenic males containing ovulin (37 kDa) tagged with GFP (26 kDa) under the control of ovulin promoter were exposed to 100μM or 1mM DBP during their development, as described above. The levels of ovulin-GFP in the male accessory glands were initially monitored based on the intensity of GFP fluorescence. In comparison to controls (Fig. 5A), males exposed to 100μM (p < 0.001) or 1mM DBP (p < 0.001) contained significantly reduced GFP fluorescence in their accessory glands (Figs. 5B and 5C), indicating significant reduction in ovulin levels even at the protein level (Fig. 5D).
FIG. 5.
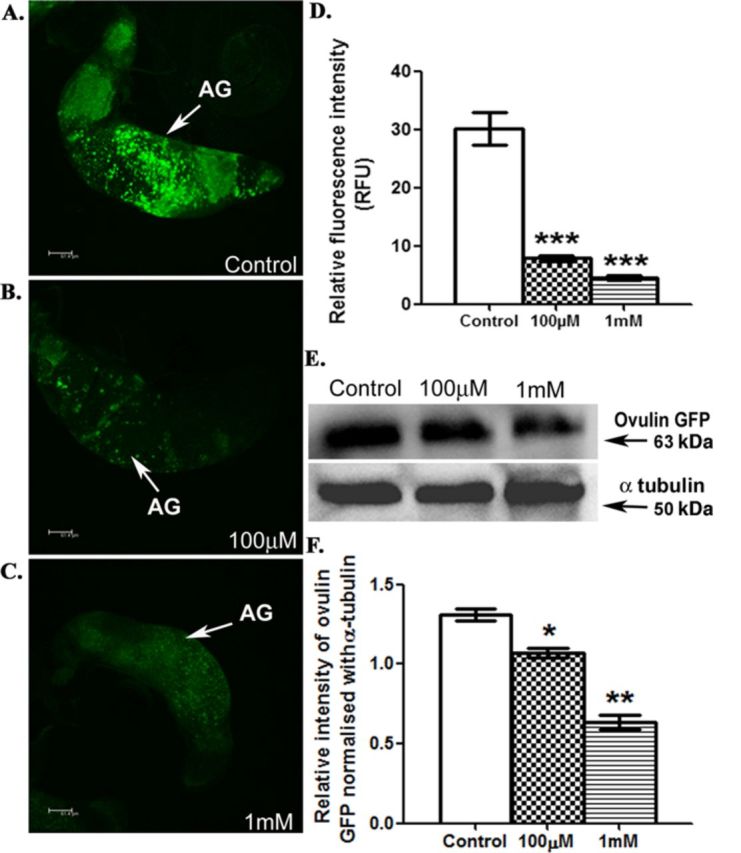
Reduced ovulin levels in the accessory glands of ovulin-GFP transgenic males developmentally exposed to DBP. To determine if changes in transcript levels are reflected at the protein level, we visualized the ovulin protein based on the GFP fluorescence in the accessory glands of (A) control, (B) males exposed to 100μM DBP, and (C) males exposed to 1mM DBP through confocal microscopy. Panel (D) indicates the significant reduction in the fluorescence intensity of ovulin-GFP, in 100μM DBP exposed males and 1mM DBP exposed males (***p < 0.001). Anti-GFP antibody detected ovulin-GFP (63 kDa) in protein samples from the male accessory glands of control and exposed males on Western blots (panel E, ovulin-GFP). Upon normalization of ovulin-GFP intensities with corresponding α-tubulin signals (panel E, α-tubulin), the levels of ovulin-GFP were found to be significantly reduced in males exposed to 100μM DBP (*p < 0.05) or 1mM DBP (**p < 0.01), when compared with those in control (panel F). The data depicted is the average of five replicates from as many independent blots.
Western blot analysis indicated similar reduction of ovulin at the protein level. The protein profile from the accessory glands of males harboring the ovulin-GFP chimera, showed reduced intensity for 63 kDa fraction corresponding to the ovulin-GFP in response to the chemical exposure, when compared with that in control (Fig. 5E, ovulin-GFP panel). Normalization of the signal intensities of ovulin-GFP, with those from α-tubulin (Fig. 5E, α-tubulin panel, as a loading control) indicated significant reduction of ovulin (18 or 51% in 100μM or 1mM DBP males, respectively) at the protein level in a concentration dependent manner (p < 0.05, p < 0.01 when exposed to 100μM, 1mM DBP, respectively; Fig. 5F).
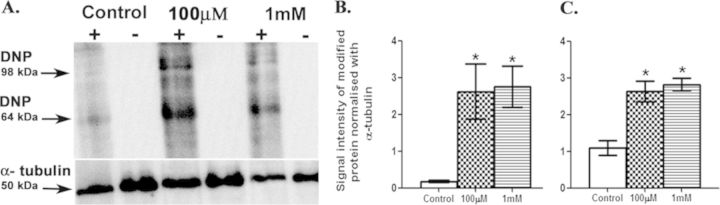
Oxidative modification of the reproductive tract proteins in males exposed to DBP
To determine if free radicals are generated in the reproductive tract in response to DBP, proteins from reproductive tracts of control as well as exposed males were probed for their oxidative modification using anti-DNP antibody. We detected two faint DNP-specific signals (at approximate molecular weight ranges of 98 and 64 kDa) in the reproductive tract protein samples from control (Fig. 6A, see “+” lane under control in the DNP panel). However, we detected similar fractions but with higher intensity in protein samples from the reproductive tracts of exposed organisms (Fig. 6A, see “+” lanes under 100μM and 1mM DBP lanes in the DNP panel). Comparison of the same upon normalization against a loading control (Fig. 6A, α-tubulin panel) and densitometry revealed a significant increase in the signal intensities of these protein fractions from exposed males (Figs. 6B and 6C; p < 0.05). The increased DNP signal intensity in exposed organisms suggests an escalation in the carbonylation of reproductive tract protein(s) in response to DBP.
FIG. 6.
Oxyblot of reproductive tract proteins from males exposed to DBP and their controls. The “+” lane represents protein samples wherein protein carbonyl functional groups were derivatized with DNPH (“+” lanes) whereas samples without DNPH served as derivatization controls (“–” lanes) for the corresponding batches. These samples were probed with anti-DNP antibody (panel A, DNP). Western blot of α-tubulin (panel A, α-tubulin) served as the loading control. Analysis of signal intensities of 98 and 64 kDa through densitometry, after normalization with loading control, revealed a dose-dependent increase in the extent of oxidative modification of 98 (panel B; *p < 0.05) and 64 kDa (panel C; *p < 0.05) in males exposed to 100μM or 1mM DBP when compared with controls. The data depicted is the average of three replicates from a minimum of three independent blots.
Exposure to DBP Affects the Expression as well as Activity of a Nuclear Receptor in Drosophila Males
As described above, our transcript analysis has shown reduced transcript levels of ERR in exposed males. To further determine if DBP exposure affects the down-stream functions of ERR, we analyzed the activity of ERR through X-gal staining of reproductive tissues from hs-GAL4-ERR.LBD; UAS-LacZ males (containing heat shock inducible GAL4 DNA binding domain fused to ERR ligand binding domain (LBD)). We observed X-gal positive cells in testes and seminal vesicles of males from control group harboring UAS-LacZ under the transcriptional regulation of hs-GAL4- ERR.LBD (Fig. 7A, control panel). However, we observed a dose-dependent decline in the staining pattern as well as intensity of the stain in males exposed to 100μM (Fig. 7B) or 1mM DBP (Fig. 7C). The number of spots (Fig. 7D) and the cumulative intensity (Fig. 7E) of the staining pattern in reproductive tracts of males exposed to 100μM (p < 0.01) or 1mM (p < 0.001) DBP were significantly lower when compared with those in control males, indicating the effect of DBP on ERR.LBD-mediated transcriptional activation.
FIG. 7.
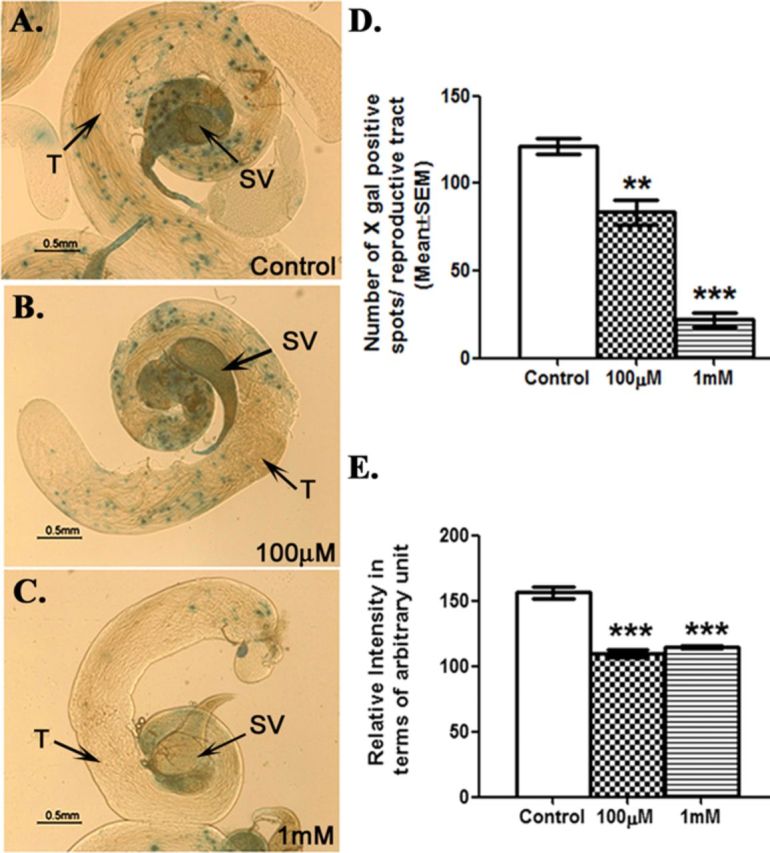
Exposure to DBP modulates the activity of estrogen related receptor (ERR) in Drosophila. Spatial patterns of ERR activity were visualized through X-gal stainingof male reproductive tract tissues from (A) control males, (B) males exposed to 100μM DBP, and (C) males exposed to 1mM DBP. Panel (D) represents the number of X-gal positive spots observed in reproductive tissues of males exposed to 100μM (**p < 0.01), and 1mM (***p < 0.001) in comparison to controls. The cumulative intensities of X-gal spots observed in the reproductive tracts of males exposed to 100μM or 1mM in comparison to control (***p < 0.001) are represented in panel (E). All experiments were repeated three times with 10–15 replicates per group.
Exposure to DBP Influences the Fate of Male Reproductive Molecules in Drosophila
Females mated to males developmentally exposed to DBP contain significantly fewer sperm in their sperm storage organs
To determine if the sperm from males exposed to DBP during development are stored in mated females at levels similar to control, Protamine-B EGFP males (3 days old), control or exposed, were allowed to mate with females in three broods and an additional female. The sperm stored in the mated females’ sperm storage organs, namely seminal receptacle and paired spermathecae, were counted, at 2 h ASM in I, IV, VII, and X mates from all the broods of both control and exposed groups. Interestingly, the number of sperm stored in seminal receptacles and/or spermathecae of first mates of exposed males significantly declined with the increasing mate order at 2 h ASM, when compared with their controls (I mate: p < 0.01, Figs. 8A and 8B; IV mate: p < 0.01, Figs. 8C and 8D; VII mate: p < 0.05, Figs. 8E and 8F; X mate: p < 0.05, Figs. 8G and 8L).
FIG. 8.
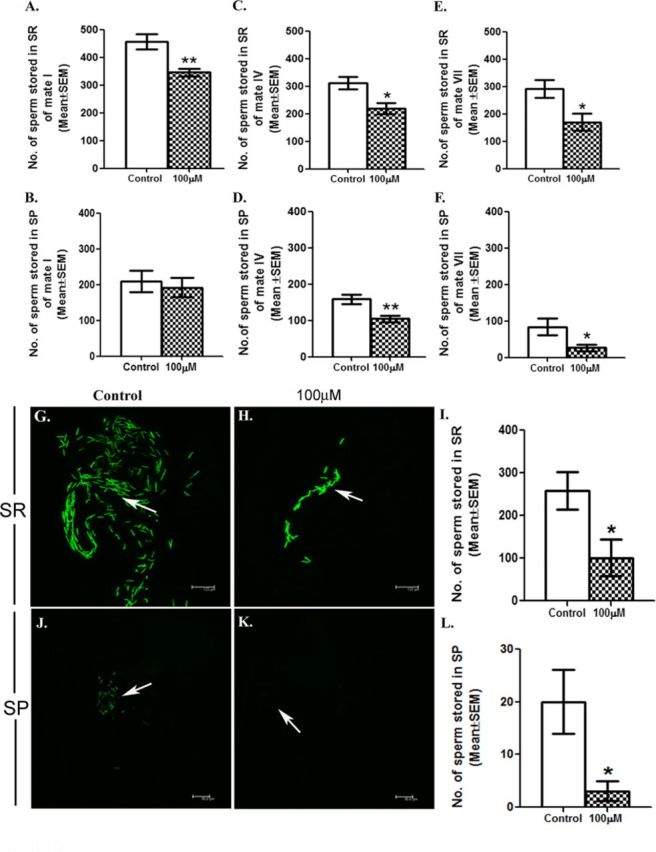
Exposure to DBP influences the fate of sperm in Drosophila. To determine the effect of DBP on the fate of sperm in the female milieu, each of the control or exposed Protamine B-EGFP male was presented with broods of three virgin females every 24 h for 3 days. The first mated female of brood1 (considered as mate I) of exposed male, had significantly fewer sperm in SR (seminal receptacle; A) at 2 h ASM whereas the sperm counts in SP (spermathecae; B) did not differ from those in mate I of control males. However, mate IV (from brood 2) and mate VII (from brood 3) of male exposed to 100μM DBP contained fewer sperms in both seminal receptacle (C and E) and spermathecae (D and F) when compared with those mates in control broods (*p < 0.05; **p < 0.01; N = 10–15/replicate). In addition, sperm in seminal receptacles (SR) from X mates of control (G) or exposed (H) Protamine B-EGFP males were counted and we observed significant reduction in the number of sperms in seminal receptacle of females mated to Protamine B-EGFP males, developmentally exposed to 100μM in comparison to control (*p < 0.05; N = 10–15; panel I). Panels (J) and (K) depict sperm in the spermathecae of X mates of control or exposed Protamine B-EGFP males, respectively. Panel (L) presents the number of sperm stored in spermathecae of females mated to exposed males, when compared with their controls (*p < 0.05; N = 10–15/replicate).
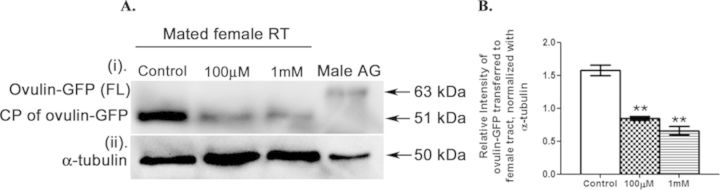
Females mated to DBP exposed males contain low levels of ovulin, a seminal protein, in their reproductive tracts at 1 h ASM
Western blotting of protein samples from reproductive tract samples of females with anti-GFP antibody detected reduced levels of ovulin-GFP in mates of exposed males (Fig. 9A; lanes 100μM or 1mM in ovulin-GFP panel) when compared with those from controls (Fig. 9A; lane control in ovulin-GFP panel) at 1 h ASM. We observed 46 or 58% reduction (p < 0.01) in ovulin levels, in 100μM or 1mM DBP mates, respectively, when compared with controls after normalization of ovulin-GFP intensities with those of α-tubulin (Fig. 9A; α-tubulin panel as loading control) through densitometry (Fig. 9B).
FIG. 9.
Ovulin in the reproductive tracts of females mated to DBP exposed males or their controls. Panel (A) represents the Western blot showing ovulin-GFP (tagged with GFP) at 1 h ASM in the reproductive tracts of females, which received seminal proteins and sperm from ovulin-GFP control males (lane control), or from males developmentally exposed to either 100μM (lane 100μM) or 1mM (lane 1mM). The blots were probed with α-tubulin (α-tubulin) as control for protein loading. Semi-quantitative analysis through densitometry revealed lower levels of ovulin in the female tracts of those mated to males exposed developmentally either to 100μM or 1mM DBP (**p < 0.01), when compared with control (panel B). The data depicted is the average of three replicates from a minimum of three independent blots.
DISCUSSION
The study was designed to develop Drosophila as a model for the assessment and understanding of male reproductive adversities by defining the organismal, biochemical, molecular as well as endocrine signatures typical to male reproductive toxicity. Male-specific endpoints of reproductive toxicity assessment in higher organisms include fertility of the female mated to exposed male, semen quality, anomalies related to development of testes, sperm production, and organ weight (Guidelines for Reproductive Toxicity Risk Assessment, 1996). Given the homology of Drosophila, in the context of male reproduction, to higher organisms (Pandey and Nichols, 2011), we hypothesized that exposure of Drosophila to a model reproductive toxicant, DBP, would result in similar endpoints. In vertebrates and mammals, adult and/or developmental exposure to DBP was shown to reduce fertility, induce testicular lesions, alter testosterone levels and cause damage to sertoli/leydig cells (Shen et al., 2011). The observed reduction in the fertility of females mated to exposed males in the present study, irrespective of the window of exposure, suggests that even in Drosophila, adult or developmental exposure leads to adverse male reproductive consequences analogous to those in higher organisms. Moreover, the observed magnitude of reduction in the fertility with a LOAEL at 1/620 of LC50 suggests that male reproductive components are more vulnerable to developmental exposure than adult exposure to DBP. The developmental exposure window encompasses gonadal development, spermatogenesis, (which starts as early as 24 h after fertilization; Hennig, 1996) and transcription of genes encoding seminal proteins (initiated during pupal stage; Chapman et al., 1995). Therefore, we focused on males exposed to DBP during their development to determine the Drosophila-based endpoints of male reproductive toxicity.
Exposure to DBP in higher organisms is known to alter the quality of semen (sperm and seminal proteins), which is critical for male fertility (Dobrzynska et al., 2011). Even in Drosophila, fertility of mated females depends on the receipt of sperm and seminal proteins (Chapman and Davies, 2004). Therefore, we analyzed two major constituents of Drosophila semen, namely sperm, and seminal fluid proteins in males exposed to DBP at the level of production in males. The observed significant reduction in the number of sperm in seminal vesicles and sperm bundles in testes of males after multiple matings suggested that exposure to DBP reduces sperm counts and hampers spermatogenesis in males, analogous to that in higher organisms (Pant et al., 2008). In addition, exposure to phthalates is known to alter the expression of more than 400 genes in various cell types of fetal rat testes (Liu et al., 2005). In the present study, our observation of significant down-/up-regulation of eight genes expressed in the reproductive tract and/or encoding seminal proteins in Drosophila males exposed to DBP, indicates that like in mammals, exposure to DBP alters the expression of genes in the reproductive tract of Drosophila. Interestingly, we observed down-regulation of CG8982 (Ovulin), CG10586 (seminase), and CG11864 (a predicted astacin-type metalloprotease), all three of which are members of the regulated proteolytic cascade reported in Drosophila seminal fluids (LaFlamme et al., 2012). Regulated proteolysis in seminal fluids influences fertility in both Drosophila and mammals (Laflamme et al., 2014). In mammals, the regulated proteolysis involving prostate-specific antigen (PSA) and the protease inhibitor PCI controls the liquefaction of semen (Suzuki et al., 2007), which in turn is used as a critical parameter for the assessment of semen quality in mammals. Therefore, the reduced expression of members of proteolysis network of Drosophila seminal fluids of males exposed to DBP may point to hampered seminal proteolytic events, in a manner analogous to delayed liquefaction of semen in higher organisms exposed to DBP. Another evidence of alteration at the molecular level in response to DBP in the present study stems from the observation of oxidative modification of proteins in the male reproductive tracts, similar to that observed in response to oxidative stress generated by the exposure to DBP in mammals (Zhou et al., 2011).
Interestingly, in higher organisms, disruption of endocrine function is another facet to DBP-mediated male reproductive toxicity. DBP has the potential to interact with nuclear receptors (NRs) such as CAR, PXR, PPARα, β, and γ, which in turn are involved in steroidal metabolism (Lapinskas et al., 2005). In the present study, significant down-regulation of dERR, a nuclear receptor, in response to DBP exposure, prompted us to analyze the activity of dERR in response to DBP. In Drosophila, dERR is the only ERR isoform with highest sequence similarity to ERRβ of the three ERRs in vertebrates (Giguere, 1999). We analyzed the activity of dERR using males that express chimeric dERR (comprising ERR ligand binding domain and GAL4 activation domain) and permit the assay of ERR function through reporter gene (LacZ) expression (Palanker et al., 2006). Significantly reduced dERR activity as evidenced by a dose-dependent decline in the intensity as well as number of X-gal positive spots in testes and seminal vesicles of hs-GAL4 ERR.LBD; UAS-LacZ males exposed to DBP and heat-shock, suggests likely disruption of endocrine function in Drosophila in response to developmental exposure to DBP. The decline in the activity of dERR might be a consequence of interaction of DBP with either the ligand binding domain of the receptor or the internal ligand itself. However, at present, we do not know if the observed decline in the activity of dERR is a direct or indirect consequence of exposure to DBP. Nevertheless, our results from transcriptional and biochemical analysis do indicate the interference of DBP at some level of dERR expression as well as the activity, likely affecting the normal functioning of the ERR in the male reproductive milieu of Drosophila. In addition, our finding suggests dERR as a potential candidate to understand the mechanisms underlying chemical-mediated endocrine disruption. Our finding supports the proposal of Hirsch et al. (2010) to use Drosophila as a model system to study endocrine disruption.
The present study using Drosophila also facilitated the toxicity assessment on the fate of male reproductive molecules. In Drosophila, sperm, upon their transfer to mated females, are stored in two sperm storage organs, namely seminal receptacle and paired spermathecae (Bloch Qazi et al., 2003). Our observation of fewer sperm in the storage organs of females mated to exposed males points to the chemical effect on the fate of sperm. Fewer sperm in storage might be a consequence of reduced transfer of sperm to females by the exposed males due to reduced sperm counts in males exposed to DBP. Alternatively, reduced sperm motility due to exposure to DBP, as in mammals, might also result in fewer sperm reaching the storage organs. However, we observed sperm being motile under fluorescence microscope but could not measure the efficiency of sperm motility due to technical limitations. Nevertheless, fewer sperm in storage reflect the observed reduction in the fertility of females mated to males exposed to DBP. Similarly, we observed reduced levels of a seminal protein, namely ovulin, in females, which have received seminal fluids from DBP exposed males. The reduced level of ovulin in mated females might be due to significantly lower production of ovulin protein observed in male and consequently less ovulin protein being transferred to the female. However, the difference in the observed magnitude of reduction in the synthesis (18%) and transfer (46%) of ovulin in 100μM exposed males/mates, indicates the effect of DBP both on the synthesis of ovulin within the male tract, as well as its transfer to the mated female. Ovulin is essential for the enhancement of ovulation and egg laying of mated females in the first 24 h ASM (Heifetz et al., 2000). Therefore, the observed reduction in the fecundity of mates of DBP exposed males at 24 h ASM, might be a consequence of reduced transfer/receipt of ovulin.
The mechanisms underlying the actions of DBP on male reproduction in Drosophila are likely to be complex and in part might parallel some of the key events associated with mode of action proposed in mammals. In rats, DBP is hydrolyzed to more active metabolite, MBP and exerts antiandrogenic effects through repression of genes involved in cholesterol transportation into mitochondria and steroidogenic enzyme activities in the fetal testis in a PPARα (peroxisome proliferator-activated receptor)-dependent manner (Plummer et al., 2013) leading to reduction in testicular testosterone production and subsequent testicular dysgenesis. In Drosophila, presence of MBP in males exposed to DBP and the reproductive toxicity of MBP even at a concentration 10 times lower than that of DBP are analogous to those events reported in mammals and thus validate Drosophila as an alternative model for screening of chemicals. However, there is no convincing ortholog for PPAR in D. melanogaster (King-Jones and Thummel, 2005). At this juncture, it is appropriate to note that ERRs are implicated in somatic PPAR signaling in mammals (Huss et al., 2004). Together, these may point to an analogous role for ERR (in the absence of PPAR) in DBP-mediated reproductive adversities in Drosophila but further studies are required to decipher the functional as well as evolutionary significance of ERR in DBP-mediated male reproductive adversity.
Apart from reproductive toxicity, DBP is reported to be neurotoxic in mammals (Li et al., 2013). Bis(2-ethylhexyl) phthalate (DEHP), another phthalate ester, is known to modulate the cholinergic synaptic transmission in Drosophila, probably by inhibiting the calcium channel activities (Ran et al., 2012). In this context, the neural circuitry underlying male sexual behavior (Stockinger et al., 2005) and cholinergic neuronal control of seminal emissions in Drosophila (Acebes et al., 2004), raise the possibility of observed reproductive adversities of DBP, in part, being mediated through its neurotoxicity. Although normal mating behavior of DBP exposed males observed in the present study negates such a possibility at the tested concentrations, future studies on neuroendocrine regulation of seminal emissions in the context of chemical exposure would help to decipher the same.
To conclude, developmental exposure to DBP or MBP hampers the male reproductive performance of Drosophila, the latter being more toxic, as in higher organisms. Analogous to those observed in mammals exposed to DBP, the observed reduction in the male reproductive performance is a consequence of reduced semen quality as exemplified by reduced sperm counts, reduced levels of seminal proteins (at the scale of transcripts/protein), and oxidative modification/ damage to the reproductive tract proteins. Further, altered expression and the activity of dERR suggest endocrine disruption in lower organisms when exposed to DBP. Moreover, exposure to DBP modulates the fate of sperm and seminal fluid proteins during/after transfer from males to females. These findings reflect the potential of Drosophila for quick and first-tier screening of innumerable environmental chemicals on their ability to hamper male fertility. In addition, Drosophila being an insect, our findings are also relevant to economically beneficial insects (Huang et al., 1999) and to design pest-control strategies. Finally, Drosophila has the potential to serve as an excellent model toward understanding chemical-mediated endocrine disruption.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Council for Scientific and Industrial Research (CSIR), New Delhi (BSC0103 to K.R.R., M.K.R.M. and fellowship to S.M.); University Grants Commission (UGC), New Delhi (GAP 155 to V.S. and C.H.R.); Science and Engineering Research Board (SERB), New Delhi (SR/FT/LS-133/2009 to K.R.R.).
AUTHOR CONTRIBUTIONS
S.M. and K.R.R. designed the experiments. S.M., A.S., C.H.R., V.S., and M.K.R.M. carried out experiments. S.M., A.S., C.H.R., M.K.R.M., and K.R.R. analyzed the results. All authors wrote and reviewed the manuscript.
Supplementary Material
Acknowledgments
We thank the Director, CSIR-IITR, Head, Embryotoxicology, CSIR-IITR for providing the research facility, Prof. John Belote, Department of Biological Sciences, University of Syracuse, Syracuse, NY, USA, for generously providing w;P{Protamine B-EGFP}flies, and Prof. M.F. Wolfner, Department of Molecular Biology and Genetics, Cornell University, Ithaca, NY, USA for providing Ovulin-GFP flies. We thank the anonymous reviewers for their invaluable suggestions in improving this manuscript.
REFERENCES
- Acebes A., Grosjean Y., Everaerts C., Ferveur J. F. Cholinergic control of synchronized seminal emissions in Drosophila. Curr. Biol. 2004;14:704–710. doi: 10.1016/j.cub.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Aitken R. J., Smith T. B., Jobling M. S., Baker M. A., De Iuliis G. N. Oxidative stress and male reproductive health. Asian J. Androl. 2014;16:31–38. doi: 10.4103/1008-682X.122203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila F. W., Sirot L. K., LaFlamme B. A., Rubinstein C. D., Wolfner M. F. Insect seminal fluid proteins: Identification and function. Annu. Rev. Entomol. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch Qazi M. C., Heifetz Y., Wolfner M. F. The developments between gametogenesis and fertilization: Ovulation and female sperm storage in Drosophila melanogaster. Dev. Biol. 2003;256:195–211. doi: 10.1016/s0012-1606(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Carlsen E., Giwercman A., Keiding N., Skakkebaek N. E. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T., Davies S. J. Functions and analysis of the seminal fluid proteins of male Drosophila melanogaster fruit flies. Peptides. 2004;25:1477–1490. doi: 10.1016/j.peptides.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Chapman T., Liddle L. F., Kalb J. M., Wolfner M. F., Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- Dobrzynska M. M., Tyrkiel E. J., Pachocki K. A. Developmental toxicity in mice following paternal exposure to Di-N-butyl-phthalate (DBP) Biomed. Environ. Sci. 2011;24:569–578. doi: 10.3967/0895-3988.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Fischer B. E., Wasbrough E., Meadows L. A., Randlet O., Dorus S., Karr T. L., Russell S. Conserved properties of Drosophila and human spermatozoal mRNA repertoires. Proc. Biol. Sci. 2012;279:2636–2644. doi: 10.1098/rspb.2012.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. M. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int. J. Androl. 2006;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Giguere V. Orphan nuclear receptors: From gene to function. Endocr. Rev. 1999;20:689–725. doi: 10.1210/edrv.20.5.0378. [DOI] [PubMed] [Google Scholar]
- Gilchrist A. S., Partridge L. Male identity and sperm displacement in Drosophila melanogaster. J. Insect Physiol. 1995;41:1087–1092. [Google Scholar]
- Hartung T. Food for thought… on cell culture. Altex. 2007;24:143–152. doi: 10.14573/altex.2007.3.143. [DOI] [PubMed] [Google Scholar]
- Heifetz Y., Lung O., Frongillo E. A., Jr, Wolfner M. F. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr. Biol. 2000;10:99–102. doi: 10.1016/s0960-9822(00)00288-8. [DOI] [PubMed] [Google Scholar]
- Hennig W. Spermatogenesis in Drosophila. Int. J. Dev. Biol. 1996;40:167–76. [PubMed] [Google Scholar]
- Hirsch H. V. B., Possidente D., Possidente B. Pb2+: An endocrine disruptor in Drosophila. Behav. Physiol. 2010;99:254–259. doi: 10.1016/j.physbeh.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Huang G. L., Sun H. W., Song Z. H. Interactions between dibutyl phthalate and aquatic organisms. Bull. Environ. Contam. Toxicol. 1999;63:759–765. doi: 10.1007/s001289901044. [DOI] [PubMed] [Google Scholar]
- Huss J. M., Torra I. P., Staels B., Giguere V., Kelly D. P. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol. Cell. Biol. 2004;24:9079–9091. doi: 10.1128/MCB.24.20.9079-9091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb J. M., DiBenedetto A. J., Wolfner M. F. Probing the function of Drosophila melanogaster accessory glands by directed cell ablation. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8093–8097. doi: 10.1073/pnas.90.17.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Jones K., Thummel C. S. Nuclear receptors—a perspective from Drosophila. Nat. Rev. 2005;6:311–323. doi: 10.1038/nrg1581. [DOI] [PubMed] [Google Scholar]
- Knez J. Endocrine-disrupting chemicals and male reproductive health. Reprod. Biomed. Online. 2013;26:440–448. doi: 10.1016/j.rbmo.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Laflamme B. A., Avila F. W., Michalski K., Wolfner M. F. A Drosophila protease cascade member, seminal metalloprotease-1, is activated stepwise by male factors and requires female factors for full activity. Genetics. 2014;196:1117–1129. doi: 10.1534/genetics.113.160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFlamme B. A., Ram K. R., Wolfner M. F. The Drosophila melanogaster seminal fluid protease “seminase” regulates proteolytic and post-mating reproductive processes. PLoS Genet. 2012;8:e1002435. doi: 10.1371/journal.pgen.1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapinskas P. J., Brown S., Leesnitzer L. M., Blanchard S., Swanson C., Cattley R. C., Corton J. C. Role of PPARalpha in mediating the effects of phthalates and metabolites in the liver. Toxicology. 2005;207:149–163. doi: 10.1016/j.tox.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Li X. J., Jiang L., Chen L., Chen H. S., Li X. Neurotoxicity of dibutyl phthalate in brain development following perinatal exposure: A study in rats. Environ. Toxicol. Pharmacol. 2013;36:392–402. doi: 10.1016/j.etap.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Liu K., Lehmann K. P., Sar M., Young S. S., Gaido K. W. Gene expression profiling following in utero exposure to phthalate esters reveals new gene targets in the etiology of testicular dysgenesis. Biol. Reprod. 2005;73:180–192. doi: 10.1095/biolreprod.104.039404. [DOI] [PubMed] [Google Scholar]
- Lung O., Wolfner M. F. Drosophila seminal fluid proteins enter the circulatory system of the mated female fly by crossing the posterior vaginal wall. Insect Biochem. Mol. Biol. 1999;29:1043–1052. doi: 10.1016/s0965-1748(99)00078-8. [DOI] [PubMed] [Google Scholar]
- Manier M. K., Belote J. M., Berben K. S., Novikov D., Stuart W. T., Pitnick S. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science. 2010;328:354–357. doi: 10.1126/science.1187096. [DOI] [PubMed] [Google Scholar]
- Mikhaylova L. M., Nguyen K., Nurminsky D. I. Analysis of the Drosophila melanogaster testes transcriptome reveals coordinate regulation of paralogous genes. Genetics. 2008;179:305–315. doi: 10.1534/genetics.107.080267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell D. J., Rorth P., Spradling A. C. slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP. Cell. 1992;71:51–62. doi: 10.1016/0092-8674(92)90265-e. [DOI] [PubMed] [Google Scholar]
- Mueller J. L., Linklater J. R., Ravi Ram K., Chapman T., Wolfner M. F. Targeted gene deletion and phenotypic analysis of the Drosophila melanogaster seminal fluid protease inhibitor Acp62F. Genetics. 2008;178:1605–1614. doi: 10.1534/genetics.107.083766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller J. L., Ripoll D. R., Aquadro C. F., Wolfner M. F. Comparative structural modeling and inference of conserved protein classes in Drosophila seminal fluid. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13542–13547. doi: 10.1073/pnas.0405579101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylchreest E., Cattley R. C., Foster P. M. Male reproductive tract malformations in rats following gestational and lactational exposure to Di(n-butyl) phthalate: An antiandrogenic mechanism. Toxicol. Sci. 1998;43:47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- Palanker L., Necakov A. S., Sampson H. M., Ni R., Hu C., Thummel C. S., Krause H. M. Dynamic regulation of Drosophila nuclear receptor activity in vivo. Development. 2006;133:3549–3562. doi: 10.1242/dev.02512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey U. B., Nichols C. D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011;63:411–436. doi: 10.1124/pr.110.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant N., Shukla M., Kumar Patel D., Shukla Y., Mathur N., Kumar Gupta Y., Saxena D. K. Correlation of phthalate exposures with semen quality. Toxicol. Appl. Pharmacol. 2008;231:112–116. doi: 10.1016/j.taap.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Plummer S. M., Dan D., Quinney J., Hallmark N., Phillips R. D., Millar M., Macpherson S., Elcombe C. R. Identification of transcription factors and coactivators affected by dibutylphthalate interactions in fetal rat testes. Toxicol. Sci. 2013;132:443–457. doi: 10.1093/toxsci/kft016. [DOI] [PubMed] [Google Scholar]
- Ran D., Cai S., Wu H., H., G. Di (2-ethylhexyl) phthalate modulates cholinergic mini-presynaptic transmission of projection neurons in Drosophila antennal lobe. Food Chem. Toxicol. 2012;50:3291–3297. doi: 10.1016/j.fct.2012.03.070. [DOI] [PubMed] [Google Scholar]
- Ravi Ram K., Ji S., Wolfner M. F. Fates and targets of male accessory gland proteins in mated female Drosophila melanogaster. Insect Biochem. Mol. Biol. 2005;35:1059–1071. doi: 10.1016/j.ibmb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Ravi Ram K., Wolfner M. F. A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 2009;106:15384–15389. doi: 10.1073/pnas.0902923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland M., Le Moal J., Wagner V., Royere D., De Mouzon J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum. Reprod. 2012;28:462–470. doi: 10.1093/humrep/des415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe R. M. Hormones and testis development and the possible adverse effects of environmental chemicals. Toxicol. Lett. 2001;120:221–232. doi: 10.1016/s0378-4274(01)00298-3. [DOI] [PubMed] [Google Scholar]
- Shen O., Wu W., Du G., Liu R., Yu L., Sun H., Han X., Jiang Y., Shi W., Hu W., et al. Thyroid disruption by di-n-butyl phthalate (DBP) and mono-n-butyl phthalate (MBP) in Xenopus laevis. PLoS One. 2011;6:e19159. doi: 10.1371/journal.pone.0019159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique H. R., Chowdhuri D. K., Saxena D. K., Dhawan A. Validation of Drosophila melanogaster as an in vivo model for genotoxicity assessment using modified alkaline Comet assay. Mutagenesis. 2005;20:285–290. doi: 10.1093/mutage/gei032. [DOI] [PubMed] [Google Scholar]
- Stockinger P., Kvitsiani D., Rotkopf S., Tirian L., Dickson B. J. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kise H., Nishioka J., Hayashi T. The interaction among protein C inhibitor, prostate-specific antigen, and the semenogelin system. Semin. Thromb. Hemost. 2007;33:46–52. doi: 10.1055/s-2006-958461. [DOI] [PubMed] [Google Scholar]
- Zhou D., Wang H., Zhang J. Di-n-butyl phthalate (DBP) exposure induces oxidative stress in epididymis of adult rats. Toxicol. Ind. Health. 2011;27:65–71. doi: 10.1177/0748233710381895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.